Abstract
Symbiotic associations between animals and chemoautotrophic bacteria crowd around hydrothermal vents. In these associations, symbiotic bacteria use chemical reductants from venting fluid for the energy to support autotrophy, providing primary nutrition for the host. At vents along the Eastern Lau Spreading Center, the partially oxidized sulfur compounds (POSCs) thiosulfate and polysulfide have been detected in and around animal communities but away from venting fluid. The use of POSCs for autotrophy, as an alternative to the chemical substrates in venting fluid, could mitigate competition in these communities. To determine whether ESLC symbioses could use thiosulfate to support carbon fixation or produce POSCs during sulfide oxidation, we used high-pressure, flow-through incubations to assess the productivity of three symbiotic mollusc genera—the snails Alviniconcha spp. and Ifremeria nautilei, and the mussel Bathymodiolus brevior—when oxidizing sulfide and thiosulfate. Via the incorporation of isotopically labelled inorganic carbon, we found that the symbionts of all three genera supported autotrophy while oxidizing both sulfide and thiosulfate, though at different rates. Additionally, by concurrently measuring their effect on sulfur compounds in the aquaria with voltammetric microelectrodes, we showed that these symbioses excreted POSCs under highly sulfidic conditions, illustrating that these symbioses could represent a source for POSCs in their habitat. Furthermore, we revealed spatial disparity in the rates of carbon fixation among the animals in our incubations, which might have implications for the variability of productivity in situ. Together, these results re-shape our thinking about sulfur cycling and productivity by vent symbioses, demonstrating that thiosulfate may be an ecologically important energy source for vent symbioses and that they also likely impact the local geochemical regime through the excretion of POSCs.
Keywords: symbiosis, hydrothermal vent, sulfur metabolism, ecosystem engineer
1. Introduction
Hydrothermal vents support dense assemblages of invertebrates, many of which rely on chemoautotrophic bacterial symbionts for nourishment. These microbial–animal associations aggregate around vent orifices so that their symbionts can use chemicals in the venting fluid for the energy to support carbon fixation [1]. The symbionts of animals from multiple phyla have been shown to use sulfide, methane and/or hydrogen from vent fluid as energy sources [2–11]. Though these are important substrates for microbial energy production [12], other reduced compounds can be present in and around venting fluid and, to date, less is known about the extent to which vent symbioses use these compounds [13–16]. Some of these, including the partially oxidized sulfur compounds (POSCs), polysulfide and thiosulfate, are produced from the abiotic and/or biological oxidation of vent-derived sulfide [13,17]. POSCs are especially abundant away from hotter venting fluids, where the concentrations of sulfide and other reductants sourced directly from the fluid have become depleted or diluted [13,17–19]. As competition among vent symbioses for vent-derived resources (manifest as competition for space around a vent) is likely to be intense, POSCs could be ecologically advantageous for organisms that can use these compounds, as it could lessen the competition for space around vigorous venting. Additionally, use of POSCs could mitigate exposure to the high temperatures and toxic chemicals (e.g. heavy metals) that accompany the chemical reductants in venting fluid, providing additional advantage for the hosts, which like all animals, exhibit some sensitivity to such conditions.
It is well known that chemoautotroph–animal symbioses from non-vent ecosystems can use POSCs for energy generation [20–23]. Moreover, some free-living, vent chemoautotrophs can use POSCs [24,25], and thiosulfate-driven autotrophy has been observed among the epibiotic symbionts of vent crustaceans [6–8,26]. While a few studies have measured thiosulfate oxidation ex hospite by the intracellular symbionts of vent mussels and clams [2,5,9,27,28], thiosulfate-driven autotrophy has not been demonstrated in an intact vent symbiosis, and the extent to which thiosulfate and other POSCs support productivity is unknown.
The distribution and abundance of POSCs in vent fields has been best characterized along the Eastern Lau Spreading Center (ELSC), which is in the southwestern Pacific near the island of Tonga. Symbiotic mollusc species from three genera dominate at these vent fields: three cryptic provannid snail species from the genus Alviniconcha, another Provannid snail I. nautilei and the mussel B. brevior [29]. Each species associates with different, phylogenetically distant lineages of symbiotic, intracellular bacteria [30–38] that are capable of supporting autotrophy using sulfide [39]. Typically, these animals form concentric patterns around vent orifices, with Alviniconcha spp. found closest to the vent, followed by a zone of I. nautilei, and finally B. brevior at the very edges of the assemblage [18,40,41]. It has been suggested that these zones form as a result of competitive interactions for vent-derived resources as well as due to differences in temperature and chemical tolerance or preference [41,42]. Thiosulfate and polysulfides (POSCs) are common in and around these concentric aggregations at concentrations up to 1000 and 400 µM, respectively, which are equal to or greater than the concentrations of sulfide and likely other reductants from venting fluid in the same areas [13,17,18]. Strikingly, the diversity and abundance of POSCs correspond to the distribution of the mollusc genera. In particular, the highest concentrations of thiosulfate in the aggregations are found over the zones of B. brevior [17,18], while the highest concentrations of polysulfides are found among Alviniconcha spp. and I. nautilei [13,18].
To determine whether these symbioses could use POSCs to support carbon fixation or produce them during sulfide oxidation, we conducted a series of shipboard incubations with all three ELSC mollusc symbioses using high-pressure, flow-through aquaria. Inline voltammetric electrodes allowed us to assess total sulfur flux through the aquaria, while the use of isotopically labelled inorganic carbon allowed us to quantify individual productivity during the incubations.
2. Material and methods
(a). Animal collections
Animals were collected from the vent fields ABE (−20°45.8′ by −176°11.5′) or Tu'i Malila (−21°59.4′ by −176°34.1′) at the ELSC by the remotely operated vehicle JASON II during the R/V Thomas G. Thompson expedition TM-235 in 2009. Alviniconcha spp., I. nautilei and B. brevior were recovered in insulated containers and, once on board, were briefly kept in 4°C seawater. Individuals responsive to touch were placed in the flow-through titanium aquaria of the high-pressure respirometry system (HPRS; electronic supplementary material, Methods and figure S1; [4,43]). For each incubation, between three and 10 individuals of each genus were placed into three separate aquaria. Animals were situated upon perforated acrylic partitions so that they were stacked vertically in the cylindrical aquaria.
(b). Incubation conditions and acclimation
Three incubations (hereafter ‘rate experiments’) were performed to compare net sulfur uptake and excretion rates, as well as carbon fixation rates, by the three mollusc genera at three different conditions: 105 µM sulfide, 300 µM thiosulfate and sulfur-free. During each rate experiment, an empty high-pressure aquarium (control) was run alongside the three animal-containing aquaria in order to account for systematic losses and enable the most robust mass-specific rate determinations. Two additional incubations (hereafter ‘variation experiments’) were performed to establish the extent of variation in carbon fixation rates among the animals within each aquarium. Variation experiments were run at 350 µM sulfide or 300 µM thiosulfate, up to twice the number of individuals per aquaria than in the rate experiments.
Animals were acclimated to the aquaria by incubating in aerated seawater at 15°C and 25 MPa for 8 h prior to the start of each experiment or treatment. Similarly, during the sulfur-free experiment, animals were acclimated with approximately 300 µM sulfide for 8 h before sulfur was stopped and isotopic tracer was added. As the sulfur-free experiment required a prolonged period without exogenous reductants, acclimation with sulfide allowed normalization of physiological states including, potentially, the accumulation of sulfur stores.
(c). Sulfur oxidation and excretion rates
To determine the net sulfur oxidation rates, as well as to detect the excretion of POSCs, the concentration of sulfur compounds in the aquaria effluent were compared to the effluent from the empty control aquarium (rate experiments) or to the concentrations in input water (variation experiments). The concentrations of total sulfide (∑H2S and HS−), thiosulfate and polysulfides were measured via voltammetric microelectrodes [15,44]. Though we were unable to quantify oxygen concentrations during the incubations at high concentrations of sulfide, the voltammetric microelectrodes (see below) were able to always detect oxygen in the aquaria effluent during all incubations (minimum detection limit is 5 µM; [13]), suggesting that oxygen was never completely depleted by the symbioses in the aquaria (see electronic supplementary material, Methods).
The input and/or effluent water was measured in a cyclical series of 30 min intervals throughout the duration of the experiment; one complete 2-h series of four intervals starting with an experimental effluent and ending with either the control effluent or input water is hereafter referred to as a ‘set’. At the start of every 15 min period, a series of 21 electrochemical scans were performed at a rate of one per approximately 2 s, resulting in 42 measurements per 30 min interval. All net sulfur oxidation rates were calculated using data obtained after 10 h or more into the experiment, which allowed at least three turnovers in the volume of water in the aquaria. To calculate the concentrations of each sulfur compound during each 30 min interval, the first 10 scans, or 24%, in each interval were removed to exclude the transitory scans that occurred after the switch to a different aquarium effluent. The concentrations resulting from the remaining scans were averaged, resulting in a single concentration value per interval (hereafter, ‘interval concentration’). To calculate mass-specific oxidation rates for each mollusc genus within a set, the interval concentration of the control effluent was subtracted from the interval concentration of the experimental effluent, which in turn was divided by the total gill mass of the animals in each aquarium and multiplied by the fluid flow rate through the aquaria in litres per hour.
(d). Sampling of experimental animals
At the conclusion of each experiment, aquaria were depressurized and animals were quickly removed, excised from their shells and weighed via a motion-compensated shipboard balance [45]. Each individual's gill weight was estimated from linear equations derived from the regression of total body mass to gill mass (electronic supplementary material, figure S2) for each genus. Gill and foot tissue were frozen at −80°C for isotopic analysis and gill tissue was homogenized and preserved in Trizol™ (Life Technologies) for nucleic acid extraction.
(e). Symbiont identities
DNA was extracted from the Trizol™-preserved tissue samples following RNA extraction via a modified manufacturer's protocol. Symbiont 16S rRNA genes from I. nautilei and B. brevior associations were amplified via PCR using universal bacterial primers [46] and directly Sanger sequenced. Because some Alviniconcha spp. from the ELSC are known to host mixed assemblages of phylogenetically distinct symbionts [34], direct sequencing of the symbiont populations was not possible for this genus. Thus, symbiont populations associated with the experimental Alviniconcha spp. were assessed via qPCR as in Beinart et al. [34]. Subsequently, a Bayesian inference phylogeny was produced with MrBayes [47] (see electronic supplementary material, Methods).
(f). Carbon stable isotopic analyses and carbon fixation rates
DIC isotopic compositions were determined at the Yale Institute of Biospheric Studies Earth System Center for Stable Isotopic Studies, using well-established protocols (see electronic supplementary material, Methods). Approximately 300 mg of symbiont-containing gill and symbiont-free foot tissue were subsampled while frozen for carbon isotopic analysis. Samples were lyophilized for 24 h then acidified with 0.1 N HCl to remove unincorporated Na13CO3. The samples were subsequently dried for 24–48 h at 50–60°C, weighed to determine dry weight, then homogenized to a fifine powder. The carbon isotopic composition and per cent carbon content were determined for foot and gill tissue samples via isotope ratio mass spectrometry (see electronic supplementary material, Methods).
To obtain the percentage of 13C incorporated during these experiments (%13Cinc), the following formula, modified from Riou et al. [48] was used
where A%g is the atomic per cent of the symbiont-containing gill tissue sample in which carbon incorporation occurs; A%f is the atomic percent of the same individual's foot tissue sample and A%w is the atomic per cent of the input water DIC containing the added isotopic label. Because foot tissue in these animals is symbiont-free and unlikely to receive appreciable quantities of translocated carbon from the gill over the short time course of these experiments, it serves to represent the initial A% of the individual in the above equation.
The weight of incorporated carbon (W13Cinc) was calculated by multiplying the %13Cinc by the dry weight of the gill tissue (DWg) and the carbon content of the sample (%C). Carbon incorporation rates (Cinc) are expressed as micromoles 13C per gram of wet tissue per hour. This was first calculated as a rate of carbon incorporation expressed as micromoles 13C per gram of dry tissue per hour (DryCinc) with the formula
where MWc is the molecular weight of 13C; and t is the duration of the experiment. DryCinc was then converted to Cinc by multiplying dry Cinc by the ratio of DWg to the wet weight of that same sample (Wg).
Additional details may be found in the electronic supplementary material, Methods.
3. Results
(a). Sulfur metabolism and carbon fixation in rate experiments
All three symbioses demonstrated net sulfide and thiosulfate uptake (figure 1 and table 1). Though sulfur concentrations were diminished by the animals during the rate experiments (relative to the control aquarium), measurable concentrations of added sulfide and thiosulfate were detected in the aquaria effluent, suggesting that sulfur compounds did not become limiting. Net sulfide oxidation rates were comparable among the three genera, though B. brevior had the highest average rate at these experimental conditions (figure 1a). Net thiosulfate oxidation rates were more varied among the three genera, with I. nautilei having almost twice the average rate of Alviniconcha spp. (figure 1b). Additionally, thiosulfate oxidation rates fluctuated more widely over the duration of the experiment than did sulfide oxidation rates (figure 1a,b). No additional sulfur compounds were detected in the effluent of the three experiments, indicating that biologically mediated sulfur transformations and excretions did not occur at these conditions.
Figure 1.
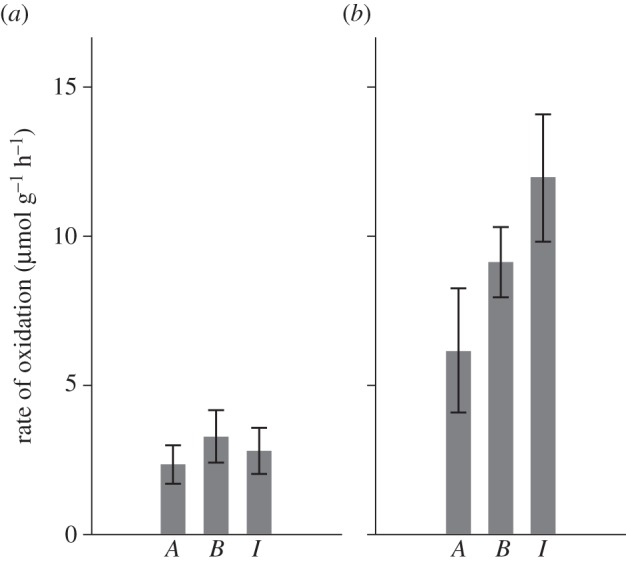
Average mass-specific net sulfur oxidation rates (micromoles of sulfur per gram of wet gill tissue per hour) ±s.d. during the sulfide (a) and thiosulfate (b) rate experiments for Alviniconcha spp. (A), B. brevior (B) and I. nautilei (I).
Table 1.
Net sulfur uptake (oxidation) and excretion by the three mollusc genera. Number of individuals (n) and the total wet weight of gill tissue for each mollusc genus in each experiment, as well as the average (min, max) concentrations of sulfide, thiosulfate and polysulfides as measured via a voltammetric electrode in the effluent from each aquaria. BDL stands for ‘below detection limit’.
| experiment | genus | n | total gill wet weight (g) | sulfide (μM)a | thiosulfate (μM)a | polysulfides (μM)a |
|---|---|---|---|---|---|---|
| sulfur-free | control | 0 | 0 | BDL | BDL | BDL |
| Alviniconcha | 5 | 15.88 | BDL | BDL | BDL | |
| I. nautilei | 5 | 18.83 | BDL | BDL | BDL | |
| B. brevior | 4 | 18.78 | BDL | BDL | BDL | |
| sulfide | control | 0 | 0 | 66 (46, 107) | BDL | BDL |
| Alviniconcha | 5 | 22.56 | 5.7 (2.7,9.7) | BDL | BDL | |
| I. nautilei | 5 | 20.49 | 5.8 (3.0, 8.6) | BDL | BDL | |
| B. brevior | 4 | 18.36 | 3.0 (1.4, 3.9) | BDL | BDL | |
| thiosulfate | control | 0 | 0 | 0.32 (BDL, 1.1) | 276 (216, 310) | BDL |
| Alviniconcha | 3 | 21.42 | 0.22 (BDL, 0.36) | 139 (BDL, 209) | BDL | |
| I. nautilei | 4 | 19.50 | 0.22 (BDL, 0.31) | 42 (BDL, 70) | BDL | |
| B. brevior | 3 | 13.46 | BDL | 140 (67 188) | BDL |
aDetection limits: sulfide and polysulfides, 0.20 μM; thiosulfate, 30 μM.
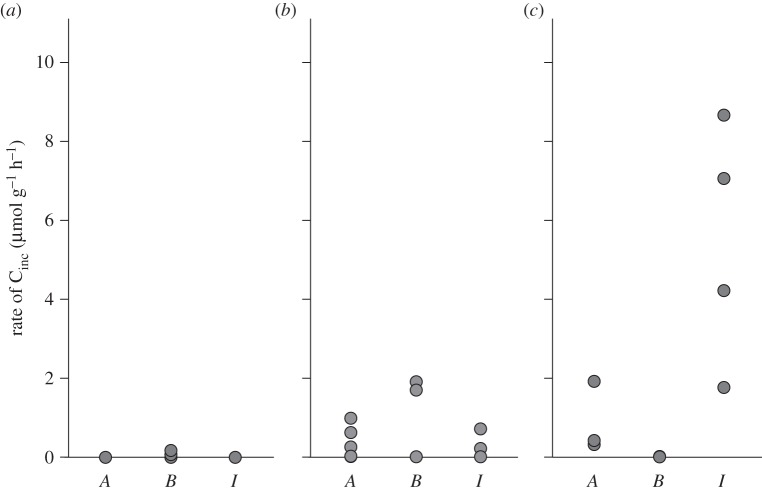
Carbon incorporation (fixation) was stimulated in the gills of individuals from all three symbiotic genera when sulfide was provided (figure 2b; electronic supplementary material, table S1). By contrast, only Alviniconcha spp. and I. nautilei individuals fixed carbon when given thiosulfate (figure 2c). Carbon incorporation rates among the genera did not differ significantly from one another during the sulfide experiment (Kruskal–Wallis, p = 0.424) (figure 2b). Among all three experiments, the greatest rates of carbon fixation occurred in I. nautilei individuals supplied thiosulfate (figure 2c), though this was not significantly different from Alviniconcha spp. individuals in that experiment (Mann–Whitney U, p = 0.114; B. brevior was omitted from this comparison as no carbon incorporation was observed in the thiosulfate treatment). Carbon fixation was not stimulated in Alviniconcha spp. and I. nautilei individuals in the sulfur-free (control) experiment, though minor carbon incorporation was detected in two of the four B. brevior individuals.
Figure 2.
Individual mass-specific carbon incorporation rates (micromoles of 13C per gram of wet gill tissue per hour) for Alviniconcha spp. (A), I. nautilei (I) and B. brevior (B) during the (a) sulfur-free, (b) sulfide and (c) thiosulfate rate experiments.
(b). Sulfur metabolism and carbon fixation during variation experiments
During the variation experiments, sulfide and thiosulfate concentrations were depleted in the effluents of the aquaria in their respective treatments (relative to the input water; table 2). As with the rate experiments, measurable concentrations of added sulfide and thiosulfate were detected in the experimental effluent of all incubations, indicating that the symbioses did not completely exhaust these compounds in the aquaria. Mass-specific uptake (oxidation) rates were comparable among treatments, though the average sulfide oxidation rate was greater than the average thiosulfate oxidation rate for all three genera (table 2). Sustained sulfur excretion was observed throughout the duration of the sulfide treatment, but not during the thiosulfate treatment (table 2). When exposed to sulfide, both Alviniconcha spp. and B. brevior released thiosulfate, at rates equivalent to 24–30% and 5–5.2% of their sulfide oxidation rates, respectively. Ifremeria nautilei released polysulfide at rates equivalent to 11–47% of its rate of sulfide oxidation.
Table 2.
Net sulfur uptake (oxidation) and excretion by the three mollusc genera during variation experiments. Number of individuals (n); the sum total wet weight of gill tissue for each mollusc genus; average (min, max) concentrations of sulfide, thiosulfate and polysulfides as measured via a voltammetric electrode in the effluent from each aquaria; average (±s.d.) rates of net oxidation or excretion (indicated as negative or positive values, respectively) as micromoles per gram of wet gill tissue per hour.
| genus | N | total gill wet weight (g) | sulfidea | thiosulfatea | polysulfidesa | |
|---|---|---|---|---|---|---|
| sulfide treatment | ||||||
| input | n.a. | n.a. | concentration (μM) | 349 (329, 387) | BDL | BDL |
| Alviniconcha | 10 | 36.85 | concentration (μM) | 21 (19, 23) | 73 (68, 77) | BDL |
| rate (μmoles g−1 h−1) | −6.8 ± 0.50 | +0.92 ± 0.05 | BDL | |||
| I. nautilei | 5 | 34.08 | concentration (μM) | 85 (76, 90) | BDL | 78 (0.2, 126) |
| rate (μmoles g−1 h−1) | −7.2 ± 0.75 | BDL | +2.2 ± 1.5 | |||
| B. brevior | 6 | 39.87 | concentration (μM) | 14 (12, 15) | 34 (30, 40) | BDL |
| rate (μmoles g−1 h−1) | −7.4 ± 0.52 | +0.10 ± 0.08 | BDL | |||
| thiosulfate treatment | ||||||
| input | n.a. | n.a. | concentration (μM) | BDL | 302 (251, 404) | BDL |
| Alviniconcha | 8 | 39.29 | concentration (μM) | BDL | 40 (30, 82) | BDL |
| rate (μmol g−1 h−1) | BDL | −5.62 ± 1.22 | BDL | |||
| I. nautilei | 10 | 50.68 | concentration (μM) | BDL | 44 (30, 60) | BDL |
| rate (μmol g−1 h−1) | BDL | −4.66 ± 0.93 | BDL | |||
| B. brevior | 6 | 45.29 | concentration (µM) | BDL | 103 (30, 162) | BDL |
| rate (µmol g−1 h−1) | BDL | −4.02 ± 0.84 | BDL | |||
aDetection limits: sulfide and polysulfides, 0.20 µM; thiosulfate, 30 µM.
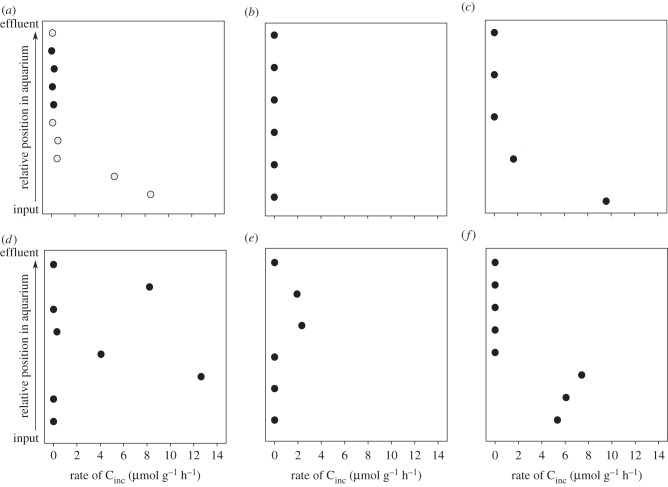
Alviniconcha spp. and I. nautilei individuals from both the sulfide and thiosulfate treatments incorporated carbon (figure 3a,c,d,f; electronic supplementary material, table S1). No carbon incorporation was observed by B. brevior in the sulfide treatment (figure 3b), though during the thiosulfate treatment two of the six B. brevior individuals incorporated carbon (figure 3e). Within both the sulfide and thiosulfate treatments, carbon incorporation rates were greatly variable among individuals. Further examination of these data suggests that the observed variability is likely related to substrate depletion (e.g. sulfur or oxygen) in the aquaria. Specifically, animals positioned in the aquaria closest to the input of water had the highest carbon fixation rates (figure 3). Additionally, individuals from these experiments demonstrated the highest rates of carbon fixation among all of the animals in this study. Alviniconcha spp. and B. brevior individuals from the thiosulfate variation experiment showed the highest rates of carbon fixation among all individuals of their genus in this study. Additionally, an individual from the sulfide variation experiment had the highest carbon fixation rate for any experimental I. nautilei.
Figure 3.
Individual mass-specific carbon incorporation rates (micromoles of 13C per gram of wet gill tissue per hour) during the variation experiments. Sulfide variation experiment with (a) Alviniconcha spp., (b) B. brevior and (c) I. nautilei; thiosulfate variation experiment with (d) Alviniconcha spp., (e) B. brevior and (f) I. nautilei. In panel (a), grey symbols indicate Alviniconcha spp. hosting ε-proteobacterial symbionts; black symbols indicate Alviniconcha spp. hosting γ-1 symbionts. All individuals are shown according to their relative position in the HPRS aquaria.
(c). Symbiont identity
Assessment of the symbiont populations through analysis of the 16S rRNA genes demonstrated that the symbionts of each host genus associated with γ-proteobacterial symbionts. As indicated by their 16S rRNA genes, there was low diversity among the symbionts of I. nautilei and B. brevior. Amplification and direct sequencing resulted in clean chromatograms with no evidence of mixed symbiont populations within individuals. Individuals of I. nautilei yielded six unique sequences that were at least 99.5% identical to one another, whereas B. brevior individuals yielded three unique sequences that were at least 98.8% identical to one another. Bayesian phylogenetic analysis (electronic supplementary material, figure S3) showed that the experimental B. brevior symbiont sequences fell in a well-supported clade consisting of symbionts from Bathymodiolus mussels and Calyptogena clams and a few free-living marine bacteria. Experimental I. nautilei symbiont sequences fell in a well-supported clade of I. nautilei symbionts from the Lau basin and other hydrothermal vent regions.
With the exception of some individuals in the sulfide variation experiment, all experimental Alviniconcha spp. were dominated by symbionts from one of the two γ-proteobacterial phylotypes (i.e. more than or equal to 93% of the detected 16S rRNA genes; electronic supplementary material, table S2) that associate with this genus in the ELSC. Most of the individuals in the rate experiments and thiosulfate variation experiment hosted mainly γ-1 symbionts; only two Alviniconcha from the sulfur-free experiment, which showed no measurable sulfur metabolism or carbon incorporation, were dominated by γ-Lau symbionts (sensu [34]). Thus, most of the results regarding Alviniconcha metabolism herein are in regards to Alviniconcha spp. hosting γ-1 symbionts. However, six of the 10 Alviniconcha in the sulfide variation experiment hosted ε-proteobacterial symbionts, and, accordingly, the rates of sulfide oxidation and thiosulfate excretion in that experiment reflect the net effect of both ε-proteobacterial and γ-1 symbionts’ metabolic activity.
4. Discussion
(a). The significance of thiosulfate oxidation for hydrothermal vent symbioses
Here, we demonstrated, for the first time in hospite, that exogenous thiosulfate drove autotrophy in animal–microbial hydrothermal vent symbioses. Two previous in vitro and ex hospite studies have shown that the intracellular symbionts of Bathymodiolus mussels and Calyptogena clams support autotrophy via thiosulfate [2,5,9,27,28]. However, as many animals produce thiosulfate from sulfide as a detoxification mechanism [49], these experiments left it ambiguous whether the symbionts were only able to use endogenous thiosulfate produced by their host, or if the intact symbioses could take up and use thiosulfate from their surroundings. The use of stable isotopically labelled inorganic carbon in our experiment revealed that three mollusc symbioses were able to support autotrophy through the uptake of exogenous thiosulfate.
Sulfide oxidation is often considered the main driver of primary productivity by chemoautotrophs at vent habitats [12]; however, the complete oxidation of thiosulfate with oxygen is comparable in energy output to the complete oxidation of HS− (standard Gibbs free energies of −738.7 and −732.6 kJ mol substrate−1, respectively [50]). In our experiments, individual thiosulfate-dependent carbon fixation rates often met or exceeded individual carbon fixation rates at a comparable sulfide concentration. Thiosulfate is non-toxic and is more stable with respect to abiotic oxidation than sulfide [51]. As a result, it may be readily concentrated within the host's or symbionts’ cells, leading to a high favourability of reaction resulting from an increased abundance of the substrate. Thus, thiosulfate has the potential to be an important energy source for some hydrothermal vent symbioses, though the extent to which the ELSC symbioses (or others) preferentially use thiosulfate remains to be determined.
Our sulfur-free experiment further emphasizes the value of using thiosulfate as a reductant in the highly variable vent environment. It has long been hypothesized that symbionts could ‘buffer’ the variability in sulfide that arises from variations in flow by using stored, intracellular elemental sulfur granules when exogenous reductants are absent [38,52]. However, for these symbioses, the absence of exogenous sulfur compounds resulted in a cessation of autotrophy (note that all animals were exposed to sulfide during the acclimation period, and thus had the opportunity to build up sulfur stores). For these symbioses, flexible use of multiple sulfur compounds, rather than use of stored compounds, may help them contend with the dynamic conditions at hydrothermal vents. Given the variability in exposure to vent fluid that symbioses likely experience due to temporal and spatial fluid dynamics, the ability to use multiple reductants (e.g. both sulfide and thiosulfate) may relieve the energy limitation that could occur if a symbiosis was exclusively dependent on energy sources found only in venting fluid.
(b). Sulfur oxidation by the mollusc symbioses at the Eastern Lau Spreading Center
It has been suggested that thiosulfate may be an especially important energy source for B. brevior, as it may enable exploitation of habitat away from the venting fluid, where conditions are tolerable but energetic resources derived directly from venting fluid are scarce [18]. Like in previous observations [39], we saw that sulfide-driven carbon incorporation by B. brevior only occurred only in the presence of lower sulfide concentrations, but not with the relatively high concentrations of sulfide in the variation experiment. This bolsters the hypothesis that physiological intolerance to the high concentrations of sulfide associated with exposure to venting fluid prevents B. brevior from inhabiting areas near vent sources. However, while we observed that B. brevior can indeed use thiosulfate to power autotrophy, only two of the nine total individuals tested exhibited carbon incorporation in the presence of thiosulfate (figures 2 and 3), suggesting an inefficient coupling between the metabolic processes. While previous studies have shown that non-vent bivalves with chemoautotrophic symbionts can support autotrophy with thiosulfate [20,21,23], the ability of B. brevior symbionts to support autotrophy with thiosulfate in situ remains to be determined.
The snails Alviniconcha spp. and I. nautilei typically live in close proximity to the venting source, where sulfide is more abundant and thiosulfate concentrations are low [13,18,41]. This correlation has led to the suggestion that sulfide is the main energy source for these snails, while thiosulfate is either not being used or is not important. Our results were congruent with a previous report of autotrophy powered by sulfide oxidation in these taxa [39], though our I. nautilei mass-specific carbon fixation rates were higher. As rates reported by Henry et al. [39] were based on the net uptake of carbon dioxide, high rates of respiration by I. nautilei may have masked higher rates of carbon fixation in their experiments. Our isotopic labelling experiments suggest that I. nautilei may be as productive as Alviniconcha spp. at our experimental conditions (i.e. when sulfide and oxygen are replete). We also observed robust coupling between thiosulfate oxidation and carbon fixation in I. nautilei and Alviniconcha spp. Consequently, the observation that I. nautilei and Alviniconcha spp. commonly inhabit regions of low thiosulfate may simply reflect their influence on the pool of thiosulfate. This supposition is supported by the observed increase in thiosulfate concentrations when Alviniconcha spp. and I. nautilei were removed from snail and mussel aggregations in situ [17].
As both sulfide and thiosulfate drove carbon incorporation by all three symbioses, binary differences in the ability to use these compounds do not appear to drive the observed differences in habitat utilization. However, the relative significance of these energy sources to each symbiotic taxon, their contribution to the overall productivity of the community and comparisons of sulfur metabolism among Alviniconcha spp. hosting different symbiont phylotypes will require further work.
(c). Variability in the carbon fixation rate and substrate limitation in the aquaria
Most previous work measuring the rates of carbon fixation by intact hydrothermal vent symbioses have been performed on low numbers of individuals [4,5,27,39], and used the resulting collective effect on the chemical composition of the seawater outflow and the total biomass to estimate mass-specific metabolic rates. Here, the use of stable isotopically labelled inorganic carbon in our aquarium seawater allowed us to examine the variability in productivity among individuals in our variation experiment incubations, where we interrogated the mass-specific rates of carbon fixation for between five and 10 individuals per genus. We found that the most productive animals in each aquarium likely accounted for much of the sulfur oxidation occurring in each aquaria. Using the molar ratios of 6.21 and 6.64 for the amount of carbon fixed per sulfide or thiosulfate [50] with an assumed 10% efficiency of energy conservation, we calculated the predicted rate of sulfur oxidation by the most productive animals in each aquarium from their individual rates of carbon fixation. In the sulfide treatments, sulfide oxidation by the two most productive individuals may have accounted for 27% and 51% of the total oxidation in the Alviniconcha spp. and I. nautilei aquaria, respectively. In the thiosulfate treatments, oxidation by the productive individuals (all other individuals showed no carbon incorporation) may have accounted for 100% and 64% (Alviniconcha spp. and I. nautilei, respectively) of the total oxidation in each aquarium.
The striking disparity in carbon fixation rates among individuals was unlikely due to biological diversity (e.g. differences in symbiont populations). The symbionts of all I. nautilei and B. brevior exhibited low diversity across all experiments. Though individuals of the Alviniconcha spp. in the sulfide treatment of the variation experiment were dominated by either ɛ-proteobacterial or γ-1 symbionts, rates of incorporation did not appear to be related to symbiont identity (figure 3a). Instead, these disparities in carbon incorporation may be due to differences in the availability of resources along the length of the aquaria. We discovered that the individuals closest to the water input incorporated the greatest amount of carbon, and those near the outflow showed no measurable carbon incorporation, indicating that the most productive individuals near the input were depleting some substrate for those downstream. This pattern was clearly observed in the variation experiments (figure 3). Because both sulfur and oxygen were not entirely depleted in the effluent, it is unclear whether these substrates, or any other substrate for that matter, were restricting the productivity of the symbioses. It is also plausible that waste products from the more productive individuals may have inhibited productivity by those located downstream, though it is unclear what those waste products might be (the predominant waste products of chemoautotrophic sulfide oxidation are oxidized sulfur compounds and hydrogen ions) [43,53].
Our results have important implications for our understanding of total productivity of assemblages of these organisms in their habitats, particularly if the observed variability in carbon fixation rates is due to sulfur limitation. Large communities of these symbioses are often found piled around hydrothermal vent orifices, often two to seven animals deep (C. Fisher 2013, personal communication). In these piles, an individual's access to sulfide (or any vent-derived substrate)—as well as its exposure to waste products from other animals—is governed by the confluence of end-member concentration, fluid flow rate and animal/microbial uptake rate. In situ populations are likely experiencing gradients in vent-derived geochemistry resulting from the net effect of biotic and abiotic factors. Our data suggest that competition for vent-derived resources, which is tied to spatial position in the fluid flow relative to other individuals, may be significant for these symbioses. Interestingly, these results also provide another perspective from which to view thiosulfate-driven autotrophy in these ecosystems. At the tops of the assemblages, where vent-derived reductants may be depleted by the activity of those below, use of an energy source that is not directly sourced from venting fluid could sustain productivity. Therefore, metabolic flexibility has the potential to relieve competition for vent-derived reductants, both within and among species.
(d). Excretion of sulfur compounds
Though links between the distribution of particular ELSC symbioses and elevated concentrations of partially oxidized sulfur may indicate a preference for that particular geochemical niche, it is also possible that such correlation may result from excretion of that compound by the associated symbioses. To address sulfur excretion by the ELSC symbioses, we measured the production of partially oxidized sulfur in our incubations. The sulfide treatment in our variation experiment showed that these symbioses have the potential to contribute to the partially oxidized sulfur pools in their environment. During the approximately 350 µM sulfide variation experiment, I. nautilei released polysulfides (as previously described in Gartman et al. [13]). We quantified mass-specific rates, showing that this excretion stoichiometrically represented up to approximately 50% of the sulfur oxidized in that aquaria. Additionally, both Alviniconcha spp. and B. brevior excreted thiosulfate, though the mass-specific rate was nine times higher in Alviniconcha spp. Because this Alviniconcha incubation included individuals hosting either ɛ-proteobacterial or γ-1 symbionts, the extent to which these phylotypes contributed to the observed excretion remains unclear. Furthermore, as our incubations were performed on intact symbioses, we are unable to discern which partner, host or symbiont, is the source of the excreted sulfur. Many invertebrates, even those without chemoautotrophic symbionts, detoxify sulfide via oxidation to thiosulfate in their mitochondria [49]. Because we did not observe sulfur excretion in the other incubation with a lower sulfide concentration (i.e. the rate experiment with approx. 105 μM sulfide), this could be the case. Additionally, B. brevior did not fix carbon when exposed to approximately 350 μM sulfide, though sulfide oxidation was observed. The sulfide concentration in this incubation is likely higher than what B. brevior would experience in situ [40–42], suggesting the possibility that it was oxidizing sulfide to thiosulfate for detoxification.
POSCs may also be the product of sulfide oxidation by the symbionts of these animals. The symbionts of the vent tubeworm Riftia pachyptila produce polysulfides during sulfide oxidation in vitro [27], which is thought to be a normal intermediate during the production of sulfur granules as it is with other sulfide oxidizers [54]. Experiments with the sulfur-oxidizing isolate Thiobacillus thioparus showed that both thiosulfate and polysulfides can be produced via sulfide oxidation when oxygen is limiting [55]. Though more than 5 μM oxygen was always detected in the effluent of the aquaria (data not shown), respiration by the high biomass in the sulfide variation experiment could have caused low local concentrations in the aquaria, resulting in high sulfide to oxygen ratios. Additionally, host physiology likely mediates the intracellular oxygen concentrations experienced by the symbionts, and it is possible that oxygen was limiting for them despite conditions in the aquaria.
Regardless of the organism of origin, net excretion of partially oxidized sulfur by these symbioses reveals a biological source for these compounds in situ. Higher polysulfide concentrations are detected around I. nautilei and Alviniconcha spp. at the ELSC, whereas higher thiosulfate concentrations are found around B. brevior [17]. It was suggested previously that these POSCs result from the abiotic oxidation of sulfide in venting fluid by aqueous iron or rocky substrate, or from biological oxidation by the symbioses. Here, we demonstrate that biological oxidation influences the presence of these sulfur compounds, ultimately affecting the local sulfur regime. As both free-living microbes and vent symbioses can use these compounds for autotrophy, biological sulfur transformations have the potential to affect the distribution and activities of many organisms within these ecosystems.
5. Conclusion
The extent to which vent symbioses can use exogenous thiosulfate to drive autotrophy remains unknown, though genomic analyses of the symbionts of vent organisms has revealed that many possess the metabolic pathway for the oxidation of sulfur, including thiosulfate [56–61]. Thiosulfate concentrations at vent habitats have only been extensively documented at the ELSC, but given the abiotic oxidation of sulfide to thiosulfate in the presence of certain metals [62,63], as well as the rapid oxidation of sulfur by many microorganisms, POSCs are likely present in these and other systems [64]. Thiosulfate-fueled autotrophy has potential ecological benefits, particularly for symbioses that cannot be near the high concentrations of reductants in venting fluid, either due to physiological intolerance to high temperatures or toxic vent chemicals or because of competitive exclusion. Additionally, flexible use of multiple reductants may help vent symbioses cope with periods of low exposure to reductants in vent fluid that result from the temporal and spatial inconsistency of these habitats. Though we showed that access to particular sulfur compounds differentially affects the productivity of these symbioses, the ability of all three coexisting genera to fuel autotrophy with both sulfide and thiosulfate implies that metabolic flexibility has important advantages in these ecosystems.
While it has long been expected that vent symbioses can alter the local geochemical regime through the uptake and oxidation of sulfide, the observed excretion of partially oxidized sulfur establishes a new mode of chemical interaction and alteration within their ecosystem. Since the discovery of hydrothermal vents, sulfide has been known to play a fundamental role in structuring and supporting vent assemblages [65]. Here, our data reveal an influence of vent symbioses on sulfur biogeochemical cycling beyond the acquisition and complete oxidation of sulfide. These symbioses may also be influencing the availability of POSCs that are of energetic value to the free-living microbes in these ecosystems. Tubeworm symbioses have previously been described as ecosystem engineers for the role they play in shaping the physical structure of their environment [66]; our data illustrate the extent to which chemoautotrophic symbioses might govern the local sulfur regime as well.
Supplementary Material
Acknowledgements
We are grateful for the crews of the RV Thomas G. Thompson and the ROV JASON II for their support. We thank A. Thomsen, R. Hussey and T. Galyean for their assistance with the sulfide measurements from our discrete samples; and G. Hunsinger from the Yale Institute of Biospheric Studies' Earth System Center for Stable Isotopic Studies.
Data accessibility
DNA sequences: GenBank accession KF780847–55. Stable isotopic data: uploaded as electronic supplementary material.
Funding statement
This material is based upon work supported by the National Science Foundation (GRF grant no. DGE-1144152 to R.A.B., OCE-0732439 to G.W.L. and IOS-1257755 as well as OCE-0732369 to P.R.G.).
References
- 1.Stewart FJF, Newton ILGI, Cavanaugh CMC. 2005. Chemosynthetic endosymbioses: adaptations to oxic–anoxic interfaces. Trends Microbiol. 13, 439–448. ( 10.1016/j.tim.2005.07.007) [DOI] [PubMed] [Google Scholar]
- 2.Belkin S, Nelson DC, Jannasch HW. 1986. Symbiotic assimilation of CO2 in the two hydrothermal vent animals, the mussel Bathymodiolus thermophilus and the tubeworm Riftia pachyptila. Biol. Bull. 170, 110–121. ( 10.2307/1541384) [DOI] [Google Scholar]
- 3.Nelson DC, Hagen KD. 1995. Physiology and biochemistry of symbiotic and free-living chemoautotrophic sulfur bacteria. Am. Zool. 35, 91–101. ( 10.1093/icb/35.2.91) [DOI] [Google Scholar]
- 4.Girguis PR, Childress JJ. 2006. Metabolite uptake, stoichiometry and chemoautotrophic function of the hydrothermal vent tubeworm Riftia pachyptila: responses to environmental variations in substrate concentrations and temperature. J. Exp. Biol. 209, 3516–3528. ( 10.1242/jeb.02404) [DOI] [PubMed] [Google Scholar]
- 5.Childress JJ, Fisher CR, Favuzzi JA, Sanders NK. 1991. Sulfide and carbon dioxide uptake by the hydrothermal vent clam, Calyptogena magnifica, and its chemoautotrophic symbionts. Physiol. Zool. 64, 1444–1470. [Google Scholar]
- 6.Ponsard J, et al. 2012. Inorganic carbon fixation by chemosynthetic ectosymbionts and nutritional transfers to the hydrothermal vent host–shrimp Rimicaris exoculata. ISME J. 7, 96–109. ( 10.1038/ismej.2012.87) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watsuji T-O, Nishizawa M, Morono Y, Hirayama H, Kawagucci S, Takahata N, Sano Y, Takai K. 2012. Cell-specific thioautotrophic productivity of epsilon-proteobacterial epibionts associated with Shinkaia crosnieri. PLoS ONE 7, e46282 ( 10.1371/journal.pone.0046282) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watsuji T-O, Nakagawa S, Tsuchida S, Toki T, Hirota A, Tsunogai U, Takai K. 2010. Diversity and function of epibiotic microbial communities on the galatheid crab, Shinkaia crosnieri. Microb. Environ. 25, 288–294. ( 10.1264/jsme2.ME10135) [DOI] [PubMed] [Google Scholar]
- 9.Fisher CR, Childress JJ, Oremland RS, Bidigare RR. 1987. The importance of methane and thiosulfate in the metabolism of the bacterial symbionts of two deep-sea mussels. Mar. Biol. 96, 59–71. ( 10.1007/BF00394838) [DOI] [Google Scholar]
- 10.Robinson JJ, Polz MF, Fiala-Medioni A, Cavanaugh CM. 1998. Physiological and immunological evidence for two distinct C1-utilizing pathways in Bathymodiolus puteoserpentis (Bivalvia: Mytilidae), a dual endosymbiotic mussel from the Mid-Atlantic Ridge. Mar. Biol. 132, 625–633. ( 10.1007/s002270050427) [DOI] [Google Scholar]
- 11.Petersen JM, et al. 2011. Hydrogen is an energy source for hydrothermal vent symbioses. Nature 476, 176–180. ( 10.1038/nature10325) [DOI] [PubMed] [Google Scholar]
- 12.Amend JP, McCollom TM, Hentscher M, Bach W. 2011. Catabolic and anabolic energy for chemolithoautotrophs in deep-sea hydrothermal systems hosted in different rock types. Geochim. Cosmochim. Acta 75, 5736–5748. ( 10.1016/j.gca.2011.07.041) [DOI] [Google Scholar]
- 13.Gartman A, et al. 2011. Sulfide oxidation across diffuse flow zones of hydrothermal vents. Aquat. Geochem. 17, 583–601. ( 10.1007/s10498-011-9136-1) [DOI] [Google Scholar]
- 14.Luther GW, III, Rozan TF, Taillefert M, Nuzzio DB, Di Meo C, Shank TD, Lutz RA, Cary SC. 2001. Chemical speciation drives hydrothermal vent ecology. Nature 410, 813–816. ( 10.1038/35071069) [DOI] [PubMed] [Google Scholar]
- 15.Luther Iii GW, Glazer BT, Hohmann L, Popp JI, Taillefert M, Rozan TF, Brendel PJ, Theberge SM, Nuzzio Donald B. 2001. Sulfur speciation monitored in situ with solid state gold amalgam voltammetric microelectrodes: polysulfides as a special case in sediments, microbial mats and hydrothermal vent waters. J. Environ. Monitor. 3, 61–66. ( 10.1039/b006499h) [DOI] [PubMed] [Google Scholar]
- 16.Schmidt C, Vuillemin R, Le Gall C, Gaill F, Le Bris N. 2008. Geochemical energy sources for microbial primary production in the environment of hydrothermal vent shrimps. Mar. Chem. 108, 18–31. ( 10.1016/j.marchem.2007.09.009) [DOI] [Google Scholar]
- 17.Mullaugh KM, et al. 2008. Voltammetric (micro)electrodes for the in situ study of Fe2+oxidation kinetics in hot springs and S2O32−production at hydrothermal vents. Electroanalysis 20, 280–290. ( 10.1002/elan.200704056) [DOI] [Google Scholar]
- 18.Waite TJ, et al. 2008. Variation in sulfur speciation with shellfish presence at a Lau Basin diffuse flow vent site. J. Shellfish Res. 27, 163–168. ( 10.2983/0730-8000(2008)27[163:VISSWS]2.0.CO;2) [DOI] [Google Scholar]
- 19.Gru C, Legoff H, Narcon S, Sarradin P-M, Caprais J-C, Lallier FH. 1998. Determination of reduced sulfur compounds by high-performance liquid chromatography in hydrothermal seawater and body fluids from Riftia pachyptila. Analyst 123, 1289–1293. ( 10.1039/a800032h) [DOI] [Google Scholar]
- 20.Dando PR, Southward AJ, Southward EC. 1986. Chemoautotrophic symbionts in the gills of the bivalve mollusc Lucinoma borealis and the sediment chemistry of its habitat. Proc. R. Soc. Lond. B 227, 227–247. ( 10.1098/rspb.1986.0021) [DOI] [Google Scholar]
- 21.Scott KM, Cavanaugh CM. 2007. CO2 uptake and fixation by endosymbiotic chemoautotrophs from the bivalve Solemya velum. Appl. Environ. Microbiol. 73, 1174–1179. ( 10.1128/AEM.01817-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giere O, Wirsen CO, Schmidt C, Jannasch HW. 1988. Contrasting effects of sulfide and thiosulfate on symbiotic CO2-assimilation of Phallodrilus leukodermatus (Annelida). Mar. Biol. 97, 413–419. ( 10.1007/BF00397771) [DOI] [Google Scholar]
- 23.Childress JJ, Fisher CR, Favuzzi JA, Arp AJ, Oros DR. 1998. The role of a zinc-based, serum-borne sulphide-binding component in the uptake and transport of dissolved sulphide by the chemoautotrophic symbiont-containing clam Calyptogena elongata. J. Exp. Biol. 179, 131–158. [Google Scholar]
- 24.Teske A, Brinkhoff T, Muyzer G, Moser DP, Rethmeier J, Jannasch HW. 2000. Diversity of thiosulfate-oxidizing bacteria from marine sediments and hydrothermal vents. Appl. Environ. Microbiol. 66, 3125–3133. ( 10.1128/AEM.66.8.3125-3133.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sievert S, Vetriani C. 2012. Chemoautotrophy at deep-sea vents: past, present, and future. Oceanography 25, 218–233. ( 10.5670/oceanog.2012.21) [DOI] [Google Scholar]
- 26.Polz MF, Robinson JJ, Cavanaugh CM, Van Dover CL. 1998. Trophic ecology of massive shrimp aggregations at a Mid-Atlantic Ridge hydrothermal vent site. Limnol. Oceanogr. 43, 1631–1638. ( 10.4319/lo.1998.43.7.1631) [DOI] [Google Scholar]
- 27.Wilmot DB, Jr, Vetter RD. 1990. The bacterial symbiont from the hydrothermal vent tubeworm Riftia pachyptila is a sulfide specialist. Mar. Biol. 106, 273–283. ( 10.1007/BF01314811) [DOI] [Google Scholar]
- 28.Nelson DC, Hagen KD, Edwards DB. 1995. The gill symbiont of the hydrothermal vent mussel Bathymodiolus thermophilus is a psychrophilic, chemoautotrophic, sulfur bacterium. Mar. Biol. 121, 487–495. ( 10.1007/BF00349457) [DOI] [Google Scholar]
- 29.Desbruyeres D, et al. 1994. Deep-sea hydrothermal communities in southwestern Pacific back-arc basins (The North Fiji and Lau Basins): composition, microdistribution and food-web. Mar. Geol. 116, 227–242. ( 10.1016/0025-3227(94)90178-3) [DOI] [Google Scholar]
- 30.Suzuki Y, et al. 2006. Host-symbiont relationships in hydrothermal vent gastropods of the genus Alviniconcha from the Southwest Pacific. Appl. Environ. Microbiol. 72, 1388–1393. ( 10.1128/AEM.72.2.1388-1393.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki Y, Sasaki T, Suzuki M, Nogi Y, Miwa T, Takai K, Nealson KH, Horikoshi K. 2005. Novel chemoautotrophic endosymbiosis between a member of the Epsilonproteobacteria and the hydrothermal-vent gastropod Alviniconcha aff. hessleri (Gastropoda: Provannidae) from the Indian Ocean. Appl. Environ. Microbiol. 71, 5440–5450. ( 10.1128/AEM.71.9.5440-5450.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki Y, Sasaki T, Suzuki M, Tsuchida S, Nealson KH, Horikoshi K. 2005. Molecular phylogenetic and isotopic evidence of two lineages of chemoautotrophic endosymbionts distinct at the subdivision level harbored in one host-animal type: the genus Alviniconcha (Gastropoda: Provannidae). FEMS Microbiol. Lett. 249, 105–112. ( 10.1016/j.femsle.2005.06.023) [DOI] [PubMed] [Google Scholar]
- 33.Suzuki Y, et al. 2006. Single host and symbiont lineages of hydrothermalvent gastropods Ifremeria nautilei (Provannidae): biogeography and evolution. Mar. Ecol. Prog. Ser. 315, 167–175. ( 10.3354/meps315167) [DOI] [Google Scholar]
- 34.Beinart RA, et al. 2012. Evidence for the role of endosymbionts in regional-scale habitat partitioning by hydrothermal vent symbioses. Proc. Natl Acad. Sci. USA 109, E3241–E3250. ( 10.1073/pnas.1202690109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dubilier N, Windoffer R, Giere O. 1998. Ultrastructure and stable carbon isotope composition of the hydrothermal vent mussels Bathymodiolus brevior and B. sp. affinis brevior from the North Fiji Basin, western Pacific. Mar. Ecol. Prog. Ser. 165, 187–193. ( 10.3354/meps165187) [DOI] [Google Scholar]
- 36.Urakawa H, Dubilier N, Fujiwara Y, Cunningham DE, Kojima S, Stahl DA. 2005. Hydrothermal vent gastropods from the same family (Provannidae) harbour ε- and γ-proteobacteria endosymbionts. Environ. Microbiol. 7, 750–754. ( 10.1111/j.1462-2920.2005.00753.x) [DOI] [PubMed] [Google Scholar]
- 37.Windoffer R, Giere O. 1997. Symbiosis of the hydrothermal vent gastropod Ifremeria nautilei (Provannidae) with endobacteria: structural analyses and ecological considerations. Biol. Bull. 193, 381–392. ( 10.2307/1542940) [DOI] [PubMed] [Google Scholar]
- 38.Stein JL, Cary SC, Hessler RR, Ohta S, Vetter RD, Childress JJ, Felbeck H. 1988. Chemoautotrophic symbiosis in a hydrothermal vent gastropod. Biol. Bull. 174, 373–378. ( 10.2307/1541963) [DOI] [Google Scholar]
- 39.Henry MS, Childress JJ, Figueroa D. 2008. Metabolic rates and thermal tolerances of chemoautotrophic symbioses from Lau Basin hydrothermal vents and their implications for species distributions. Deep Sea Res. I Oceanogr. Res. Papers 55, 679–695. ( 10.1016/j.dsr.2008.02.001) [DOI] [Google Scholar]
- 40.Podowski EL, Moore TS, Zelnio KA, Luther GW, Fisher CR. 2009. Distribution of diffuse flow megafauna in two sites on the Eastern Lau Spreading Center, Tonga. Deep-Sea Res. I 56, 2041–2056. ( 10.1016/j.dsr.2009.07.002) [DOI] [Google Scholar]
- 41.Podowski EL, Ma S, Luther Iii GW, Wardrop D, Fisher CR. 2010. Biotic and abiotic factors affecting distributions of megafauna in diffuse flow on andesite and basalt along the Eastern Lau Spreading Center, Tonga. Mar. Ecol. Prog. Ser. 418, 25–45. ( 10.3354/meps08797) [DOI] [Google Scholar]
- 42.Sen A, Becker EL, Podowski EL, Wickes LN, Ma S, Mullaugh KM, Hourdez S, Luther GW, Fisher CR. 2013. Distribution of mega fauna on sulfide edifices on the Eastern Lau Spreading Center and Valu Fa Ridge. Deep Sea Res. I Oceanogr. Res. Papers 72, 48–60. ( 10.1016/j.dsr.2012.11.003) [DOI] [Google Scholar]
- 43.Girguis PR, Childress JJ, Freytag JK, Klose K, Stuber R. 2002. Effects of metabolite uptake on proton-equivalent elimination by two species of deep-sea vestimentiferan tubeworm, Riftia pachyptila and Lamellibrachia cf luymesi: proton elimination is a necessary adaptation to sulfide-oxidizing chemoautotrophic symbiont. J. Exp. Biol. 205, 3055–3066. [DOI] [PubMed] [Google Scholar]
- 44.Brendel PJ, Luther GW. 1995. Development of a gold amalgam voltammetric microelectrode for the determination of dissolved Fe, Mn, O2, and S (-II) in porewaters of marine and freshwater sediments. Environ. Sci. Technol. 29, 751–761. ( 10.1021/es00003a024) [DOI] [PubMed] [Google Scholar]
- 45.Childress JJ, Mickel TJ. 1980. A motion compensated shipboard precision balance system. Deep Sea Res. A Oceanogr. Res. Papers 27, 965–970. ( 10.1016/0198-0149(80)90008-4) [DOI] [Google Scholar]
- 46.Lane DJ. 1991. 16S/23S rRNA sequencing. In Nucleic acid techniques in bacterial systematics (eds Stackebrandt E, Goodfellow M.), pp. 115–175. New York, NY: Wiley and Sons. [Google Scholar]
- 47.Huelsenbeck JP, Ronquist FR. 2001. MRBAYES: Bayesian inference of phylogeny. Bioinformatics 17, 754–755. ( 10.1093/bioinformatics/17.8.754) [DOI] [PubMed] [Google Scholar]
- 48.Riou V, Halary S, Duperron S, Bouillon S, Elskens M, Bettencourt R, Santos RS, Dehairs F, Colaco A. 2008. Influence of CH4 and H2S availability on symbiont distribution, carbon assimilation and transfer in the dual symbiotic vent mussel Bathymodiolus azoricus. Biogeosciences 5, 1681–1691. ( 10.5194/bg-5-1681-2008) [DOI] [Google Scholar]
- 49.Grieshaber MK, Volkel S. 1998. Animal adaptations for tolerance and exploitation of poisonous sulfide. Annu. Rev. Physiol. 60, 33–53. ( 10.1146/annurev.physiol.60.1.33) [DOI] [PubMed] [Google Scholar]
- 50.Kelly DP. 1999. Thermodynamic aspects of energy conservation by chemolithotrophic sulfur bacteria in relation to the sulfur oxidation pathways. Arch. Microbiol. 171, 219–229. ( 10.1007/s002030050703) [DOI] [Google Scholar]
- 51.O'Brien DJ, Birkner FB. 1977. Kinetics of oxygenation of reduced sulfur species in aqueous solution. Environ. Sci. Technol. 11, 1114–1120. ( 10.1021/es60135a009) [DOI] [Google Scholar]
- 52.Vetter RD. 1985. Elemental sulfur in the gills of three species of clams containing chemoautotrophic symbiotic bacteria: a possible inorganic energy storage compound. Mar. Biol. 88, 33–42. ( 10.1007/BF00393041) [DOI] [Google Scholar]
- 53.Girguis PR, Childress JJ. 1998. H+ equivalent elimination by the tube-worm Riftia pachyptila. Cahiers Biol. Mar. 39, 295–296. [Google Scholar]
- 54.Dahl C, Prange A. 2006. Bacterial sulfur globules: occurrence, structure and metabolism. In Microbiology monographs (ed. Shively J.), pp. 21–51. Berlin, Germany: Springer. [Google Scholar]
- 55.van den Ende FP, Gemerden HV. 1993. Sulfide oxidation under oxygen limitation by a Thiobacillus thioparus isolated from a marine microbial mat. FEMS Microbiol. Ecol. 13, 69–77. ( 10.1016/0168-6496(93)90042-6) [DOI] [Google Scholar]
- 56.Kleiner M, Petersen JM, Dubilier N. 2012. Convergent and divergent evolution of metabolism in sulfur-oxidizing symbionts and the role of horizontal gene transfer. Curr. Opin. Microbiol. 15, 621–631. ( 10.1016/j.mib.2012.09.003) [DOI] [PubMed] [Google Scholar]
- 57.Newton ILG, et al. 2007. The Calyptogena magnifica chemoautotrophic symbiont genome. Science 315, 998–1000. ( 10.1126/science.1138438) [DOI] [PubMed] [Google Scholar]
- 58.Kuwahara H, et al. 2007. Reduced genome of the thioautotrophic intracellular symbiont in a deep-sea clam, Calyptogena okutanii. Curr. Biol. 17, 881–886. ( 10.1016/j.cub.2007.04.039) [DOI] [PubMed] [Google Scholar]
- 59.Robidart JC, Bench SR, Feldman RA, Novoradovsky A, Podell SB, Gaasterland T, Allen EE, Felbeck H. 2008. Metabolic versatility of the Riftia pachyptila endosymbiont revealed through metagenomics. Environ. Microbiol. 10, 727–737. ( 10.1111/j.1462-2920.2007.01496.x) [DOI] [PubMed] [Google Scholar]
- 60.Nakagawa S, et al. 2013. Allying with armored snails: the complete genome of gammaproteobacterial endosymbiont. ISME J. 8, 40–51. ( 10.1038/ismej.2013.131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gardebrecht A, et al. 2011. Physiological homogeneity among the endosymbionts of Riftia pachyptila and Tevnia jerichonana revealed by proteogenomics. ISME J. 6, 766–776. ( 10.1038/ismej.2011.137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pyzik AJ, Sommer SE. 1981. Sedimentary iron monosulfides: kinetics and mechanism of formation. Geochim. Cosmochim. Acta 45, 687–698. ( 10.1016/0016-7037(81)90042-9) [DOI] [Google Scholar]
- 63.Santos A, Dos M, Stumm W. 1992. Reductive dissolution of iron(III) (hydr)oxides by hydrogen sulfide. Langmuir 8, 1671–1675. ( 10.1021/la00042a030) [DOI] [Google Scholar]
- 64.Luther GW, Findlay AJ, Macdonald DJ, Owings SM, Hanson TE, Beinart RA, Girguis PR. 2011. Thermodynamics and kinetics of sulfide oxidation by oxygen: a look at inorganically controlled reactions and biologically mediated processes in the environment. Front. Microbiol. 2, 62 ( 10.3389/fmicb.2011.00062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fisher CR, Takai K, Le Bris N. 2007. Hydrothermal vent ecosystems. Oceanography 20, 14–23. ( 10.5670/oceanog.2007.75) [DOI] [Google Scholar]
- 66.Mullineaux LS, Peterson CH, Micheli F, Mills SW. 2003. Successional mechanism varies along a gradient in hydrothermal fluid flux at deep-sea vents. Ecol. Monogr. 73, 523–542. ( 10.1890/02-0674) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
DNA sequences: GenBank accession KF780847–55. Stable isotopic data: uploaded as electronic supplementary material.