Abstract
Worm lizards (Amphisbaenia) are burrowing squamates that live as subterranean predators. Their underground existence should limit dispersal, yet they are widespread throughout the Americas, Europe and Africa. This pattern was traditionally explained by continental drift, but molecular clocks suggest a Cenozoic diversification, long after the break-up of Pangaea, implying dispersal. Here, we describe primitive amphisbaenians from the North American Palaeocene, including the oldest known amphisbaenian, and provide new and older molecular divergence estimates for the clade, showing that worm lizards originated in North America, then radiated and dispersed in the Palaeogene following the Cretaceous-Palaeogene (K-Pg) extinction. This scenario implies at least three trans-oceanic dispersals: from North America to Europe, from North America to Africa and from Africa to South America. Amphisbaenians provide a striking case study in biogeography, suggesting that the role of continental drift in biogeography may be overstated. Instead, these patterns support Darwin and Wallace's hypothesis that the geographical ranges of modern clades result from dispersal, including oceanic rafting. Mass extinctions may facilitate dispersal events by eliminating competitors and predators that would otherwise hinder establishment of dispersing populations, removing biotic barriers to dispersal.
Keywords: evolution, dispersal, adaptive radiation, mass extinction
1. Introduction
Amphisbaenians, or worm lizards, are a bizarre group of squamates specialized for life as subterranean predators [1]. Adaptations for underground locomotion include a robust skull to plough through the Earth [2]; an elongate body and reduced limbs to move through tunnels [2,3] and scales arranged in rings, allowing amphisbaenians to use an earthworm-style, accordion-like movement to crawl. Amphisbaenians live and hunt in mostly in darkness, and so their eyes are reduced [3,4], but the auditory apparatus is adapted to detect low-frequency vibrations made by prey [5,6]. Although the clade's origins have been debated [7], molecular analyses group them with Lacertidae [8–12], indicating that similarities with snakes and other long-bodied squamates are convergent.
While worm lizards are unusual in many respects, the clade's biogeography is especially puzzling. The limited locomotor abilities of amphisbaenians should limit dispersal, yet worm lizards occur widely throughout the tropics and subtropics including the Americas, Europe, the Middle East and Africa [1,13]. As amphisbaenians rarely leave their burrows, their presence on either side of the Atlantic has been read as evidence that the clade originated and spread prior to the break-up of Pangaea [14–16], which began in the Jurassic with the formation of the North Atlantic ca 180–190 Ma [17] and concluded in the Cretaceous with the formation of the South Atlantic ca 100 Ma [17].
Surprisingly, molecular divergence dating suggests that the Amphisbaenia diversified relatively recently [13], with the crown originating in the mid-Cretaceous (109 Ma), a major diversification in the Palaeogene (56–40 Myr ago), and South American amphisbaenids separating from African forms at 40 Ma [13]—long after the formation of the South Atlantic. If so, Amphisbaenian biogeography cannot result from continental drift, and must result from dispersal via rafting [13]. However, previous attempts to examine the timing of amphisbaenian radiation and dispersal [13] have largely overlooked fossils, which provide critical data for constraining the time and place of diversification. Here, we use fossil and molecular data to show that the clade originated in North America and radiated in the Cenozoic but earlier than previously thought, soon after the Cretaceous-Palaeogene (K-Pg) mass extinction.
2. Systematic palaeontology
Squamata Oppel 1811 sensu Estes et al. 1988
Amphisbaenia Gray 1844 sensu Estes et al. 1988
Rhineuridae Vanzolini 1951
Archaerhineura mephitis gen et sp. nov.
Diagnosis. Rhineurid with the following, unique combination of characters: eight dentary teeth, enlarged adductor fossa extending below last tooth; resembles Plesiorhineura and differs from later rhineurids in having a dentary coronoid process extending above the coronoid in lateral view.
Holotype. Yale Peabody Museum of Natural History, Princeton University collections (YPM-PU) 18627, right dentary (figure 1d–f).
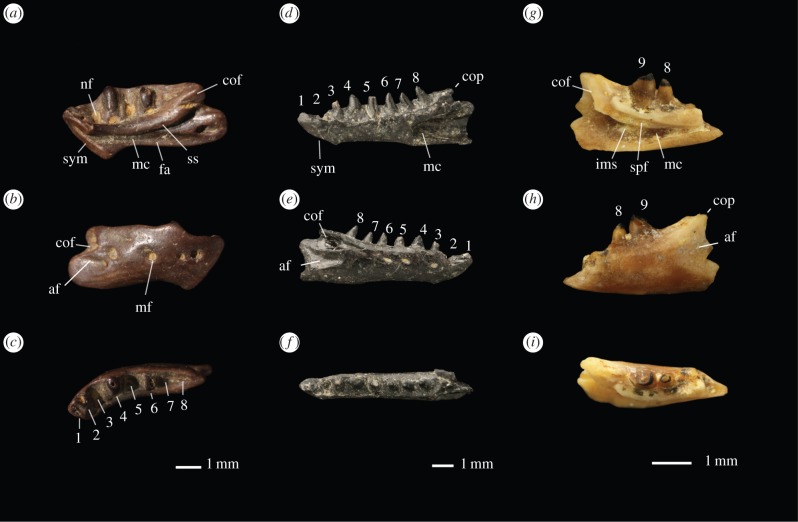
Figure 1.
Fossils of Palaeocene Amphisbaenia from North America. (a–c) Chthonophis subterraneus, AMNH 30799, holotype right dentary: (a) medial view, (b) dorsal view and (c) lateral view. (d–f) Archaerhineura mephitis, YPM 18627, holotype right dentary: (d) lateral view, (e) medial view and (f) dorsal view. (g–i) Oligodontosaurus cf. wyomingensis, YPM-PU 16777 left dentary: (g) lateral view, (h) medial view and (i) dorsal view. Abbreviations: af, adductor fossa; cof, coronoid facet; cop, coronoid process; fa, facet for articular; mc, Meckelian canal; mf, mental foramina; nf, nutrient foramen; spf, splenial facet; sym, symphysis.
Etymology. Genus name from the Greek archaios (ancient) and Rhineura (Florida worm lizard). Species name from the Greek mephitis (skunk).
Locality and horizon. Fritz Quarry, Polecat Bench Formation, Park County, Wyoming, USA. The Fritz Quarry is assigned to the Tiffanian 5 land mammal age [18] Late Palaeocene (approximately 58–57 Myr ago).
Description and comparisons. Archaerhineura possesses eight teeth, a plesiomorphy shared with Dyticonastis and Spathorhynchus; the first and fourth are enlarged, a derived feature of rhineurids. Attachment is subacrodont. The dentary symphysis closes around the Meckelian groove as in other rhineurids. The jaw tapers anteriorly and has a ventral bulge posteriorly; both are rhineurid characters. The Meckelian canal is closed, and posteriorly, the dentary ventral margin wraps up around the splenial, both rhineurid characters. Laterally, the jaw retains a deep adductor fossa, as in Plesiorhineura, but it extends anteriorly below the last tooth, an autapomorphy of Archaerhineura. As in other rhineurids, the coronoid process is low. There is a recess on the lateral surface of the dentary to receive the coronoid anterolateral process, as in other rhineurids, however, it suggests an extremely narrow anterolateral process, another feature distinguishing Archaerhineura from Plesiorhineura. The coronoid process of the dentary is exposed dorsal to the coronoid where a distinct lip of bone lies above the coronoid recess; this feature occurs in Plesiorhineura, but not in other rhineurids.
Chthonophidae new taxon
Chthonophis subterraneus gen et sp. nov.
Holotype. Right dentary, American Museum of Natural History (AMNH) 30799 (figure 1a–c).
Etymology. Genus: Greek chthonios (beneath the earth) + ophis (snake). Species name from the Latin sub- (beneath) + terra (earth).
Locality and horizon. Maastrichtian-Palaeocene Bug Creek Anthills, Fort Union Formation, Montana, USA. The Bug Creek Anthills are an earliest Palaeocene (Puercan 1) fauna with reworked Cretaceous fossils [19]. AMNH 30799 is most likely Palaeocene, given that the assemblage primarily contains Palaeocene fossils, and that intensively studied Maastrichtian squamate assemblages from western North America [20–22] lack amphisbaenians.
Diagnosis. Characterized by the following unique combination of characters: unrestricted Meckelian canal, dentary coronoid process absent, seven to eight teeth, anterior teeth small but posterior teeth enlarged; extremely short, deep mandible that is strongly U-shaped in ventral view, hypertrophied symphysis projecting ventrally in lateral view.
Description and comparisons. Chthonophis is represented by a right dentary (figure 1). Teeth and the posterior margin of the jaw are rounded, suggesting the specimen was partially digested prior to burial, but the salient anatomical features are visible. The dentary is 6.2 mm long, making Chthonophis larger than most other North American amphisbaenians, extant or fossil. Compared to other Amphisbaenia, the dentary is deep, measuring 2.4 mm deep at the back of the tooth row, approximately 40% of its length. In dorsal view, the dentary is bowed, which would have produced a U-shaped mandible; in other Amphisbaenia the mandible is more V-shaped.
In lateral view, the margin has a scalloped appearance that is unique to Chthonophis. Five mental foramina are present, and are enlarged as in other Amphisbaenia. Caudodorsally the mandible bears a shallow adductor fossa, a feature of amphisbaenians and other lacertoids. Caudally, the dentary bears a facet for an anterior process of the overlapping coronoid, as in Rhineuridae and Lacertidae. As in rhineurids, the dentary lacks a coronoid process.
Medially, the dentary symphysis has a large ventral accessory facet, an amphisbaenian synapomorphy. The symphysis is V-shaped in medial view, as in Bipes. The ventral facet is much longer than the dorsal facet, and extends ventrally to create a distinctive chin.
Four teeth attach in pleurodont fashion, with positions for three to four more. Posterior teeth are greatly enlarged as in other Amphisbaenia, but anterior teeth are reduced. Below the teeth is a subdental ridge overhanging the Meckelian canal; as in other Amphisbaenia the shelf is massively constructed. The Meckelian canal is deep and broad, whereas the groove is constricted and shallow in Bipedidae and Amphisbaeniformes, and the canal is enclosed in Rhineuridae. In extant Amphisbaenia the restricted Meckelian groove is associated with reduction or loss of the splenial; presumably the broad Meckelian groove here corresponds to an unreduced splenial. A splenial articular facet runs along the ventral margin of dentary to the middle of the tooth row.
Oligodontosauridae Estes 1975
Oligodontosaurus cf. wyomingensis Gilmore 1942
Diagnosis. Characterized by the following unique combination of characters: nine teeth, last tooth hypertrophied, tooth apices pigmented; angular slot absent, dentary coronoid process present but short and narrow, coronoid hooked in lateral view.
Holotype. YPM-PU 14246, left mandible.
Referred material. YPM-PU 18627 (figure 1g,i).
Locality and horizon. Holotype: Tiffanian Princeton Quarry, Polecat Bench Fm, Park County, Wyoming, USA. Referred specimen: Medicine Rocks, Tongue River Fm, Carter County, Montana, USA. The Medicine Rocks are referred to the Torrejonian 3 land mammal age/middle Palaeocene, approximately 62 Ma.
Description and comparisons. The holotype of Oligodontosaurus wyomingensis [23] is lost, but a second, older specimen [24], YPM-PU 16777, is described here. This specimen, a left dentary, is incomplete but indicates a short, deep jaw, as in the holotype. As in other Amphisbaenia, teeth are large and unicuspid, with smooth enamel; attachment is subacrodont. Tooth apices are pigmented, as in some anguids [25]. The subdental ridge is deep anteriorly, but tapers below the last tooth. The Meckelian groove is narrow with a distinct lip below. Posteriorly, the intramandibular septum lies below the last tooth, as in Amphisbaeniformes, however the articular slot seen in Amphisbaeniformes is absent. A dentary coronoid process is present, as in Amphisbaeniformes, but it is small. Posterolaterally, the dentary bears an adductor fossa. The type in [23] shows additional amphisbaenian features: the first tooth is enlarged, and anterior teeth are procumbent. The presence of a coronoid process of the dentary and the posteriorly positioned intramandibular septum indicate Amphisbaeniformes affinities, while absence of the angular slot suggests a basal position in Amphisbaeniformes.
3. Material and methods
Phylogenetic analysis was conducted using a combination of molecular and morphological data. For molecular analysis, we used 16S, BDNF, CMOS, ND2 and RAG1 from a previous alignment [12], with poorly aligned segments of 16S removed using Gblocks. The dataset includes 73 extant amphisbaenians and 14 outgroups, including rhynchocephalians, archosaurs and mammals, to sample the major lepidosaur clades and provide multiple calibration points. This approach allows inclusion of two robust, root-ward calibrations (lizard-archosaur divergence and tuatara-lizard divergence), a robust calibration in the outgroup (the marsupial-placental split) and use of a well understood and well constrained root prior (the mammal–reptile divergence). Bayesian phylogenetic analyses were conducted using PhyloBayes 3.3f [26] with CAT + GTR + G as substitution model. Convergence was assessed using the bpcomp function in PhyloBayes.
Placement of fossils was conducted using a matrix derived from the AToL matrix [7] (see the electronic supplementary material). Uninformative characters were removed and new characters and taxa were added for a total of 308 characters and 51 taxa. Character descriptions, character–taxon matrix and full results are given in the electronic supplementary material. Analysis was performed using PAUP* v. 4.0 b10 [27] under parsimony, with the molecular topology used as a backbone constraint tree; Goloboff's implied weighting was used to improve resolution and accuracy [28].
Molecular clock analyses were performed using PhyloBayes 3.3f [26]. Based on the placement of fossil taxa in the combined analysis, we used fossil calibrations to perform two clock analyses (see the electronic supplementary material): one analysis using soft-bounded minima and maxima calibrations and another using only hard-bounded minima and the root prior. Soft-bounded analyses used four maximum constraints (crown archosaurs, crown therians, crown lepidosaurs, crown squamates) and a single maximum [13] for the Caribbean clade. We also implemented a series of minimum calibrations (see the electronic supplementary material). For the hard bounded only-minima analyses these four maxima were excluded.
Optimal molecular clock analyses were performed under the autocorrelated Cox-Ingersoll-Ross (CIR) model [29], using exclusively hard-bounded minima, a birth–death prior to model the distribution of the node ages, a root prior defined using a Gamma distribution with an average of 325 Ma and an s.d. of 10 million years (see the electronic supplementary material). Multiple validatory analyses were performed and result in minimal changes in estimated divergence times. These investigated the effect of using different molecular clock models (other autocorrelated models and rate uncorrelated models), alternative root priors (including a permissive exponential prior of average 325 Ma), the use of soft-bounded minima and maxima, and investigation of the prior used (i.e. analyses performed without data). Finally, we investigated the effect of using alternative calibrations. We, therefore performed analyses that used only non-amphisbaenian calibrations and analyses that rather than calibrating the clock using fossils implied that the driver for the evolution of Amphisbaenia was vicariance (i.e. the break-up of Pangaea). The chosen vicariance event was the connection of the North and South Atlantic at 100 Ma [17]. Convergence of molecular clock analyses was investigated comparing results from different runs. The sampling was terminated when median ages were less than 2 Ma from each other in 90% of the nodes.
4. Results
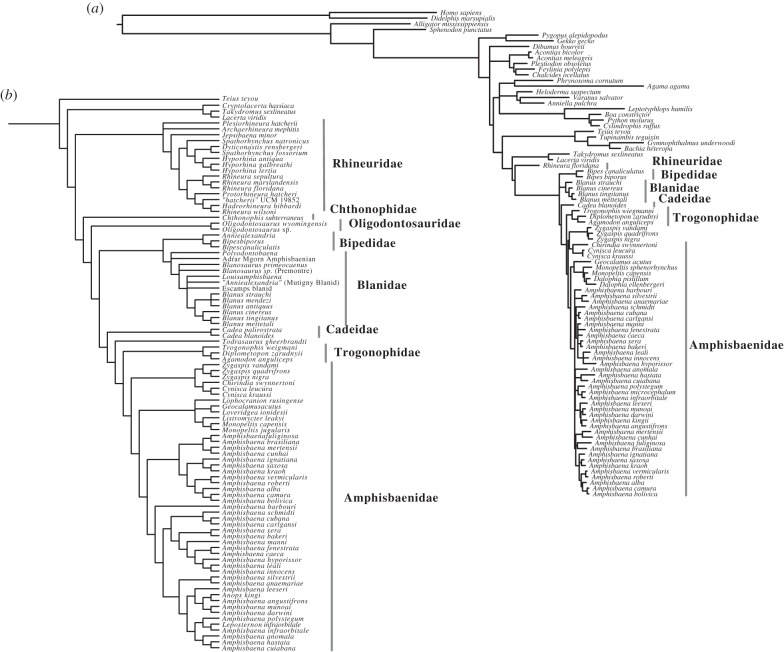
Analysis of morphological data using molecular constraints recovers the Rhineuridae as the oldest split within Amphisbaenia, as found previously [7,10,12,13,30]. Within Rhineuridae, the Palaeocene Archaerhineura and Plesiorhineura lie at the base of a Palaeogene radiation, which ultimately dwindles to the single extant species, Rhineura floridana. The remaining Amphisbaenia form a clade that is sister to the Rhineuridae. The deepest divergence of this clade is Chthonophis. One node up, Oligodontosaurus, previously placed in Rhineuridae [14], branches off.
All other Amphisbaenia form a clade, here named Amphisbaeniformes. The basal divergence in this clade is a grouping of Bipedidae and Blanidae. The early Eocene Anniealexandria is recovered as a bipedid, making it the oldest (indeed, the only) fossil member of the family. Blanidae was found to include a range of fossil forms from the Cenozoic of Europe.
The remaining families, Cadeidae, Trogonophidae and Amphisbaenidae, form a clade, with trogonophids and amphisbaenids forming a clade originating in Africa, Afrobaenia, as in previous molecular [12,13,30] and morphological [7] analyses; Todrasaurus gheerbrandti, from the Palaeocene of Africa, may represent a stem member of this lineage. Listromycter leakyi and Lophocranion rusingensis, from the African Miocene [31] are found to represent crown members of the Amphisbaenidae. As found previously [12,13,30], we recover a radiation endemic to South America.
Molecules and morphology disagree about the placement of several taxa. Molecules place Bipes and Blanus in a clade (with poor support), whereas morphology recovers Blanus outside all other Amphisbaeniformes. This topology would result in a slightly younger divergence for Amphisbeaniformes but would not alter the basic conclusions of our paper in terms of the timing and biogeography of the clade. Morphology and molecules also clash in that morphology unites keel-headed forms (Cadea, Anops kingii and Geocalamus) in a clade and shovel-headed forms (Monopeltis, Leposternon) in another. Molecules show that their similarities are the result of convergent evolution of specialized burrowing strategies.
Our analysis fails to recover the Eocene lizard Cryptolacerta hassiaca [32] as sister to Amphisbaenia. Instead, several derived features, including superciliary osteoderms and roofed over supratemporal fenestrae, place it with Lacertidae (figure 2b). However, placement of Cryptolacerta on the amphisbaenian stem would not alter the conclusions presented below.
Figure 2.
Phylogenetic analysis of Amphisbaenia. (a) Results of Bayesian analysis of five genes (16S, BDNF, CMOS, ND2 and RAG1) using CAT-GTR in PhyloBayes 3.3f. (b) Results of morphological analysis, using molecular data as a constraint.
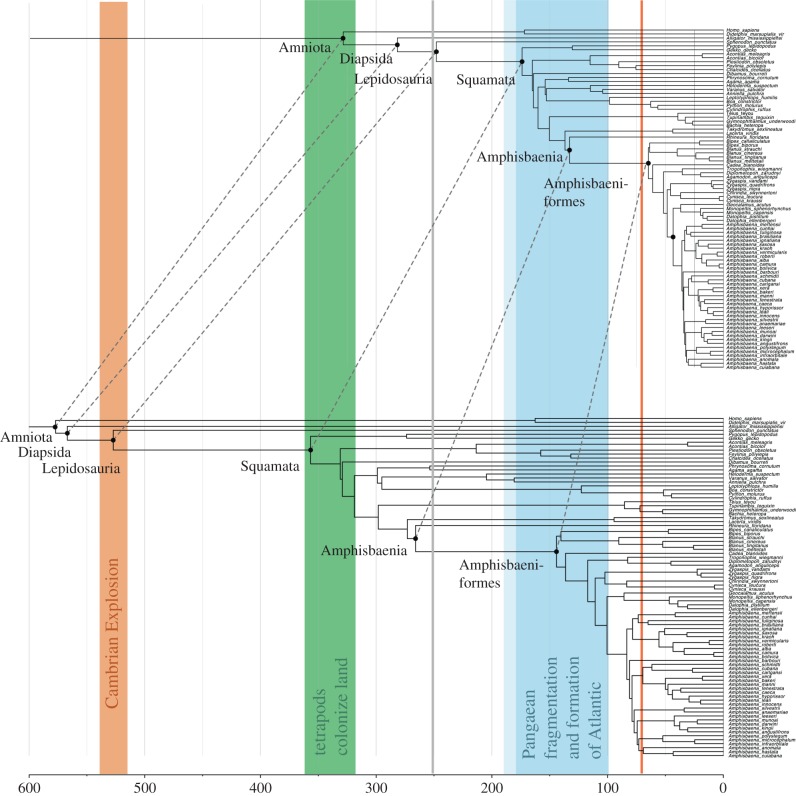
The results of our molecular clock analysis (figure 3) suggest that crown Squamata originated in the Jurassic; Lacertibaenia (Amphisbaenia + Lacertidae) originate near the Jurassic–Cretaceous boundary. Although crown Amphisbaenia originate in the Early Cretaceous, the origins of the extant diversity lie in the Palaeocene radiation of Amphisbaeniformes—which includes Blanidae, Bipedidae, Cadeidae and Afrobaenia between 0.4 and 5 Ma after the Chicxulub event. The split between African and South American Amphisbaenidae occurs ca 43 Ma, and crown Amphisbaenia emerges in the Early Cretaceous. Similar results are found using outgroup calibrations alone. Attempts to use vicariant calibrations (figure 4) produce unreasonably old dates for lizard diversification.
Figure 3.
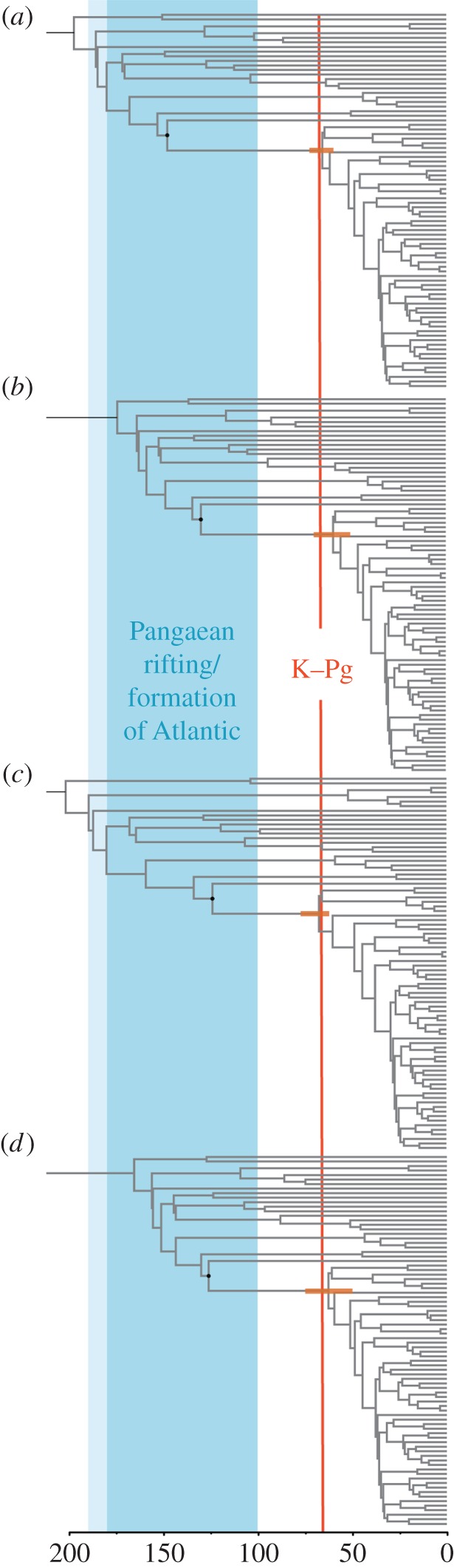
Divergence dates for molecular clock analyses, showing post K–Pg radiation. (a) CIR hard bounds, fossil minima only, (b) CIR soft bounds, minima and maxima, (c) uncorrelated gamma multipliers, fossil minima only, and (d) CIR soft bounds, minima and maxima, only calibrations external to Amphisbaenia. Red line and blue band represent the K–Pg boundary and break-up of Pangaea, respectively. Red vertical bars indicate 95% CIs for diversification of the main radiation of Amphisbaenia, the Amphisbaeniformes. In all cases, the 95% CI overlaps the K–Pg boundary consistent with post-Cretaceous diversification of Amphisbaenia, but fails to overlap the formation of the Atlantic Ocean, rejecting (p < 0.05) the hypothesis that vicariance drove amphisbaenian diversification.
Figure 4.
Comparison of fossil- and vicariance-calibrated molecular divergence estimates. (a) Calibrated using fossil minima and maxima, (b) calibrated using the connection of the North and South Atlantic ca 100 Ma to date the divergence of African and South American Amphisbaenia.
5. Discussion
The inclusion of fossils refines our understanding of the timing of amphisbaenian radiation and dispersal, shedding light on the processes driving these events. Previous molecular estimates put the diversification and initial dispersal of the Amphisbaeniformes in the Late Palaeocene and Early Eocene, greater than 9 Ma after the K–Pg extinctions [13]. Our results show that diversification and dispersal began almost immediately after the K–Pg event, suggesting that these events were somehow connected to the extinction.
Initial diversification of Amphisbaenia in the Cretaceous implies survival of multiple lineages across the K–Pg boundary (figure 5). Fossil evidence shows that squamates suffered severe extinctions at the K–Pg boundary [22], but the survival of several amphisbaenians is unsurprising given their unusual ecology. Amphisbaenians would have been largely insulated against the effects of the Chicxulub impact by their subterranean habitus and diet of soil-dwelling invertebrates. Living below ground would confer a degree of protection against either cooling [33] or a thermal pulse [34], and low light levels [33] would be a trivial problem for animals that exist in perpetual darkness. Critically, the subterranean environment contains invertebrates that feed on decaying plant matter, e.g. earthworms and termites, and so detritus-feeding invertebrates would have continued to provide food even in the absence of primary productivity. A similar mechanism may explain high survival rates in freshwater ecosystems, where the food chain is based on detritus [35].
Figure 5.
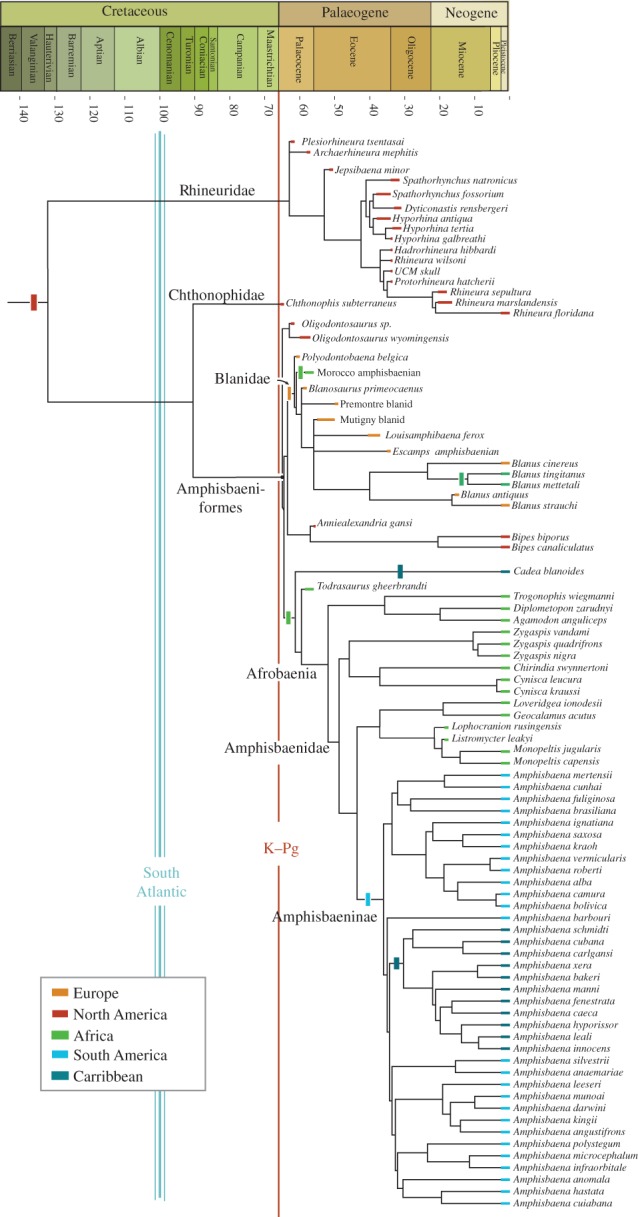
Phylogeny of Amphisbaenia. The basal position of the rhineurids and the Palaeocene Chthonophis and Oligodontosaurus are consistent with a North American origin of Amphisbaenia. The rapid appearance of new lineages in the Palaeocene documents post-Chicxulub radiation. The radiation is associated with multiple dispersal events—colonization of Europe by Blanidae, Africa by Afrobaenia and South America by Amphisbaenidae. Strict consensus of 76 trees, based on an analysis of 48 taxa and 310 characters using a molecular tree as a backbone constraint. See the electronic supplementary material for details.
The rapid Palaeogene diversification of Amphisbaeniformes and Rhineuridae appears to be an opportunistic radiation of the survivors of the extinction, as seen among mammals [36], birds [37] and teleosts [38]. This pattern of radiation has been proposed to characterize lizards as well [22] but our study is, to our knowledge, the first to provide both fossil and molecular evidence for post-Cretaceous radiation in a reptilian clade.
The absence of Amphisbaenia in the Cretaceous, which are predicted on the basis of molecules, represents the most striking discrepancy between the fossil and molecular data. There are no definitive amphisbaenian fossils from the Mesozoic [7,39], but the three lineages found in the early Palaeocene–Chthonophidae, Rhineuridae, and Oligodontosauridae—are derived, consistent with a Cretaceous origin of the crown. In particular, the appearance of the highly derived Chthonophis in the earliest Palaeocene, more than 53 000 years after the K–Pg event [40] supports a Cretaceous origin for the crown. A possible explanation is that Cretaceous amphisbaenians had restricted geographical ranges, or were restricted to particular habitats, then expanded their ranges in the Palaeocene, increasing their odds of being discovered. Similar patterns are seen in booid snakes [22], ungulates and primates [41], and cryptobranchid [42] and amphiumid [43] salamanders, all of which were absent or rare in the Cretaceous, but widespread in the Palaeocene. The most likely explanation is that the K–Pg extinction eliminated competitors and predators, allowing amphisbaenians and others to expand their ranges.
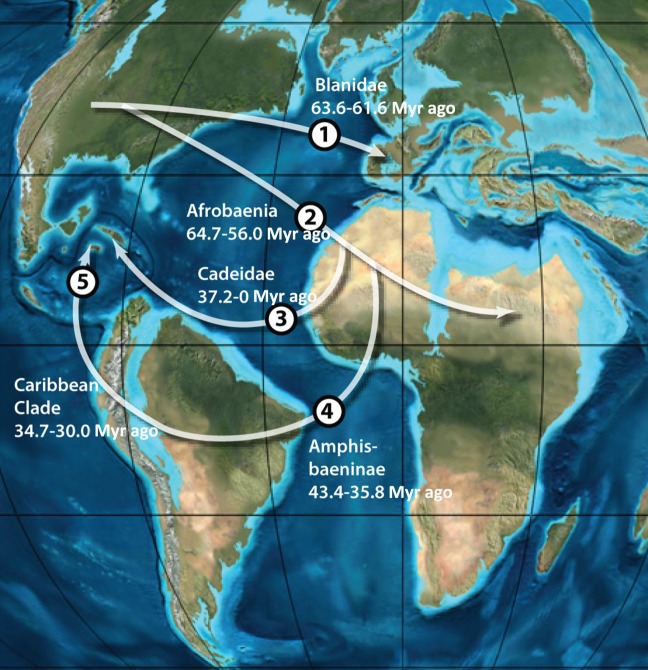
The geographical origin of the Amphisbaenia has been unclear [13]. With the inclusion of fossils, it can be seen that the three deepest branches of the Amphisbaenia—Rhineuridae, Chthonophidae and Oligodontosauridae—are endemic to North America, supporting a North American origin for the crown (figure 5). Following the K–Pg extinction, a series of long-distance dispersal events occurred (figure 6). The first is the dispersal of Blanidae from North America to Europe in the Palaeocene [44]. In addition to extant Blanus, this lineage includes a range of fossil amphisbaenians (figure 5), documenting a Palaeogene radiation following colonization. An amphisbaenian from the Palaeocene of Morocco [45] may document initial dispersal of blanids to Africa, which occurs again in the Neogene (figure 5).
Figure 6.
Oceanic dispersal of Amphisbaenia. Multiple oceanic dispersal events are implied by the phylogeny recovered here: (1) dispersal of Blanidae from North America to Europe; (2) dispersal of stem Afrobaenia from North America to Africa; (3) dispersal of Cadeidae to Cuba (either from Africa, as shown, or else from North America); (4) dispersal of Amphisbaeninae to South America; and (5) dispersal of Amphisbaeninae through the Caribbean.
The second dispersal event is the dispersal of amphisbaenians to Africa. Todrasaurus [45] from Morocco [45] shows that this event occurred by the end of the Palaeocene (figure 5). African colonization led to a major radiation, the Afrobaenia. Unlike the morphologically conservative Rhineuridae and Blanidae, Afrobaenia show high disparity—evolving round-headed, shovel-headed and keel-headed forms—together with high diversity, making them a true adaptive radiation. The tropics of Africa may have provided more niches, or the Afrobaenia may have exploited key innovations such as the ossified extracolumella.
Subsequently, the African radiation crossed the South Atlantic in the Eocene. This clade, the Amphisbaeninae, is the most diverse clade of Amphisbaenia, and staged its own adaptive radiation, which paralleled the African radiation by evolving round-headed, shovel-headed and keel-headed morphs. Subsequent dispersal and radiation occurred as amphisbaenines colonized the Caribbean.
The origins of Cadeidae are unclear [13]. Cadea is endemic to Cuba and therefore represents a fourth dispersal event, rafting either from North America or Africa. North America is closer, but the existence of related forms in the Palaeocene of Africa favours an African origin for Cadeidae.
While amphisbaenian biogeography has traditionally been interpreted in terms of Pangaean break-up [14,16], fossils and molecules (figure 5) both show that amphisbaenians first became widespread in the Palaeogene. Even when estimated using outgroup calibrations alone, clock results are consistent with the fossil record. An external, fossil-calibrated analysis therefore rejects vicariance as a driving mechanism for amphisbaenian biogeography, but demonstrates that estimates using relaxed molecular clocks (both uncorrelated and autocorrelated) and the chosen fossil calibrations are internally consistent. By comparison, when the African/South American split is calibrated to Gondwanan break-up at 100 Ma (figure 4), basal splits within Squamata are pushed down into the Permian and Carboniferous, the squamata-rhynchocephalian divergence occurs during the Cambrian Explosion, and diapsids and amniotes emerge in the Ediacaran. These results reject a vicariant model for Amphisbaenia and illustrate the issues associated with using an insufficient number of calibrations in molecular clock analyses. More generally these results show why vicariant models need to be tested rather than assumed with molecular clocks.
If amphisbaenian diversification occurred in the Palaeogene, then vicariance, while intuitively appealing, must be eliminated as a mechanism for explaining the clade's distribution. Instead, the clade must have dispersed to occupy its present range. Potential dispersal mechanisms include land bridges and oceanic rafting. High latitude land bridges linked North America and Europe in the Palaeogene, but would have been impassible to ectotherms until warming at the Palaeocene–Eocene boundary opened these corridors to cold intolerant species [46,47]. This warming occurs after dispersal of Blanidae in Europe in the mid-Palaeocene [44], making rafting a more likely explanation. No land bridges existed to Africa, or South America, however; both were island continents (figure 6). If we eliminate both vicariance and land bridges as options, then amphisbaenian distribution can only be explained by oceanic rafting. Amphisbaenians are not highly mobile animals, and yet their peculiar lifestyle may have preadapted them to long-distance dispersal.
While it seems improbable that burrowing reptiles would cross oceans, they may have done so as passengers on floating islands—rafts of trees, bound together by roots and soil, that are sometimes seen adrift at sea [48,49]. More frequently, individual trees can fall off eroding cliffs into the sea, or fall into rivers where they may be swept downstream and out to sea. Soil bound in roots of individual trees or rafts could potentially carry amphisbaenians out to sea, burrow and all. Given their low metabolic rates [50], amphisbaenians could survive without food [13] for the days to weeks required [48,49] for the wind to push a raft or tree across the sea. Consistent with this hypothesis, the dispersal routes found here (figure 5) follow trade winds and currents, which trend east towards Europe and North Africa from North America, and west towards South America and the Caribbean from central Africa. The odds of any particular tree or raft resulting in a successful dispersal may be millions to one, but with millions of trees washed out to sea over millions of years, the odds could be relatively high. Furthermore, the occurrence of amphisbaenians on offshore islands, including Cuba, Puerto Rico, Hispaniola [13] and Fernando de Noronha [51], shows unequivocally that worm lizards disperse over water. Similar patterns of dispersal in burrowing squamates are seen in dibamids [52] and scolecophidians [53].
In the case of Amphisbaenia, we suggest that the K–Pg extinction facilitated dispersal. Mass extinctions would not alter the probability of an oceanic crossing, but could change the odds of colonists becoming successfully established. The presence of competitors and predators may form a barrier to dispersal. In addition to physical barriers to dispersal, such as oceans, mountains or climate, predators and especially competitors may form a biotic barrier. Consistent with this hypothesis, islands with species-poor faunas are more susceptible to invasive species than those with diverse faunas [54]. If so, then in the wake of a major extinction event such as the K–Pg event, the odds of successful colonization following dispersal should increase, because there are few competitors or predators to prevent dispersing populations from becoming established, reducing the biotic barrier. The distribution of amphisbaenians and other taxa may, therefore, have more to do with asteroid impacts than shifting continents.
Amphisbaenians are uniquely specialized, even bizarre organisms. Yet in terms of their biogeography, worm lizards are of interest not because they are unusual, but because they may provide a model for understanding biogeography. Following the discovery of continental drift, biogeography has been influenced by the paradigm of vicariance [55], in which modern distributions are explained via Pangaean fragmentation [14,16]. However, evidence from fossils and molecules show that many of the clades that are diverse and widespread today originated and spread only after the Chicxulub impact at 66 Ma [22,56–58]. Like Amphisbaenidae, the Iguanidae, Agamidae, Varanidae, Lacertidae, Booidea and Caenophidia radiate and become widespread in the Cenozoic [22], requiring oceanic dispersal. Among the more striking examples is the Fijian iguana Brachylophus; its close relationship to New World species [7,59] implies trans-Pacific dispersal from the Americas to the South Pacific. Similarly, the existence of iguanas in Madagascar implies oceanic dispersal [60].
These patterns are not unique to reptiles. Cenozoic radiations of placental mammals [57], birds [56,61,62] and freshwater fishes [58] cannot owe their distributions to the Mesozoic fragmentation of Pangaea, and must have become widespread because of dispersal. For instance, the presence of carnivorans and primates in Madagascar [63], rodents [64,65] and primates [64] in South America, and rodents in Australia [66] can only be explained by oceanic dispersal. Birds fly, and in light of evidence for Paleogene radiation [61,62] there is little reason to think that continental drift played a role in their biogeography. Amphibians show patterns of vicariance in the distribution of early lineages, but multiple trans-oceanic dispersals in the Cenozoic appear to explain the distribution of recent groups [67].
We suggest that dispersal, perhaps favoured by biotic changes and radiations following the K–Pg event, is probably the primary mechanism behind the distribution of modern clades of squamates and other groups. Some lineages do have ‘Gondwanan’ distributions. In Amphisbaenidae, a ‘Gondwanan’ distribution probably reflects the facts that the Atlantic was narrow in the Palaeogene, facilitating dispersal, and that the Gondwanan continents contain most of the world's tropics, such that clades restricted to warm environments will tend to be most diverse in Africa and South America. Other groups of lizards and many other organisms may show ‘Gondwanan’ distributions for these reasons, and not because of vicariance.
In conclusion, amphisbaenians appear to have radiated and dispersed in the Palaeogene in response to the K–Pg extinction. Worm lizards managed to cover long distances and to cross oceans despite their unusual, subterranean habits, and perhaps because of them. Together, fossils, skeletons and DNA tell a remarkable story of how small burrowing reptiles once travelled vast distances across oceans. This evidence provides a striking example in support for the hypothesis of Darwin [68] and Wallace [48], that the distribution of many living species is explained by long-distance dispersal across oceans. The patterns seen here suggest that mass extinctions may play a role in driving dispersal, because the elimination of predators and competitors provides the opportunity for species to become established in new habitats.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank the staff of the Yale Peabody Museum for specimen access, and Mark Norell, Carl Mehling and the staff of the American Museum of Natural History for specimen access and loans. Thanks also to Isabelle Kruta for scanning Blanus, to Bhart-Anjan Bhullar for scanning Chthonophis and for discussions and to Ron Blakey for use of map imagery.
Funding statement
This work was supported by DBI-0905765 and DEB-1441719 to R.A.P. and EF-0334966 and DEB-0132227 to J.A.G.
References
- 1.Pianka ER, Vitt LJ. 2003. Lizards: windows to the evolution of diversity. Berkeley, CA: University of California Press. [Google Scholar]
- 2.Gans C. 1975. Tetrapod limblessness: evolution and functional corollaries. Am. Zool. 15, 455–467. [Google Scholar]
- 3.Gans C. 1978. The characteristics and affinities of the Amphisbaenia. Trans. Zool. Soc. Lond. 34, 347–416. ( 10.1111/j.1096-3642.1978.tb00376.x) [DOI] [Google Scholar]
- 4.Foureaux G, Egami MI, Jared C, Antoniazzi MM, Gutierre RC, Smith RL. 2010. Rudimentary eyes of squamate fossorial reptiles (Amphisbaenia and Serpentes). Anat. Rec. 293, 351–357. ( 10.1002/ar.21021) [DOI] [PubMed] [Google Scholar]
- 5.Gans C, Wever EG. 1972. The ear and hearing in Amphisbaenia (Reptilia). J. Exp. Zool. 179, 17–34. ( 10.1002/jez.1401790103) [DOI] [Google Scholar]
- 6.Gans C, Wever EG. 1975. The amphisbaenian ear: Blanus cinereus and Diplometopon zarudnyi. Proc. Natl Acad. Sci. USA 72, 1487–1490. ( 10.1073/pnas.72.4.1487) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gauthier J, Kearney M, Maisano JA, Rieppel O, Behlke A. 2012. Assembling the squamate tree of life: perspectives from the phenotype and the fossil record. Bull. Yale Peabody Mus. 53, 3–308. ( 10.3374/014.053.0101) [DOI] [Google Scholar]
- 8.Vidal N, Hedges SB. 2005. The phylogeny of squamate reptiles (lizards, snakes, and amphisbaenians) inferred from nine nuclear protein-coding genes. C. R. Biol. 328, 1000–1008. ( 10.1016/j.crvi.2005.10.001) [DOI] [PubMed] [Google Scholar]
- 9.Fry BG, et al. 2006. Early evolution of the venom system in lizards and snakes. Nature 439, 584–588. ( 10.1038/nature04328) [DOI] [PubMed] [Google Scholar]
- 10.Hedges SB, Vidal N. 2009. Lizards, snakes, and amphisbaenians. In The timetree of life (eds Hedges SB, Kumar S.), pp. 383–389. Oxford, UK: Oxford University Press. [Google Scholar]
- 11.Vidal N, Hedges SB. 2009. The molecular evolutionary tree of lizards, snakes, and amphisbaenians. C. R. Biol. 332, 129–1139. ( 10.1016/j.crvi.2008.07.010) [DOI] [PubMed] [Google Scholar]
- 12.Pyron RA, Burbrink FT, Wiens JJ. 2013. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evol. Biol. 13, 93 ( 10.1186/1471-2148-13-93) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vidal N, Azvolinsky A, Cruaud C, Hedges SB. 2008. Origin of tropical American burrowing reptiles by transatlantic rafting. Biol. Lett. 4, 115–118. ( 10.1098/rsbl.2007.0531) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kearney M. 2003. Systematics of the Amphisbaenia (Lepidosauria: Squamata) based on morphological evidence from recent and fossil forms. Herpetol. Monogr. 17, 1–74. ( 10.1655/0733-1347(2003)017[0001:SOTALB]2.0.CO;2) [DOI] [Google Scholar]
- 15.Macey JR, Papenfuss TJ, Kuehl JV, Fourcade HM, Boore JL. 2004. Phylogenetic relationships among amphisbaenian reptiles based on complete mitochondrial genomic sequences. Mol. Phylogenet. Evol. 33, 22–31. ( 10.1016/j.ympev.2004.05.003) [DOI] [PubMed] [Google Scholar]
- 16.Hembree DI. 2006. Amphisbaenian paleobiogeography: evidence of vicariance and geodispersal patterns. Palaeogeogr. Palaeoclimatol. Palaeoecol. 235, 340–354. ( 10.1016/j.palaeo.2005.11.006) [DOI] [Google Scholar]
- 17.Veevers J. 2004. Gondwanaland from 650–500 Ma assembly through 320 Ma merger in Pangea to 185–100 Ma breakup: supercontinental tectonics via stratigraphy and radiometric dating. Earth Sci. Rev. 68, 1–132. ( 10.1016/j.earscirev.2004.05.002) [DOI] [Google Scholar]
- 18.Lofgren DL, Lillegraven JA, Clemens WA, Gingerich PD, Williamson TE. 2004. Paleocene biochronology: the Puercan through Clarkforkian land mammal ages. In Late Cretaceous and Cenozoic mammals of North America: biostratigraphy and geochronology (ed. Woodburne MO.), p. 376 New York, NY: Columbia University Press. [Google Scholar]
- 19.Lofgren DL. 1995. The Bug Creek problem and the Cretaceous-Tertiary transition at McGuire Creek, Montana. Univ. Calif. Publ. Geol. Sci. 140, 1–185. [Google Scholar]
- 20.Estes R. 1964. Fossil vertebrates from the Late Cretaceous Lance Formation, Eastern Wyoming. Univ. Calif. Publ. Geol. Sci. 49, 1–180. [Google Scholar]
- 21.Gao K-Q, Fox RC. 1996. Taxonomy and evoluton of Late Cretaceous lizards (Reptilia: Squamata) from Western Canada. Bull. Carnegie Mus. Nat. Hist. 33, 1–107. [Google Scholar]
- 22.Longrich NR, Bhullar A-BS, Gauthier J. 2012. Mass extinction of lizards and snakes at the Cretaceous-Paleogene boundary. Proc. Natl Acad. Sci. USA 109, 21 396–21 401. ( 10.1073/pnas.1211526110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Estes R. 1975. Lower vertebrates from the Fort Union Formation, Late Paleocene, Big Horn Basin, Wyoming. Herpetologica 31, 365–385. [Google Scholar]
- 24.Estes R. 1976. Middle Paleocene lower vertebrates from the Tongue River Formation, Southeastern Montana. J. Paleontol. 50, 500–520. [Google Scholar]
- 25.Gauthier JA. 1982. Fossil xenosaurid and anguid lizards from the early Eocene Wasatch Formation, southeast Wyoming, and a revision of Anguoidea. Contrib. Geol. Univ. Wyom. 21, 17–54. [Google Scholar]
- 26.Lartillot N, Lepage T, Blanquart S. 2009. PhyloBayes 3: a Bayesian software package for phylogenetic reconstruction and molecular dating. Bioinformatics 25, 2286–2288. ( 10.1093/bioinformatics/btp368) [DOI] [PubMed] [Google Scholar]
- 27.Swofford DL. 2002. Paup*: phylogenetic analysis using parsimony (*and other methods). Sunderland, MA: Sinauer Associates. [Google Scholar]
- 28.Goloboff PA, Carpenter JM, Arias JS, Esquivel DRM. 2008. Weighting against homoplasy improves phylogenetic analysis of morphological data sets. Cladistics 24, 758–773. ( 10.1111/j.1096-0031.2008.00209.x) [DOI] [Google Scholar]
- 29.Lepage T, Bryant D, Philippe H, Lartillot N. 2007. A general comparison of relaxed molecular clock models. Mol. Biol. Evol. 24, 2669–2680. ( 10.1093/molbev/msm193) [DOI] [PubMed] [Google Scholar]
- 30.Kearney M, Stuart BL. 2004. Repeated evolution of limblessness and digging heads in worm lizards revealed by DNA from old bones. Proc. R. Soc. Lond. B 271, 1677–1683. ( 10.1098/rspb.2004.2771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Charig AJ, Gans C. 1990. Two new amphisbaenians from the lower Miocene of Kenya. Bull. Br. Mus. Nat. Hist. 46, 19–36. [Google Scholar]
- 32.Müller J, Hipsley CA, Head JJ, Kardjilov N, Hilger A, Wuttke M, Reisz RR. 2011. Eocene lizard from Germany reveals amphisbaenian origins. Nature 473, 364–367. ( 10.1038/nature09919) [DOI] [PubMed] [Google Scholar]
- 33.Alvarez LW, Alvarez W, Asaro F, Michel HV. 1980. Extraterrestrial cause for the Cretaceous-Tertiary extinction. Science 208, 1095–1108. ( 10.1126/science.208.4448.1095) [DOI] [PubMed] [Google Scholar]
- 34.Robertson DS, McKenna MC, Toon OB, Hope S, Lillegraven JA. 2004. Survival in the first hours of the Cenozoic. Geol. Soc. Am. Spec. Bull. 116, 11–12. [Google Scholar]
- 35.Sheehan PM, Fastovsky DE. 1992. Major extinctions of land-dwelling vertebrates at the Cretaceous-Tertiary boundary, Eastern Montana. Geology 20, 556–560. () [DOI] [Google Scholar]
- 36.Alroy J. 1999. The fossil record of North American mammals: evidence for a Palaeocene evolutionary radiation. Syst. Biol. 48, 107–118. ( 10.1080/106351599260472) [DOI] [PubMed] [Google Scholar]
- 37.Feduccia A. 1995. Explosive evolution in Tertiary birds and mammals. Science 267, 637–638. ( 10.1126/science.267.5198.637) [DOI] [PubMed] [Google Scholar]
- 38.Friedman M. 2010. Explosive morphological diversification of spiny-finned teleost fishes in the aftermath of the end-Cretaceous extinction. Proc. R. Soc. B 277, 1675–1683. ( 10.1098/rspb.2009.2177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kearney M. 2003. The phylogenetic position of Sineoamphisbaena hexatabularis reexamined. J. Vert. Paleontol. 23, 394–403. ( 10.1671/0272-4634(2003)023[0394:TPPOSH]2.0.CO;2) [DOI] [Google Scholar]
- 40.Renne PR, Deino AL, Hilgen FJ, Kuiper KF, Mark DF, Mitchell WS, III, Morgan LE, Mundil R, Smit J. 2013. Time scales of critical events around the Cretaceous-Paleogene boundary. Science 339, 684–687. ( 10.1126/science.1230492) [DOI] [PubMed] [Google Scholar]
- 41.Clemens WA. 2002. Evolution of the mammalian fauna across the Cretaceous-Tertiary boundary in northeastern Montana and other areas of the Western Interior. Geol. Soc. Am. 361, 217–245. [Google Scholar]
- 42.Naylor BG. 1981. Cryptobranchid salamanders from the Paleocene and Miocene of Saskatchewan. Copeia 1981, 76–86. ( 10.2307/1444042) [DOI] [Google Scholar]
- 43.Gardner JD. 2003. The fossil salamander Proamphiuma cretacea Estes (Caudata; Amphiumidae) and relationships within the Amphiumidae. J. Vert. Paleontol. 23, 769–782. ( 10.1671/1828-4) [DOI] [Google Scholar]
- 44.Folie A, Smith R, Smith T. 2013. New amphisbaenian lizards from the Early Paleogene of Europe and their implications for the early evolution of modern amphisbaenians. Geol. Belgica 16, 227–235. [Google Scholar]
- 45.Augé M, Rage J-C. 2006. Herpetofaunas from the Upper Paleocene and Lower Eocene of Morocco. Ann. Paléontol. 92, 235–253. ( 10.1016/j.annpal.2005.09.001) [DOI] [Google Scholar]
- 46.Koch PL, Zachos JC, Gingerich PD. 1992. Correlation between isotope records in marine and continental carbon reservoirs near the Paleocene-Eocene boundary. Nature 358, 319–322. ( 10.1038/358319a0) [DOI] [Google Scholar]
- 47.Smith T, Rose KD, Gingerich PD. 2006. Rapid Asia–Europe–North America geographic dispersal of earliest Eocene primate Teilhardina during the Paleocene–Eocene thermal maximum. Proc. Natl Acad. Sci. USA 103, 11 223–11 227. ( 10.1073/pnas.0511296103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wallace AR. 1902. Island life, or, the phenomena and causes of insular faunas and floras: including a revision and attempted solution of the problem of geological climates. New York, NY: Macmillan. [Google Scholar]
- 49.Houle A. 1998. Floating islands: a mode of long-distance dispersal for small and medium-sized terrestrial vertebrates. Divers. Distrib. 4, 201–216. [Google Scholar]
- 50.Kamel S, Gatten RE., Jr 1983. Aerobic and anaerobic activity metabolism of limbless and fossorial reptiles. Physiol. Zool. 19, 419–429. [Google Scholar]
- 51.Pregill G. 1984. Durophagous feeding adaptations in an amphisbaenid. J. Herpetol. 18, 186–191. ( 10.2307/1563747) [DOI] [Google Scholar]
- 52.Townsend TM, Leavitt DH, Reeder TW. 2011. Intercontinental dispersal by a microendemic burrowing reptile (Dibamidae). Proc. R. Soc. B 278, 20102598 ( 10.1098/rspb.2010.2598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vidal N, et al. 2010. Blindsnake evolutionary tree reveals long history on Gondwana. Biol. Lett. 6, 558–561. ( 10.1098/rsbl.2010.0220) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Helmus MR, Mahler DL, Losos JB. 2014. Island biogeography of the Anthropocene. Nature 513, 543–546. ( 10.1038/nature13739) [DOI] [PubMed] [Google Scholar]
- 55.Wiley EO. 1988. Vicariance biogeography. Annu. Rev. Ecol. Syst. 19, 513–542. ( 10.1146/annurev.es.19.110188.002501) [DOI] [Google Scholar]
- 56.Ericson PGP, et al. 2006. Diversification of Neoaves: integration of molecular sequence data and fossils. Biol. Lett. 2, 543–547. ( 10.1098/rsbl.2006.0523) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.dos Reis M, Inoue J, Hasegawa M, Asher RJ, Donoghue PCJ, Yang Z. 2012. Phylogenomic datasets provide both precision and accuracy in estimating the timescale of placental mammal phylogeny. Proc. R. Soc. B 279, 3491–3500. ( 10.1098/rspb.2012.0683) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Friedman M, Keck BP, Dornburg A, Eytan RI, Martin CH, Hulsey CD, Wainwright PC, Near TJ. 2013. Molecular and fossil evidence place the origin of cichlid fishes long after Gondwanan rifting. Proc. R. Soc. B 280, 20131733 ( 10.1098/rspb.2013.1733) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Townsend TM, Mulcahy DG, Noonan BP, Sites JW, Kuczynski CA, Wiens JJ, Reeder TW. 2011. Phylogeny of iguanian lizards inferred from 29 nuclear loci, and a comparison of concatenated and species-tree approaches for an ancient, rapid radiation. Mol. Phylogenet. Evol. 61, 363–380. ( 10.1016/j.ympev.2011.07.008) [DOI] [PubMed] [Google Scholar]
- 60.Vences M. 2004. Origin of Madagascar's extant fauna: a perspective from amphibians, reptiles and other non-flying vertebrates. Ital. J. Zool. 71, 217–228. ( 10.1080/11250000409356639) [DOI] [Google Scholar]
- 61.Jarvis ED, et al. 2014. Whole-genome analyses resolve early branches in the tree of life of modern birds. Science 346, 1320–1331. ( 10.1126/science.1253451) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mitchell KJ, Llamas B, Soubrier J, Rawlence NJ, Worthy TH, Wood J, Lee MS, Cooper A. 2014. Ancient DNA reveals elephant birds and kiwi are sister taxa and clarifies ratite bird evolution. Science 344, 898–900. ( 10.1126/science.1251981) [DOI] [PubMed] [Google Scholar]
- 63.Yoder AD, Burns MM, Zehr S, Delefosse T, Veron G, Goodman SM, Flynn JJ. 2003. Single origin of Malagasy Carnivora from an African ancestor. Nature 421, 734–737. ( 10.1038/nature01303) [DOI] [PubMed] [Google Scholar]
- 64.Poux C, Chevret P, Huchon D, De Jong WW, Douzery EJ. 2006. Arrival and diversification of caviomorph rodents and platyrrhine primates in South America. Syst. Biol. 55, 228–244. ( 10.1080/10635150500481390) [DOI] [PubMed] [Google Scholar]
- 65.Rowe DL, Dunn KA, Adkins RM, Honeycutt RL. 2010. Molecular clocks keep dispersal hypotheses afloat: evidence for trans-Atlantic rafting by rodents. J. Biogeogr. 37, 305–324. ( 10.1111/j.1365-2699.2009.02190.x) [DOI] [Google Scholar]
- 66.Rowe KC, Reno ML, Richmond DM, Adkins RM, Steppan SJ. 2008. Pliocene colonization and adaptive radiations in Australia and New Guinea (Sahul): multilocus systematics of the old endemic rodents (Muroidea: Murinae). Mol. Phylogenet. Evol. 47, 84–101. ( 10.1016/j.ympev.2008.01.001) [DOI] [PubMed] [Google Scholar]
- 67.Pyron RA. 2014. Biogeographic analysis in amphibians reveals both ancient continental vicariance and recent oceanic dispersal. Syst. Biol. 63, 779–797. ( 10.1093/sysbio/syu042) [DOI] [PubMed] [Google Scholar]
- 68.Darwin CR. 1859. The origin of species. London, UK: John Murray. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.