Abstract
Sex-chromosome differentiation was recently shown to vary among common frog populations in Fennoscandia, suggesting a trend of increased differentiation with latitude. By rearing families from two contrasted populations (respectively, from northern and southern Sweden), we show this disparity to stem from differences in sex-determination mechanisms rather than in XY-recombination patterns. Offspring from the northern population display equal sex ratios at metamorphosis, with phenotypic sexes that correlate strongly with paternal LG2 haplotypes (the sex chromosome); accordingly, Y haplotypes are markedly differentiated, with male-specific alleles and depressed diversity testifying to their smaller effective population size. In the southern population, by contrast, a majority of juveniles present ovaries at metamorphosis; only later in development do sex ratios return to equilibrium. Even at these later stages, phenotypic sexes correlate only mildly with paternal LG2 haplotypes; accordingly, there are no recognizable Y haplotypes. These distinct patterns of gonadal development fit the concept of ‘sex races’ proposed in the 1930s, with our two populations assigned to the ‘differentiated’ and ‘semi-differentiated’ races, respectively. Our results support the suggestion that ‘sex races’ differ in the genetic versus epigenetic components of sex determination. Analysing populations from the ‘undifferentiated race’ with high-density genetic maps should help to further test this hypothesis.
Keywords: gonadal development, sex ratio, XY recombination, epigenetic sex determination
1. Introduction
In contrast with the strict and stable genotypic sex determination (GSD) that characterizes birds and mammals, the mechanisms of sex determination in ectothermic vertebrates are generally quite labile and may include important epigenetic components. Epigenetics is meant here in its broadest sense (sensu [1,2]), referring to a phenotypic differentiation triggered by non-genetic cues, be they intrinsic (e.g. positional) or extrinsic (e.g. environmental or social). Purely environmental sex determination (ESD) has been documented in several fish and non-avian reptiles (e.g. [3–7]). Sex chromosomes in these groups are often homomorphic, partly due to frequent turnovers (e.g. [8]) and partly to occasional events of XY recombination (e.g. [9]). These two processes are non-exclusive [10,11], both being possibly mediated by occasional events of sex reversal induced by environmental interactions [12,13]. In amphibians, all species investigated so far present a genetic component to sex determination (as supported by co-segregation of sex with genetic markers; reviewed in [14]), sometimes with temperature effects, but cytogenetically differentiated sex chromosomes occur in less than 4% of species [14]. Particularly frequent transitions have been reported in ranid frogs, where different chromosome pairs have been co-opted for sex determination depending on species [15]. Temperature effects have been documented in a few species, mostly consisting of masculinization of XX individuals at high temperatures (e.g. [16]); sex-reversed XX males tend to produce female-biased clutches (e.g. [17,18]).
The common frog Rana temporaria, widespread from Spain to Northern Norway and from sea level to more than 2500 m.a.s.l. [19], appears as a good model to investigate interactions between genes and environment. Its sex-determination system had already raised interest in the early twentieth century, with the description by Witschi [16,20] of ‘sex races’, correlating with climatic zones. In the so-called ‘differentiated race’, assigned to boreal and alpine climates, juveniles present equal sex ratios at metamorphosis, with well-differentiated testes or ovaries. In the ‘undifferentiated race’, found in the milder climate of southern England, Netherlands and central Germany down to the Jura Mountains, all juveniles present ovaries at metamorphosis; only later in development do some froglets replace ovaries by testes. In the ‘semi-differentiated’ race, found in intermediate climatic conditions, variable proportions of females, males and sometimes hermaphrodites are found at metamorphosis. Piquet [21] provided laboratory evidence for temperature effects on sex determination and hypothesized sex races to differ in the underlying mechanisms of sex determination, being pure GSD in the differentiated race, but comprising epigenetic effects in the undifferentiated one. Evidence for genetic effects has been gathered from populations of Fennoscandia and Switzerland, where several markers display a clear association with sex, consistent with male heterogamety. However, the strength of the association varies between populations and families [22–24]. Those markers fall into linkage group 2 (LG2, which also includes the LG15 of Cano et al. [25]). Environmental effects on sex determination in nature are supported by the strong fluctuations in sex ratios documented in some subarctic populations, with evidence for sex-reversed XX males [23,26,27] and possibly XY females [12,22].
Rodrigues et al. [28] recently found sex differentiation at LG2 to differ among populations along a 1500 km latitudinal transect in Sweden, seemingly with a latitudinal trend: differentiation was strongest in the northern-boreal population of Ammarnäs (with high FST between sexes, heterozygote excess in males and male-specific alleles and haplotypes) but null in the southernmost population of Tvedöra (nemoral climate). Other populations displayed intermediate patterns, with an apparently bimodal distribution of males: some clustered on their own, while others were genetically undistinguishable from females. It is tempting to interpret this intriguing pattern in light of Witschi's [20] and Piquet's [21] suggestions of sex races: the northern population (Ammarnäs), with clear GSD, would belong to the differentiated race, whereas the southern population (Tvedöra), with no sign of GSD (i.e. possibly pure ESD), would belong to the undifferentiated race. Intermediate populations would present a mix of ESD and GSD families, and belong to the semi-differentiated race. This hypothesis is formalized in figure 1 (adapted from [28]). There are, however, alternative interpretations to the empirical trend documented by Rodrigues et al. [28]. An obvious one is that all populations harbour the same GSD system, with the same master sex-determination gene on LG2, but differ in the patterns of recombination. The northern population (Ammarnäs), for instance, might have fixed a large inversion on the Y chromosome, preventing XY recombination in males, while X haplotypes would recombine more freely with non-inverted Y haplotypes in the southern population (Tvedöra); the two types of Y chromosomes would segregate in intermediate populations.
Figure 1.
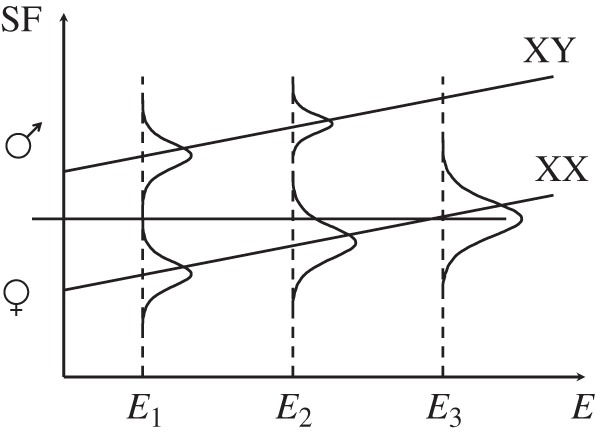
Hypothetical norms of reaction for XX and XY genotypes, with sex modelled as a threshold trait. The amount of a sex factor SF (e.g. a male hormone) produced by a given genotype increases with the environmental gradient E (e.g. temperature). For the environmental range considered, the XY genotype always produces enough of the sex factor to lie above the threshold (horizontal straight line), so that all XY individuals develop as males. At low environmental values (E1), the amount of sex factor produced by the XX genotype always lies below the threshold, so that all XX individuals develop as females; sex determination is thus purely genetic (GSD). As the environmental gradient increases, an increasing proportion of XX individuals exceed the threshold, thus developing into ‘sex-reversed’ males. As a result of sex-ratio selection, the frequency of XY individuals progressively diminishes. At the extreme (E3), all individuals are XX and sex determination becomes purely environmental (ESD). Adapted from [28].
In the present paper, we test between these two alternative hypotheses, by screening families from the two populations of Ammarnäs and Tvedöra for patterns of LG2 recombination, sex linkage and offspring sex ratios. The specific predictions stemming from our two hypotheses are straightforward: if, on the one hand, the differences in population genetics result from differences in the patterns of XY recombination, then the LG2 map should be very short (close to 0.0 cM) in males from Ammarnäs, but significantly larger in males from Tvedöra. In this latter population, association with sex should vary with markers, the strongest link being found for markers closest to the SD locus. If, on the other hand, differences are due to the sex-determination system being genotypic in Ammarnäs, versus epigenetic in Tvedöra, then we expect a perfect association with sex in the former population, but none in the latter. Furthermore, if these two populations indeed fit Witschi’s differentiated versus undifferentiated races, respectively, then we expect juveniles from Ammarnäs to present either testes or ovaries in equal proportions at metamorphosis, but only ovaries for those from Tvedöra, with some individuals replacing ovaries by testes later in development.
2. Material and methods
(a). Field sampling and husbandry
Frogs were sampled during the 2013 breeding season from the two populations of Tvedöra (55°42′0.85″ N, 13°25′50.91″ E; nemoral climate with broad-leaved deciduous forests) and Ammarnäs (65°58′12.60″ N, 16°12′43.80″ E; northern-boreal climate with a subalpine vegetation of conifers and birches). Eleven mating pairs were caught in Tvedöra between 16 and 20 April, and 20 mating pairs in Ammarnäs between 17 and 20 May. Individual pairs were kept overnight in 11 l plastic boxes with grass tufts and half-filled with pond water, allowing them to lay a clutch. On the next day, adults were sampled for buccal cells with sterile cotton swabs [29], then released at the place of capture. A total of 12 clutches—six from Tvedöra (T1 to T6) and six from Ammarnäs (A1 to A6)—were collected and brought to outdoor facilities at the Lausanne University campus. Each family was raised in 525 l tanks until tadpoles reached metamorphosis, exposed to outdoor climatic conditions (temperature, humidity, rain and sunlight). Tanks were randomized with respect to population origin. Within one week of metamorphosis (stage 43 [30]), 40 offspring from each of the 12 families (referred to as ‘metamorphs’ hereafter) were anaesthetized in 0.2% ethyl3-aminobenzoate methanesulfonate salt solution (MS222), then dropped in 70% ethanol for euthanasia and preservation at −20°C. The remaining offspring were maintained in outdoor tanks and fed crickets, fruitflies (Drosophila) and mealworms. When reaching about 2 cm snout–vent length (stage 45 [30]), these juveniles (referred to as ‘froglets’ hereafter) were anaesthetized, euthanized and conserved in ethanol. Metamorphs and froglets were dissected under a binocular microscope in order to determine phenotypic sex based on gonad morphology. Ovaries in common frogs develop from the whole gonadal primordia into a large whitish/yellowish structure with distinct lobes, and a characteristic granular aspect conferred by the many oocytes embedded in the cortex [31]. By contrast, testes develop from the anterior part of the gonadal primordia only (the posterior part degenerates) into a small oblong structure, with a smooth cortex covered with melanic spots [32]. In case of doubt, gonads were considered as undifferentiated and sex was not assigned (NA).
(b). Microsatellite amplifications and analyses
After overnight treatment with 10% proteinase K (QIAgen) at 56°C, DNA was extracted from hindleg tissues (metamorphs and froglets) and buccal swabs (adults) using a QIAgen DNeasy kit and a BioSprint 96 workstation (QIAgen), which resulted in 200 μl Buffer AE (QIAgen) DNA elution. The same 13 sex-linked markers used by Rodrigues et al. [24,28] were amplified by polymerase chain reaction (PCR). Electronic supplementary material, table S1 provides information on primers (GenBank accession numbers, repeat motifs, primer sequences, range of allele sizes and references) and the two multiplex mixes used. PCR reactions were performed with a total volume of 10 µl, including 3 µl of extracted DNA, 3 µl of QIAgen Multiplex Master Mix 2×, and 0.05 to 0.7 µl of labelled forward primer and unlabelled reverse primer (see electronic supplementary material, table S1). PCRs were conducted on Perkin Elmer 2700 machines using the following thermal profile: 15 min of Taq polymerase activation at 95°C, followed by 35 cycles including denaturation at 94°C for 30 s, annealing at 57°C for 1 min 30 s and elongation at 72°C for 1 min, ending the PCR with a final elongation of 30 min at 60°C. PCR products for genotyping were run on an automated ABI Prism 3100 sequencer (Applied Biosystems, Foster City, CA, USA) and alleles were scored on GeneMapper v. 4.0 (Applied Biosystems).
(c). Statistical analysis
Fixation indices (gene diversity HS, FST between sexes, FIS within sexes) were calculated with Fstat v. 2.9.4, updated from [33] based on the 20 adult pairs from Ammarnäs and 11 adult pairs from Tvedöra. Principal component analyses (PCA) were performed with Pcagen v. 2.0, updated from [34], with input files generated by Create v. 1.33 [35].
Sex-specific recombination rates were estimated independently from the Ammarnäs and Tvedöra families using Crimap v. 2.4 [36]. The twopoint option was used to identify marker pairs with a LOD score exceeding 3.0, the all option to generate loci order, the build option to calculate the distances between loci (centimorgans, cM) and the flip option to test the robustness of loci order. Sex-specific recombination maps were plotted using Mapchart v. 2.2 [37].
Family and population-wide sex-ratio biases among metamorphs and froglets were tested with binomial tests, or Pearson's χ2 tests when sample size n exceeded 100. Correlations between paternal haplotypes and offspring phenotypic sex were tested with Fisher's exact test, or Pearson's χ2 test when sample size n exceeded 100; they were quantified by ϕ2, an index of association ranging from 0 to 1, obtained as χ2/n.
Sex haplotypes could be phased in Ammarnäs thanks to the strong sex differences in allelic frequencies and the absence of male recombination (see Results). X and Y haplotypes were analysed separately for gene diversity (i.e. expected heterozygosity HS) and differentiation (FST), and plotted along the main factors of a principal component analysis (Fstat v. 2.9.4 [33]; Pcagen v. 2.0 [34]). The genetic diversity index θ was calculated from HS as θ = ((1 − HS)−2 − 1)/2, assuming a stepwise mutation model [38]. At neutral equilibrium, the θ value for locus i is expected to reflect the effective population size Ne, mutation rate μi and number of copies per breeding pair ci: θi = ciNeμi. Thus, values for X-linked and Y-linked markers should represent ¾ and ¼ of autosomal values, respectively, assuming similar effective population sizes and mutation rates, and absence of recombination.
3. Results
(a). Population genetics
In line with the results of Rodrigues et al. [28], the two populations differed markedly in terms of sex differentiation at LG2, which was strong and significant in Ammarnäs (FST = 0.108, p ∼ 0.01) but absent in Tvedöra (FST = −0.0005, p ∼ 0.8). Similarly, FIS was strongly negative in the males from Ammarnäs (FIS = −0.235), but slightly positive in females from this population (FIS = +0.029), as well as in both sexes from Tvedöra (FIS = +0.066 in males, +0.072 in females). This is illustrated by the results of Structure and Pcagen analyses (figure 2): males and females from Tvedöra are randomly allocated to the two Structure groups and mixed within a single cluster in Pcagen analysis. By contrast, adults from Ammarnäs are allocated to two well-differentiated clusters that perfectly match phenotypic sexes, except for one male (A17M), which shows mixed assignment to the male and female groups. This individual lacked male-specific alleles at three loci, but also harboured unique alleles at two others. It was found in amplexus with a normal XX female, but its fertility is unknown, as no clutch from this pair was retained for laboratory rearing.
Figure 2.
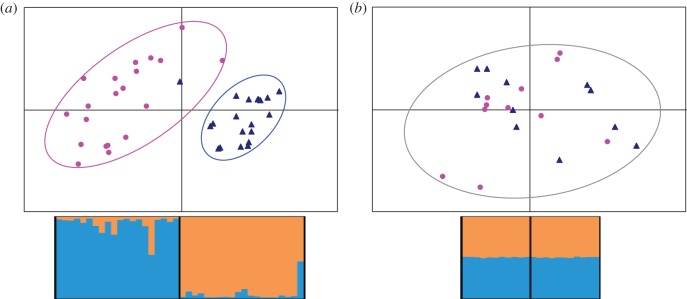
Plots from Pcagen and Structure analyses of LG2. (a) In Ammarnäs, males (blue triangles) and females (pink dots) form clearly differentiated clusters in Pcagen analyses (upper panel) and are assigned to different clusters by Structure: individuals on the left (females) are assigned to the orange cluster, and those on the right (males) to the blue cluster. The male outlier is A17M. (b) In Tvedöra, males and females group into the same Pcagen cluster (upper panel) and are randomly assigned to the blue and orange group by Structure.
(b). Recombination maps
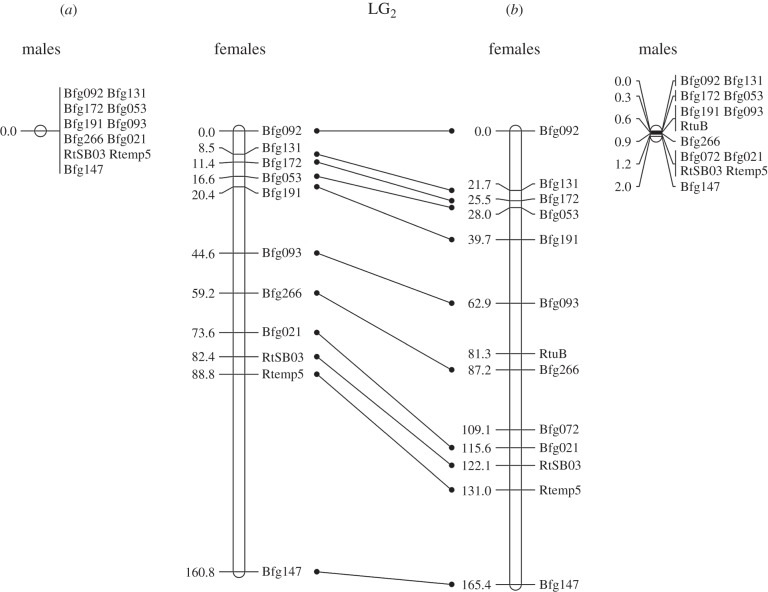
The patterns of recombination differed strongly between sexes (figure 3), with much longer maps in females (160.8 and 165.4 cM in Ammarnäs and Tvedöra, respectively) than in males (0.0 and 2.0 cM, respectively). Altogether we only identified four events of recombination in males (out of a total of 594 offspring genotyped with 13 markers), spread among three families of Tvedöra (T2, T3 and T4). The difference between populations was not significant (p = (323/594)4 = 0.087, one-sided combinatorial test, probability that all four recombination events occur among the 323 offspring from Tvedöra). The maps from both Ammarnäs and Tvedöra (figure 3) showed the exact same loci order as found in Swiss families [24], although two loci, Bfg072 and RtuB, could not be placed on the Ammarnäs map.
Figure 3.
LG2 recombination map from (a) Ammarnäs and (b) Tvedöra. Units are in Kosambi cM, and homologies are shown between the two populations with bars at the centre.
(c). Family sex ratios
Sex-ratio patterns differed markedly between the two populations (table 1). In Ammarnäs, 70% of offspring (167/240) presented well-differentiated gonads at metamorphosis, with some variance among families, however: no offspring of family A6, for instance, could be sexed at this stage. The other families provided enough sexed offspring for proper testing and displayed equal sex ratios, except for family A2, with a significant female bias (p = 0.0015; binomial test), but also 13 unassigned individuals. As a result, the sex ratio among sexed metamorphs was slightly female biased at the population level (66 males, 101 females; χ2 = 7.34; dl = 1; p < 0.01). However, this trend disappears (χ2 = 2.69) if the 13 unassigned offspring from family A2 are considered as males, as their genotypes indicate (see below). Sex ratios in other families also remain equal when assigning all offspring with undifferentiated gonads to their genotypic sex (most often male; table 1). All froglets (31/31) could be sexed unambiguously; there were too few individuals per family for proper testing, but sex ratio did not differ significantly from even at the population level (12 males and 19 females; p = 0.28, binomial test).
Table 1.
Sex ratios and sex linkage at metamorph and froglet stages in families from Ammarnäs (A1 to A6) and Tvedöra (T1 to T6), with population totals. M, F, NA: number of offspring with male, female or undifferentiated gonads, respectively. χ2, p-val: χ2 and p-values associated with deviation from equal sex ratio and from a random association to sex. ϕ2 indicates the strength of association to sex. Sex ratios at the metamorph stage in Ammarnäs were also tested after assigning all offspring with undifferentiated gonads to their genotypic sex (most often male); corresponding values are indicated by χ2NA and p-valNA. n.s.: not significant.
| metamorph stage |
froglet stage |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| sex ratio |
sex linkage |
sex ratio |
sex linkage |
|||||||||||||||
| M | F | NA | χ2 | p-val | χ2NA | p-valNA | χ2 | p-val | ϕ2 | M | F | NA | χ2 | p-val | χ2 | p-val | ϕ2 | |
| A1 | 12 | 22 | 6 | 2.94 | n.s.a | 0.4 | n.s.a | 29.96 | ***b | 0.88 | 2 | 7 | 0 | 2.78 | n.s.a | 9.00 | *b | 1.00 |
| A2 | 5 | 22 | 13 | 10.70 | **a | 0.4 | n.s.a | 27.00 | ***b | 1.00 | 1 | 2 | 0 | 0.33 | n.s.a | 3.00 | n.s.b | 1.00 |
| A3 | 12 | 17 | 11 | 0.86 | n.s.a | 3.6 | n.s.a | 29.00 | ***b | 1.00 | 5 | 2 | 0 | 1.29 | n.s.a | 7.00 | *b | 1.00 |
| A4 | 17 | 22 | 1 | 0.64 | n.s.a | 0.4 | n.s.a | 39.00 | ***b | 1.00 | 0 | 1 | 0 | 1.00 | — | — | — | — |
| A5 | 20 | 18 | 2 | 0.11 | n.s.a | 0.1 | n.s.a | 34.18 | ***b | 0.90 | 4 | 3 | 0 | 0.14 | n.s.a | 7.00 | *b | 1.00 |
| A6 | 0 | 0 | 40 | — | — | 0.4 | n.s.a | — | — | — | 0 | 4 | 0 | 4.00 | n.s.a | — | — | — |
| Ammarnäs | 66 | 101 | 73 | 7.34 | **c | 1.35 | n.s.c | 158.88 | ***c | 0.95 | 12 | 19 | 0 | 1.58 | n.s.a | 31.00 | ***b | 1.00 |
| T1 | 0 | 40 | 0 | 40.00 | ***a | NA | NA | 0.00 | n.s.b | 0.00 | 1 | 10 | 0 | 7.36 | *a | 1.93 | n.s.b | 0.18 |
| T2 | 1 | 4 | 35 | 1.80 | n.s.a | NA | NA | 5.00 | n.s.b | 1.00 | 7 | 0 | 0 | 7.00 | *a | 0.00 | n.s.b | 0.00 |
| T3 | 4 | 36 | 0 | 25.60 | ***a | NA | NA | 5.43 | *b | 0.14 | 12 | 3 | 0 | 5.40 | *a | 5.10 | n.s.b | 0.34 |
| T4 | 4 | 35 | 1 | 24.60 | ***a | NA | NA | 2.96 | n.s.b | 0.08 | 10 | 8 | 4 | 0.22 | n.s.a | 3.38 | n.s.b | 0.19 |
| T5 | 9 | 29 | 2 | 10.50 | **a | NA | NA | 8.58 | **b | 0.23 | 11 | 8 | 1 | 0.47 | n.s.a | 19.00 | ***b | 1.00 |
| T6 | 6 | 27 | 7 | 13.40 | ***a | NA | NA | 5.40 | *b | 0.16 | 5 | 2 | 1 | 1.29 | n.s.a | 7.00 | *b | 1.00 |
| Tvedöra | 24 | 171 | 45 | 111.00 | ***c | NA | NA | 21.74 | ***c | 0.11 | 46 | 31 | 6 | 2.92 | n.s.a | 29.79 | ***b | 0.39 |
aBinomial test.
bFisher's exact test.
cχ2 test.
In Tvedöra, 81% of offspring (195/240) presented well-differentiated gonads at metamorphosis, also with some variance among families: in family T2, for instance, only 13% of offspring (5/40) could be sexed. The other families provided enough sexed offspring for proper testing and displayed strong and highly significant female biases. As a result, sex ratio was highly biased at the population level (24 males and 171 females; χ2 = 111, dl = 1, p < 0.0001). This result remains highly significant (χ2 = 43.4, p < 0.0001) if all 45 unsexed offspring are assigned to the male category. At the froglet stage, 93% of offspring (77/83) could be sexed; sex ratios were equal at the population level (46 males and 31 females; p = 0.11, binomial test), but significantly biased at the family level, either towards males (families T2 and T3) or towards females (family T1).
(d). Sex linkage
The patterns of sex linkage also differed markedly between the two populations (table 1). In Ammarnäs, association with paternal LG2 haplotypes was strong and highly significant already at metamorphosis in all five families where offspring could be phenotypically sexed (ϕ2 values ranging 0.88 to 1.0). The two cases showing imperfect association (A1 and A5, ϕ2 = 0.88 and 0.90, respectively) were due to one sex-reversed XY daughter in each. Among froglets, association was perfect (ϕ2 = 1) in all four families where offspring of both sexes were obtained. At the population level, association was strong and highly significant both among metamorphs (ϕ2 = 0.95; χ2 = 159, dl = 1, 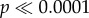 ) and among froglets (ϕ2 = 1; p = 7 × 10−9; Fisher's exact test).
) and among froglets (ϕ2 = 1; p = 7 × 10−9; Fisher's exact test).
In Tvedöra, this association varied markedly between families and developmental stages. At metamorphosis, ϕ2 varied from 0 to 0.23 (discounting family T2 where only five offspring could be sexed), with a mild but significant sex linkage in three families (T3, T5 and T6). As a result, sex linkage was weak but highly significant at the population level (ϕ2 = 0.11, p < 0.001). In froglets, ϕ2 values were both larger on average and more variable (ranging 0 to 1). Sex linkage was complete (ϕ2 = 1) and significant in two families (T5 and T6). Deviations from perfect linkage in other families stemmed from many instances of XX females and XY males. At the population level, association was highly significant, though much lower than in Ammarnäs (ϕ2 = 0.39 versus 1.00).
(e). Phasing X and Y haplotypes
The X and Y haplotypes could be phased in males from Ammarnäs, thanks to the absence of male recombination and strong sex differences in allelic frequencies, combined with information on offspring phenotypic sexes. This allowed identification of a limited set of highly similar Y haplotypes. On the Pcagen projection (figure 4), these Y haplotypes are well differentiated from the male X haplotypes; the latter perfectly co-localize with XX females (which indirectly corroborates our X and Y assignments in males), with however a larger variance due to their haploid state. The Y haplotype of male A17M takes an intermediate position between the X and Y clusters. Excluding this individual, gene diversity is about three times lower on the Y than on the X (HS = 0.29 versus 0.69, averaged over 13 loci), and θ values seven times lower (1.75 versus 12.32). Such phasing could not be performed in Tvedöra, where there was no evidence for male-specific alleles or distinct Y haplotypes among the 11 males. Even the males from the four families showing a significant correlation between paternal genotypes and offspring phenotypic sexes did not share similar alleles or haplotypes.
Figure 4.
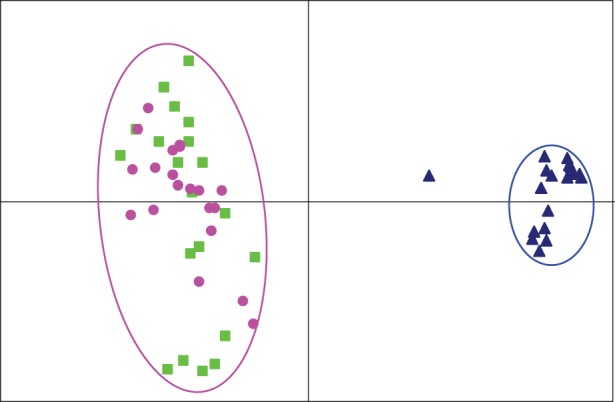
Plots from Pcagen analyses of LG2 in Ammarnäs, with phased male haplotypes. The Y haplotypes (blue triangles) cluster on their own, while the male X haplotypes (green squares) co-localize with the female genotypes (pink dots). The Y outlier is A17M.
4. Discussion
Families from the two populations under study displayed very similar sex-specific rates of recombination on LG2. The only notable difference concerned the male map, which was 0.0 cM in Ammarnäs and 2.0 cM in Tvedöra. Even though the difference is very small (and not significant from our limited sample), a limited rate of male recombination still has the potential to contribute to the mix of X and Y alleles observed in Tvedöra.
More striking differences, however, were found in the association between paternal LG2 haplotypes and offspring phenotypic sexes. All families from Ammarnäs displayed large and highly significant ϕ2 values; only two XY females were found among the 240 metamorphs, and none among froglets or adults. This parallels the strong XY differentiation at the population level, with highly differentiated X and Y haplotypes (figure 4), suggesting that XY females do not contribute significantly to reproduction. The absence of XY recombination is also supported by the much lower θ values obtained for Y than for X (1.75 versus 12.32), pointing to the action of Hill–Robertson interferences in addition to the threefold drop in effective population size. One reproductive male (A17M) had a mixed Y haplotype, suggestive of a past event of XY recombination (male-specific alleles were lacking at three loci), but possibly also indicating an immigrant from a distant population (two loci harboured unique alleles). In Tvedöra, by contrast, patterns were highly heterogeneous, with relatively large and significant ϕ2 values in a few families, but no association in others. Even families with significant ϕ2 values presented some mismatches between phenotypic sex and paternal LG2 haplotypes, suggesting frequent occurrence of ‘sex-reversed’ XX males and XY females. If the latter reproduce, the ensuing XY recombination should be sufficient to prevent XY differentiation (the ‘fountain of youth’ [12]) and probably contributes to the complete overlap in allelic frequencies at the population level (figure 2). This situation is highly reminiscent of the Swiss populations investigated by Rodrigues et al. [24], which also displayed a large variance among families in the association between offspring sex and paternal LG2 haplotype, together with a complete overlap in allelic frequencies, and no differentiated Y haplotypes. Importantly, these results oppose the simple alternative hypothesis formulated in §1, according to which sex determination would be purely epigenetic in the southern population. A genetic sex determinant also occurs on LG2 in Tvedöra, but differs from that found in Ammarnäs in being weaker and variable in strength among families.
In addition, we also found strong differences in family and population sex ratios. They were equal at metamorphosis in all Ammarnäs families, except for a slight female bias in A2. This latter family, moreover, also contained 13 offspring with undifferentiated gonads, which were all males according to their LG2 haplotype; assigning these 13 offspring to their genotypic sex makes biases vanish both in this family and at the population level. By contrast, families from Tvedöra displayed strong and highly significant female biases at metamorphosis. Population-level sex ratio returned to even at the froglet stage, but some biases remained at the family level, suggesting multigenic or environmental contributions to sex determination. Although experimental tanks might have slightly differed in terms of local conditions (e.g. density, food or temperature), we do not expect this to affect our conclusions, due to randomization (the differences in the patterns of gonadal development mostly occurred between populations, not between families within populations). Similarly, differential mortality is unlikely to have played a role; this would imply mortality to be sex biased in families from Tvedöra but not from Ammarnäs, and in a very specific way, being biased towards males before metamorphosis, then towards females after metamorphosis. We find more parsimonious the suggestion that offspring from these two populations fit the distinct patterns of gonadal development already documented for this species [16,20,21]. Thus, we tentatively assign Ammarnäs to Witschi's [20] ‘differentiated race’, in which offspring present either testes or ovaries in equal proportion at metamorphosis, and Tvedöra to the ‘semi-differentiated race’, characterized by a female bias at metamorphosis, but also some juveniles already with testes.
When combined with sex-linkage data, these contrasted patterns of gonadal development furthermore support a link between Witschi's ‘sex races’ and the mechanisms of sex determination; specifically, as already hypothesized by Piquet [21], these races might differ in the genetic versus epigenetic components of sex determination. Accordingly, the ‘differentiated race’, such as found in Ammarnäs, would be characterized by strong genetic sex determinants, with XX and XY genotypes lying far apart each side of the threshold (figure 1), leading to an early and unambiguous differentiation into either a male or a female phenotype. Sex reversals and ensuing XY recombination would be absent or sufficiently rare that Y haplotypes are well differentiated at the population level. By contrast, the ‘semi-differentiated race’, such as found in Tvedöra and possibly in the Swiss populations investigated by Rodrigues et al. [24], would be characterized by a weaker genetic component (i.e. XX and XY genotypes closer to the threshold), making sex determination vulnerable to random effects or environmental factors such as temperature. The frequent occurrence of sex reversals and ensuing sex-chromosome recombination in XY females would prevent the differentiation of X and Y haplotypes.
It is worth noting, however, that the genetic component of sex determination also varies in strength among families within populations. Such polymorphism might actually account for the bimodal distribution of male genotypes documented in several mid-boreal populations by Rodrigues et al. [28]. Indeed, if some of the Y alleles segregating in a population are strong enough to entirely prevent XY individuals from developing into females, then they will generate families of non-recombining haplotypes that will progressively diverge from local X haplotypes. Furthermore, families also seem to differ in the timing of sex determination: whatever their ultimate phenotypic sex, offspring from families with a weak sex determinant tend to develop ovaries first, which are later replaced by testes in some individuals. This suggests a genetic difference in the sex-determination pathway between the differentiated and undifferentiated races, which could be the actual upstream gene, its robustness to environmental variation or the interactions of genes in the downstream pathway.
A potential role of phylogeography was suggested to account for the latitudinal trend in sex-chromosome differentiation across Fennoscandia [28]: two divergent eastern and western mtDNA-lineages of R. temporaria meet south of Fennoscandia [39], raising the possibility that the trend documented reflects a divergence between lineage-specific systems of sex determination. However, the point must also be made that the distribution of Witschi's sex races fits climatic gradients [20], while that of mitochondrial lineages fits roads of postglacial recolonization (e.g. [40]). If our present hypothesis of a link with Witschi's sex races holds true, then the patterns of sex-chromosome differentiation should be independent of phylogeographic lineages. This is worth testing through further investigations on populations from different lineages and climatic zones.
It should be clear from our results that such ‘sex races’ are not to be seen as discrete entities, but as a continuum, underlaid by a cline in the strength of allelic effects (similar to the one found, for example, in the silverside Menidia menidia [41,42]), where alleles contributing strong effects are preferentially found in harsh and unpredictable environments, and those with weak effects in milder and more predictable environments, though with a segregating polymorphism among families within populations.
5. Conclusion and perspectives
The present study provides several important new insights on the intriguing sex-determination system of common frogs. First, we show that among-population differences in sex-chromosome differentiation [28] do no stem from differences in XY recombination, but in the mechanisms of sex determination. Second, by analysing the patterns of gonadal development, we provide support for a link between sex-chromosome differentiation [28] and Witschi's sex races. Third, we substantiate the view that these sex races differ in the genetic versus epigenetic component of sex determination. In the northern population (assigned to the differentiated race), the phasing of sex haplotypes enabled us to quantify a diversity drop on Y chromosomes, probably to stem from Hill–Robertson interferences. In the southern population (assigned to the semi-differentiated race), we could document a variance in sex ratios among families, together with a variance in the association between offspring phenotypic sex and paternal LG2 haplotype, pointing to within-population polymorphism at the sex-determining locus.
Extrapolating from our data, the ‘undifferentiated race’ (described from central and southern Germany, Netherlands and southern England [20]) would have sex determined mostly or entirely epigenetically. Such populations would be worth investigating in detail to test our present hypothesis; the specific prediction being that, in such populations, not only do all offspring present ovaries at metamorphosis, but the phenotypic sex of froglets is completely uncorrelated with parental haplotypes.
Linkage groups other than LG2 should of course also be tested, in order to exclude a contribution of alternative genetic factors mapping to different chromosomes. Rodrigues et al. [24] did not find any sex association with linkage groups other than LG2, despite very low male recombination over the whole genome, but analyses should be furthered with a higher density genetic map (e.g. with RAD Seq markers), in order to exclude alternative genetic components with more confidence. It would also be interesting to perform gene expression analyses, in order to provide further evidence of differences in the sex determination cascade between the differentiated and undifferentiated races, for example in terms of gene expression timing or gene interactions.
It is worth noting that similar polymorphisms in sex-determination mechanisms have been suggested for other ranid frogs; in a population of Rana nigromaculata, for instance, sex was shown to co-segregate with paternal chromosome-4 haplotypes in some families, but not in others, which furthermore showed ‘very irregular sex ratios’ [43]. Moreover, similar polymorphisms in the patterns of gonadal development, with differentiated, undifferentiated and semi-differentiated types, have been described for other species of frogs (e.g. [44,45]). Extending investigations to a wider taxonomic range might provide important insights on the evolution of sex determination in amphibians.
Supplementary Material
Acknowledgements
We thank Juha Merilä and Svante Winberg for help with getting experimentation permits, Johan Elmberg for sharing information on sampling locations, and the Ammarnäs research station, Philippe Walter and Ludovic Dutoit for help on the field and sheltering. We also thank Paris Veltsos for comments on the manuscript.
Ethics statement
Capture permits were delivered by the prefectures of Skåne and Västerbotten counties for Tvedöra (522-363-2013) and Ammarnäs (522-3396-2013). An additional permit was delivered for Ammarnäs as part of the nature reserve of Vindelfjällen (521-3407-2013). Ethical permits were delivered by the Swedish Board of Agriculture for Tvedöra (M 19-13) and Ammarnäs (A 10-13), and by the Service de la consommation et des affaires vétérinaires of the Canton Vaud, Switzerland (authorization 2287).
Data accessibility
Microsatellite genotypes (electronic supplementary material, table S2) are available from the Dryad Digital Repository at doi:10.5061/dryad.4j169.
Author contributions
N.R. and N.P. conceived the study; N.R., Y.V. and J.L. did the field sampling; Y.V. carried out the laboratory work; N.R., Y.V. and N.P. carried out the genotyping and statistical analyses; N.P. drafted the manuscript, which was improved by N.R. All authors gave final approval for publication.
Funding statement
This study was supported by the Swiss National Science Foundation (grant no. 31003A_129894 to N.P.).
Conflict of interests
We have no competing interests.
References
- 1.Holliday R. 1990. DNA methylation and epigenetic inheritance. Phil. Trans. R. Soc. Lond. B 326, 329–338. ( 10.1098/rstb.1990.0015) [DOI] [PubMed] [Google Scholar]
- 2.Beukeboom LW, Perrin N. 2014. The evolution of sex determination. Oxford, UK: Oxford University Press. [Google Scholar]
- 3.Charnier M. 1966. Action de la température sur la sex-ratio chez l'embryon d’Agama agama (Agamidae, Lacertilien). C. R. Séances Soc. Biol. l'Ouest Africain 160, 620–622. [PubMed] [Google Scholar]
- 4.Pieau C. 1974. Différenciation du sexe en fonction de la température chez les embryons d’Emys orbicularis L. (Chélonien); effets des hormones sexuelles. Annal. Embryol. Morph. 7, 365–394. [Google Scholar]
- 5.Bull JJ, Vogt RC. 1979. Temperature-dependant sex determination in turtles. Science 206, 1186–1188. ( 10.1126/science.505003) [DOI] [PubMed] [Google Scholar]
- 6.Conover DO. 1984. Adaptive significance of temperature-dependent sex determination in a fish. Am. Nat. 123, 297–313. ( 10.1086/284205) [DOI] [Google Scholar]
- 7.Warner DA, Shine R. 2008. The adaptive significance of temperature-dependent sex determination in a reptile. Nature 451, 566–568. ( 10.1038/nature06519) [DOI] [PubMed] [Google Scholar]
- 8.Volff JN, Nanda I, Schmid B, Schartl M. 2007. Governing sex determination in fish: regulatory putches and ephemeral dictators. Sex Dev. 1, 85–99. ( 10.1159/000100030) [DOI] [PubMed] [Google Scholar]
- 9.Stöck M, et al. 2011. Ever-young sex chromosomes in European tree frogs. PLoS Biol. 9, e1001062 ( 10.1371/journal.pbio.1001062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stöck M, Savary R, Betto-Colliard C, Biollay S, Jourdan-Pineau H, Perrin N. 2013. Low rates of X-Y recombination, not turnovers, account for homomorphic sex chromosomes in several diploid species of Palearctic green toads (Bufo viridis subgroup). J. Evol. Biol. 26, 674–682. ( 10.1111/jeb.12086) [DOI] [PubMed] [Google Scholar]
- 11.Van Doorn GS. 2014. Patterns and mechanisms of evolutionary transitions between genetic sex-determining systems. Cold Spring Harbor Perspect. Biol. 6, a017681 ( 10.1101/cshperspect.a017681) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perrin N. 2009. Sex reversal: a fountain of youth for sex chromosomes? Evolution 63, 3043–3049. ( 10.1111/j.1558-5646.2009.00837.x) [DOI] [PubMed] [Google Scholar]
- 13.Grossen C, Neuenschwander S, Perrin N. 2011. Temperature-dependent turnovers in sex-determination mechanisms: a quantitative model. Evolution 65, 64–78. ( 10.1111/j.1558-5646.2010.01098.x) [DOI] [PubMed] [Google Scholar]
- 14.Eggert C. 2004. Sex determination: the amphibian models. Reprod. Nutr. Dev. 44, 539–549. ( 10.1051/rnd:2004062) [DOI] [PubMed] [Google Scholar]
- 15.Miura I. 2007. An evolutionary witness: the frog Rana rugosa underwent change of heterogametic sex from XY male to ZW female. Sex Dev. 1, 323–331. ( 10.1159/000111764) [DOI] [PubMed] [Google Scholar]
- 16.Witschi E. 1929. Studies on sex differentiation and sex determination in amphibians. III. Rudimentary hermaphroditism and Y chromosome in Rana temporaria. J. Exp. Zool. 54, 157–223. ( 10.1002/jez.1400540202) [DOI] [Google Scholar]
- 17.Crew F. 1921. Sex-reversal in frogs and toads—a review of the recorded cases of abnormality of the reproductive system and an account of a breeding experiment. J. Genet. 11, 141–181. ( 10.1007/BF02983047) [DOI] [Google Scholar]
- 18.Miura I. 1994. Sex chromosome differentiation in the Japanese brown frog, Rana japonica. I. Sex-related heteromorphism of the distribution pattern of constitutive heterochromatin in chromosome no. 4 of the Wakuya population. Zool. Sci. 11, 797–806. [Google Scholar]
- 19.Gasc JP, et al. 1997. Atlas of amphibians and reptiles in Europe. Paris, France: Societas Europaea Herpetologica & Museum National d'Histoire Naturelle (IEGB/SPN). [Google Scholar]
- 20.Witschi E. 1930. Studies on sex differentiation and sex determination in amphibians. IV. The geographical distribution of the sex races of the European grass frog (Rana temporaria, L.). J. Exp. Zool. 56, 149–165. ( 10.1002/jez.1400560202) [DOI] [Google Scholar]
- 21.Piquet J. 1930. Détermination du sexe chez les Batraciens en fonction de la température. Rev. Suisse Zool. 37, 173–281. [Google Scholar]
- 22.Matsuba C, Miura I, Merilä J. 2008. Disentangling genetic versus. environmental causes of sex determination in the common frog, Rana temporaria. BMC Genet. 9, 3 ( 10.1186/1471-2156-9-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alho JS, Matsuba C, Merilä J. 2010. Sex reversal and primary sex ratios in the common frog (Rana temporaria). Mol. Ecol. 19, 1763–1773. ( 10.1111/j.1365-294X.2010.04607.x) [DOI] [PubMed] [Google Scholar]
- 24.Rodrigues N, Betto-Colliard C, Jourdan-Pineau H, Perrin N. 2013. Within-population polymorphism of sex-determination systems in the common frog (Rana temporaria). J. Evol. Biol. 26, 1569–1577. ( 10.1111/jeb.12163) [DOI] [PubMed] [Google Scholar]
- 25.Cano JM, Li MH, Laurila A, Vilkki J, Merilä J. 2011. First-generation linkage map for the common frog Rana temporaria reveals a sex linkage group. Heredity 107, 530–536. ( 10.1038/hdy.2011.39) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alho JS, Herczeg G, Merilä J. 2008. Female biased sex ratios in subarctic common frogs. J. Zool. 275, 57–63. ( 10.1111/j.1469-7998.2007.00409.x) [DOI] [Google Scholar]
- 27.Matsuba C, Alho JS, Merilä J. 2010. Recombination rate between sex chromosomes depends on phenotypic sex in the common frog. Evolution 64, 3634–3637. ( 10.1111/j.1558-5646.2010.01076.x) [DOI] [PubMed] [Google Scholar]
- 28.Rodrigues N, Merilä J, Patrelle C, Perrin N. 2014. Geographic variation in sex-chromosome differentiation in the common frog (Rana temporaria). Mol. Ecol. 23, 3409–3418. ( 10.1111/mec.12829) [DOI] [PubMed] [Google Scholar]
- 29.Broquet T, Berset-Brändli L, Emaresi G, Fumagalli L. 2007. Buccal swab allow efficient and reliable microsatellite genotyping in amphibians. Conserv. Genet. 8, 509–511. ( 10.1007/s10592-006-9180-3) [DOI] [Google Scholar]
- 30.Gosner KL. 1960. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16, 183–190. [Google Scholar]
- 31.Ogielska M, Kotusz A. 2004. Pattern and rate of ovary differentiation with reference to somatic development in anuran amphibians. J. Morphol. 259, 41–54. ( 10.1002/jmor.10162) [DOI] [PubMed] [Google Scholar]
- 32.Haczkiewicz K, Ogielska M. 2013. Gonadal differentiation in frogs: how testes become shorter than ovaries. Zool. Sci. 30, 125–134. ( 10.2108/zsj.30.125) [DOI] [PubMed] [Google Scholar]
- 33.Goudet J. 1995. FSTAT (version 1.2): a computer program to calculate F-statistics. J. Hered. 86, 485–486. [Google Scholar]
- 34.Goudet J. 1999. PCAGEN, a program to perform a principal component analysis (PCA) on genetic data (version 1.2). Lausanne, Switzerland: Population Genetics Laboratory, University of Lausanne. [Google Scholar]
- 35.Coombs JA, Letcher BH, Nislow KH. 2008. CREATE: a software to create input files from diploid genotypic data for 52 genetic software programs. Mol. Ecol. Resour. 8, 578–580. ( 10.1111/j.1471-8286.2007.02036.x) [DOI] [PubMed] [Google Scholar]
- 36.Green P, Falls K, Crook S. 1990. Documentation for CRIMAP, version 2.4. St. Louis, MO: Washington University School of Medicine. [Google Scholar]
- 37.Voorrips RE. 2002. MapChart: software for graphical presentation of linkage maps and QTLs. J. Hered. 93, 77–78. ( 10.1093/jhered/93.1.77) [DOI] [PubMed] [Google Scholar]
- 38.Kimura M, Ohta T. 1975. Distribution of allelic frequencies in a finite population under stepwise production of neutral alleles. Proc. Natl Acad. Sci. USA 72, 2761–2764. ( 10.1073/pnas.72.7.2761) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palo JU, Schmeller DS, Laurila A, Primmer CR, Kuzmin SL, Merilä J. 2004. High degree of population subdivision in a widespread amphibian. Mol. Ecol. 13, 2631–2644. ( 10.1111/j.1365-294X.2004.02269.x) [DOI] [PubMed] [Google Scholar]
- 40.Vences M, et al. 2013. Radically different phylogeographies and patterns of genetic variation in two European brown frogs, genus Rana. Mol. Phylogenet. Evol. 15, 34–40. ( 10.1006/mpev.1999.0738) [DOI] [PubMed] [Google Scholar]
- 41.Conover DO, Heins SW. 1987. Adaptive variation in environmental and genetic sex determination in a fish. Nature 326, 496–498. ( 10.1038/326496a0) [DOI] [PubMed] [Google Scholar]
- 42.Lagomarsino IV, Conover DO. 1993. Variation in environmental and genotypic sex-determining mechanisms across a latitudinal gradient in the fish, Menidia menidia. Evolution 47, 487–494. ( 10.2307/2410066) [DOI] [PubMed] [Google Scholar]
- 43.Nishioka M, Sumida M. 1994. The position of sex-determining genes in the chromosomes of Rana nigromaculata and Rana brevipoda. Sci. Rep. Lab. Amphibian Biol. Hiroshima Univ. 13, 51–97. [Google Scholar]
- 44.Gramapurohit NP, Shanbhag BA, Saidapur SK. 2000. Pattern of gonadal sex differentiation, development, and onset of steroidogenesis in the frog, Rana curtipes. Gen. Comp. Endocrinol. 119, 256–264. ( 10.1006/gcen.2000.7513) [DOI] [PubMed] [Google Scholar]
- 45.Saidapur SK, Gramapurohit NP, Shanbhag BA. 2001. Effect of sex steroids on gonadal differentiation and sex reversal in the frog, Rana curtipes. Gen. Comp. Endocrinol. 124, 115–123. ( 10.1006/gcen.2001.7699) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Microsatellite genotypes (electronic supplementary material, table S2) are available from the Dryad Digital Repository at doi:10.5061/dryad.4j169.