Abstract
Ornamentation of parents poses a high risk for offspring because it reduces cryptic nest defence. Over a century ago, Wallace proposed that sexual dichromatism enhances crypsis of open-nesting females although subsequent studies found that dichromatism per se is not necessarily adaptive. We tested whether reduced female ornamentation in a sexually dichromatic species reduces the risk of clutch depredation and leads to adaptive parental roles in the red-capped plover Charadrius ruficapillus, a species with biparental incubation. Males had significantly brighter and redder head coloration than females. During daytime, when visually foraging predators are active, colour-matched model males incurred a higher risk of clutch depredation than females, whereas at night there was no difference in depredation risk between sexes. In turn, red-capped plovers maintained a strongly diurnal/nocturnal division of parental care during incubation, with males attending the nest largely at night when visual predators were inactive and females incubating during the day. We found support for Wallace's conclusion that reduced female ornamentation provides a selective advantage when reproductive success is threatened by visually foraging predators. We conclude that predators may alter their prey's parental care patterns and therefore may affect parental cooperation during care.
Keywords: Wallace's hypothesis, sexual dichromatism, ornamentation, adaptive parental care, Charadrius, natural selection
1. Background
Sexual dichromatism is common among animals [1–4]. Ornamentation in one sex but not the other is generally attributed to differences in intrasexual competition for mating opportunities, with competition and ornamentation usually higher among males than females [5,6]. However, bright ornamentation may incur costs if it makes the bearer more vulnerable to predators. Therefore, predation is a powerful agent of natural selection that modulates ornamentation within a population [7]. If sexual selection favours bright males, natural selection may reduce conspicuousness in females, who usually look after the offspring, and hence reduce nest detectability for visual predators [8–12], resulting in sexual dichromatism. Wallace first stated in 1891 that ‘whenever the male is gay and conspicuous and the nest is open so as to expose the sitting bird to view, the female is of dull or obscure colours’ ([12], p. 124). Given the prominence of this statement in evolutionary biology, it is surprising that there is a lack of experimental studies to explicitly test the key implication of Wallace's assumption: reduced female ornamentation leads to lower predation risk for the offspring.
Predation plays an important role in the life history of prey species, especially with regard to breeding, because offspring (eggs and young) are vulnerable to predators [13–15]. Thus, many breeding and parental care strategies have evolved to cope with predation [16–18]. Predation risk is a particular threat for ground-dwelling prey species because predators gain easy access to offspring and parents [19,20]. Moreover, many ground-dwelling species rely upon crypsis/camouflage as the primary form of defence, thus successful breeding often involves avoiding detection by predators [21–24]. By contrast, bright coloration of parents may put the offspring at risk and reduce reproductive success [10,11,25].
Birds arguably provide the most stunning examples of sexual dichromatism and bright coloration among vertebrates [26,27], and hence have featured prominently in studies aimed at conceiving the theory of natural and sexual selection [6,12]. It has been suggested that male-like ornamentation is the developmental default state in birds and it is believed female drabness evolves by repression of ornamentation [28]. Shorebirds (plovers and sandpipers) are ground-nesting birds in which often both sexes attend the nest [29,30]. Generally, at lower latitudes with a clear night/day rhythm, males contribute more to nocturnal incubation duties, whereas females predominantly perform diurnal incubation [31–32] (but see [33]). It has been hypothesized that the brighter coloration of males leads to the predominance of nocturnal incubation by this sex. When visually foraging diurnal predators dominate, the shorebird incubation schedule could have evolved as a behavioural adaptation to minimize the risk of detection of the nest by predators, although this hypothesis remains untested.
We investigated the adaptiveness of dichromatism and parental incubation roles in the red-capped plover (Charadrius ruficapillus), a socially monogamous shorebird in Australia [34,35]. Red-capped plovers are an ideal model system to test Wallace's prediction of adaptive female coloration because (i) they appear sexually dichromatic to the human eye, with males possessing a bright red colour and females a dull orange colour on their heads [34,35], (ii) they nest in open habitat, and (iii) clutch depredation is high [34,36]. Importantly, red-capped plovers have evolved in environments with predominantly visually foraging predators such as little ravens (Corvus mellori). Using visual categorization by a human observer, digital photography and spectrophotometry to account for the different visual systems of the main predators, we show first that male red-capped plovers have brighter head coloration than females. Second, we test experimentally whether the duller female coloration reduces the risk of clutch depredation. Finally, we show that schedule of biparental incubation in this sexually dichromatic species is adaptive with regard to clutch depredation, with brightly coloured males largely restricted to incubation at night when visual predators are inactive, whereas duller-coloured females attend the nest during the daytime.
2. Material and methods
(a). General fieldwork
Fieldwork was carried out at Cheetham Wetlands (37°53′ S, 144°47′ E; 420 ha) and adjacent Truganina Swamp (37°52′ S, 144°48′ E; 148 ha), southwest of Melbourne in Victoria, Australia. The study area is a highly modified wetland, dominated by saltmarsh vegetation communities, with strongly restricted human entry [37]. Approximately 200 pairs of red-capped plovers breed in the study area. Sexes were identified based on a combination of behavioural and morphological characteristics, including courtship, size and plumage [35]. The study encompassed three breeding seasons, from July 2008 to May 2011.
Nests of marked red-capped plovers were located by spotting the incubating parent from a car or on foot. Hatching dates were estimated using the flotation method [38]. Nest fate was determined by re-visiting the nests at the predicted hatching dates and during camera maintenance on nests that had video monitoring systems installed (see below). An experiment (electronic supplementary material, experiment S1) was conducted to ascertain that little raven and red fox (Vulpes vulpes) were the primary egg predators during day and night, respectively, and also to determine the sensory cues used by these predators to detect eggs.
(b). Sexual dichromatism
Head colour is the most conspicuous plumage trait in red-capped plovers to a human observer. Two different measurements of head colour were collected. ‘Brightness’ of head colour referred to the difference in the shade of red colour present on the head, where males possessed a bright red colour and females a dull orange colour. ‘Redness’ referred to the varying amounts of red and orange present on the heads of males and females, respectively (figure 1). These two measurements were collected on birds in the hand or from a distance in the field, using (i) visual estimates, (ii) standardized photography and (iii) spectrophotometry.
Figure 1.
The scale used to make ‘visual estimates' of the redness present on the heads of adult male and female red-capped plovers.
Visual estimates involved the categorization of redness according to a predetermined ordinal scale (figure 1). Captured and wild-living marked birds were classified according to this scale each time they were encountered, either in the hand or in the field. This simple scale offered a practical way of estimating head colour for a substantial number of wild-living birds throughout the breeding season (n = 81). We also checked whether redness in heads changed within individuals across the season by examining whether individual birds changed categories with age or over the season. Second, we used a standardized photography technique to calibrate the visual estimates of head colour and to test for differences in brightness of male and female head colour. This technique involved a light-impervious box containing an 18% grey reference card as background. Pictures of captured birds were taken using a Nikon Coolpix P90 digital camera with the white balance switched off and flash forced. Captured birds were held inside the light box and their heads were photographed from each of three angles (top and both sides). The images obtained were analysed at the scale of the pixel, using Adobe Photoshop v. CS5, which produced a mean grey value for each individual ranging from 0 (black) to 255 (white), after converting all colours in the image to a shade of grey [39].
To further evaluate brightness of head colour, and also to investigate the possibility of ultraviolet (UV) reflection from the head and other parts of the body, spectrophotometry was used on captured birds. This was necessary because avian predators may detect UV reflection of red-capped plover plumage [27,40]. Spectrophotometry provides quantitative measurements for colours and their reflective properties, which can then be plotted on a ‘long short versus medium ultraviolet’ (LSMU) colour space in which bird hue is the angle clockwise from the top and chroma is the distance from the origin [41,42]. The LSMU colour space has been widely used in bird coloration studies as it provides a graphical representation of colours based on avian vision and the wavelengths of peak sensitivity of the types of cone cells present in the bird eye [42]. We used a high-sensitivity (300–700 nm) Ocean Optics USB2000+ portable spectrometer with an Ocean Optics PX-2 lamp for illuminating samples via a bifurcated fibre-optic probe with a probe shield, which excludes ambient light. This spectrometer was used on six captured males and six captured females. The probe was used on the bird's body parts that potentially displayed sexual dichromatism, namely the crown, chest and back.
(c). Head colour and depredation risk
We assessed experimentally whether depredation risk of clutches was associated with head coloration of male and female nest attendants. Four male and four female models of incubating red-capped plovers (henceforth ‘models’) were used to assess differences in rates of detection and egg predation by predators associated with head coloration. These models were cast from the material Hydro-stone (United States Gypsum Company, Chicago, IL, USA), a durable type of odourless gypsum cement with low water absorption properties, which is generally suitable for outdoor sculptures with fine detail. A professional sculptor modelled the posture of an incubating plover parent based on images of an incubating bird photographed from various aspects with graph paper in the background, to provide an accurate size scale. Male and female models were identical in size and only differed in coloration. To maximize the difference in coloration, male models mimicked the brightest red of head colour measured by the spectrometer and female models mimicked the dullest orange of head colour measured. Different colours of matt paint (non-UV-reflecting) were selected to match the original colours of the birds as determined by spectrophotometry. These were mixed in various proportions to acquire the exact quantitative measurements of the true colours by comparing paint samples and true colours in the LSMU colour space. Models of the same sex were checked for consistency using the spectrophotometer and were identical in all measurements.
Following a weathering period of two weeks, models were then placed at locations in Cheetham Wetlands (February–April 2011), beside a small scrape containing two common quail (Coturnix coturnix) eggs to mimic real plover nests [43]. The eggs were placed next to the bird models because naturally incubating birds leave the nest when a predator is nearby, leaving the eggs exposed [34,35]. To determine day and night detection rates by predators we checked models at dawn and dusk, leaving them exposed for 12 h periods. Models were exposed for a total of 25 days and nights, resulting in 200 independent samples (100 day and 100 night) for each of the two treatments (male/females). Models were positioned at least 200 m apart, in randomly chosen locations in open salt pan habitat (65% of 102 real red-capped plover nests located during the study period occurred in this habitat) and moved to different locations every 12 h to avoid spatial habituation by predators. The disappearance of the eggs was regarded as predation (the only cause of non-flood-related egg loss; M.A.W. 2013, unpublished data). No eggs in this experiment were lost to flooding.
(d). Parental care division during incubation
Automatic video monitoring systems were used on 12 nests to observe nesting red-capped plovers, 24 h per day over 3–4 consecutive days to distinguish sex roles and describe parental care. The systems featured low-profile, low-light, black and white cameras with infrared illuminators (25 × 45 mm). The camera was placed approximately 300 mm from the nest. Birds readily returned to nests after installation or maintenance (once per day). Sexes were distinguished by being marked with a small plastic flag on either the right (males) or left leg (females). Data were coded to indicate the presence/absence of each sex undertaking incubation duties at the nest, over a 24 h cycle.
(e). Statistical analysis
(i). Sexual dichromatism
To test for differences in brightness between male and female head colour, we conducted an independent t-test using mean grey values obtained from standard digital photographs. Mean grey values were also used to assess differences in redness among the three head colour categories of males and females in a one-way analysis of variance (ANOVA). Paired t-tests were conducted on the mean grey values of males and females captured multiple times within a breeding season, especially those that were captured early, mid and/or late in the breeding season, were compared to determine whether the brightness/redness of head colour changed across a breeding season. The visual scales of individuals that were captured in multiple breeding seasons were compared to determine whether the brightness/redness of head colour changed across years. An analysis of similarity (ANOSIM) was used on the quantitative colour measurements obtained from the spectrometer to assess the similarity between brightness of male and female head colour. All the above tests were conducted in R v. 2.15.2 [44].
(ii). Head colour and depredation risk
We modelled the probability of clutch depredation using generalized (i) linear models (GLM) and (ii) mixed models (GLMM) with the ID of the model incubator fitted as a random effect. Both sets of models produced near-identical results, suggesting negligible impact of model ID, and we therefore present only the results of the GLM. The presence/absence of depredation was modelled as a binomial distribution using logistic regression. Two predictor variables were included to examine the key hypothesis that head colour influences predation. First, the sex of the plover model (male or female) and the time of day (either night or day) were entered as two categorical variables, each with two levels. To assess support for the hypothesis that clutch depredation rate is higher for males than females during daytime, an interaction term was fitted between sex and time. For this sex-by-time interaction, male during daytime was set as the reference category. We also added two other variables previously associated with depredation risk of red-capped plover nests in this population to the initial models: (i) distance to closest vegetation and (ii) distance to water (measured in metres) [36].
An information theoretic approach [45] was used to assess support for candidate models including all combinations of the four predictor variables and the sex-by-time interaction. Models were ranked using Akaike's information criterion adjusted for small samples (AICc). This approach does not provide p-values but rather assesses the level of support from the data for the current model compared with the most highly ranked model using AICc differences (Δi) and associated model weights (wi) [45]. Models with Δi-values less than 2 have substantial support, whereas those with Δi > 10 have little or no support [45]. A model is regarded as ‘clearly’ the best among the candidate set where wi > 0.9. When no model has wi > 0.9, there is support for multiple candidate models [45]. In the latter case, we used model averaging of parameter estimates to provide estimates of the strength, direction and uncertainty of parameters. Model averaging was undertaken using the MuMIn package in R . 2.15.2 [44].
(iii). Parental care division during incubation
To assess the extent to which males and females partition incubation over the diel period, we used logistic regression with a generalized additive mixed modelling (GAMM) framework. The proportion of time that males or females spent at the nest per hour was modelled as a binomial distribution, with a categorical predictor variable indicating the sex of the individual at the nest (i.e. male or female). Time was included as a continuous smoothed term to allow nonlinear changes in the rate of occupancy by males and females over the diel period. An interaction term between time since dawn and sex was fitted to allow a separate response curve for males and females, respectively. ‘Nest’ was included as a random effect to account for repeated observations of the same nest over time [46]. GAMMs were fitted using the mgcv package in R v. 2.15.2 [44].
3. Results
(a). Sexual dichromatism
Intersexual variation in head colour was examined based on ‘brightness’ of head colour, with males possessing a bright red colour and females a dull orange colour. Mean grey values from digital photographs revealed sexual dichromatism, with males having brighter head colours than females (independent t-test, t24 = 29.910, p < 0.001). To classify intrasexual variation in head coloration in the field (‘visual scale’) and test for temporal consistency we divided males and females into six head colour categories based on their ‘redness’ (amount of red present) assessed by a human observer (figure 1). Head colour categories of females and males used in visual estimates differed significantly from each other when mean grey values were assessed from photographs for a small sample of plovers (females: one-way ANOVA, F1,13 = 6.131, p = 0.028; n = 14; males: one-way ANOVA, F1,10 = 7.251, p = 0.041; n = 11), suggesting the visual scale was reliable. Mean grey values for redness of head colour did not change significantly over the breeding season (females: paired t-test, t13 = 2.021, p = 0.08, n = 14; males: paired t-test, t10 = 1.125, p = 0.12, n = 11). Similarly, the visual estimates of males and females captured in multiple breeding seasons indicated no change in redness of head colour across years (53 females and 28 males were monitored and none changed visual scales across or within seasons).
Spectrometric measurements of male and female plumage were plotted on an LSMU colour space (figure 2). As with mean grey values, males showed consistently more chromatic head coloration than females (ANOSIM, R = 0.76, p ≤ 0.001) whereas the measurements of back and chest plumage were overlapping (back: ANOSIM, R = 0.06, p = 0.22; chest: ANOSIM, R = 0.11, p = 0.18). Importantly, none of the body parts showed evidence of ultraviolet reflection, meaning that the dichromatism and coloration could be entirely sensed by visual systems of mammalian and avian predators alike.
Figure 2.
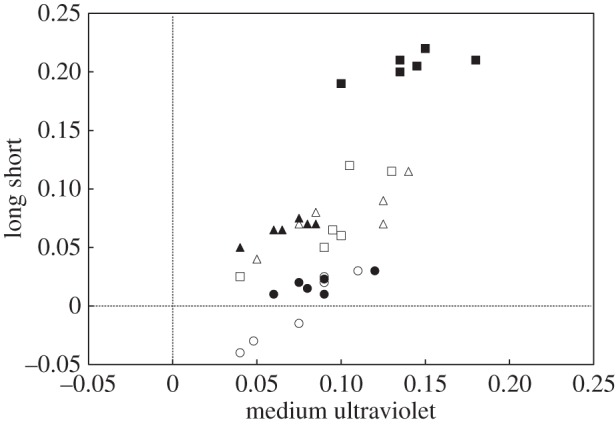
Spectrophotometric analysis of male and female plumage, as displayed on a ‘long short’ versus ‘medium ultraviolet' (LSMU) wavelength colour space. The parts of the body are: squares, head; triangles, back; circles, chest. Males are indicated in black shapes and females in white shapes.
(b). Head colour and depredation risk
Monitoring artificial nests with quail eggs, we found that little raven and red fox were the only egg predators of red-capped plovers, with ravens being mainly responsible for depredation during the day and foxes exclusively being responsible for depredation at night (electronic supplementary material, experiment S1). To test whether there was a difference in depredation risk according to sexual dichromatism, we placed quail eggs (which broadly resemble plover eggs in size and shape) next to male and female artificial models of red-capped plovers. These artificial models only differed in their head coloration, and we recorded depredation during night and day. Overall, 64% of 800 eggs next to artificial models were depredated. The four top-ranked statistical models included sex, time and the sex-by-time interaction (combined wi = 1; see electronic supplementary material, table S2). The best statistical model contained three terms: sex, time and sex-by-time interaction (d.f. = 4, D2 = 26%, wi = 0.53). However, since there was not a single statistical model with overwhelming support (i.e. wi > 0.9), we calculated averaged estimates of coefficients (c) and standard errors by model averaging. Model averaging provided further support for the importance of the sex-by-time interaction. All levels of the sex-by-time interaction differed from the reference level (male artificial model during the day), with the largest difference in depredation risk between male and female artificial models during the day (c = −3.68 ± 0.42, z = 8.80; male artificial model during day versus male artificial model during night: c = −0.88 ± 0.40, z = 2.21; male artificial model during day versus female artificial model during night: c = 1.05 ± 0.39, z = 2.66). By contrast, clutches near male and female artificial plover models experienced similar rates of depredation during the night (figure 3; electronic supplementary material, table S2).
Figure 3.
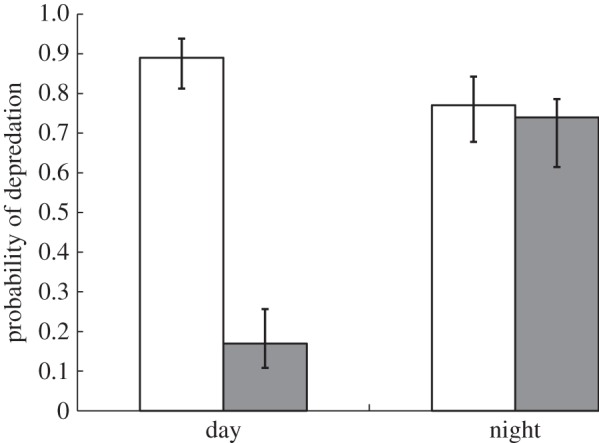
Depredation of quail eggs placed next to male (white) and female (grey) artificial models of red-capped plovers during night and day (see also electronic supplementary material, table S2).
(c). Parental care division during incubation
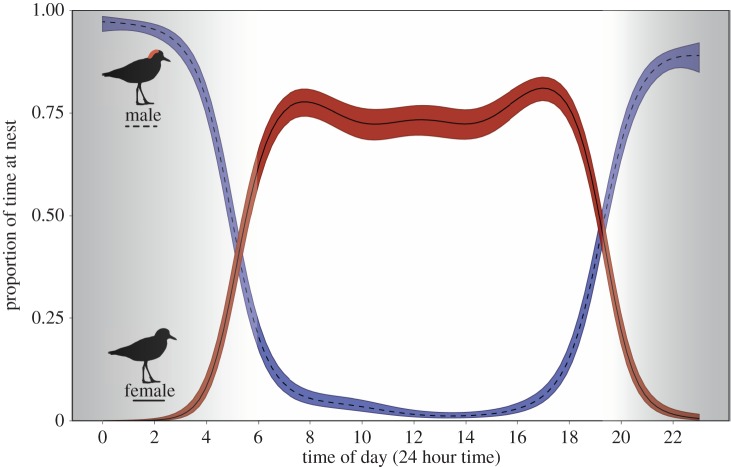
We used automated video systems to monitor incubator attendance during day and night for 72–96 consecutive hours per nest. GAMMs showed that both male and female nest attendance displayed significant relationships with time of day (GAMM, smoothed term for males: edf = 7.015, F = 61.57, p = <0.001; smoothed term for females: edf = 6.878, F = 28.28, p = <0.001, adj R2 = 0.68). Males and females strictly partitioned incubation over the diel period; males attended the nest during the night, while females attended the nest during daytime (figure 4).
Figure 4.
Probability of red-capped plover males (dashed) and females (solid) (±s.e.) attending the nest throughout the 24 h cycle, averaged across 12 nests. Background colour refers to night and day periods (dark periods in dark grey, light periods in light grey).
4. Discussion
Our study provides two important findings. First, using models with mimicked male/female coloration, we found support for Wallace's hypothesis that female coloration in an open-nesting species is adaptive and provides better cryptic nest defence than male coloration. Our experiment revealed that nest concealment during the day was more compromised by male than by female nest attendance, and therefore drab female coloration is adaptive and contributes to cryptic nest defence in red-capped plovers. Several studies have suggested that sexual dichromatism is closely linked with vulnerability to depredation [47–49] (but see [11]). Importantly, males and females may differ in a large number of characteristics (including behaviour) and none of the previous studies has tested rigorously that differences in coloration alone are responsible for a higher clutch depredation rate. For example, Martin & Badyaev [11] examined the association between predation rates of clutches and dichromatism in North American finch and warbler species, and found that not dichromatism itself but female brightness was associated with clutch depredation risk. This suggests that natural selection strongly affects female coloration.
Second, the threat of visually foraging predators was also associated with adaptive parental care behaviour. At daytime when visual predators are the main threat to the survival of the nest, the more cryptic females almost exclusively attended the nests. By contrast, brightly coloured males avoided attending the nest during this period and rather took over parental care at night when visual predators were not active. Visually foraging predators are likely to reinforce this strict incubation schedule if the main visual cue used by egg predators is the incubating parent (i.e. predators can detect incubators before incubators can detect predators, either because of structural complexity of habitats or because of the relative visual abilities of incubators and predators). We argue that high depredation risk by visual predators may answer the question of why in many other Charadrius species the bright males are more likely to incubate at night [31–33]. Such an adaptation requires clear day/night differences, which are available in lower latitudes during the breeding season but not at high arctic latitudes with permanent daylight during the breeding season [50–52]. Interestingly, male and female red-capped plovers exhibit large dull brown melanoid patches on the top of the head that are surrounded by brightly coloured ornaments at the neck and forehead (figure 1). These dull patches are variable in size, but larger in females than in males, and may help to reduce the detectability by aerial predators.
Wallace predicted that sexual dichromatism should be particularly strong in species (such as the red-capped plover) that nest in the open compared with those species that nest in habitat that helps conceal the nest. Previous studies of the relationship between nest concealment, sexual dichromatism and nest attendance in birds have found mixed support for this hypothesis. Soler & Moreno [10] tested whether nesting habitat explains sexual dichromatism in European passerines. Neither parental division during incubation (biparental versus female only) nor nest type (open versus cavity) explained sexual dichromatism. However, in support of the predictions made by Wallace, males in cavity-nesting species were more brightly coloured than males of open-nesting species, whereas open-nesting females had more cryptic coloration than females nesting in cavities [10].
Increased risk of depredation associated with brighter coloration can strongly influence reproductive success, leading to the evolution of numerous anti-predator adaptations [53–56]. The incubation schedule of red-capped plovers appears to serve as an anti-predator defence against diurnal visual predators such as little ravens. Parental coloration and incubation schedule, however, had no effect on predation risk by red foxes. Our sensory cue experiment demonstrated that foxes largely detected nests based on olfactory cues, whereas ravens detected nests based on visual cues (electronic supplementary material, experiment S1). Red foxes were introduced to the study area less than 200 years ago [57], and dichromatism of red-capped plovers appears ill suited to deal with the threat of this new mammalian predator. Other bird species exhibit sophisticated anti-predator defence mechanisms against mammals. A study by Reneerkens et al. [58] revealed that ground-nesting red knots (Calidris canutus) change the composition of their preening wax from highly detectable monoesters to less volatile diesters during egg incubation and hatching, to reduce their smell and thereby reduce detection risk by mammalian predators. Anti-predator adaptations often evolve as a result of long-term exposure to a particular predator or group of predators, and it is unknown whether red-capped plovers have a defence mechanism against mammalian predators similar to that of red knots. Cryptic nest defence is expected to evolve in response to the local predator community. Therefore, we strongly suggest that future studies on nest defence mechanisms need to take into account the sensory abilities of the main predators.
Bright coloration may also make the adults themselves more vulnerable to predation [59]. Attacks on breeding parents by local predators seem to be rare. Over 6 years of studying red-capped plovers we have never witnessed attacks by foxes or little ravens on incubating parents. However, the sexual dichromatism in red-capped plovers persists year-round and adults are likely to be targeted by other predators than nest predators, such as raptors. If detection probability affects the success of a predation attempt, bright ornamentation could conceivably increase mortality risk for plovers. Alternatively, coloration could have evolved to advertise profitability to predators if it reflects the escape abilities of the prey, and sexual dichromatism then would reflect a higher predation risk for females than for males [59].
This study has outlined adaptations of a dichromatic species to predators that are more able to detect the brighter sex, but the origins of dichromatism in the species remain unclear. To fully understand the evolution of sexual dichromatism, it is necessary to understand why male red-capped plovers are brightly coloured. The bright red coloration of neck and crown in males may represent a sexually (or socially) selected trait, and future research should test explicitly for benefits in sexual and social selection. Coloration in red-capped plovers is highly variable in males and females (figure 1). Interestingly, we found that individual males and females were remarkably conserved in the amount of red present on their heads both across the season and between seasons, with individual plovers always remaining in the same visual estimate category. This suggests that head coloration is genetically determined. A genetic correlation or sexual selection operating in both males and females [8] could explain why females remain colourful despite the strong selection pressures by clutch depredation.
Various other selection pressures may influence the incubation schedule of open-nesting birds. Nest attendance by males and females in biparentally incubating birds has been linked to energetic demands [30,60]. Incubation shift work needs food resources available for both sexes during the day and night, and prey are available for red-capped plovers in Australia throughout the day and night [35] (M.A.W. 2013, unpublished results). Parental division during incubation has also been linked to environmental harshness. In a recent study, Vincze et al. [31] identified ambient temperature in 10 Kentish and snowy plover populations (C. alexandrinus and C. nivosus, respectively) as the main predictor of incubation behaviour and schedule. They found that although there was a general pattern of male nocturnal and female diurnal incubation, high ambient temperatures (above 40°C) increased the male contribution to incubate during the day, especially around midday when it was very hot. At the same time, nest attendance by either parent increased with temperature, and the authors concluded that this increased cooperation was necessary to prevent the eggs from overheating. Interestingly, male plovers showed higher flexibility to respond to environmental differences than females, who generally incubated during daylight and dawn hours only. Depredation risk by visual predators or sexual dichromatism was not among the predictor variables tested by Vincze et al. [31]. Ambient temperatures at our study site in southern Australia seldom rise above 40°C, but red-capped plovers can also be found at lower latitudes with harsher climate, where temperature constraints might lead to a modulation of the incubation pattern.
5. Conclusion
Being conspicuous can change reproductive success. Here, we show that sexually dichromatic red-capped plovers differ in their conspicuousness to and detectability by egg predators, and in turn use an adaptive incubation schedule that might have co-evolved with their natural diurnal predator community to increase the survival prospects of their clutches. Future studies on cooperation during care in species with biparental care need to take into account detectability and depredation risk associated with male and female care as an important constraint associated with parental behaviour.
Supplementary Material
Acknowledgements
We thank Jessica Bywater, Renee Mead, Adam Cardilini, Stephanie Lomas and Christine Connelly for their assistance in data collection. We are grateful to Anne Charmantier, Ken Kraaijeveld and Mark Elgar for providing constructive comments that helped to improve the manuscript. Lucinda Brash sculptured the bird models. We also thank Parks Victoria staff from Cheetham Wetlands, Point Cook and Dr William Steele of Melbourne Water for allowing access to the study sites.
Ethics statement
Research was carried out in accordance with regulations of Deakin University Animal Welfare Committee permit nos. A44/2008 and A25-2009, Department of Sustainability and Environment permit nos. 10004586 and 10004948, and relevant Australian Bird and Bat Banding Authority permits and project approvals.
Data accessibility
Data are available at doi:10.5061/dryad.p66jt.
Funding statement
K.B.E., M.A.W., D.G.N. and J.A.E. were supported by funds from M. A. Ingram Trust and Hermon Slade Foundation. G.S.M. was supported by funding from BirdLife Australia and C.K. was supported by a Marie Curie Intra-European postdoctoral fellowship.
Author contributions
K.B.E., M.A.W., G.S.M. and J.A.E. designed the study. K.B.E., M.A.W. and J.A.E. collected the data. D.G.N., K.B.E., M.A.W. and C.K. analysed the data and interpreted the results. K.B.E., C.K., D.G.N. and M.A.W. wrote the draft manuscript. All authors contributed to subsequent revisions and approved the final version of the manuscript.
Conflict of interests
There are no competing interests for any of the authors.
References
- 1.Bell RC, Zamudio KR. 2012. Sexual dichromatism in frogs: natural selection, sexual selection and unexpected diversity. Proc. R. Soc. B 279, 4687–4693. ( 10.1098/rspb.2012.1609) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kemp DJ. 2007. Female butterflies prefer males bearing bright iridescent ornamentation. Proc. R. Soc. B 274, 1043–1047. ( 10.1098/rspb.2006.0043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llopart A, Elwyn S, Coyne JA. 2002. Fruitflies (communication arising): pigmentation and mate choice in Drosophila. Nature 419, 360 ( 10.1038/419360a) [DOI] [PubMed] [Google Scholar]
- 4.Price JJ, Eaton MD. 2014. Reconstructing the evolution of sexual dichromatism: current color diversity does not reflect past rates of male and female change. Evolution 68, 2026–2037. ( 10.1111/evo.12417) [DOI] [PubMed] [Google Scholar]
- 5.Andersson M. 1994. Sexual selection. Princeton, NJ: Princeton University Press. [Google Scholar]
- 6.Darwin C. 1871. The descent of man, and selection in relation to sex. London, UK: John Murray. [Google Scholar]
- 7.Endler JA. 1980. Natural selection on color patterns in Poecilia reticulata. Evolution 34, 76–91. ( 10.2307/2408316) [DOI] [PubMed] [Google Scholar]
- 8.Amundsen T. 2000. Why are female birds ornamented? Trends Ecol. Evol. 15, 149–155. ( 10.1016/S0169-5347(99)01800-5) [DOI] [PubMed] [Google Scholar]
- 9.Clutton-Brock T. 2009. Sexual selection in females. Anim. Behav. 77, 3–11. ( 10.1016/j.anbehav.2008.08.026) [DOI] [Google Scholar]
- 10.Soler JJ, Moreno J. 2012. Evolution of sexual dichromatism in relation to nesting habits in European passerines: a test of Wallace's hypothesis. J. Evol. Biol. 25, 1614–1622. ( 10.1111/j.1420-9101.2012.02544.x) [DOI] [PubMed] [Google Scholar]
- 11.Martin TE, Badyaev AV. 1996. Sexual dichromatism in birds: importance of nest predation and nest location for females versus males. Evolution 50, 2454–2460. ( 10.2307/2410712) [DOI] [PubMed] [Google Scholar]
- 12.Wallace AR. 1891. Natural selection and tropical nature: essays on descriptive and theoretical biology. London, UK: Macmillan and Company. [Google Scholar]
- 13.Liesenjohann M, Liesenjohann T, Trebaticka L, Haapakoski M, Sundell J, Ylönen H, Eccard J. 2011. From interference to predation: type and effects of direct interspecific interactions of small mammals. Behav. Ecol. Sociobiol. 65, 2079–2089. ( 10.1007/s00265-011-1217-z) [DOI] [Google Scholar]
- 14.Lima SL. 1998. Nonlethal effects in the ecology of predator–prey interactions. BioScience 48, 25–34. ( 10.2307/1313225) [DOI] [Google Scholar]
- 15.Sofaer HR, Sillett TS, Peluc SI, Morrison SA, Ghalambor CK. 2013. Differential effects of food availability and nest predation risk on avian reproductive strategies. Behav. Ecol. 24, 698–707. ( 10.1093/beheco/ars212) [DOI] [Google Scholar]
- 16.Ghalambor CK, Peluc SI, Martin TE. 2013. Plasticity of parental care under the risk of predation: how much should parents reduce care? Biol. Lett. 9, 20130154 ( 10.1098/rsbl.2013.0154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki TN. 2011. Parental alarm calls warn nestlings about different predatory threats. Curr. Biol. 21, R15–R16. ( 10.1016/j.cub.2010.11.027) [DOI] [PubMed] [Google Scholar]
- 18.Grovenburg TW, Monteith KL, Klaver RW, Jenks JA. 2012. Predator evasion by white-tailed deer fawns. Anim. Behav. 84, 59–65. ( 10.1016/j.anbehav.2012.04.005) [DOI] [Google Scholar]
- 19.Hare JF, Campbell KL, Senkiw RW. 2014. Catch the wave: prairie dogs assess neighbours’ awareness using contagious displays. Proc. R. Soc. B 281, 20132153 ( 10.1098/rspb.2013.2153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isaksson D, Wallander J, Larsson M. 2007. Managing predation on ground-nesting birds: the effectiveness of nest exclosures. Biol. Conserv. 136, 136–142. ( 10.1016/j.biocon.2006.11.015) [DOI] [Google Scholar]
- 21.Bókony V, Liker A, Székely T, Kis J. 2003. Melanin-based plumage coloration and flight displays in plovers and allies. Proc. R. Soc. Lond. B 270, 2491–2497. ( 10.1098/rspb.2003.2506) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colwell MA, Meyer JJ, Hardy MA, McAllister SE, Transou AN, Levalley RR, Dinsmore SJ. 2011. Western snowy plovers Charadrius alexandrinus nivosus select nesting substrates that enhance egg crypsis and improve nest survival. Ibis 153, 303–311. ( 10.1111/j.1474-919X.2011.01100.x) [DOI] [Google Scholar]
- 23.Stoddard MC. 2012. Mimicry and masquerade from the avian visual perspective. Curr. Zool. 58, 630–648. [Google Scholar]
- 24.Stevens M, Merilaita S. 2009. Animal camouflage: current issues and new perspectives. Phil. Trans. R. Soc. B 364, 423–427. ( 10.1098/rstb.2008.0217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haskell DG. 1996. Do bright colors at nests incur a cost due to predation? Evol. Ecol. 10, 285–288. ( 10.1007/BF01237685) [DOI] [Google Scholar]
- 26.Hill GE, McGraw KJ. 2006. Bird coloration: mechanisms and measurements. Cambridge, MA: Harvard University Press. [Google Scholar]
- 27.Stoddard MC, Prum RO. 2011. How colorful are birds? Evolution of the avian plumage color gamut. Behav. Ecol. 22, 1042–1052. ( 10.1093/beheco/arr088) [DOI] [Google Scholar]
- 28.Kraaijeveld K. 2014. Reversible trait loss: the genetic architecture of female ornaments. Annu. Rev. Ecol. Evol. Syst. 45, 159–177. ( 10.1146/annurev-ecolsys-120213-091550) [DOI] [Google Scholar]
- 29.Colwell MA. 2010. Shorebird ecology, conservation, and management. Los Angeles, CA: University of California Press. [Google Scholar]
- 30.Cresswell W, Holt S, Reid JM, Whitfield DP, Mellanby RJ. 2003. Do energetic demands constrain incubation scheduling in a biparental species? Behav. Ecol. 14, 97–102. ( 10.1093/beheco/14.1.97) [DOI] [Google Scholar]
- 31.Vincze O, et al. 2013. Local environment but not genetic differentiation influences biparental care in ten plover populations. PLoS ONE 8, e60998 ( 10.1371/journal.pone.0060998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burns F, McCulloch N, Dos Remedios N, Bolton M, Szekely T. 2013. Sex differences in incubation behaviour but not mortality risk in a threatened shorebird. Ibis 155, 877–880. ( 10.1111/ibi.12071) [DOI] [Google Scholar]
- 33.St Clair JJH, Herrmann P, Woods R, Székely T. 2010. Female-biased incubation and strong diel sex-roles in the two-banded plover Charadrius falklandicus. J. Ornithol. 151, 811–816. ( 10.1007/s10336-010-0517-9) [DOI] [Google Scholar]
- 34.Geering ADW, Agnew L, Harding S. 2007. Shorebirds of Australia. Collingwood, Australia: CSIRO Publishing. [Google Scholar]
- 35.Marchant S, Higgins PJ. 1993. Handbook of Australian, New Zealand and Antarctic birds: volume 2: raptors to lapwings. Melbourne, Australia: Oxford University Press. [Google Scholar]
- 36.Lomas SC, Whisson DA, Maguire GS, Tan LX, Guay P-J, Weston MA. 2014. The influence of cover on nesting red-capped plovers: a trade-off between thermoregulation and predation risk? Vic. Nat. 131, 115–127. [Google Scholar]
- 37.Antos MJ, Ehmke GC, Tzaros CL, Weston MA. 2007. Unauthorised human use of an urban coastal wetland sanctuary: current and future patterns. Landsc. Urban Planning 80, 173–183. ( 10.1016/j.landurbplan.2006.07.005) [DOI] [Google Scholar]
- 38.Liebezeit JR, et al. 2007. Assessing the development of shorebird eggs using the flotation method: species-specific and generalized regression models. Condor 109, 32–47. ( 10.1650/0010-5422(2007)109[32:ATDOSE]2.0.CO;2) [DOI] [Google Scholar]
- 39.Stevens M, Párraga CA, Cuthill IC, Partridge JC, Troscianko TS. 2007. Using digital photography to study animal coloration. Biol. J. Linnean Soc. 90, 211–237. ( 10.1111/j.1095-8312.2007.00725.x) [DOI] [Google Scholar]
- 40.Endler JA. 1990. On the measurement and classification of colour in studies of animal colour patterns. Biol. J. Linnean Soc. 41, 315–352. ( 10.1111/j.1095-8312.1990.tb00839.x) [DOI] [Google Scholar]
- 41.Starling M, Heinsohn R, Cockburn A, Langmore NE. 2006. Cryptic gentes revealed in pallid cuckoos Cuculus pallidus using reflectance spectrophotometry. Proc. R. Soc. B 273, 1929–1934. ( 10.1098/rspb.2006.3490) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Endler JA, Mielke PW. 2005. Comparing entire colour patterns as birds see them. Biol. J. Linnean Soc. 86, 405–431. ( 10.1111/j.1095-8312.2005.00540.x) [DOI] [Google Scholar]
- 43.Maguire GS, Stojanovic D, Weston MA. 2009. Conditioned taste aversion reduces fox depredation on model eggs on beaches. Wildl. Res. 36, 702–708. ( 10.1071/WR09123) [DOI] [Google Scholar]
- 44.R Development Core Team. 2012. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 45.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn New York, NY: Springer. [Google Scholar]
- 46.Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. New York, NY: Springer. [Google Scholar]
- 47.Götmark F, Hohlfält A. 1995. Bright male plumage and predation risk in passerine birds: are males easier to detect than females? Oikos 74, 475–484. ( 10.2307/3545993) [DOI] [Google Scholar]
- 48.Huhta E, Rytkönen S, Solonen T. 2003. Plumage brightness of prey increases predation risk: an among-species comparison. Ecology 84, 1793–1799. ( 10.2307/3449999) [DOI] [Google Scholar]
- 49.Møller AP, Nielsen JT. 2006. Prey vulnerability in relation to sexual coloration of prey. Behav. Ecol. Sociobiol. 60, 227–233. ( 10.1007/s00265-006-0160-x) [DOI] [Google Scholar]
- 50.Piersma T, Lindström A, Drent RH, Tulp I, Jukema J, Morrison RIG, Reneerkens J, Schekkerman H, Visser GH. 2003. High daily energy expenditure of incubating shorebirds on high arctic tundra: a circumpolar study. Funct. Ecol. 17, 356–362. ( 10.1046/j.1365-2435.2003.00741.x) [DOI] [Google Scholar]
- 51.Tulp I, Schekkerman H. 2006. Time allocation between feeding and incubation in uniparental arctic-breeding shorebirds: energy reserves provide leeway in a tight schedule. J. Avian Biol. 37, 207–218. ( 10.1111/j.2006.0908-8857.03519.x) [DOI] [Google Scholar]
- 52.Wallander J. 2003. Sex roles during incubation in the common ringed plover. Condor 105, 378–381. ( 10.1650/0010-5422(2003)105[0378:srdiit]2.0.co;2) [DOI] [Google Scholar]
- 53.Martin TE. 1993. Nest predation and nest sites. BioScience 43, 523–532. ( 10.2307/1311947) [DOI] [Google Scholar]
- 54.Amat JA, Masero JA. 2004. How Kentish plovers Charadrius alexandrinus, cope with heat stress during incubation. Behav. Ecol. Sociobiol. 56, 26–33. ( 10.1007/s00265-004-0758-9) [DOI] [Google Scholar]
- 55.Lack D. 1968. Ecological adaptations for breeding in birds. London, UK: Chapman & Hall. [Google Scholar]
- 56.Nilsson SG. 1984. The evolution of nest-site selection among hole-nesting birds: the importance of nest predation and competition. Ornis Scand. 15, 167–175. ( 10.2307/3675958) [DOI] [Google Scholar]
- 57.Rolls EC. 1969. They all ran wild: the story of pests on the land in Australia. Sydney, Australia: Angus and Robertson. [Google Scholar]
- 58.Reneerkens J, Piersma T, Damsté JSS. 2005. Switch to diester preen waxes may reduce avian nest predation by mammalian predators using olfactory cues. J. Exp. Biol. 208, 4199–4202. ( 10.1242/jeb.01872) [DOI] [PubMed] [Google Scholar]
- 59.Badyaev AV, Hill GE. 2003. Avian sexual dichromatism in relation to phylogeny and ecology. Annu. Rev. Ecol. Evol. Syst. 34, 27–49. ( 10.2307/30033768) [DOI] [Google Scholar]
- 60.Chaurand T, Weimerskirch H. 1994. Incubation routine, body mass regulation and egg neglect in the blue petrel Halobaena caerulea. Ibis 136, 285–290. ( 10.1111/j.1474-919X.1994.tb01097.x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available at doi:10.5061/dryad.p66jt.