Abstract
Changes in biogeochemical cycles and the climate system due to human activities are expected to change the quantity and quality of plant litter inputs to soils. How changing quality of fresh organic matter (FOM) might influence the priming effect (PE) on soil organic matter (SOM) mineralization is still under debate. Here we determined the PE induced by two 13C-labeled FOMs with contrasting nutritional quality (leaf vs. stalk of Zea mays L.). Soils from two different forest types yielded consistent results: soils amended with leaf tissue switched faster from negative PE to positive PE due to greater microbial growth compared to soils amended with stalks. However, after 16 d of incubation, soils amended with stalks had a higher PE than those amended with leaf. Phospholipid fatty acid (PLFA) results suggested that microbial demand for carbon and other nutrients was one of the major determinants of the PE observed. Therefore, consideration of both microbial demands for nutrients and FOM supply simultaneously is essential to understand the underlying mechanisms of PE. Our study provided evidence that changes in FOM quality could affect microbial utilization of substrate and PE on SOM mineralization, which may exacerbate global warming problems under future climate change.
Human activities such as land use change and fossil fuel combustion are expected to directly and indirectly alter plant community structure and the quantity and quality of plant litter1,2. Previous studies have shown that leaf nitrogen (N) concentration tends to decrease and leaf lignin concentration tends to increase in response to elevated atmospheric CO23,4. As fresh organic matter (FOM) inputs are delivered to the soil, its decomposition and soil organic matter (SOM) mineralization are two primary pathways of soil C cycling. Therefore, if the quality of FOM is changed, it may affect (i) FOM decomposition and soil respiration at the ecosystem scale5,6,7,8, and (ii) the intensity of priming effect (PE) on SOM mineralization9,10. A considerable amount of research has examined the first potential consequence. However, less is known about how changing quality of FOM may influence PE on SOM mineralization.
PE is defined as the changes in SOM mineralization after the inputs of exogenous substrate9. Our current understanding of the impacts of FOM quality on PE is still under debate. The addition of higher quality substrate (indicated by lower C:N ratio and lower lignin content) has been found to lead to a greater11, an equal12, or a lower positive PE on SOM mineralization13,14 than the addition of lower quality substrate. At present, there are two theories to explain the underlying mechanisms of positive PE under FOM addition: Theory I: FOM serves as an energy source for microorganisms to synthesize extracellular enzymes capable of degrading recalcitrant SOM, facilitating SOM mineralization, i.e. the “co-metabolism” theory15; and Theory II: FOM stimulates soil microbial growth and aggravates nutrients limitation, increasing the mineralization of SOM which contains higher amount of N and other nutrients than FOM, i.e. the “N-mining” theory9,16. Based on the first theory, we should expect that FOM with an optimized C:N ratio matching microbial demands and with more labile C would be the most efficient to stimulate microbial activity and positive PE17. The optimized C:N ratio for microbes is believed to be around 20, calculated by dividing microbial C:N ratio (10) by the C assimilation yield of microbial biomass (0.5)18,19. Hence, under natural conditions, leaf residues with a C:N ratio closer to 20 and lower lignin content would be expected to yield a larger PE than stem or root residues with a higher C:N ratio and higher lignin content. On the contrary, based on the second theory, FOM with higher C:N ratio would lead to higher N limitation and greater PE on SOM mineralization. Of course, these two mechanisms do not necessarily have to be alternative, but may happen simultaneously. However, those contradictory findings of PE induced by FOM with different quality may be because one mechanism dominates over the other in different cases17. Nevertheless, both theories highlight the potentially important role of soil microbial community in regulating PE on SOM mineralization. If we understand how the microbial community responds to different FOM addition while studying PE, we may be able to explain the contradiction and predict the effects of FOM quality on PE.
The quality of FOM may influence soil microbial community structure because microbes exhibit substrate preference20. For example, FOM with higher C:N ratio and higher lignin content tended to increase the relative abundance of fungi and actinomycetes which are adapted to nutrient poor environments21,22, whereas FOM of higher quality has been found to facilitate the growth of Gram negative bacteria23. Therefore, the magnitude and direction of PE may be altered by changing quality of FOM due to the modification of microbial community structure. However, our understanding is still limited by the scarce information on microbial responses to different FOM addition. Here we used 13C-labeled FOM to track C flow from FOM to microbial phospholipid fatty acids (PLFA) and to quantify PE on SOM mineralization simultaneously. Leaf and stalk litter from 13C-labelled Zea mays L. were used as FOM of different chemical quality based on their contrasting C:N ratio, nutrient content, and lignin content. We hypothesized that stalk (i.e. lower quality FOM) would induce higher positive PE based on the “N-mining” theory because its high C:N ratio means it would lead to more severe N limitation. The objectives of this study were to: (i) determine how the quality of FOM affects PE and which one of the above mentioned mechanism was predominant; (ii) investigate whether the quality of FOM modifies the structure and substrate utilization pattern of soil microbial community; and (iii) address the possible links between soil microorganisms and PE. Two forest soils (Larch Plantation soil and Secondary Forest soil) with different resource history and soil properties were used to test the generality of our findings.
Results
CO2 production and priming effect
For both soils, microbial respiration of FOM amended soils was significantly higher than that of control (P < 0.01) (Fig. 1). Temporal dynamics of CO2 production were similar in two soils (Fig. 1). During 0 ~ 9 d of the incubation, leaf treatment had higher total CO2 respiration, higher SOM-derived CO2, but lower FOM-derived CO2 than stalk treatment. During 9 ~ 30 d of the incubation, total CO2 respiration and FOM-derived CO2 were higher under stalk treatment than under leaf treatment, but SOM-derived CO2 was similar under both FOM treatments. During 30 ~ 105 d of the incubation, there was no difference in FOM-derived CO2 between the two treatments, but SOM-derived CO2 under stalk treatment was significantly higher than that under leaf treatment (Fig. 1, Table 1). At the end of the 105 d incubation, cumulative CO2 production under stalk treatment was 2.18% and 7.69% higher than under leaf treatment for Larch Plantation soil and Secondary Forest soil, respectively (Table 1). For Larch Plantation soil, cumulative SOM mineralization was not different between treatments, but for Secondary Forest soil, cumulative SOM mineralization under stalk treatment was 3.57% higher (P < 0.05) than that under leaf treatment (Table 1).
Figure 1.
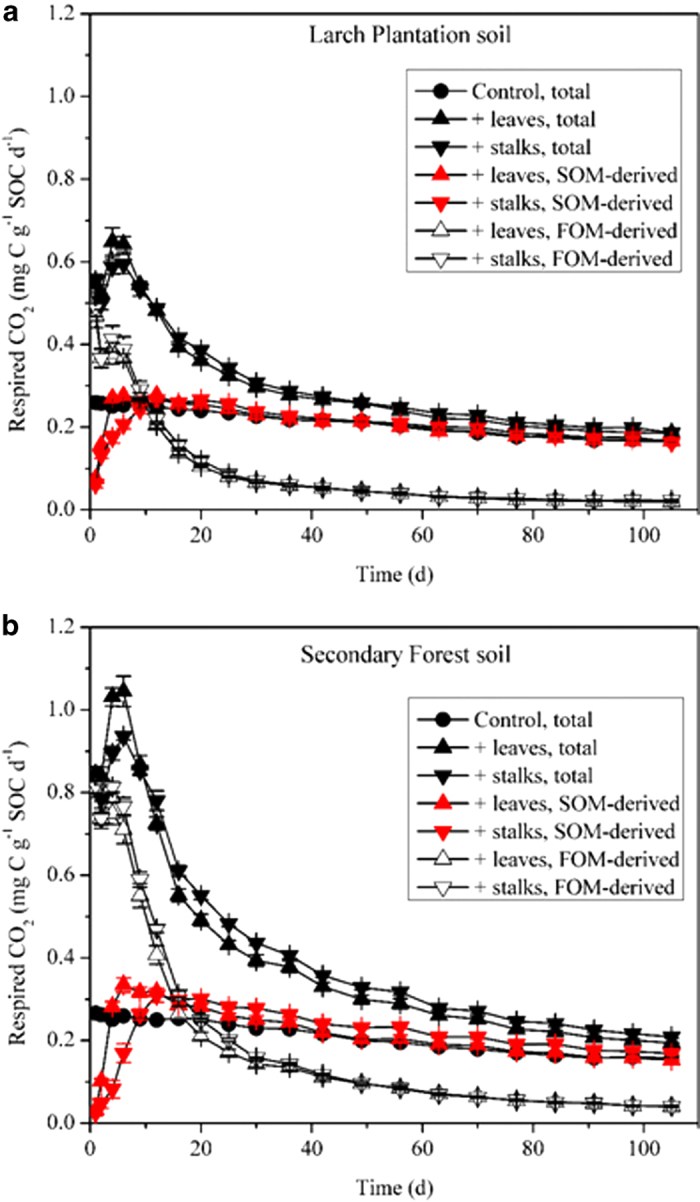
Dynamics of total, soil organic matter (SOM)-derived, and fresh organic matter (FOM)-derived CO2 flux (mg C g-1 SOC d-1) over the 105-day incubation for Larch Plantation soil (a) and Secondary Forest soil (b) which were treated with different FOMs. Means ± 1SD (n = 4) are shown.
Table 1. Cumulative CO2 production, soil organic matter (SOM) mineralization and fresh organic matter (FOM) mineralization during 0~9 d, 9~30 d, 30~105 d, and the whole period of the incubation.
|
Larch Plantation soil |
Secondary Forest soil |
|||
|---|---|---|---|---|
| +leaves | +stalks | +leaves | +stalks | |
| CO2 production (mg C kg-1 soil) | ||||
| 0~9 d | 171.13a | 161.78b | 367.46a | 338.38b |
| 9~30 d | 266.91b | 277.65a | 510.62b | 557.11a |
| 30~105 d | 560.89b | 581.29a | 895.06b | 971.00a |
| 0~105 d | 998.93b | 1020.72a | 1773.14b | 1866.49a |
| 0~9 d | 0.21a | 0.16b | 0.22a | 0.12b |
| 9~30 d | 0.53a | 0.54a | 0.59a | 0.61a |
| 30~105 d | 1.44b | 1.49a | 1.43b | 1.59a |
| 0~105 d | 2.18a | 2.19a | 2.24b | 2.32a |
| FOM mineralization (% of added FOM) | ||||
| 0~9 d | 14.86a | 15.70a | 14.15b | 14.93a |
| 9~30 d | 13.01b | 14.29a | 12.87b | 14.82a |
| 30~105 d | 12.37a | 12.95a | 13.38a | 13.67a |
| 0~105 d | 40.25a | 42.93a | 40.40b | 43.43a |
Different letters in the same line denote significant differences (P < 0.05) between two FOM treatments based on Independent-Samples T Test (n = 4).
Temporal dynamics of relative PE were similar for both FOM treatments and the two soil types throughout the incubation period: all started with a short phase of strong negative PE and then went into a long phase of relatively stable positive PE (Fig. 2). Stalk treatment generally had larger variations of PE than leaf treatment for both soils, with a longer negative PE (9 days for stalk treatment and 2 days for leaf treatment) in the first phase and a stronger (P < 0.05) positive PE in the later phase (16 ~ 105 d) (Fig. 2). The cumulative PE after 105 d incubation was positive for both treatments and both soils, and was higher in stalk treatment than in leaf treatment (Fig. 3).
Figure 2.
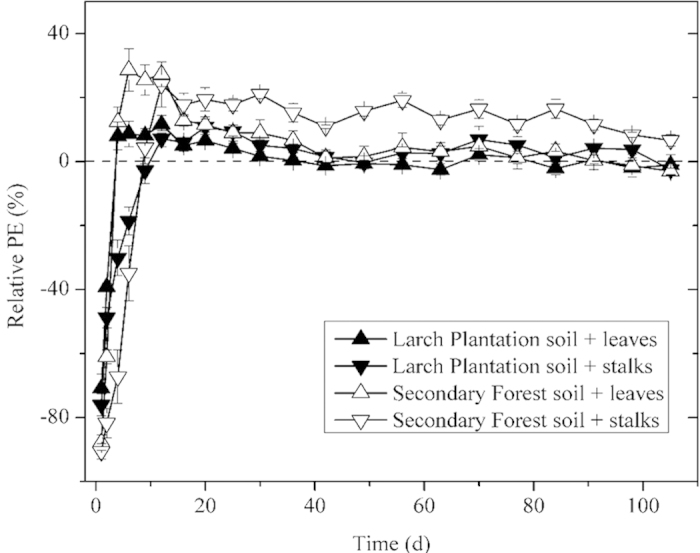
Temporal dynamics of relative priming effect (PE) for different fresh organic matter (FOM) treatments over the incubation period. Results are means ± 1SD (n = 4) on every single time-point.
Figure 3.
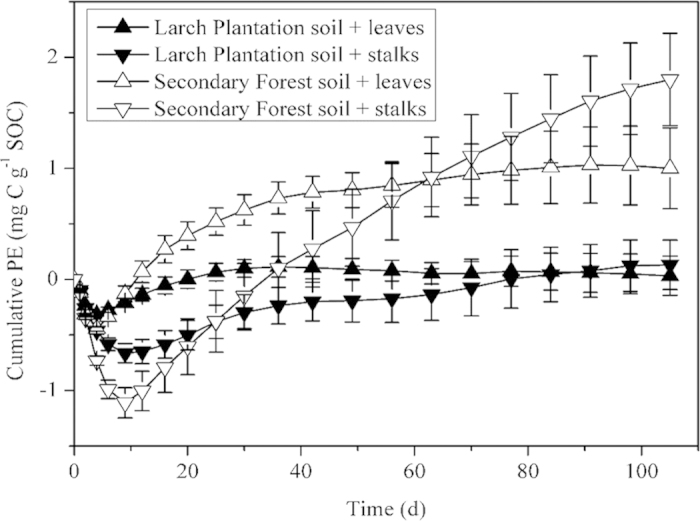
Changes of cumulative priming effect (PE) for different fresh organic matter (FOM) treatments with the incubation time. Means ± 1SD (n = 4) are shown.
Microbial community structure and 13C incorporation into PLFA
FOM addition significantly increased the concentration of both total PLFAs and the PLFAs associated with each microbial taxonomic grouping (P < 0.05) at both sampling times (Table 2). Bacteria to fungi ratio was not significantly affected by FOM addition in most cases (P > 0.05). The relative abundance of bacteria and fungi was enhanced (P < 0.05), whereas the relative abundance of actinomycetes was decreased (P < 0.05) by FOM addition (Table 2). However, there was no difference between the two FOM treatments (P > 0.05) (Table 2). PCA analysis showed soil microbial community structure was significantly affected by FOM addition, soil type, and incubation time, but was not different (P > 0.05) between the two FOM treatments, indicated by communities with the closest scores on the PC1 and PC2 (Fig. 4, Table S1).
Table 2. Concentration (nmol g-1 soil) and relative abundance (%) of selected phospholipid fatty acid (PLFA) groups in fresh organic matter (FOM) treated soils on 0 d, 20 d, and 105 d of the incubation.
| Larch Plantation soil |
Secondary Forest soil |
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total PLFAs | G+ | G- | Bacteria | Fungi | Actinomycetes | Total PLFAs | G+ | G- | Bacteria | Fungi | Actinomycetes | ||||||||||||
| PLFA concentration (nmol g-1 soil) | |||||||||||||||||||||||
| Initial | 74.76 | 25.17 | 15.38 | 42.47 | 13.74 | 7.74 | 104.00 | 32.50 | 22.78 | 57.72 | 19.81 | 9.98 | |||||||||||
| 20d | |||||||||||||||||||||||
| Control | 63.61c | 21.04c | 13.06c | 35.69c | 11.67c | 6.34c | 94.10c | 30.34c | 20.63c | 52.99c | 17.23c | 9.02c | |||||||||||
| +leaves | 119.19a | 40.69a | 24.37a | 68.35a | 22.95a | 10.49a | 181.30a | 60.45a | 37.06a | 102.14a | 36.27a | 14.43a | |||||||||||
| +stalks | 101.26b | 34.32b | 21.91b | 58.91b | 19.53b | 8.61b | 161.35b | 54.30b | 33.51b | 92.04b | 31.84b | 12.26b | |||||||||||
| 105d | |||||||||||||||||||||||
| Control | 67.14b | 22.87b | 13.40b | 38.14b | 11.38b | 7.02b | 96.35b | 32.68b | 18.94b | 54.02b | 16.34b | 10.04b | |||||||||||
| +leaves | 102.52a | 37.55a | 19.39a | 60.28a | 18.60a | 8.74a | 141.05a | 50.18a | 27.41a | 81.53a | 25.34a | 11.85a | |||||||||||
| +stalks | 104.95a | 37.22a | 20.72a | 61.22a | 19.15a | 9.57a | 147.67a | 51.75a | 28.68a | 84.37a | 26.60a | 13.01a | |||||||||||
| PLFA relative abundance (%) | |||||||||||||||||||||||
| Initial | — | 33.65 | 20.58 | 56.80 | 18.39 | 10.34 | — | 31.26 | 21.92 | 55.52 | 19.05 | 9.59 | |||||||||||
| 20d | |||||||||||||||||||||||
| Control | — | 33.07a | 20.54a | 56.11b | 18.35b | 9.97a | — | 32.26b | 21.93a | 56.34a | 18.30b | 9.59a | |||||||||||
| +leaves | — | 34.15a | 20.44a | 57.34a | 19.25a | 8.80b | — | 33.34a | 20.44b | 56.34a | 20.00a | 7.96b | |||||||||||
| +stalks | — | 33.90a | 21.64a | 58.18a | 19.29a | 8.50b | — | 33.66a | 20.77b | 57.05a | 19.73a | 7.60b | |||||||||||
| 105d | |||||||||||||||||||||||
| Control | — | 34.06b | 19.94a | 56.79b | 16.97b | 10.46a | — | 33.92c | 19.65a | 56.07c | 16.96b | 10.42a | |||||||||||
| +leaves | — | 36.64a | 18.92b | 58.81a | 18.14a | 8.52b | — | 35.58a | 19.44a | 57.82a | 19.97a | 8.39b | |||||||||||
| +stalks | — | 35.46a | 19.75a | 58.33a | 18.26a | 9.11b | — | 35.04b | 19.42a | 57.14b | 18.01a | 8.81b | |||||||||||
Letters in the same column for each sampling time denote significant differences (p < 0.05) between FOM treatments (n = 4). -, not available.
Figure 4.
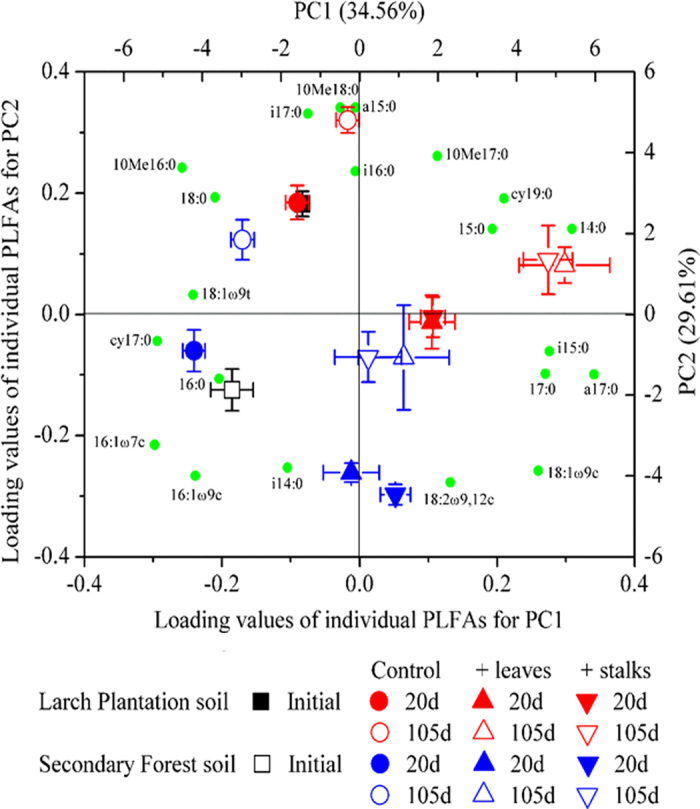
Principal components analysis (PCA) of phospholipid fatty acid (PLFA) relative abundance (%) as affected by fresh organic matter (FOM) treatments at 20 d and 105 d of the incubation for both soils. Both score plot of treatments and loading values of individual PLFAs are shown.
The distribution of FOM-derived 13C incorporation into PLFAs was strongly affected by FOM treatment, soil type, and incubation time (Fig. 5, Table S1). PC1 and PC2 explained 36.89% and 24.33% of the total variance of 13C distribution among PLFAs, respectively. Actinomycetes (10Me16:0; 10Me17:0; 10Me18:0) showed a strong positive loading on PC1 while fungi (18:2ω9,12c; 18:1ω9c; 18:1ω9t) showed a negative one (Fig. 5). For both sampling times, 13C distribution proportion within Gram-positive bacteria (Table 3) and common PLFAs (16:0 and 18:0, data not shown) under leaf treatment was higher (P < 0.05) than that under stalk treatment. In contrast, 13C distribution proportion within fungi and actinomycetes under stalk treatment tended to be higher (P < 0.05) than that under leaf treatment for both soils (Table 3). The highest proportion of FOM-derived C to total C was found in fungal PLFAs (Table 3). The proportion of FOM-derived C to total C in PLFAs of bacteria and fungi generally decreased with incubation time, whereas that of actinomycetes was significantly higher (P < 0.05) at 105 d of the incubation than at 20 d (Table 3).
Figure 5.
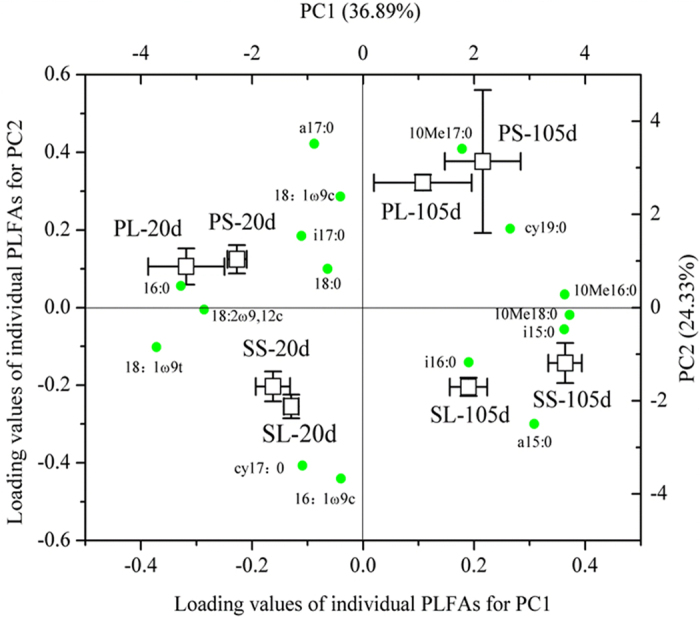
Principal components analysis (PCA) of 13C distribution among phospholipid fatty acids (PLFAs) (relative 13C incorporation into each individual PLFA (%)) as affected by fresh organic matter (FOM) treatments at 20 d and 105 d of the incubation. Both score plot of treatments and loading values of individual PLFAs are shown. PL, Larch Plantation soil+leaves; PS, Larch Plantation soil+stalks; SL, Secondary Forest soil+leaves; SS, Secondary Forest soil+stalks.
Table 3. Proportion of fresh organic matter (FOM) derived C to total C and 13C distribution among selected phospholipid fatty acid (PLFA) groups in FOM treated soils on 0 d, 20 d, and 105 d of the incubation.
|
Larch Plantation soil |
Secondary Forest soil |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G+ | G- | Bacteria | Fungi | Actinomycetes | G+ | G- | Bacteria | Fungi | Actinomycetes | ||||||
| Proportion of FOM-derived C to total C in PLFAs (%) | |||||||||||||||
| 20d | |||||||||||||||
| +leaves | 5.37a | 4.96a | 5.22a | 9.66b | 1.80b | 9.67a | 10.70a | 10.03a | 15.69a | 5.87a | |||||
| +stalks | 4.78b | 4.77a | 4.78b | 10.40a | 2.55a | 7.68b | 9.70b | 8.39b | 16.32a | 6.82a | |||||
| 105d | |||||||||||||||
| +leaves | 4.49a | 3.81a | 4.27a | 6.62a | 4.08a | 9.17a | 9.02a | 9.12a | 12.13a | 7.66b | |||||
| +stalks | 4.27a | 3.74a | 4.09a | 7.64a | 4.96a | 9.40a | 9.18a | 9.33a | 12.38a | 9.27a | |||||
| 13C distribution among PLFA groups (%)* | |||||||||||||||
| 20d | |||||||||||||||
| +leaves | 25.39a | 12.67b | 38.06a | 26.96b | 2.29b | 25.12a | 15.09a | 40.20a | 26.19b | 3.91b | |||||
| +stalks | 23.94a | 13.84a | 37.78a | 30.97a | 3.35a | 22.69b | 15.66a | 38.36b | 30.26a | 4.86a | |||||
| 105d | |||||||||||||||
| +leaves | 29.64a | 12.06a | 41.70a | 22.63b | 6.51a | 30.36a | 14.68a | 45.05a | 21.78a | 6.39b | |||||
| +stalks | 27.18b | 12.34a | 39.52a | 26.20a | 8.57a | 30.61a | 14.73a | 45.34a | 22.22a | 8.12a | |||||
Letters in the same column for each sampling time denote significant differences (P < 0.05) between FOM treatments (n = 4). Note: The proportion of FOM-derived C to total C in each microbial group (e.g. bacteria, fungi) was calculated as: 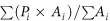 ; 13C distribution proportion in each microbial group (e.g. bacteria, fungi) was calculated as:
; 13C distribution proportion in each microbial group (e.g. bacteria, fungi) was calculated as: 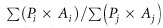 ; where Pi and Ai are the proportion of FOM-derived C to total C and the abundance of each PLFA for a special microbial group (e.g. bacteria, fungi), respectively; while Pj and Aj (j = 1, 2∙∙∙∙∙∙16) are those for all 16 PLFAs detected.
; where Pi and Ai are the proportion of FOM-derived C to total C and the abundance of each PLFA for a special microbial group (e.g. bacteria, fungi), respectively; while Pj and Aj (j = 1, 2∙∙∙∙∙∙16) are those for all 16 PLFAs detected.
*As the results of common saturated PLFAs (16:0, 18:0), which were not assigned to a taxonomic group (bacteria, fungi or actinomycetes), were not shown, the sum of these % was less than 100%.
Discussion
For both soils and both treatments, FOM addition generally reduced SOM mineralization (negative PE) during the early stage of the incubation, and stimulated SOM mineralization (positive PE) after that (Fig. 1, Fig. 2). These temporal variations of PE agree with previous reports14,24. Negative PE has often been explained as the result of “preferential substrate utilization”, meaning that the preference of microorganisms for substrates switched from relatively recalcitrant SOM to amended FOM24,25. Negative PE has been found previously when highly labile substances such as glucose or sucrose was added15. For plant residue addition, a recent study suggested negative PE only happened when high quality grass litter was added but not when low quality bracken litter was added14. The discrepancy between our results and the above mentioned study may be attributed to differences in the land cover/land use history of the soils utilized in our work. The forest soils we employed in this study receive litterfall and root inputs that are relatively low quality and characterized by high C:N ratio and lignin content. When we added the higher quality, more labile FOM amendments to these soils, the microbial community preferentially consumed these amendments and caused a negative PE on SOM mineralization. After the initial short period of negative PE, positive PE began and lasted till the end of the incubation (Fig. 2). The dynamic of positive PE, i.e. an initial large pulse followed by a smaller and more prolonged flux, is consistent with most previous findings26,27. The initial pulse has been explained as effect of soluble C input leached from plant residues27.
It is interesting to note that high quality FOM (leaf) treatment switched from negative PE to positive PE faster than low quality FOM (stalk) did; however, its superiority only persisted for a very short period (4 ~ 12 days) and then stalk treatment showed higher PE than leaf treatment (Fig. 2). We believe this is because microbial biomass increased during the negative PE period until they had largely consumed the resources associated with the added FOM. As FOM resources became depleted, microbes had to increase utilization of SOM to meet their energy and nutrient requirements, resulting in a positive PE. For high quality FOM addition, microbial biomass grew faster, requiring less time to reach positive PE. This hypothesis can be supported by the greater microbial growth on the 20 d of the incubation under leaf treatment (Table 2). Later, PE got into a stable period and stalk treatment stimulated a greater positive relative and cumulative PE than leaf treatment did (Fig. 2, Fig. 3). As introduced above, this phenomenon is consistent with the “nutrient mining” theory (Theory II) because low quality FOM would lead to more severe nutrient limitation and thereby greater PE, but not with the “co-metabolism” theory (Theory I) which suggests that high quality FOM would yield greater PE due to more sufficient energy for the synthesis of SOM-degrading enzymes. Therefore, our results supported the dominance of “nutrients mining” mechanism during the stable positive PE period. However, for the short period when leaf treatment had higher positive PE than stalk treatment, “co-metabolism” theory may be predominant.
Based on PLFA analyses, we found that both the size and structure of the soil microbial community were significantly altered by FOM addition (Fig. 4, Table 2). During both sampling events, FOM addition stimulated the growth of total microbial biomass and every microbial group (Table 2), which is consistent with many previous findings28,29. However, we found the bacteria to fungi ratio was not changed significantly by FOM addition, suggesting relative stable microbial community structure. For the relative abundance, we found a significant increase of bacteria and fungi, whereas the relative abundance of actinomycetes declined due to FOM addition, which indicated that FOM addition was more beneficial for bacteria and fungi.
Table 4. Chemical and physical characteristics of soils. Mean values are shown (n = 4).
| Larch Plantation soil | Secondary Forest soil | |
|---|---|---|
| SOC (%) | 3.31 | 4.48 |
| TN (%) | 0.31 | 0.38 |
| C:N | 10.67 | 11.69 |
| δ13CV-PDB (%o) | -26.61 | -27.22 |
| pH (H2O) | 5.5 | 5.8 |
| SWC (%, dry basis) | 42.4 | 50.5 |
| Water-stable aggregate size fraction (%) | ||
| Macroaggregate (250 ~ 2000 μm) | 16.21 | 32.54 |
| Microaggregate (53 ~ 250 μm) | 37.70 | 37.99 |
| Silt plus clay particles (<53 μm) | 46.09 | 29.47 |
| Forest C input (litterfall+fine root) (g m-2 y-1)* | 229.4 | 594.3 |
| Basal respiration (mg C kg-1 soil d-1) | 8.03 | 11.04 |
| SOC-specific basal respiration (mg C kg-1 SOC d-1) | 242.6 | 246.5 |
| Microbial biomass (mg C kg-1 soil) | 529.6 | 727.9 |
| NO3--N (mg kg-1 soil) | 28.03 | 29.81 |
| NH4+-N (mg kg-1 soil) | 7.25 | 9.12 |
SOC, soil organic carbon; TN, total nitrogen; SWC, soil water content.
*Forest C input was reported by Yang et al. (2010) ref. 39.
At 20 d of the incubation, PLFAs of bacteria, fungi and actinomycetes under leaf treatment were all higher than those under stalk treatment (Table 2). However, this difference did not last till the end of the incubation (Table 2). While leaf addition stimulated more growth of microbial biomass, none of the relative abundance of bacteria, fungi, and actinomycetes was different between the two FOM treatments, implying minimal effect of FOM quality on microbial composition. Nevertheless, we found that the proportions of FOM-derived C to total C in fungal and actinomycetic PLFAs were generally higher under stalk treatment than under leaf treatment at 20 d of the incubation (Table 3). In contrast, the proportion of FOM-derived C to total C in bacterial PLFAs (especially Gram positive bacteria) was much higher under leaf treatment (Table 3). These findings suggested that fungi and actinomycetes were better adapted to nutrient poor environment than bacteria, which has been reported before14,30.
Bacteria contributed more to the metabolism of fresh organic substrate as indicated by the higher 13C distribution proportion in bacterial PLFAs than other microbial groups under both treatments (Table 3), which is in accord with many previous findings31,32,33 and could mainly be attributed to the higher relative abundance of bacteria in these soils (Table 2). However, the higher proportion of FOM-derived C to total C in fungal PLFAs (Table 3) suggested that fungi was more efficient in using fresh organic substrate than bacteria and actinomycetes, which has also been reported before34. Gram-positive and Gram-negative bacteria did not show difference in using FOM-derived C. In contrast to the other microbial groups, actinomycetes had higher proportion of FOM-derived C to total C with increasing incubation time, indicating their competitive advantage under nutrient poor conditions after exhaustion of labile substrate.
At 20 d and 105 d of the incubation, positive PE was found to be higher under stalk treatment than under leaf treatment (Fig. 2). However, microbial biomass was found to be higher under leaf treatment than under stalk treatment at 20 d of the incubation and had no difference at 105 d of the incubation (Table 2). In addition, microbial community composition was not different between the two FOM treatments, indicated by the relative abundance of PLFA (Fig. 4, Table 2). These results together suggested that microbial biomass and microbial structure were not determinants of PE. This is contrary to the “co-metabolism” theory because if this mechanism existed, higher microbial biomass would have caused higher PE since microbes at the same time would use more SOM. Our results suggested that some FOM-stimulated microbes might use FOM only and did not necessarily use more SOM and cause positive PE.
Results of 13C distribution in PLFA biomarkers further suggested that fungi were responsible for using FOM but not promoting PE, while bacteria were the group responsible for positive PE. First, for bacteria, we found their biomass increased significantly in response to leaf and stalk addition (Table 2), but only a small proportion of the increased C was from 13C-labelled FOM (Table 3). So the increased C must be from SOM, meaning potential positive PE on SOM mineralization. The higher proportion of FOM-derived C to total C under leaf treatment was also consistent with lower PE we observed under leaf treatment (Fig. 2, Fig. 3, Table 3). Therefore, it is reasonable to speculate that bacteria are the microbial group responsible for positive PE. This viewpoint has been proposed before26, but some evidence did not support this claim28,34. Secondly, for fungi, we also found an increase of their biomass by FOM addition, especially by leaf treatment (Table 2). However, a high proportion of the increase was from 13C-labelled FOM (Table 3), meaning fungi may not be the major contributor to the increase of SOM mineralization and positive PE. Finally, for actinomycetes, their abundance was less than 10% of total microbial abundance (indicated by PLFA) in our studied soils (Table 2); hence their role in regulating PE should be minor. The average C:N ratio of bacteria, fungi, and actinomycetes is 5, 12, and 5 respectively35. We believe the reason for higher contribution to positive PE by bacteria is that they are more N-limited and they need N from SOM once their abundance increases. Fungi and actinomycetes have been found to acclimatize to N-limiting conditions much better than bacteria21,36. Of course, fungi and actinomycetes may also contribute to positive PE because the optimum substrate C:N ratios for them (as explained above) are still lower than C:N ratios of added FOM. These results further supported the “nutrient mining” theory, which purports that the more nutrient limited group of microbes will be responsible for positive PE.
In summary, we propose a conceptual model to explain the underlying mechanisms of relative PE during the incubation of our soils (Fig. 6). Addition of leaf and stalk FOM first caused negative relative PE for a short period of time (less than 10 days, A in Fig. 6) due to preferential substrate utilization, and then positive PE prevailed till the end of the incubation. It took less time for leaf treatment to switch from negative PE to positive PE than for stalk treatment because the increase of microbial biomass stimulated by FOM addition was higher under leaf treatment. With increasing microbial biomass, microbes at the same time used more SOM (co-metabolism) and caused positive PE. During this short period (B in Fig. 6), positive PE was higher under leaf treatment than under stalk treatment because microbes were mainly C limited37 and leaf FOM had higher labile C, stimulating higher microbial growth. Quickly after this initial boom of microbial biomass, microbes became limited by other nutrients such as N and had to use SOM for these nutrients (nutrients mining). Then positive PE reached a stable state (C in Fig. 6) and was higher under stalk treatment than under leaf treatment because the C:N ratio of stalk was higher, causing higher N limitation. Bacteria were the microbes mainly responsible for positive PE during this period (C in Fig. 6) because they were more limited by N. If future climate change reduces the quality of litter3,4, positive PE may be increased due to “microbial N mining” from SOM, causing higher SOM mineralization and CO2 emission fluxes and exacerbating global warming problems. It should be noted that although this conceptual model is applicable to both soils we studied, the generality of this model requires further assessment on account of the limitation of our approach: the fact that we used two parts of the same plants but not the same part of a plant grown either in “current climatic conditions (e.g. standard CO2 conditions” or in “future climatic conditions (e.g. increased CO2 conditions)”.
Figure 6.
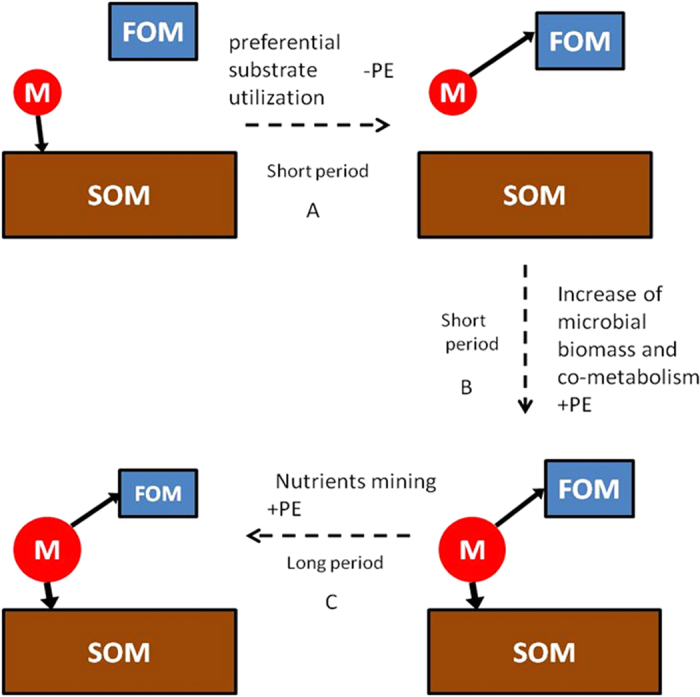
Conceptual model showing the temporal dynamics of the priming effect (PE) and its underlying mechanisms during the incubation of our studied soils. The solid arrows represent the tendency of microbes to utilize different substrates; the dotted arrows show the underlying mechanisms of priming. “M” in red circles represents soil microbes; FOM represents fresh organic matter; and SOM represents soil organic matter. They are for illustrative purposes only and do not represent actual shape or size.
In conclusion, the two soils examined in this study showed consistent results: negative PE appeared in the early period after FOM addition, which suggested that soil microbes preferentially utilized more labile substrate. Soils amended with leaf tissue switched faster from negative PE to positive PE than soils amended with stalk tissue, due to greater microbial growth stimulated by leaf FOM during this short period. After the exhaustion of easily available nutrients, positive PE was higher under stalk treatment than under leaf treatment from approximately 16 d until the end of the incubation. During this period of stable positive PE, microbial biomass and microbial community structure did not seem to be the determinants of PE. Instead, microbial C:N ratios and microbial demand for carbon and other nutrients were major determinants of PE. If more carbon was needed, most microbes preferentially used FOM with more labile C, which would not induce positive PE; and if more N was needed, they had to use SOM with higher N content, which would cause positive PE. Therefore, our results suggested that FOM quality and microbial demands acted together to determine PE. Future studies should consider incorporating this effect of litter quality on PE into C cycling models for better understanding and predicting ecosystem responses to climate change.
Methods
Study area and soils
Soils were collected from the 0–15 cm depth increment in a 60-year-old natural secondary forest, and a 35- to 45-year-old larch plantation located at the Qingyuan Experimental Station, Liaoning Province, China (41°51′ N, 124°54′ E, 500 ~ 1100 m above sea level). For detailed descriptions of the Secondary Forest and Larch Plantation sites, see Yang et al.38. Soils at both sites are typical brown forest soils with a silty loam texture and are classified as Udalfs according to the second edition of US Soil Taxonomy38. The chemical and physical properties of the soils are shown in Table 4. At each site, six soil cores (15 cm × 15 cm, 0-15 cm) were randomly collected after removal of surface plant litter and then bulked together. Fresh soils were passed through a 2 mm sieve and visible plant residues were removed prior to storage at 4 °C for later measurements.
Production of 13C-labeled plant material
Zea mays L. (yellow field maize, Paymaster Hybrid 8951) was grown under field conditions at the USDA/ARS Rice Research Unit in Beaumont, Texas. A portion of one row of corn at the grain-filling portion of the life cycle was covered with a transparent chamber (3 m × 0.9 m × 2.4 m, L × W × H) constructed with PVC tubing and polyethylene film, and then labeled with 50 L of 99.3 atom % 13CO2 delivered continuously between 1200-1600 hours, after which the chamber was removed. Maize plants continued to grow in the field until they were harvested 28 days post-labeling. Leaves and stalks were separated from the whole plant, dried at 50 °C, and then finely milled (passed through a 2 mm screen) prior to incubations. Stalks had higher C:N ratio and higher lignin content than leaves (Table 5), and were therefore considered as lower quality FOM.
Table 5. Chemical characteristics of fresh organic matter (FOM) used in soil incubations.
| C (%) | N (%) | C:N | Klason-lignin (%) | Ash (%) | δ13CV-PDB (%o) | |
|---|---|---|---|---|---|---|
| Stalks | 44.8 | 1.0 | 44.8 | 11.4 | 4.0 | 132.6 |
| Leaves | 43.0 | 1.5 | 29.4 | 8.6 | 9.6 | 142.9 |
Incubation design
For the incubation, six treatments (2 soil types×3 FOM treatments) with 8 replicates were set up: Larch Plantation soil control (without FOM addition), Larch Plantation soil+leaves, Larch Plantation soil+stalks; Secondary Forest soil control, Secondary Forest soil+leaves, Secondary Forest soil+stalks. Four replicates were used to measure soil CO2 efflux and δ13CO2 (n = 4); the other four replicates were prepared for destructive sampling at 20 d of the incubation to quantify soil microbial community structure. We checked the respiration rate in the flasks used for destructive sampling and those used for PE during the initial 3 times of air sampling (i.e. 1 d, 2 d and 4 d after FOM addition), and found there was no difference (P > 0.05) between them. The rates of C added as FOM to each of the two soil types were chosen to mimic rates of annual C input (litterfall+fine root) into top 0-15 cm soils under field conditions (see Table 4)39: calculated 0.691 g C kg−1 dry soil for Larch Plantation soils and 1.905 g C kg−1 dry soil for Secondary Forest soils for our incubation.
First, 200 g fresh soil at ambient field moisture content was added to a 1.0 L Mason jar. The jar was sealed with Parafilm® M (Bemis Company, Neenah, WI) during incubation to minimize evaporation without affecting gas exchange. Soils were pre-incubated for 10 days at 20 °C to stabilize the disturbance of previous soil preparation and to recover microbial activity that may have been diminished during the storage period. Then, FOM was added to pre-incubated soils and mixed to distribute the material homogeneously. This procedure was also conducted in controls although no FOM was added. These jars were then incubated for 105 days (post FOM addition) at 20 °C in darkness. Soil water content was maintained at original soil moisture throughout the incubation by daily addition of deionized water as necessary.
Soil CO2 efflux and δ13CO2
Four replicates of each treatment were utilized to measure soil CO2 efflux and δ13CO2 for the entire duration of the incubation experiment. The accumulation method was used to quantify CO2 production40. Each jar was first flushed with CO2-free air for 1 h to reduce headspace CO2 concentration to < 10 ppm, then hermetically sealed for 6 h with a silicone stopper fitted with two three-way stopcocks (the highest CO2 concentration was about 2800 ppm after air accumulation, thus the environment in the jar would not be anoxic). After that, 150 ml gas sample was taken from each jar with a syringe through the three-way stopcock and injected into an evacuated aluminum foil airbag (200 ml). A gas substitution procedure was conducted to avoid gas exchange during sampling: 150 ml CO2-free air was injected to the jar using the syringe; the syringe was gently pumped up and down for 5 times; and then gas samples were taken. The decrease of CO2 concentration due to gas substitution was corrected before statistical analysis. CO2 concentrations were determined at 1, 2, 4, 6, 9, 12, 16, 20, 25, 30, 36, 42, 49, 56, 63, 70, 77, 84, 91, 98 and 105 day after FOM addition. Gas samples were analyzed for CO2 concentration and δ13CO2 values within 24 h after sampling using a carbon dioxide isotope analyzer (CCIA-36d-EP; LGR, Mountain View, CA, USA). The reproducibility and accuracy of the analytical procedure checked with reference gas (361.5 ppm, with a δ13C value of -8.935) were better than 1.0 ppm and 0.4 ppm for CO2 concentration, whereas 0.4%o and 0.2%o for δ13CO2, respectively (n = 4).
Soil sampling and chemical analysis
Before treatments and after pre-incubation, four samples of each soil type were collected to measure microbial biomass C (MBC), NO3−-N and NH4+-N concentrations. In addition, four subsamples of each treatment were sampled at 20 d and 105 d after FOM addition; these soil samples were freeze-dried and ground with a ball mill (Retsch MM200; Haan, Germany) for analysis of phosopholipid fatty acids (PLFAs).
The concentration of C and N in initial bulk soil and FOM were determined by an element analyzer (Model CN, vario Macro Elementar, GmbH, Germany). Klason-lignin content of FOMs was measured according to the gravimetric method of Theander and Westerlund41. The δ13C signatures of SOC and FOM were determined using a stable isotope ratio mass spectrometer (Thermo Finnigan, DELTA Plus XP) interfaced with an elemental analyzer (Flash EA 1112). MBC was determined by fumigation-extraction method42.
PLFA analyses
Soil samples before treatments (after pre-incubation) and at 20 d and 105 d after treatments were analyzed for PLFA using a modified Bligh-Dyer method28. Briefly, 5 g of freeze-dried soil was extracted with phosphate buffer-chloroform-methanol (0.8:1:2), and then the phospholipids were separated and eluted with methanol on a silicic acid column. PLFAs were mildly derivatized to fatty acid methyl esters (FAMEs) with alkaline methanol. Methyl nonadecanoate (19:0, Sigma-Aldrich, St. Louis, MO, USA) was used as the internal standard. The concentrations of FAMEs were identified using a Thermo Finnigan Trace GC-MS System, and the 13C values of FAMEs were determined using a Thermo Scientific Trace GC Ultra attached to a Finnigan MAT 253 IRMS, as described by Rubino et al31.
For the identification of soil microbial community composition, the following PLFA designations were used: Gram-positive bacteria i14:0, a15:0, i15:0, i16:0, a17:0, i17:0; Gram-negative bacteria 17:0cy, 19:0cy, 16:1ω7c, 16:1ω9c; actinomycetes 10Me16:0, 10Me17:0, 10Me18:0; and fungi 18:1ω9c, 18:1ω9t and 18:2ω9,12 c. Short or odd-chain saturated PLFAs (14:0, 15:0, 17:0) were considered non-specific bacterial markers. The term “bacteria” in this paper refers to Gram-positive bacteria, Gram-negative bacteria and also these non-specific bacteria collectively. Common saturated PLFAs (16:0, 18:0) were not assigned to a taxonomic group but were considered as additional measures of total microbial biomass33,43,44. Soil microbial community structure was investigated using relative PLFA abundance (mol%).
Among the total 21 PLFAs identified, only 16 of those were present in sufficient amount for accurate isotopic analysis. Hence, 5 PLFAs without sufficient amount (14:0, 15:0, 17:0, i14:0 and 16:1ω7c) were not included in subsequent calculations. The proportion of FOM-derived C to total C in each PLFA (Pi) was determined using the following equation31:
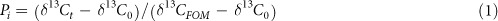 |
where δ13Ct and δ13C0 are the δ13C values (%o) of individual PLFA in the “FOM+soil” treatments and control, respectively; δ13CFOM is the δ13C (%o) of the labeled FOM.
The proportion of FOM-derived C to total C in each microbial group (e.g. bacteria, fungi) was calculated as:
 |
13C distribution proportion in each microbial group (e.g. bacteria, fungi) was calculated as:
 |
where Pi and Ai are the proportion of FOM-derived C to total C and the abundance (accounting for molecular C content) of each PLFA for a special microbial group (e.g. bacteria, fungi), respectively; while Pj and Aj (j = 1,2,∙∙∙∙∙∙16) are those for all 16 PLFAs.
Calculations
To calculate the contribution of added FOM to CO2 respiration, a two end-member mixing model was used:
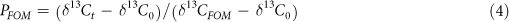 |
where PFOM is the proportion of FOM-derived CO2; δ13Ct and δ13C0 are the δ13C values (%o) of respired CO2 in the “FOM+soil” treatments and control, respectively; δ13CFOM is the δ13C value (%o) of FOM.
PE induced by the addition of FOM was calculated by comparing the amount of SOC-derived CO2 under FOM treatments with the amount of CO2 under control15, according to the following equation:
 |
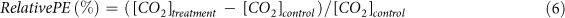 |
where, [CO2]treatment and [CO2]control represent SOM-derived CO2 efflux (or cumulative CO2 respiration) in the “FOM+soil” treatments and control, respectively.
Cumulative CO2 production, cumulative SOM mineralization, cumulative FOM mineralization, and cumulative PE for a specific time span could be estimated by integrating CO2 efflux, SOM-derived CO2 efflux, FOM-derived CO2 efflux, and absolute PE over time respectively.
Statistical analyses
Statistical analyses were carried out using the SPSS 16.0 package (SPSS, Chicago, IL, USA). Homogeneity of variances of data was tested prior to analyses and data were log-transformed when necessary (P < 0.05). Repeated measures analysis of variance (ANOVA) were used to examine differences in CO2 respiration (including total CO2, SOM-derived CO2 and FOM-derived CO2) and PE (including relative PE and cumulative PE) with time among FOM treatments. One-way ANOVA or Independent-Samples T Test was used to analyze the effects of FOM treatments on the cumulative CO2 respiration for a specific time span, PLFA abundance, proportion of FOM-derived C in each PLFA groups and 13C incorporation into each PLFA groups for each sampling time and each soil respectively. Impacts of treatments on microbial community structure (PLFA relative abundances) and 13C distribution among PLFAs (relative FOM-derived 13C incorporation into each individual PLFA) were assessed by principal components analysis (PCA). Two-way ANOVA was used to test the effects of FOM quality, sampling time, and their interactions on the first component (PC1) and the second component (PC2) of PCA. An α value of 0.05 was chosen to indicate statistical significance.
Author Contributions
H.W., W.H.X. and E.B. conceived and designed the experiments. H.W., G.Q.H., T.W.B., and P.J. performed the experiments. H.W., G.Q.H. and E.B. analyzed the data. H.W., T.W.B. and E.B. wrote the manuscript; other authors provided editorial advice.
Additional Information
How to cite this article: Wang, H. et al. Quality of fresh organic matter affects priming of soil organic matter and substrate utilization patterns of microbes. Sci. Rep. 5, 10102; doi: 10.1038/srep10102 (2015).
Acknowledgments
This work was supported by the National Basic Research Program of China (973 Program; 2014CB954400), the National Natural Science Foundation of China (41175138), the State Key Laboratory of Forest and Soil Ecology, the Doctoral Scientific Research Foundation of Liaoning Province (20121083), and by USDA National Institute of Food and Agriculture (Hatch Project 1003961).
References
- García-Palacios P., Maestre F. T., Bardgett R. D. & de Kroon H. Plant responses to soil heterogeneity and global environmental change. J. Ecol. 100, 1303–1314 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post W. M. & Kwon K. C. Soil carbon sequestration and land-use change: processes and potential. Glob. Change Biol. 6, 317–327 (2000). [Google Scholar]
- Norby R. J., Cotrufo M. F., Ineson P., O'Neill E. G. & Canadell J. G. Elevated CO2, litter chemistry, and decomposition: a synthesis. Oecologia 127, 153–165 (2001). [DOI] [PubMed] [Google Scholar]
- Liu L. L., King J. S. & Giardina C. P. Effects of elevated atmospheric CO2 and tropospheric O3 on nutrient dynamics: decomposition of leaf litter in trembling aspen and paper birch communities. Plant Soil 299, 65–82 (2007). [Google Scholar]
- Aerts R. Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79, 439–449 (1997). [Google Scholar]
- Hobbie S. E. et al. Tree species effects on decomposition and forest floor dynamics in a common garden. Ecology 87, 2288–2297 (2006). [DOI] [PubMed] [Google Scholar]
- Strickland M. S., Osburn E., Lauber C., Fierer N. & Bradford M. A. Litter quality is in the eye of the beholder: initial decomposition rates as a function of inoculum characteristics. Funct. Ecol. 23, 627–636 (2009). [Google Scholar]
- Cleveland C. C. et al. Litter quality versus soil microbial community controls over decomposition: a quantitative analysis. Oecologia 174, 283–294 (2014). [DOI] [PubMed] [Google Scholar]
- Blagodatskaya Е. & Kuzyakov Y. Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: critical review. Biol. Fert. Soils 45 (2008). [Google Scholar]
- Gul S. & Whalen J. Plant life history and residue chemistry influences emissions of CO2 and N2O from soil perspectives for genetically modified cell wall mutants. Crit. Rev. Plant Sci. 32, 344–368 (2013). [Google Scholar]
- Conde E. et al. The impacts of inorganic nitrogen application on mineralization of 14C-labelled maize and glucose, and on priming effect in saline alkaline soil. Soil Biol. Biochem. 37, 681–691 (2005). [Google Scholar]
- Guenet B., Neill C., Bardoux G. & Abbadie L. Is there a linear relationship between priming effect intensity and the amount of organic matter input? Appl. Soil Ecol. 46, 436–442 (2010). [Google Scholar]
- Zhang W. D. & Wang S. L. Effects of NH4+and NO3- on litter and soil organic carbon decomposition in a Chinese fir plantation forest in South China. Soil Biol. Biochem. 47, 116–122 (2012). [Google Scholar]
- Potthast K., Hamer U. & Makeschin F. Impact of litter quality on mineralization processes in managed and abandoned pasture soils in Southern Ecuador. Soil Biol. Biochem. 42, 56–64 (2010). [Google Scholar]
- Kuzyakov Y., Friedel J. K. & Stahra K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 32, 1485–1498 (2000). [Google Scholar]
- Fontaine S., Mariotti A. & Abbadie L. The priming effect of organic matter: a question of microbial competition? Soil Biol. Biochem. 35, 837–843 (2003). [Google Scholar]
- Chen R. et al. Soil C and N availability determine the priming effect: microbial N mining and stoichiometric decomposition theories. Glob. Change Biol. 20, 2356–2367 (2014). [DOI] [PubMed] [Google Scholar]
- Manzoni S., Trofymow J. A., Jackson R. B. & Porporato A. Stoichiometric controls on carbon, nitrogen, and phosphorus dynamics in decomposing litter. Ecol. Monogr. 80, 89–106 (2010). [Google Scholar]
- Recous S., Robin D., Darwis D. & Mary B. Soil inorganic N availability: effect on maize residue decomposition. Soil Biol. Biochem. 27, 1529–1538 (1995). [Google Scholar]
- Nicolardot B., Bouziri L., Bastian F. & Ranjard L. A microcosm experiment to evaluate the influence of location and quality of plant residues on residue decomposition and genetic structure of soil microbial communities. Soil Biol. Biochem. 39, 1631–1644 (2007). [Google Scholar]
- Eskelinen A., Stark S. & Männistö M. Links between plant community composition, soil organic matter quality and microbial communities in contrasting tundra habitats. Oecologia 161, 113–123 (2009). [DOI] [PubMed] [Google Scholar]
- Gul S., Whalen J. K., Ellis B. E. & Grayston S. J. Plant residue chemistry impacts soil processes and microbial community structure: a study with Arabidopsis thaliana cell wall mutants. Appl. Soil Ecol. 60, 84–91 (2012). [Google Scholar]
- Bastian F., Bouziri L., Nicolardot B. & Ranjard L. Impact of wheat straw decomposition on successional patterns of soil microbial community structure. Soil Biol. Biochem. 41, 262–275 (2009). [Google Scholar]
- Kuzyakov Y. & Bol R. Sources and mechanisms of priming effect induced in two grassland soils amended with slurry and sugar. Soil Biol. Biochem. 38, 747–758 (2006). [Google Scholar]
- Werth M. & Kuzyakov Y. 13C fractionation at the root-microorganisms-soil interface: a review and outlook for partitioning studies. Soil Biol. Biochem. 42, 1372–1384 (2010). [Google Scholar]
- Nottingham A. T., Griffiths H., Chamberlain P. M., Stott A. W. & Tanner E. V. J. Soil priming by sugar and leaf-litter substrates: a link to microbial groups. Appl. Soil Ecol. 42, 183–190 (2009). [Google Scholar]
- Rasmussen C., Southard R. J. & Horwath W. R. Soil mineralogy affects conifer forest soil carbon source utilization and microbial priming. Soil Sci. Soc. Am. J. 71, 1141–1150 (2007). [Google Scholar]
- Wang Q., Wang S., He T., Liu L. & Wu J. Response of organic carbon mineralization and microbial community to leaf litter and nutrient additions in subtropical forest soils. Soil Biol. Biochem. 71, 13–20 (2014). [Google Scholar]
- Rousk J. & Bååth E. Fungal and bacterial growth in soil with plant materials of different C/N ratios. Fems Microbiol. Ecol. 62, 258–267 (2007). [DOI] [PubMed] [Google Scholar]
- Fioretto A., Papa S., Pellegrino A. & Fuggi A. Decomposition dynamics of Myrtus communis and Quercus ilex leaf litter: mass loss, microbial activity and quality change. Appl. Soil Ecol. 36, 32–40 (2007). [Google Scholar]
- Rubino M. et al. Carbon input belowground is the major C flux contributing to leaf litter mass loss: evidences from a 13C labelled-leaf litter experiment. Soil Biol. Biochem. 42, 1009–1016 (2010). [Google Scholar]
- Dungait J. A. J. et al. Variable responses of the soil microbial biomass to trace concentrations of 13C-labelled glucose, using 13C-PLFA analysis. Eur. J. Soil Sci. 62, 117–126 (2011). [Google Scholar]
- Moore-Kucera J. & Dick R. P. Application of 13C-labeled litter and root materials for in situ decomposition studies using phospholipid fatty acids. Soil Biol. Biochem. 40, 2485–2493 (2008). [Google Scholar]
- Fontaine S. et al. Fungi mediate long term sequestration of carbon and nitrogen in soil through their priming effect. Soil Biol. Biochem. 43, 86–96 (2011). [Google Scholar]
- Strickland M. S. & Rousk J. Considering fungal:bacterial dominance in soils - Methods, controls, and ecosystem implications. Soil Biol. Biochem. 42, 1385–1395 (2010). [Google Scholar]
- Fierer N., Schimel J. P. & Holden P. A. Variations in microbial community composition through two soil depth profiles. Soil Biol. Biochem. 35, 167–176 (2003). [Google Scholar]
- Demoling F., Figueroa D. & Bååth E. Comparison of factors limiting bacterial growth in different soils. Soil Biol. Biochem. 39, 2485–2495 (2007). [Google Scholar]
- Yang K., Shi W. & Zhu J. J. The impact of secondary forests conversion into larch plantations on soil chemical and microbiological properties. Plant Soil. 368, 535–546 (2013). [Google Scholar]
- Yang K., Zhu J. J., Zhang M., Yan Q. L. & Sun O. J. Soil microbial biomass carbon and nitrogen in forest ecosystems of Northeast China: a comparison between natural secondary forest and larch plantation. J. Plant Ecol. 3, 175–182 (2010). [Google Scholar]
- Garcia-Pausas J. & Paterson E. Microbial community abundance and structure are determinants of soil organic matter mineralisation in the presence of labile carbon. Soil Biol. Biochem. 43, 1705–1713 (2011). [Google Scholar]
- Theander O. & Westerlund E. A. Studies on dietary fiber. 3. Improved procedures for analysis of dietary fiber. J. Agr. Food Chem. 34, 330–336 (1986). [Google Scholar]
- Vance E. D., Brookes P. C. & Jenkinson D. S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 19, 703–707 (1987). [Google Scholar]
- Waldrop M. P. & Firestone M. K. Microbial community utilization of recalcitrant and simple carbon compounds: impact of oak-woodland plant communities. Oecologia 138, 275–284 (2004). [DOI] [PubMed] [Google Scholar]
- McMahon S. K., Williams M. A., Bottomley P. J. & Myrold D. D. Dynamics of microbial communities during decomposition of carbon-13 labeled ryegrass fractions in soil. Soil Sci. Soc. Am. J. 69, 1238–1247 (2005). [Google Scholar]


