Abstract
Leptospirosis is a neglected zoonosis caused by pathogenic Leptospira. In this study, we characterized the virulence of isolate B3-13S obtained from a wild mouse (Mus musculus) captured in New Caledonia, subsequently identified as a bacterium belonging to the L. borgpetersenii serogroup Ballum. Hamsters were infected with an intraperitoneal injection of 2 × 108 bacteria, resulting in severe histopathological organ damages consistent with tissue lesions previously observed with other strains. Hamsters were also injected with 1 × 108 or 5 × 107 bacteria and animals that recovered showed renal carriage of leptospires in concentrations similar to the bacterial load quantified in mouse kidneys, with urinary shedding of bacteria up to 4 weeks postinfection. The serogroup Ballum is increasingly reported in human leptospirosis, and these results highlight the use of the B3-13S isolate for the development of models resulting in either severe acute or chronic forms of the infection, allowing for better characterization of its pathogenesis.
Leptospirosis is a widespread zoonosis caused by pathogenic spirochetes of the genus Leptospira. Severe forms in humans include multiple organ failures with renal, hepatic, and pulmonary failures, possibly leading to death. Pathogens are transmitted to humans by direct or indirect contact with contaminated urine from mammalian reservoir hosts.
Although rats and mice are major maintenance hosts, they present with mostly asymptomatic infections and with subsequent elimination of the pathogens from all organs except the kidneys.1 Indeed, bacteria levels are maintained through chronic infection of the renal proximal tubules, although bacteria are subsequently shed through urine for the entire lifetime of the animal.2 Different host–serovar associations seem ubiquitous as typically observed for rats (commonly Rattus norvegicus and R. rattus) with serovars Icterohaemorrhagiae and Copenhageni, and for mice (Mus musculus and other Mus species) with the serogroup Ballum.3 Icterohaemorrhagiae is the serogroup most often implicated in human infection.4 However, epidemiological studies in the Caribbean and Pacific regions suggest an increased contribution of the serogroup Ballum to human leptospirosis on a level comparable to that of Icterohaemorrhagiae serogroup.5,6 In New Caledonia, leptospires of the Ballum serogroup are involved in less than 5% of human cases,7 with mice comprising the main asymptomatic carrier of this serogroup.8 Herein, using histological and molecular techniques, we characterized both severe acute and chronic infections in the golden Syrian hamster with the strain B3-13S of L. borgpetersenii serogroup Ballum, isolated from a wild mouse captured in New Caledonia.8
OF1 mice (Mus musculus) and golden Syrian hamsters (Mesocricetus auratus), whose genitors originated from Charles River Laboratories, were bred at Institut Pasteur in New Caledonia. Animal manipulations were conducted according to the guidelines of the Animal Care and Use Committees of the Institut Pasteur and followed European Recommendation 2007/526/EC.
The B3-13S isolate was obtained from the Ellinghausen–McCullough–Johnson–Harris (EMJH)-culture from the kidneys of a wild mouse (M. musculus) in 2009 in New Caledonia, and was subsequently characterized as L. borgpetersenii belonging to serogroup Ballum.8 Bacteria were cultured in a liquid EMJH medium as previously described.9 Cell concentration was determined using a Petroff–Hausser counting chamber (Hausser Scientific, Horsham, PA). Virulence of bacteria was maintained by intraperitoneally injecting hamsters, and reisolating leptospires from the blood.
Hamsters 6- to 8-week-old were injected with three doses of B3-13S (5 × 107, 1 × 108, or 2 × 108 bacteria, determined after preliminary tests) to reproduce acute or chronic infection. Mice were injected with 1 × 108 B3-13S to produce typical chronic infection. For ethical reasons, nonreactive animals were considered dead and euthanized by atlanto-occipital dislocation after anesthesia with chloroform. For chronic experimental infection, animals were euthanized at 21 or 28 days postinfection.
After dissection, we conducted gross macroscopic examination of the organs. For histological observations, organs were harvested and fixed in 10% neutral buffered formalin, and tissues were then stained by hematoxylin-erythrosin (HE), Masson's trichrome, or Alcian Blue staining. Leptospires were visualized after silver impregnation following the Warthin–Starry (WS) protocol modified with pyrocatechol.10
Total DNA was extracted from samples using QIAamp DNA Mini Kit (Qiagen GmbH, Hilden, Germany). For renal tissue, 25 mg samples were placed into MagNA Lyser Green Bead tubes (Roche Applied Sciences, Auckland, New Zealand) containing 360 μL lysis buffer, and disrupted and homogenized using the MagNA Lyser Instrument (Roche Diagnostic Ltd., Rotkreuz, Switzerland). Urinary excretion of leptospires was evaluated in urine collected from the bladder or by collecting pieces of wood shaving in cages of B3-13S-infected animals at 21 or 28 days postinfection. Shaving samples were vortexed into 2 mL phosphate buffered saline (PBS). Following the proteinase K incubation step, and after washing, the eluted DNA was quantified by spectrophotometry (NanoDrop 2000; ThermoFisher, Wilmington, DE). PCR targeting the lfb1 gene11 was carried out on a LightCycler 2.0 using the LightCycler FastStart DNA Master SYBR Green I (Roche Applied Science) with primers purchased from Eurogentec (Seraing, Belgium). A standard curve obtained from serial 10-fold dilutions of known numbers of leptospires was used for absolute quantification. Results were expressed as the number of Leptospira genome equivalents per microgram of DNA extracted from kidney tissue. Statistical studies were performed using GraphPad Prism v4 (GraphPad Software Inc., La Jolla, CA) to analyze the difference in bacterial load between animals (unpaired t test).
Hamsters were infected with twofold serial dilutions of 2 × 108 (N = 17 individuals), 1 × 108 (N = 17), or 5 × 107 (N = 4) virulent B3-13S, and survival was monitored until 21 days. Fatal outcome was observed for all hamsters within 4–6 days after inoculation with 2 × 108 bacteria, whereas 41% and 50% animals survived until 21 days after infection with 1 × 108 and 5 × 107 bacteria, respectively.
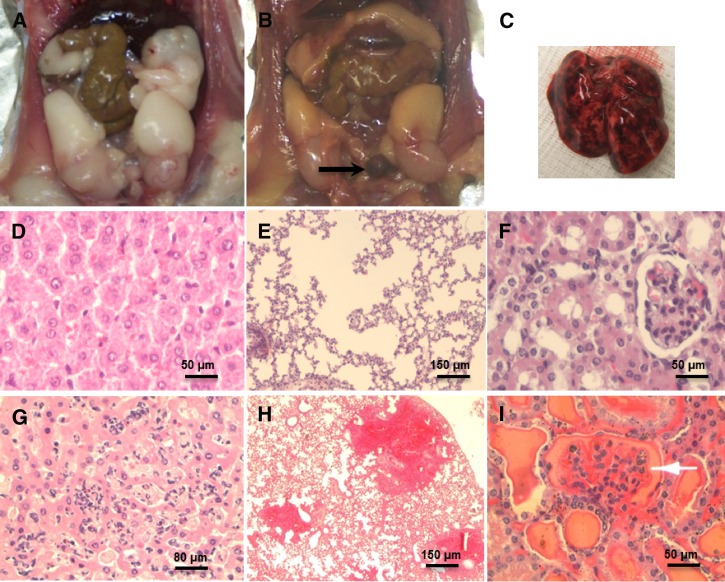
Tissue lesions of hamsters lethally infected with B3-13S were then examined. Necropsy revealed icterus and blood in the urinary bladder (Figure 1B). Massive hemorrhages in the respiratory tract and pulmonary tissues were also observed in some hamsters (Figure 1C). Sinusoidal congestion and infiltration of inflammatory cells in liver of infected hamsters associated with hepatocytic necrosis were observed (Figure 1G). Alveolar edema and foci of intensive hemorrhages were observed in lungs of infected animals (Figure 1H). Diffuse congestion and intense hemorrhages in renal tubules and glomeruli of hamsters were also seen at day 5 postinfection (Figure 1I).
Figure 1.
Acute lesions in hamsters experimentally infected with 2 × 108 B3-13S. (A) Peritoneal cavity of a control hamster. (B) Icterus with yellowish adipose tissues and blood accumulation in the bladder (arrow) of hamster deceased 5 days after B3-13S infection. (C) Massive pulmonary hemorrhage of lungs in an infected hamster. (D) Normal hepatocytic architecture (HE [hematoxylin-erythrosin], ×400). (E) Regular alveolar structure in lungs of noninfected animals (HE, ×100). (F) Normal tubules and glomerulus in kidneys (HE, ×400). (G) necrotic hepatocytes associated with sinusoidal congestion and infiltration of inflammatory cells in liver of B3-13S-infected animals 5 days postinfection (HE, ×400). (H) Edema and foci of intensive hemorrhages in lungs 5 days postinfection (HE, ×100). (I) Intense hemorrhages in tubules and glomeruli (arrow) in kidneys of infected animals (HE, ×400).
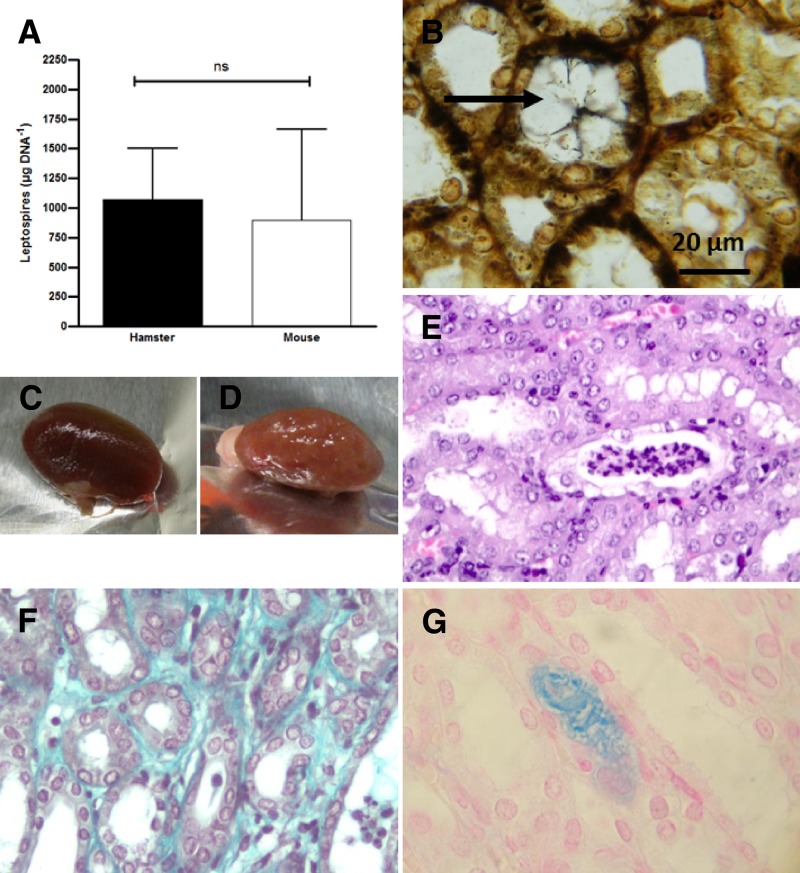
We also examined surviving hamsters infected by inoculation of 1 × 108 or 5 × 107 B3-13S, and compared infectious characteristics to the chronically infected mice. Localized carriage in kidneys and subsequent shedding of leptospires in urine were evaluated by molecular approaches and histology from samples collected at 28 days postinfection. Quantitative PCR confirmed the presence of leptospires in kidneys of all animals with detection of leptospiral DNA in renal tissues 4 weeks postinfection (Figure 2A). Results showed no difference in bacterial load between infected hamsters (∼1,000 Leptospira genomes/μg DNA tissue) and mice (∼900 Leptospira genomes/μg DNA tissue). Shedding of leptospires in urine was also confirmed by quantitative polymerase chain reaction (qPCR) from urine or from bedding collected from hamster cages (not shown). Argentic staining also confirmed the presence of intact spirochetes in the kidneys of hamsters until 28 days postinfection (Figure 2B). Clusters of bacteria were found localized in the central zone of the lumen of the proximal tubules.
Figure 2.
Carriage of B3-13S leptospires and lesions in the kidneys of hamsters 28 days postinfection. (A) Bacterial load in kidneys of mice and hamsters infected with 1 × 108 virulent B3-13S, quantified at 28 days postinfection by quantitative polymerase chain reaction (qPCR) assays. Results expressed as Leptospira genomes per microgram of DNA extracted from kidneys. Values are means ± SEMs (n = 3). ns = nonsignificant. (B) Spirochetes in the kidneys of hamsters 28 days after infection; a large cluster of leptospires is indicated in the central zone of a tubular lumen by an arrow (WS, ×1,000). (C) Kidney from a control hamster. (D) Kidney from a surviving hamster 28 days after infection. (E) Tubular infiltration of polynuclear neutrophils and lymphocytes with necrotic cells in infected hamster (HE, ×400). (F) Collagen in interstitial space (Masson's trichrome, ×1,000). (G) Intense staining of mucopolysaccharides in a tubule (Alcian Blue, ×1,000).
Histological changes were also investigated in the renal tissue of hamsters at 28 days postinfection. Contrasting with lethal cases, kidneys did not show any congestion in gross observation, but rather, abnormal pallor and sclerosis (Figure 2D). These observations correlated with histological changes showing a crenulated capsule (not shown). Infiltration of polynuclear neutrophils and lymphocytes associated with necrotic elements in the tubular structures (Figure 2E) supports acute or subacute tubulointerstitial nephritis. Evidence of interstitial fibrosis was demonstrated by staining collagen in the interstitium using Masson's trichrome (Figure 2F). Alcian Blue staining showed intensive coloration of mucopolysaccharides in the lumen of some kidney tubules (Figure 2G).
Rodents are regarded as principal reservoirs of leptospires,1,2 and their close proximity to inhabited areas in developing and tropical countries constitutes a favorable condition for human contamination.8,12 The serogroup Ballum, the principal reservoir of which is mice, is increasingly reported in human infection.5,6 Here, we characterized the virulence of a Ballum strain B3-13S, which was isolated from a wild mouse captured in New Caledonia, using hamster model. We described tissue lesions during both the acute and chronic stages of infection. Lethal leptospirosis of hamsters infected with the L. interrogans serovar Icterohaemorrhagiae strain Verdun was previously described13 and, similarly, led to fatality within 4–6 days, as was also observed for the Ballum B3-13S isolate. This contrasts with the mean period before onset of death observed with another serogroup, Ballum strain 4E, in the same animal model, which died between 8 and 18 days postinfection.14 However, bacterial concentration of B3-13S greater than 1 × 108 leptospires was required to induce lethality in all hamsters; this contrasts with lethality observed from doses lower than 1 × 102 for Ballum strain 4E.14 The B3-13S-infected hamsters revealed severe hemorrhaging, as well as edema and congestion in liver, lung, and kidney tissue at the time of death, similar to lesions observed with other strains.13,15
Hamsters that survived acute disease developed chronic infection with the B3-13S isolate. Indeed, WS staining and specific qPCR techniques confirmed the presence of Leptospira in the kidneys of infected hamsters at 28 days postinfection. Recently, Zuerner and others described the chronic infection of hamsters with one particular isolate of L. borgpetersenii serovar Hardjo.16 In that study, leptospires established renal colonization, and hamsters remained asymptomatic with chronic renal infections until 30 days after inoculation. The bacterial load was quantified and no difference was observed between mice and hamsters infected with B3-13S. Previous studies quantified the bacterial load in kidneys of animals infected with virulent strains of serovar Copenhageni.17 It is noteworthy that acute infection models showed higher concentrations of leptospires (> 2.4 × 104 genomes/μg DNA) compared with reservoir rats (50–825/μg DNA). The bacterial load in the kidneys of chronic infection models was closer to the range observed in those of reservoir rats. A lower Leptospira burden seems representative of chronic infection compared with the higher load observed in acute infection.
During chronic infection, hamsters presented with no clinical signs although renal lesions were observed with inflammatory infiltrates characteristic of interstitial nephritis at 28 days postinfection, a condition also associated with chronic leptospirosis in experimentally infected carrier animals including rats, mice, pigs, cattle, and dogs.18–22 Interestingly, hamsters infected with virulent strains of leptospires and developing rapid acute lethal infection presented with severe damage of tubular epithelia with cell swelling, whereas hamsters presenting advanced signs of the disease (10 days postinfection) exhibited multifocal moderate nephritis with infiltrates of leukocytes and histiocytes.23 During chronic infection, interstitial nephritis is commonly associated with fibrosis. Using Masson's trichrome staining, we also recorded evidence of intensive interstitial fibrosis in hamster kidneys during chronic leptospirosis. This was associated with observed tubular atrophy related to necrotic epithelial cells. Interstitial nephritis evolving into tubular atrophy and renal fibrosis was also seen in chronically infected animals, such as dogs infected with serovar Canicola and rats infected with serovar Icterohaemorrhagiae.2
Histological staining allowed for visualization of Leptospira localization in the kidneys of infected animals. Large clusters of spirochetes were confined into the central area of the tubular lumen in infected hamsters. Using another Ballum strain, colonization of renal tissues by leptospires was found exclusively in the tubules of hamsters.16 In contrast, description of the close proximity of leptospire layer to tubular epithelium was reported using WS staining in infected wild, long-tailed field mouse (Apodemus sylvaticus), suggesting attachment of the leptospires to the tubular wall.24 Scanning electron microscopy also revealed leptospires attached to the inner tubular surface in kidneys of rats infected with virulent serovar Copenhageni.1 Aggregation of pathogenic leptospires has been previously observed in vitro when cultured in fresh water for 110 days, and was proposed as a possible survival mechanism in the natural environment.25 Aggregates of leptospires may be assimilated to biofilms, with Ristow and others reporting evidence supporting the formation of biofilm composed of pathogenic leptospires surrounded by an extracellular matrix.26 Interestingly, endogenous expression of a major sialic acid was reported in a virulent serovar Copenhageni.27 Herein, Alcian Blue staining of kidney sections of B3-13S-infected hamsters showed massive mucopolysaccharidic structures that may include sialic acids in the tubular lumen. These components could be produced by tubular cells in the chronically infected animals as a potential host response. This mechanism may be related to the formation of biofilm-like structures possibly contributing to the maintenance of bacteria in the kidneys of host reservoirs.
This study, therefore, confirms the utility of the B3-13S isolate in exploring mechanisms of leptospirosis. The development of accurate models for both acute and chronic infection is critical for pursuing robust research on the disease. Such research could have significant implications for public health in tropical and subtropical regions where human leptospirosis infection is considered a significant and growing morbidity risk among vulnerable populations.
ACKNOWLEDGMENTS
We are grateful to Yannick Rougier for his help with the histological part of the study, especially for the use of Masson's trichrome. Warm thanks is also extended to the staff of Pathological Anatomic Laboratory, especially Evelyne Tuheiava and Lucie Garcin, for their skillful technical assistance and Erika Noebel for medical and scientific writing.
Footnotes
Financial support: The research position of Mariko Matsui was financed by the Government of New Caledonia. This Leptospirosis Research program was cofunded by the French Ministry of Research and Technology, Institut Pasteur de Nouvelle-Calédonie, and Institut Pasteur de Paris.
Authors' addresses: Mariko Matsui, Louise Roche, Marie-Estelle Soupé-Gilbert, and Cyrille Goarant, Leptospirosis Research and Expertise Unit, Institut Pasteur de Nouvelle-Calédonie, Noumea, New Caledonia, E-mails: mmatsui@pasteur.nc, louisette.roche@gmail.com, msoupe@pasteur.nc, and cgoarant@pasteur.nc. Martine Roudier and Vincent Moniquet, Anatomic Pathology Laboratory, Territorial Hospital Centre of New Caledonia, Noumea Cedex, New Caledonia, E-mails: martine.roudier@ventana.roche.com and vincent.moniquet@yahoo.fr.
References
- 1.Athanazio DA, Silva EF, Santos CS, Rocha GM, Vannier-Santos MA, McBride AJ, Ko AI, Reis MG. Rattus norvegicus as a model for persistent renal colonization by pathogenic Leptospira interrogans. Acta Trop. 2008;105:176–180. doi: 10.1016/j.actatropica.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 2.Monahan A, Callanan J, Nally J. Host-pathogen interactions in the kidney during chronic leptospirosis. Vet Pathol. 2009;46:792–799. doi: 10.1354/vp.08-VP-0265-N-REV. [DOI] [PubMed] [Google Scholar]
- 3.Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, Levett PN, Gilman RH, Willig MR, Gotuzzo E, Vinetz JM. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;3:757–771. doi: 10.1016/s1473-3099(03)00830-2. [DOI] [PubMed] [Google Scholar]
- 4.Picardeau M. Diagnosis and epidemiology of leptospirosis. Med Mal Infect. 2013;43:1–9. doi: 10.1016/j.medmal.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Herrmann-Storck C, Postic D, Lamaury I, Perez JM. Changes in epidemiology of leptospirosis in 2003–2004, a two El Nino Southern Oscillation period, Guadeloupe archipelago, French West Indies. Epidemiol Infect. 2008;136:1407–1415. doi: 10.1017/S0950268807000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thornley CN, Baker MG, Weinstein P, Maas EW. Changing epidemiology of human leptospirosis in New Zealand. Epidemiol Infect. 2002;128:29–36. doi: 10.1017/s0950268801006392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goarant C, Laumond-Barny S, Perez J, Vernel-Pauillac F, Chanteau S, Guigon A. Outbreak of leptospirosis in New Caledonia: diagnosis issues and burden of disease. Trop Med Int Health. 2009;14:926–929. doi: 10.1111/j.1365-3156.2009.02310.x. [DOI] [PubMed] [Google Scholar]
- 8.Perez J, Brescia F, Becam J, Mauron C, Goarant C. Rodent abundance dynamics and leptospirosis carriage in an area of hyper-endemicity in New Caledonia. PLoS Negl Trop Dis. 2011;5:e1361. doi: 10.1371/journal.pntd.0001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faine S, Adler B, Bolin C, Perolat P. Leptospira and Leptospirosis. 2nd ed. Melbourne, Australia: MedSci; 1999. [Google Scholar]
- 10.Wuscher N, Huerre M. Method of Warthin-Starry modified with pyrocatechol: interest for revealing spirochetes and infectious agents [Méthode de Warthin-Starry modifiée au pyrocatechol: intérêt pour la mise en évidence des spirochètes et agents infectieux] Revue française d'histotechnologie. 1993;6:5–8. [Google Scholar]
- 11.Merien F, Portnoi D, Bourhy P, Charavay F, Berlioz-Arthaud A, Baranton G. A rapid and quantitative method for the detection of Leptospira species in human leptospirosis. FEMS Microbiol Lett. 2005;249:139–147. doi: 10.1016/j.femsle.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Ivanova S, Herbreteau V, Blasdell K, Chaval Y, Buchy P, Guillard B, Morand S. Leptospira and rodents in Cambodia: environmental determinants of infection. Am J Trop Med Hyg. 2012;86:1032–1038. doi: 10.4269/ajtmh.2012.11-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsui M, Rouleau V, Bruyère-Ostells L, Goarant C. Gene expression profiles of immune mediators and histopathological findings in animal models of leptospirosis: comparison between susceptible hamsters and resistant mice. Infect Immun. 2011;79:4480–4492. doi: 10.1128/IAI.05727-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diniz JA, Felix SR, Bonel-Raposo J, Seixas Neto AC, Vasconcellos FA, Grassmann AA, Dellagostin OA, Aleixo JA, da Silva EF. Highly virulent Leptospira borgpetersenii strain characterized in the hamster model. Am J Trop Med Hyg. 2011;85:271–274. doi: 10.4269/ajtmh.2011.11-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Praditpornsilpa K, Sangjun N, Kittikowit W, Phulsuksombati D, Avihingsanon Y, Kansanabuch T, Tungsanga K, Eiam-Ong S. Alleviation of renal and pulmonary injury by immunomodulation in leptospirosis: hamster model. J Med Assoc Thai. 2006;89:S178–S187. [PubMed] [Google Scholar]
- 16.Zuerner RL, Alt DP, Palmer MV. Development of chronic and acute golden Syrian hamster infection models with Leptospira borgpetersenii serovar Hardjo. Vet Pathol. 2012;49:403–411. doi: 10.1177/0300985811409252. [DOI] [PubMed] [Google Scholar]
- 17.Chagas-Junior AD, da Silva CL, Soares LM, Santos CS, Athanazio CDMS, Dos Reis DA, Cruz MG, McBride FW, McBride AJ. Detection and quantification of Leptospira interrogans in hamster and rat kidney samples: immunofluorescent imprints versus real-time PCR. PLoS ONE. 2012;7:e32712. doi: 10.1371/journal.pone.0032712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pezzolato M, Maina E, Lonardi S, Bozzetta E, Grassi F, Scanziani E, Radaelli E. Development of tertiary lymphoid structures in the kidneys of pigs with chronic leptospiral nephritis. Vet Immunol Immunopathol. 2012;145:546–550. doi: 10.1016/j.vetimm.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 19.Santos CS, Macedo JO, Bandeira MS, Chagas AD, Jr., McBride AJ, McBride FW, Reis MG, Athanazio D. Different outcomes of experimental leptospiral infection in mouse strains with distinct genotypes. J Med Microbiol. 2010;59:1101–1106. doi: 10.1099/jmm.0.021089-0. [DOI] [PubMed] [Google Scholar]
- 20.Scanziani E, Crippa L, Giusti AM, Luini M, Pacciarini ML, Tagliabue S, Cavalletti E. Leptospira interrogans serovar Sejroe infection in a group of laboratory dogs. Lab Anim. 1995;29:300–306. doi: 10.1258/002367795781088261. [DOI] [PubMed] [Google Scholar]
- 21.Tucunduva de Faria M, Athanazio DA, Goncalves Ramos EA, Silva EF, Reis MG, Ko AI. Morphological alterations in the kidney of rats with natural and experimental Leptospira infection. J Comp Pathol. 2007;137:231–238. doi: 10.1016/j.jcpa.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Yener Z, Keles H. Immunoperoxidase and histopathological examinations of leptospiral nephritis in cattle. J Vet Med A Physiol Pathol Clin Med. 2001;48:441–447. doi: 10.1046/j.1439-0442.2001.00355.x. [DOI] [PubMed] [Google Scholar]
- 23.Silva EF, Santos CS, Athanazio DA, Seyffert N, Seixas FK, Cerqueira GM, Fagundes MQ, Brod CS, Reis MG, Dellagostin OA, Ko AI. Characterization of virulence of Leptospira isolates in a hamster model. Vaccine. 2008;26:3892–3896. doi: 10.1016/j.vaccine.2008.04.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Twigg GI, Cox PJ. The distribution of leptospires in the kidney tubules of some British wild mammals. J Wildl Dis. 1976;12:318–321. doi: 10.7589/0090-3558-12.3.318. [DOI] [PubMed] [Google Scholar]
- 25.Trueba G, Zapata S, Madrid K, Cullen P, Haake D. Cell aggregation: a mechanism of pathogenic Leptospira to survive in fresh water. Int Microbiol. 2004;7:35–40. [PubMed] [Google Scholar]
- 26.Ristow P, Bourhy P, Kerneis S, Schmitt C, Prevost MC, Lilenbaum W, Picardeau M. Biofilm formation by saprophytic and pathogenic leptospires. Microbiology. 2008;154:1309–1317. doi: 10.1099/mic.0.2007/014746-0. [DOI] [PubMed] [Google Scholar]
- 27.Ricaldi JN, Matthias MA, Vinetz JM, Lewis AL. Expression of sialic acids and other nonulosonic acids in Leptospira. BMC Microbiol. 2012;12:161. doi: 10.1186/1471-2180-12-161. [DOI] [PMC free article] [PubMed] [Google Scholar]