Abstract
We have previously shown that anti–dengue virus nonstructural protein 1 (anti-DENV NS1) antibodies cross-react with endothelial cells, and several autoantigens have been identified. This study shows that the antibody levels against these self-proteins are higher in sera from patients with dengue hemorrhagic fever (DHF) than those in control sera. Anti–protein disulfide isomerase (PDI) and anti–heat shock protein 60 (anti-HSP60) IgM levels correlated with both anti–endothelial cells and anti-DENV NS1 IgM titers. A cross-reactive epitope on the NS1 amino acid residues 311–330 (P311–330) had been predicted. We further found that there were higher IgM and IgG levels against P311–330 in DHF patients' sera than those in the control sera. In addition, correlations were observed between anti-PDI with anti-P311–330 IgM and IgG levels, respectively. Therefore, our results indicate that DENV NS1 P311–330 is a major epitope for cross-reactive antibodies to PDI on the endothelial cell surface, which may play an important role in DENV infection–induced autoimmunity.
Introduction
Dengue disease is caused by arthropod-borne dengue virus (DENV) belonging to the genus Flavivirus of the family Flaviviridae. Dengue disease encompasses a spectrum of clinical features, ranging from a mild febrile illness to life-threatening dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS).1–3 With increased international travel and climate change, dengue is spreading beyond its usual tropical and subtropical boundaries. Dengue is therefore a globally relevant disease that requires greater understanding of the underlying pathogenetic mechanisms.4–8 There are no effective strategies for the prevention of dengue disease. Furthermore, no dengue vaccine is yet available, although several candidate vaccines are currently being evaluated.9–17
Multiple mechanisms are involved in the pathogenesis of dengue disease. The increased severity of secondary infection is associated with antibody-dependent enhancement (ADE) of infection in which FcγR engagement by antibody–virus complexes facilitates DENV entry into FcγR-bearing cells.18–21 DENV infection also induces aberrant immune responses, overproduction of cytokines and chemokines, hemophagocytosis, and apoptotic cell death.22–26 The clinical manifestations of DHF/DSS are characterized by abnormalities of hemostasis and vascular permeability.27–29 The plasma leakage of DHF is believed to be immune mediated. Hemorrhagic symptoms of DHF/DSS include thrombocytopenia, coagulopathy, and vasculopathy, which are related to the dysfunction of endothelial cells and platelets.3,30
Previous studies demonstrated that antibodies present in sera from dengue patients showed cross-reactivity with platelets and endothelial cells.31,32 The percentages of endothelial cells reactive with sera from DHF/DSS patients were higher than that with acute phase dengue fever (DF) patient sera. Sera collected in the convalescent phase showed a decrease in endothelial cell–binding activity when compared with those collected in the acute phase. Studies using sera from patients with Japanese encephalitis virus (JEV) and enterovirus (EV71) infections showed endothelial cell-binding levels similar to those of the normal controls.32 Furthermore, antibodies directed against DENV NS1 were responsible for the cross-reactivity.33–39 Several endothelial cell autoantigens, including ATP synthase β chain, protein disulfide isomerase (PDI), heat shock protein 60 (HSP60), and vimentin, recognized by anti-DENV NS1 antibodies have been identified, which have been confirmed expressing on cell surface.40 A cross-reactive epitope on the NS1 located between amino acid residues 311–330 (P311–330) reduced the binding activity of sera from patients with DHF with endothelial cells and platelets.40–42 This study shows the correlation of patient antibodies specific for DENV NS1 protein and the autoantigens on endothelial cells. DENV NS1 P311–330-specific antibodies cross-reactive with PDI on the endothelial cell surface may play an important role in DENV infection–induced autoimmunity, which provides insights into DENV vaccine development and therapeutic strategies.
Materials and Methods
Patient sera.
Dengue patient sera were provided by Dr. N. Hung (Department of Dengue Hemorrhagic Fever, Children's Hospital No. 1, Ho Chi Minh City, Vietnam). The clinical diagnosis of DF and DHF was based on the 1997 criteria of the World Health Organization (WHO)43 and parental or guardian informed consent was obtained as described previously (in Patients, Materials and Methods).44,45 Sera were collected one to four times after 3–10 days of fever onset from each individual to give a total of five samples from two DF patients and 43 samples from 15 DHF patients. One DF patient was infected with DENV1 in a secondary infection, the other one with unknown serotype primary infection. Among the DHF patients, two were infected with DENV1, one with DENV2, seven with DENV3, three with DENV4, and two with unknown serotype. One patient with DENV4 and one with unknown serotype were diagnosed with primary infections, and the other DHF patients were diagnosed with secondary infections (Supplemental Table 1). DENV infections in the patients were studied by viral envelope and membrane (E/M)–specific capture IgM ELISA and/or NS1 serotype–specific IgG ELISA to differentiate between primary and secondary infections at the Center for Disease Control, Department of Health, Taipei, Taiwan. A one-step SYBR Green I real-time RT-PCR (QuantiTect SYBR Green RT-PCR kit; Qiagen, Hilden, Germany) was performed using the Mx4000TM quantitative PCR system (Stratagene, La Jolla, CA) to detect and differentiate DENV serotypes in acute-phase serum samples, which was performed at the Center for Disease Control as described previously.7,46 Sera from healthy volunteers were obtained from Tainan, a dengue-endemic area in Taiwan.
Cell culture.
Human microvascular endothelial cell line-1 (HMEC-1) was obtained from the Centers for Disease Control, Atlanta, GA,47 and passaged in 10-cm culture plates containing Medium 200 supplemented with low serum growth supplement (LSGS) composed of 2% fetal bovine serum, 1 μg/mL hydrocortisone, 10 ng/mL epidermal growth factor, 3 ng/mL basic fibroblast growth factor, and 10 μg/mL heparin (Cascade Biologics, Portland, OR).
Antibody-cell-binding assay.
For the experiments, HMEC-1 cells were detached with 1,000 U/mL trypsin and 0.5 mM EDTA. The cell suspension was then incubated at 5 × 105 per tube with sera from patients with DHF (1:50 dilution) at room temperature for 1 hour. After washing three times with phosphate-buffered saline (PBS), the cells were incubated with fluorescein isothiocyanate (FITC)–conjugated anti-human IgM or IgG for 1 hour at 4°C and analyzed using flow cytometry (FACSCalibur; Becton Dickinson Biosciences, San Jose, CA).
ELISA.
Microtiter plates were coated with 1 μg/well DENV NS1, PDI, vimentin, HSP60, or synthetic peptides at 4°C overnight, and then blocked with 5% skimmed milk for 2 hours at 37°C. After washing with 0.05% PBS-Tween 20, 1:25 dilutions of sera from patients with DF or DHF were added and incubated at 4°C overnight. After three washes with 0.05% PBS-Tween 20, horseradish peroxidase (HRP)–conjugated anti-human IgM and IgG were added and incubated for 2 hours at 37°C. The preparations were then washed with 0.05% PBS-Tween 20 and incubated with 3,3′,5,5′-tetramethylbenzidine (TMB) or 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS), and the absorbance was measured using an EMax microplate reader (Molecular Devices, Sunnyvale, CA) at 450 nm for TMB and 405 nm for ABTS.
Recombinant DENV NS1 protein was derived by expression in Escherichia coli.35 Briefly, the full-length DENV2 (New Guinea C strain) NS1 cDNA was cloned to pRSET B expression vector (Invitrogen, Carlsbad, CA) to produce a pRSET-DENVNS1 plasmid, which was then introduced into E. coli BL21(DE3)pLysS strain (Invitrogen). The recombinant NS1 protein was induced by a 2 mM final concentration of isopropyl β-d-thiogalactoside (IPTG) and purified with Ni2+-chelating chromatography (Amersham Bioscience, Uppsala, Sweden) in TE buffer (50 mM Tris-HCl, pH 8.0, and 2 mM EDTA) containing 8 M urea. A single band was detected by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the protein sequence was confirmed. Synthetic peptides, P311–330 (residues 311–330, N′[H]-WCCRSCTLPPLRYRGEDGCW-C′[OH]) and P211–225 (residues 211–225, N′[H]-KIEKASFIEVKSCHW-C′[OH]) were obtained from Sigma-Genosys (Cashmere Scientific, Taiwan).
Statistical analysis.
Because of multiple experiments on the sera from a single patient or control, a linear mixed model was used to adjust for the intra-individual correlations among the repeated tests in comparing antibody levels between different groups.48 Statistical analyses were performed using SAS procedure PROC MIXED. Spearman's rank correlation test was used to assess the correlation between DENV NS1–specific antibodies or target protein–specific antibodies and the anti–endothelial cell antibodies in each serum sample with GraphPad Prism for Windows (version 4, GraphPad Software). Differences were considered statistically significant at P < 0.05.
Results
Presence of anti-target protein antibodies in sera from patients with DHF.
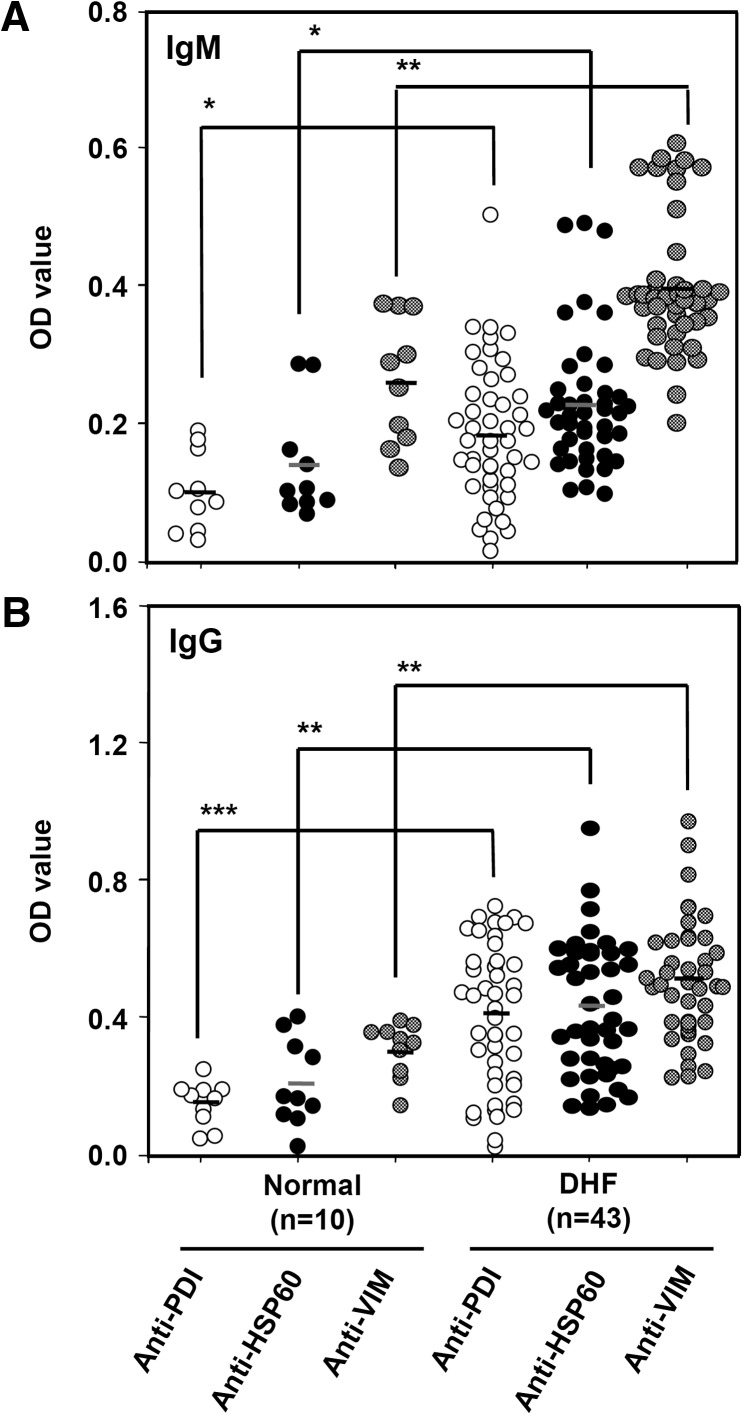
Previous studies from our laboratory showed the presence of anti–endothelial cell autoantibodies in sera from dengue patients, and anti-DENV NS1 antibodies, at least in part, accounted for the cross-reactivity.32 Several target proteins on the endothelial cell surface recognized by anti-DENV NS1 antibodies were identified, including ATP synthase β chain, PDI, HSP60, and vimentin.40 To validate the clinical implications of these target proteins, the antibody titers against these target proteins were determined in sera from patients with DHF as compared with control sera. Least-square means were performed using an SAS mixed model to calculate the difference between DHF and normal groups. The results showed that both IgM and IgG levels of anti-PDI, anti-HSP60, and anti-vimentin antibodies were higher in sera from patients with DHF than in the control sera (Figure 1). Patient serum samples were further classified into four groups according to the collection time. There was no significant difference in either IgM or IgG levels of anti-PDI, anti-HSP60, and anti-vimentin between different collection times (Supplemental Figure 1). Because of small sample size in some serotype groups, it remains to be determined whether there is any significant difference between different serotypes of infection. The antibody titer to ATP synthase was not determined because the purified protein is not commercially available.
Figure 1.
Antibody titers against protein disulfide isomerase (PDI), heat shock protein 60 (HSP60), and vimentin in sera from patients with dengue hemorrhagic fever (DHF). The IgM (A) and IgG (B) levels against PDI, HSP60, and vimentin (1 μg/well) in patient sera (1:25 dilution) were compared with normal control sera as determined by ELISA. The OD values were obtained after correction by subtracting the OD values of the secondary antibody alone group (i.e., anti-human IgG- or IgM-HRP). * P < 0.05; ** P < 0.01; *** P < 0.001 as compared with normal control.
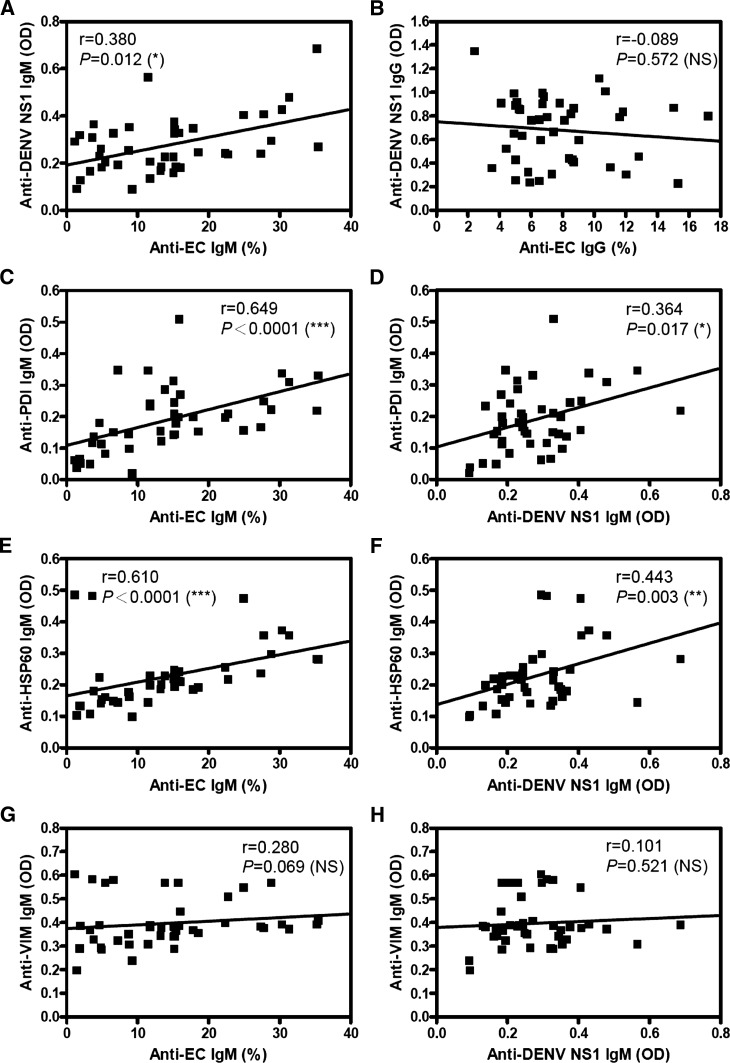
Correlation of anti–endothelial cell and anti-DENV NS1 IgM levels with anti-target protein antibodies in sera from patients with DHF.
The percentage of endothelial cells reactive with patient serum IgM positively correlated with anti-DENV NS1 IgM titers (Figure 2A). However, the anti–endothelial cell IgG levels did not correlate with anti-DENV NS1 IgG titers (Figure 2B). Furthermore, anti-PDI and anti-HSP60 IgM levels correlated positively with both anti–endothelial cell IgM titers (Figure 2C and E) and anti-DENV NS1 IgM titers (Figure 2D and F). However, anti-vimentin IgM levels did not correlate with anti–endothelial cell (Figure 2G) or anti-DENV NS1 IgM titers (Figure 2H). None of the IgG levels showed positive correlations in any of these groups (Supplemental Figure 2). There was no correlation between anti-PDI, anti-HSP60, or anti-vimentin IgM and IgG levels with anti-DENV NS1 IgM and IgG titers in normal control sera (data not shown).
Figure 2.
Correlation of antibody titers in dengue hemorrhagic fever (DHF) patient sera against endothelial cells and dengue virus nonstructural protein 1 (DENV NS1) with target proteins. The correlation of IgM (A) and IgG (B) titers against DENV NS1 (1 μg/well) in patient sera (1:25 dilution) as determined by ELISA with anti–human microvascular endothelial cell line-1 (anti-HMEC-1) cells (1:50 dilution) as determined by flow cytometry was analyzed using Spearman's rank correlation test. The correlation of anti-HMEC-1 IgM as determined by flow cytometry (C, E, G) and anti-DENV NS1 IgM titers as determined by ELISA (D, F, H) with anti-PDI (C, D), anti-HSP60 (E, F), and anti-vimentin (G, H) as determined by ELISA was analyzed using Spearman's rank correlation test. * P < 0.05; ** P < 0.01; *** P < 0.001 are indicated for significant correlation. NS = no significant difference.
Correlation of anti–endothelial cell IgM and anti-DENV NS1 P311–330 IgM levels in sera from patients with DHF.
Using homologous sequence alignment, a cross-reactive epitope on the NS1 amino acid residues 311–330 (P311–330) was predicted.40 The results showed higher IgM and IgG titers against P311–330 in sera from patients with DHF than those in the control sera (Figure 3A and B). The anti-DENV NS1 antibodies in sera from patients with DHF were used as positive control, and the anti-P211–225 antibodies were used as negative control. There was no significant difference in both IgM and IgG titers of anti-P311–330 and anti-NS1 between different collection days (Supplemental Figure 3). Furthermore, consistent with our previous finding using a different source of patient sera,42 the percentage of endothelial cells reactive with patient serum IgM correlated with anti-P311–330 IgM titers (Figure 3C).
Figure 3.
Antibody titers against P311–330 in sera from patients with dengue hemorrhagic fever (DHF). The IgM (A) and IgG (B) titers against P311–330 peptides (1 μg/well) in patient sera (1:25 dilution) were compared with normal control sera as determined by ELISA. Dengue virus nonstructural protein 1 (DENV NS1) was used as a positive control and P211–225 was used as a negative control. The OD values were obtained after correction by subtracting the OD values of the secondary antibody alone group. (C) The correlation of anti-human microvascular endothelial cell line-1 (anti-HMEC-1) IgM as determined by flow cytometry and anti-P311–330 IgM as determined by ELISA was analyzed using Spearman's rank correlation test. * P < 0.05; ** P < 0.01; *** P < 0.001 as compared with normal control. NS = no significant difference.
Although higher anti-PDI and anti-HSP60 IgM and IgG antibody levels in sera from patients with DF were comparable with those in sera from patients with DHF, anti-P311–330 titers in sera from patients with DHF were higher than those in sera from patients with DF (Table 1). Anti-P211–225 antibodies were used as negative control. We also used NS1 protein from drosophila (provided by CTK Biotech, San Diego, CA) to further confirm the binding of patient sera IgG antibodies to NS1. The result was similar to that using recombinant DENV NS1 protein derived by expression in E. coli (Supplemental Figure 4).
Table 1.
Anti-PDI, anti-HSP60, anti-P311-330, and anti-P211-225 antibody responses in DF and DHF patient sera
| Normal | DF | DHF | |
|---|---|---|---|
| Anti-PDI IgM | 0.086 ± 0.050† | 0.194 ± 0.104* | 0.189 ± 0.102* |
| Anti-PDI IgG | 0.158 ± 0.063 | 0.298 ± 0.085** | 0.413 ± 0.210*** |
| Anti-HSP60 IgM | 0.141 ± 0.080 | 0.235 ± 0.058* | 0.227 ± 0.095* |
| Anti-HSP60 IgG | 0.223 ± 0.124 | 0.464 ± 0.310* | 0.436 ± 0.191** |
| Anti-P311–330 IgM | 0.108 ± 0.058 | 0.143 ± 0.038 | 0.238 ± 0.111**‡ |
| Anti-P311–330 IgG | 0.158 ± 0.063 | 0.287 ± 0.080** | 0.519 ± 0.419** |
| Anti-P211–225 IgM | 0.082 ± 0.028 | 0.087 ± 0.016 | 0.095 ± 0.021 |
| Anti-P211–225 IgG | 0.087 ± 0.035 | 0.096 ± 0.024 | 0.101 ± 0.033 |
Anti-PDI = anti-protein disulfide isomerase; anti-HSP60 = anti-heat shock protein 60; DF = dengue fever; DHF = dengue hemorrhagic fever.
P < 0.05;
P < 0.01;
P < 0.001 as compared with normal control.
The antibody levels were detected by ELISA. The means of OD values were analyzed with a linear mixed model using the SAS procedure PROC MIXED to compare antibody levels between different groups.
P < 0.05 as compared with DF.
Correlation of anti-PDI IgM and IgG with anti-DENV NS1 P311–330 IgM and IgG levels in sera from patients with DHF.
The correlations between anti-PDI and anti-HSP60 antibody levels with anti-P311-330 titers were further determined. Results showed that anti-PDI IgM and IgG levels correlated positively with anti-P311–330 IgM and IgG levels, respectively (Figure 4A and B). However, anti-HSP60 IgM and IgG levels did not correlate with anti-P311–330 IgM and IgG levels (Figure 4C and D). These results indicate a potential role of anti-P311–330 antibodies cross-reactive with endothelial cell surface PDI in DENV-induced autoimmunity.
Figure 4.
Correlation of anti–protein disulfide isomerase (anti-PDI) and heat shock protein 60 (HSP60) antibody levels in dengue hemorrhagic fever (DHF) patient sera with anti-P311–330. The IgM (A, C) and IgG (B, D) titers against PDI (A, B) and HSP60 (C, D) (1 μg/well) in sera from patients with DHF (1:25 dilution) as determined by ELISA with anti-P311–330 IgM and IgG as determined by ELISA were analyzed using Spearman's rank correlation test. * P < 0.05; *** P < 0.001 are shown for significant correlation. NS = no significant difference.
Discussion
Anti-DENV NS1 antibodies recognize several endothelial cell surface proteins, and a major cross-reactive epitope is located within NS1 amino acid residues 311–330.40 In this study, patient sera were analyzed for correlations of anti–endothelial cell, anti-target proteins, and anti-DENV NS1 P311–330 antibody levels. Results showed that P311–330-specific antibodies cross-reactive with PDI on the endothelial cell surface may play an important role in DENV infection–induced autoimmunity. A previous study reported that sera from patients with DF and DHF showed similar anti-NS1 antibody responses.49 Notably, it is the anti–endothelial cell32 and anti-P311–330 (Table 1) antibody levels that are different between DF and DHF.
A number of viruses, such as human immunodeficiency virus, human hepatitis B and C viruses, human cytomegalovirus, herpes simplex virus, Epstein–Barr virus, and SARS coronavirus, have been reported to elicit autoimmunity,50–57 and mechanisms of molecular mimicry are involved in several virus-associated autoimmune responses or diseases.58–62 Studies from our laboratory also suggest a mechanism of molecular mimicry in which antibodies directed against NS1 cross-react with endothelial cells and platelets, causing damage and dysfunction, which may be related to certain clinical features of dengue disease.63 In this study, anti-DENV NS1 IgM titers correlate with anti-PDI and anti-HSP60 IgM, but not with anti-vimentin IgM, which might reflect the sequence homology between target proteins and P311–330 of DENV NS1.
Most of the observed positive correlations in this clinical investigation of sera from patients with DHF were of the IgM isotype. This may be partly related to the observations that although the average IgG titers of anti-target proteins, anti-DENV NS1, and anti-P311-330 were higher than IgM (Figure 1B versus 1A, 2B versus 2A y axis, and 3B versus 3A), the anti–endothelial cell IgG level was actually lower than IgM (Figure 2B versus 2A x axis). Nevertheless, the role of IgG cannot be totally ruled out. Both anti-PDI IgM and IgG levels correlated with anti-P311–330 IgM and IgG, which suggest that epitope-specific antibodies reactive with PDI autoantigen are not restricted to the IgM isotype. The only significant difference in antibody levels between secondary and primary infections was the anti-DENV NS1 IgG (P = 0.0075). For anti-DENV NS1 IgM, anti-PDI, anti-HSP60, anti-vimentin, and anti-P311–330 IgM and IgG, there were no significant differences between secondary and primary infections of DHF patients (data not shown).
In addition to endothelial cells, platelets also show autoantibody reactivity with dengue patient sera.31 An inverse correlation between platelet count and platelet-associated IgG or IgM levels was found during the acute phase of secondary dengue infections.64 PDI on the platelet surface was shown to be recognized by anti-DENV NS1 antibodies. Anti-DENV NS1 antibodies inhibited PDI activity and platelet aggregation, and both inhibitory effects were prevented when anti-DENV NS1 antibodies were preabsorbed with PDI.41 PDI may regulate the activation of integrin αIIbβ3, the fibrinogen receptor, which is required for the late stage of platelet aggregation. Therefore, the inhibitory effect of anti-DENV NS1 on the activation of integrin αIIbβ3 may occur by antibody recognition of platelet surface PDI thereby blocking its active sites and interfering with PDI function.65 We further found that endothelial cell surface PDI, which causes integrin activation, is involved in DENV entry, and DENV infection further increases PDI surface expression at later time points.66 A recent report showed that the binding of anti-NS1 antibody to PDI on endothelial cells induced heme oxygenase (HO)-1 expression, which reduced anti-NS1-induced endothelial cell apoptosis.67
Taken together, DENV NS1 P311–330-specific antibodies cross-reactive with cell surface PDI may play an important role in DENV-induced autoimmunity. DENV NS1, which is expressed on the infected cell surface,68 may serve as a vaccine candidate because of its inability to promote ADE.15,16 The characterization of cross-reactive epitopes is important for NS1-based DENV vaccine development and therapeutic strategies to avoid potentially harmful autoimmune complications.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Robert Anderson for critical reading of this manuscript.
Footnotes
Financial support: This study was supported by grants NSC101-2325-B006-006 and NSC102-2325-B006-006 from Ministry of Science and Technology, Taiwan.
Authors' addresses: Hsien-Jen Cheng and Yueh-Hsia Luo, Institute of Basic Medical Sciences, National Cheng Kung University Medical College, Tainan, Taiwan, E-mails: s5890103@nckualumni.org.tw and s5890118@nckualumni.org.tw. Shu-Wen Wan, Hsiao-Sheng Liu, and Yee-Shin Lin, Department of Microbiology and Immunology, National Cheng Kung University Medical College, Tainan, Taiwan, E-mails: iamwanwan@gmail.com, a713@mail.ncku.edu.tw, and yslin1@mail.ncku.edu.tw. Chiou-Feng Lin, Department of Microbiology and Immunology, College of Medicine, Taipei Medical University, Taipei, Taiwan, and Institute of Clinical Medicine, National Cheng Kung University Medical College, Tainan, Taiwan, E-mail: cflin@mail.ncku.edu.tw. Shan-Tair Wang, Institute of Gerontology, National Cheng Kung University Medical College, Tainan, Taiwan, E-mail: wifetz@gmail.com. Nguyen Thanh Hung, Department of Dengue Hemorrhagic Fever, Children's Hospital No. 1, Ho Chi Minh City, Vietnam, E-mail: drthanhhung@gmail.com. Ching-Chuan Liu and Tzong-Shiann Ho, Department of Pediatrics, National Cheng Kung University Medical College, Tainan, Taiwan, E-mails: ccliu@mail.ncku.edu.tw and tzong.ho@gmail.com. Trai-Ming Yeh, Department of Medical Laboratory Science and Biotechnology, National Cheng Kung University Medical College, Tainan, Taiwan, E-mail: today@mail.ncku.edu.tw.
Reprint requests: Yee-Shin Lin, Departments of Microbiology and Immunology, National Cheng Kung University Medical College, 1 University Road, Tainan 701, Taiwan, E-mail: yslin1@mail.ncku.edu.tw.
References
- 1.Halstead SB. Dengue. Curr Opin Infect Dis. 2002;15:471–476. doi: 10.1097/00001432-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Halstead SB. Dengue. Lancet. 2007;370:1644–1652. doi: 10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- 3.Green S, Rothman A. Immunopathological mechanisms in dengue and dengue hemorrhagic fever. Curr Opin Infect Dis. 2006;19:429–436. doi: 10.1097/01.qco.0000244047.31135.fa. [DOI] [PubMed] [Google Scholar]
- 4.Kyle JL, Harris E. Global spread and persistence of dengue. Annu Rev Microbiol. 2008;62:71–92. doi: 10.1146/annurev.micro.62.081307.163005. [DOI] [PubMed] [Google Scholar]
- 5.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, Hunsperger E, Kroeger A, Margolis HS, Martinez E, Nathan MB, Pelegrino JL, Simmons C, Yoksan S, Peeling RW. Dengue: a continuing global threat. Nat Rev Microbiol. 2010;8:S7–S16. doi: 10.1038/nrmicro2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin CC, Huang YH, Shu PY, Wu HS, Lin YS, Yeh TM, Liu HS, Liu CC, Lei HY. Characteristic of dengue disease in Taiwan: 2002–2007. Am J Trop Med Hyg. 2010;82:731–739. doi: 10.4269/ajtmh.2010.09-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simmons CP, Farrar JJ, Nguyen vV, Wills B. Dengue. N Engl J Med. 2012;366:1423–1432. doi: 10.1056/NEJMra1110265. [DOI] [PubMed] [Google Scholar]
- 9.Whitehead SS, Blaney JE, Durbin AP, Murphy BR. Prospects for a dengue virus vaccine. Nat Rev Microbiol. 2007;5:518–528. doi: 10.1038/nrmicro1690. [DOI] [PubMed] [Google Scholar]
- 10.Webster DP, Farrar J, Rowland-Jones S. Progress towards a dengue vaccine. Lancet Infect Dis. 2009;9:678–687. doi: 10.1016/S1473-3099(09)70254-3. [DOI] [PubMed] [Google Scholar]
- 11.Thomas SJ, Endy TP. Critical issues in dengue vaccine development. Curr Opin Infect Dis. 2011;24:442–450. doi: 10.1097/QCO.0b013e32834a1b0b. [DOI] [PubMed] [Google Scholar]
- 12.Coller BA, Clements DE. Dengue vaccines: progress and challenges. Curr Opin Immunol. 2011;23:391–398. doi: 10.1016/j.coi.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, Dulyachai W, Pengsaa K, Wartel TA, Moureau A, Saville M, Bouckenooghe A, Viviani S, Tornieporth NG, Lang J. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet. 2012;380:1559–1567. doi: 10.1016/S0140-6736(12)61428-7. [DOI] [PubMed] [Google Scholar]
- 14.Durbin AP, Wright PF, Cox A, Kagucia W, Elwood D, Henderson S, Wanionek K, Speicher J, Whitehead SS, Pletnev AG. The live attenuated chimeric vaccine rWN/DEN4Δ30 is well-tolerated and immunogenic in healthy flavivirus-naïve adult volunteers. Vaccine. 2013;31:5772–5777. doi: 10.1016/j.vaccine.2013.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dayan GH, Garbes P, Noriega F, de Sadovsky ADI, Rodrigues PM, Giuberti C, Dietze R. Immunogenicity and safety of a recombinant tetravalent dengue vaccine in children and adolescents ages 9–16 years in Brazil. Am J Trop Med Hyg. 2013;89:1058–1065. doi: 10.4269/ajtmh.13-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wan SW, Lin CF, Wang S, Chen YH, Yeh TM, Liu HS, Anderson R, Lin YS. Current progress in dengue vaccines. J Biomed Sci. 2013;20:37. doi: 10.1186/1423-0127-20-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halstead SB. Identifying protective dengue vaccines: guide to mastering an empirical process. Vaccine. 2013;31:4501–4507. doi: 10.1016/j.vaccine.2013.06.079. [DOI] [PubMed] [Google Scholar]
- 18.Halstead SB, O'Rourke EJ. Antibody-enhanced dengue virus infection in primate leukocytes. Nature. 1977;265:739–741. doi: 10.1038/265739a0. [DOI] [PubMed] [Google Scholar]
- 19.Halstead SB. Pathogenesis of dengue: challenges to molecular biology. Science. 1988;239:476–481. doi: 10.1126/science.3277268. [DOI] [PubMed] [Google Scholar]
- 20.Goncalvez AP, Engle RE, St Claire M, Purcell RH, Lai CJ. Monoclonal antibody-mediated enhancement of dengue virus infection in vitro and in vivo and strategies for prevention. Proc Natl Acad Sci USA. 2007;104:9422–9427. doi: 10.1073/pnas.0703498104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balsitis SJ, Williams KL, Lachica R, Flores D, Kyle JL, Mehlhop E, Johnson S, Diamond MS, Beatty PR, Harris E. Lethal antibody enhancement of dengue disease in mice is prevented by Fc modification. PLoS Pathog. 2010;6:e1000790. doi: 10.1371/journal.ppat.1000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martina BEE, Koraka P, Osterhaus ADME. Dengue virus pathogenesis: an integrated view. Clin Microbiol Rev. 2009;22:564–581. doi: 10.1128/CMR.00035-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herrero LJ, Zakhary A, Gahan ME, Nelson MA, Herring BL, Hapel AJ, Keller PA, Obeysekera M, Chen W, Sheng KC, Taylor A, Wolf S, Bettadapura J, Broor S, Dar L, Mahalingam S. Dengue virus therapeutic intervention strategies based on viral, vector and host factors involved in disease pathogenesis. Pharmacol Ther. 2013;137:266–282. doi: 10.1016/j.pharmthera.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Lei HY, Yeh TM, Liu HS, Lin YS, Chen SH, Liu CC. Immunopathogenesis of dengue virus infection. J Biomed Sci. 2001;8:377–388. doi: 10.1007/BF02255946. [DOI] [PubMed] [Google Scholar]
- 25.Lei HY. Transient hemophagocytic activity in dengue immunopathogenesis. J Formos Med Assoc. 2009;108:595–598. doi: 10.1016/s0929-6646(09)60379-x. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen DG. The relationship of interacting immunological components in dengue pathogenesis. Virol J. 2009;6:211–217. doi: 10.1186/1743-422X-6-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clyde K, Kyle JL, Harris E. Recent advances in deciphering viral and host determinants of dengue virus replication and pathogenesis. J Virol. 2006;80:11418–11431. doi: 10.1128/JVI.01257-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srikiatkhachorn A. Plasma leakage in dengue haemorrhagic fever. Thromb Haemost. 2009;102:1042–1049. doi: 10.1160/TH09-03-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Basu A, Chaturvedi UC. Vascular endothelium: the battlefield of dengue viruses. FEMS Immunol Med Microbiol. 2008;53:287–299. doi: 10.1111/j.1574-695X.2008.00420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin CF, Lei HY, Liu CC, Liu HS, Yeh TM, Wang ST, Yang TI, Sheu FC, Kuo CF, Lin YS. Generation of IgM anti-platelet autoantibody in dengue patients. J Med Virol. 2001;63:143–149. [PubMed] [Google Scholar]
- 32.Lin CF, Lei HY, Shiau AL, Liu CC, Liu HS, Yeh TM, Chen SH, Lin YS. Antibodies from dengue patient sera cross-react with endothelial cells and induce damage. J Med Virol. 2003;69:82–90. doi: 10.1002/jmv.10261. [DOI] [PubMed] [Google Scholar]
- 33.Falconar AK. The dengue virus nonstructural-1 protein (NS1) generates antibodies to common epitopes on human blood clotting, integrin/adhesin proteins and binds to human endothelial cells: potential implications in haemorrhagic fever pathogenesis. Arch Virol. 1997;142:897–916. doi: 10.1007/s007050050127. [DOI] [PubMed] [Google Scholar]
- 34.Falconar AK. Antibody responses are generated to immunodominant ELK/KLE-type motifs on the nonstructural-1 glycoprotein during live dengue virus infections in mice and humans: implications for diagnosis, pathogenesis, and vaccine design. Clin Vaccine Immunol. 2007;14:493–504. doi: 10.1128/CVI.00371-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin CF, Lei HY, Shiau AL, Liu HS, Yeh TM, Chen SH, Liu CC, Chiu SC, Lin YS. Endothelial cell apoptosis induced by antibodies against dengue virus nonstructural protein 1 via production of nitric oxide. J Immunol. 2002;169:657–664. doi: 10.4049/jimmunol.169.2.657. [DOI] [PubMed] [Google Scholar]
- 36.Lin CF, Chiu SC, Hsiao YL, Wan SW, Lei HY, Shiau AL, Liu HS, Yeh TM, Chen SH, Liu CC, Lin YS. Expression of cytokine, chemokine, and adhesion molecules during endothelial cell activation induced by antibodies against dengue virus nonstructural protein 1. J Immunol. 2005;174:395–403. doi: 10.4049/jimmunol.174.1.395. [DOI] [PubMed] [Google Scholar]
- 37.Lin CF, Lei HY, Liu CC, Liu HS, Yeh TM, Anderson R, Lin YS. Patient and mouse antibodies against dengue virus nonstructural protein 1 cross-react with platelets and cause their dysfunction or depletion. Am J Infect Dis. 2008;4:69–75. [Google Scholar]
- 38.Liu IJ, Chiu CY, Chen YC, Wu HC. Molecular mimicry of human endothelial cell antigen by autoantibodies to nonstructural protein 1 of dengue virus. J Biol Chem. 2011;286:9726–9736. doi: 10.1074/jbc.M110.170993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wan SW, Lin CF, Yeh TM, Liu CC, Liu HS, Wang S, Ling P, Anderson R, Lei HY, Lin YS. Autoimmunity in dengue pathogenesis. J Formos Med Assoc. 2013;112:3–11. doi: 10.1016/j.jfma.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 40.Cheng HJ, Lin CF, Lei HY, Liu HS, Yeh TM, Luo YH, Lin YS. Proteomic analysis of endothelial cell autoantigens recognized by anti-dengue virus nonstructural protein 1 antibodies. Exp Biol Med. 2009;234:63–73. doi: 10.3181/0805-RM-147. [DOI] [PubMed] [Google Scholar]
- 41.Cheng HJ, Lei HY, Lin CF, Luo YH, Wan SW, Liu HS, Yeh TM, Lin YS. Anti-dengue virus nonstructural protein 1 antibodies recognize protein disulfide isomerase on platelets and inhibit platelet aggregation. Mol Immunol. 2009;47:398–406. doi: 10.1016/j.molimm.2009.08.033. [DOI] [PubMed] [Google Scholar]
- 42.Wan SW, Lin CF, Chen MC, Lei HY, Liu HS, Yeh TM, Liu CC, Lin YS. C-terminal region of dengue virus nonstructural protein 1 is involved in endothelial cell cross-reactivity via molecular mimicry. Am J Infect Dis. 2008;4:85–91. [Google Scholar]
- 43.World Health Organization . Dengue Hemorrhagic Fever: Diagnosis, Treatment, Prevention and Control. 2nd ed. Geneva, Switzerland: World Health Organization; 1997. [Google Scholar]
- 44.Hung NT, Lei HY, Lan NT, Lin YS, Huang KJ, Lien LB, Lin CF, Yeh TM, Ha DQ, Huong VTQ, Chen LC, Huang JH, My LY, Liu CC, Halstead SB. Dengue hemorrhagic fever in infants: a study of clinical and cytokine profiles. J Infect Dis. 2004;189:221–232. doi: 10.1086/380762. [DOI] [PubMed] [Google Scholar]
- 45.Hung NT, Lan NT, Lei HY, Lin YS, Lien LB, Huang KJ, Lin CF, Ha DQ, Huong VTQ, My LT, Yeh TM, Huang JH, Liu CC, Halstead SB. Volume replacement in infants with dengue hemorrhagic fever/dengue shock syndrome. Am J Trop Med Hyg. 2006;74:684–691. [PubMed] [Google Scholar]
- 46.Shu PY, Chang SF, Kuo YC, Yueh YY, Chien LJ, Sue CL, Lin TH, Huang JH. Development of group- and serotype-specific one-step SYBR green I real-time reverse transcription-PCR assay for dengue virus. J Clin Microbiol. 2003;41:2408–2416. doi: 10.1128/JCM.41.6.2408-2416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ades EW, Candal FJ, Swerlick RA, George VG, Summers S, Bosse DC, Lawley TJ. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol. 1992;99:683–690. doi: 10.1111/1523-1747.ep12613748. [DOI] [PubMed] [Google Scholar]
- 48.Galbraith S, Daniel JA, Vissel B. A study of clustered data and approaches to its analysis. J Neurosci. 2010;30:10601–10608. doi: 10.1523/JNEUROSCI.0362-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shu PY, Chen LK, Chang SF, Yueh YY, Chow L, Chien LJ, Chin C, Lin TH, Huang JH. Dengue NS1-specific antibody responses: isotype distribution and serotyping in patients with dengue fever and dengue hemorrhagic fever. J Med Virol. 2000;62:224–232. doi: 10.1002/1096-9071(200010)62:2<224::aid-jmv14>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 50.Zandman-Goddard G, Shoenfeld Y. HIV and autoimmunity. Autoimmun Rev. 2002;1:329–337. doi: 10.1016/s1568-9972(02)00086-1. [DOI] [PubMed] [Google Scholar]
- 51.Wickham S, Carr DJ. Molecular mimicry versus bystander activation: herpetic stromal keratitis. Autoimmunity. 2004;37:393–397. doi: 10.1080/08916930410001713106. [DOI] [PubMed] [Google Scholar]
- 52.Lin YS, Lin CF, Fang YT, Kuo YM, Liao PC, Yeh TM, Hwa KY, Shieh CC, Yen JH, Wang HJ, Su IJ, Lei HY. Antibody to severe acute respiratory syndrome (SARS)-associated coronavirus spike protein domain 2 cross-reacts with lung epithelial cells and causes cytotoxicity. Clin Exp Immunol. 2005;141:500–508. doi: 10.1111/j.1365-2249.2005.02864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Molina V, Shoenfeld Y. Infection, vaccines and other environmental triggers of autoimmunity. Autoimmunity. 2005;38:235–245. doi: 10.1080/08916930500050277. [DOI] [PubMed] [Google Scholar]
- 54.Maya R, Gershwin ME, Shoenfeld Y. Hepatitis B virus (HBV) and autoimmune disease. Clin Rev Allergy Immunol. 2008;34:85–102. doi: 10.1007/s12016-007-8013-6. [DOI] [PubMed] [Google Scholar]
- 55.Buskila D. Hepatitis C-associated rheumatic disorders. Rheum Dis Clin North Am. 2009;35:111–123. doi: 10.1016/j.rdc.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 56.Pohl D. Epstein-Barr virus and multiple sclerosis. J Neurol Sci. 2009;286:62–64. doi: 10.1016/j.jns.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 57.Varani S, Frascaroli G, Landini MP, Söderberg-Nauclér C. Human cytomegalovirus targets different subsets of antigen-presenting cells with pathological consequences for host immunity: implications for immunosuppression, chronic inflammation and autoimmunity. Rev Med Virol. 2009;19:131–145. doi: 10.1002/rmv.609. [DOI] [PubMed] [Google Scholar]
- 58.Zhao ZS, Granucci F, Yeh L, Schaffer PA, Cantor H. Molecular mimicry by herpes simplex virus-type 1: autoimmune disease after viral infection. Science. 1998;279:1344–1347. doi: 10.1126/science.279.5355.1344. [DOI] [PubMed] [Google Scholar]
- 59.McClain MT, Heinlen LD, Dennis GJ, Roebuck J, Harley JB, James JA. Early events in lupus humoral autoimmunity suggest initiation through molecular mimicry. Nat Med. 2005;11:85–89. doi: 10.1038/nm1167. [DOI] [PubMed] [Google Scholar]
- 60.Fujinami RS, von Herrath MG, Christen U, Whitton JL. Molecular mimicry, bystander activation, or viral persistence: infections and autoimmune disease. Clin Microbiol Rev. 2006;19:80–94. doi: 10.1128/CMR.19.1.80-94.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim B, Kaistha SD, Rouse BT. Viruses and autoimmunity. Autoimmunity. 2006;39:71–77. doi: 10.1080/08916930500484708. [DOI] [PubMed] [Google Scholar]
- 62.Sfriso P, Ghirardello A, Botsios C, Tonon M, Zen M, Bassi N, Bassetto F, Doria A. Infections and autoimmunity: the multifaceted relationship. J Leukoc Biol. 2009;87:385–395. doi: 10.1189/jlb.0709517. [DOI] [PubMed] [Google Scholar]
- 63.Lin YS, Yeh TM, Lin CF, Wan SW, Chuang YC, Hsu TK, Liu HS, Liu CC, Anderson R, Lei HY. Molecular mimicry between virus and host and its implications for dengue disease pathogenesis. Exp Biol Med. 2011;236:515–523. doi: 10.1258/ebm.2011.010339. [DOI] [PubMed] [Google Scholar]
- 64.Saito M, Oishi K, Inoue S, Dimaano EM, Alera MT, Robles AM, Estrella BD, Jr, Kumatori A, Moji K, Alonzo MT, Buerano CC, Matias RR, Morita K, Natividad FF, Nagatake T. Association of increased platelet-associated immunoglobulins with thrombocytopenia and the severity of disease in secondary dengue virus infections. Clin Exp Immunol. 2004;138:299–303. doi: 10.1111/j.1365-2249.2004.02626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen MC, Lin CF, Lei HY, Lin SC, Liu HS, Yeh TM, Anderson R, Lin YS. Deletion of the C-terminal region of dengue virus nonstructural protein 1 (NS1) abolishes anti-NS1-mediated platelet dysfunction and bleeding tendency. J Immunol. 2009;183:1797–1803. doi: 10.4049/jimmunol.0800672. [DOI] [PubMed] [Google Scholar]
- 66.Wan SW, Lin CF, Lu YT, Lei HY, Anderson R, Lin YS. Endothelial cell surface expression of protein disulfide isomerase activates β1 and β3 integrins and facilitates dengue virus infection. J Cell Biochem. 2012;113:1681–1691. doi: 10.1002/jcb.24037. [DOI] [PubMed] [Google Scholar]
- 67.Immenschuh S, Rahayu P, Bayat B, Saragih H, Rachman A, Santoso S. Antibodies against dengue virus nonstructural protein-1 induce heme oxygenase-1 via a redox-dependent pathway in human endothelial cells. Free Radic Biol Med. 2013;54:85–92. doi: 10.1016/j.freeradbiomed.2012.10.551. [DOI] [PubMed] [Google Scholar]
- 68.Rothman AL. Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nat Rev Immunol. 2011;11:532–543. doi: 10.1038/nri3014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.