Abstract
A major theme in evolutionary and ecological physiology of terrestrial vertebrates encompasses the factors underlying the evolution of endothermy in birds and mammals and interspecific variation of basal metabolic rate (BMR). Here, we applied the experimental evolution approach and compared BMR in lines of a wild rodent, the bank vole (Myodes glareolus), selected for 11 generations for: high swim-induced aerobic metabolism (A), ability to maintain body mass on a low-quality herbivorous diet (H) and intensity of predatory behaviour towards crickets (P). Four replicate lines were maintained for each of the selection directions and an unselected control (C). In comparison to C lines, A lines achieved a 49% higher maximum rate of oxygen consumption during swimming, H lines lost 1.3 g less mass in the test with low-quality diet and P lines attacked crickets five times more frequently. BMR was significantly higher in A lines than in C or H lines (60.8, 56.6 and 54.4 ml O2 h−1, respectively), and the values were intermediate in P lines (59.0 ml O2 h−1). Results of the selection experiment provide support for the hypothesis of a positive association between BMR and aerobic exercise performance, but not for the association of adaptation to herbivorous diet with either a high or low BMR.
Keywords: endothermy, food habits, aerobic metabolism, experimental evolution
1. Introduction
Successful performance of vital animal functions—such as resource acquisition, maintaining homeostasis, growth or reproduction—depends on a complex network of physiological processes. However, each of these processes involves conversion of energy, and therefore the rate of energy metabolism can be used as a unifying quantitative measure of organismal functioning [1,2]. Obvious sources of variation in the rate of metabolism are changes in body and ambient temperature and the level of physical activity, which result in instantaneous changes in metabolic rate. Therefore, the basal rate of metabolism (BMR), which is measured in resting animals at standardized thermal conditions [1], has received special attention as a trait suitable for interspecific comparisons. Consequently, questions about the factors underlying the huge interspecific variation in BMR have become a major theme in evolutionary and ecological physiology of terrestrial vertebrates [1].
At the macroevolutionary scale, the most striking difference in BMR is between ‘endotherms’ (birds or mammals) and ‘ectotherms’ (reptiles). Benefits of endothermy, which allows maintaining a high body temperature by means of metabolic heat production, are easy to identify. However, evolution of the high level of BMR in birds and mammals, which translates to at least an order of magnitude higher costs of maintenance in comparison to ectothermic reptiles, is puzzling and the selection mechanisms that have led to evolution of such an energetically wasteful strategy remain subject to a vivid discussion (reviews: [1–17]). According to the ‘aerobic capacity model’—one of the main hypotheses—high BMR in endotherms evolved as a correlated response to selection for increased locomotor performance fuelled by aerobic metabolism [18]. Testing the basic assumption of the model—that BMR is positively correlated with aerobic capacity (maximum rate of oxygen consumption)—has been a motivation for many comparative, experimental, quantitative genetic and conceptual studies, but the issue is not resolved (recent reviews: [13,17,19,20]).
At the level of interspecific comparisons within birds and mammals, many studies have focused on the associations between BMR and food habits (e.g. [1,21–29]). Predation and herbivory are the two most basic, but also opposite, food habit strategies available. Evolutionary selection for one of these strategies has a profound effect on other behavioural, physiological and morphological traits. However, the relationship between the expected BMR and either of these strategies is unclear; in both cases, one can provide theoretical and empirical arguments for a relatively low or high BMR [26–29].
The majority of research on hypothetical correlates of BMR has been based on comparative analyses or intraspecific phenotypic correlations, but more recently quantitative genetic analyses (e.g. [30–34]) and selection experiments [2,35–41] have been recognized as powerful tools in such studies (but see [19,20,42,43] for discussion of limitations of these tools).
Here, we applied the experimental evolution approach and asked: ‘how would BMR in a particular species change in response to controlled selection for traits that comparative analyses have indicated as plausible triggers for the evolution of interspecific variation in BMR?’ To this end, we designed a multidirectional artificial selection experiment, with lines of bank voles, selected in three directions (figure 1): increased maximum rate of exercise-induced aerobic metabolism (A), ability to grow on a low-quality herbivorous diet (H) and intensity of predatory behaviour (P) [44]. In this paper, we present a comparison of the level of BMR of voles from lines selected for 11 generations with that of unselected, control lines (C). Based on results from our earlier quantitative genetic analyses, we predicted that BMR will increase both in lines selected for high swim-induced aerobic metabolism [32] and in lines selected for herbivorous capability [33]. Because of a close connection between predatory propensity and locomotor activity, shown also in other selection experiments [45], and in line with the aerobic capacity model of the evolution of endothermy [16,46], we predicted that BMR will also increase in lines selected for increased predatory behaviour.
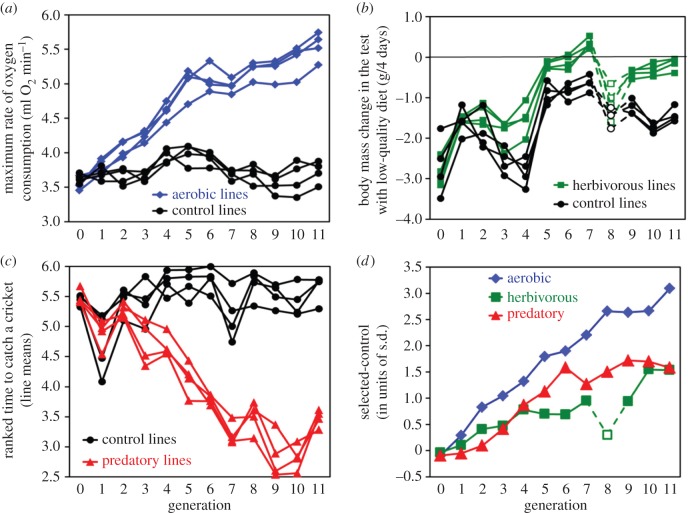
Figure 1.
Direct phenotypic responses to 11 generations of selecting bank voles towards (a) high swim-induced aerobic metabolism, (b) herbivorous capability measured as ability to maintain body mass in a test with low-quality diet, (c) predatory propensity measured as ranked time to attack a cricket, and (d) comparison of the cumulative effects of selection in the three directions expressed as a difference between the means of four selected (in each direction) and four control lines (expressed in units of phenotypic standard deviation). In generation 8, the food used in selection trial in the ‘herbivorous’ lines was different than in other generations, which resulted in the irregular pattern (marked with dashed lines and open symbols on graphs (b) and (d)). (Online version in colour.)
2. Material and methods
(a). Animals and the selection experiment
This work was performed on bank voles (Myodes = Clethrionomys glareolus Schreber 1780) from generation 11 of a multivariate artificial selection experiment. The rationale, history and protocols of the ongoing selection experiment have been presented in our earlier work [44] and in the electronic supplementary material of this paper. Briefly, selection was applied based on the following criteria: high aerobic metabolism (A)—the maximum 1 min rate of oxygen consumption (V̇O2swim), achieved during 17 min of swimming at 38°C; herbivorous capability (H)—body mass change in a 4 day trial, during which voles were fed a low-quality, herbivorous diet (made of dried grass and flour); and predatory behaviour (P)—ranked time to catch a live cricket in a 10 min trial (ranks 1–5: cricket caught in 0.5, 1, 3, 6 or 10 min, respectively; rank 6: cricket not caught). The measurements of swim-induced aerobic metabolism and the predatory behaviour tests were performed on adults (about 75–95 days old), and the tests with low-quality diet on young, still growing animals (32–36 days). All the trait values used as selection criteria were mass-adjusted (residuals from ANCOVA including also other covariates and cofactors). Four replicate lines for each selection direction and an unselected control (C) were maintained (to allow valid tests of the effects of selection [47]), with 15–20 reproducing families in each of the 16 lines (which avoided excess inbreeding). The selection was applied mostly within families, but if more than 16 families were available, families in which all individuals scored below the line mean were excluded.
In generation 11, differences between all selection directions and the unselected C lines were highly significant (ANCOVA mixed nested models, with lines as a random effect nested in fixed selection factor, and random family effect nested in lines: p < 0.0001). V̇O2swim was 49% higher in A lines (mean ± s.d.: 333.1 ± 39.2 ml O2 h−1; n = 824) in comparison to C lines (223.37 ± 31.83 ml O2 h−1; n = 98; figure 1a). Voles from H lines were nearly able to maintain body mass during the 4 day test with low-quality diet (body mass loss: 0.15 ± 0.83 g; n = 1019), whereas those from C lines lost more mass (1.42 ± 0.826 g; n = 107; figure 1b). In P lines, 75.3% of individuals attacked a cricket in at least one of the tests and the ranked time to catch a cricket averaged 3.46 ± 1.81 (n = 837), whereas in C lines only 14.4% of individuals behaved as predators, and the ranked time to catch averaged 5.64 ± 0.94 (n = 837; figure 1c).
The animals were maintained in standard plastic mouse cages with sawdust bedding, at a constant temperature (20 ± 1°C) and photoperiod (16 L : 8 D; light phase starting at 2.00). Water and food (a standard rodent food: 24% protein, 3% fat, 4% fibre; Labofeed H, Kcynia, Poland) were provided ad libitum. More detailed information about the housing conditions and animal welfare is provided in the electronic supplementary material.
Measurements of BMR were performed on 313 individuals of both sexes from all 16 lines: 22–28 from each of the A lines, 21–22 from each of the P lines and 14–18 from each of the C and H lines. Animals were chosen randomly from 12 to 15 families per line, with the condition that no more than two males and two females from a full-sibling family were chosen. Individuals were born in litters one to four of a given female (only six were from the fourth litter, and in statistical analyses they were merged with the third litter), and they were not used in the test with low-quality diet. The animals were adults at ages ranging from 69 to 155 days (mostly 90–130 days; mean 109 days), which allowed us to determine if BMR changed with age.
(b). Measurement of basal metabolic rate
Measurements of BMR were performed similarly as described in Labocha et al. [48]. Details of the respirometric set-up, the measurement protocol and calculation of the rate of oxygen consumption (V̇O2) are described in the electronic supplementary material. Briefly, animals were weighed and placed in respirometric chambers without water or food (BMR is defined as minimum resting metabolism at post-absorptive state [1]). Two types of chambers were used: glass 550 ml with 300 ml min−1 air flow rate or plastic 850 ml with 350 ml min−1 air flow rate. The chambers were placed in a climate-controlled room at 28°C (at the lower side of thermal neutral zone [49]). Only dim red lights were left on in the room.
To check if hypothetical differences in BMR are not associated only with a particular time of day, the BMR trials were performed in three ‘timing’ groups (the actual timing varied ±0.45 h from the following values): ‘night’ (20.30–06.00), ‘morning’ (06.00–14.00) and ‘afternoon’ (09.00–20.30). In the morning and night groups, the chambers were connected to the respirometric system at the start of the trial, and measurements lasted until the end of their measurement period. When voles from the afternoon group started the trial, the respirometric system continued to record data for the morning group. Therefore, their chambers were connected to the system only at 14.00. Thus, in the afternoon group, V̇O2 was not measured during the initial 5 h, which is typically considered a period of acclimatization and fasting not included in BMR trials (cf. [48]).
Of the 313 voles measured, 15 died (all from A lines). These incidents were not caused by fasting, because sometimes death occurred at the beginning of the trials. The size of respirometric chambers was large enough to allow free movement (animals were not force-constrained; see the electronic supplementary material). Animals did not suffer from inadequate ventilation, because even at moments of intensive activity CO2 concentration did not exceed 1.2%, and nearly all the time was below 0.6%, which causes no adverse effects in burrowing rodents. Apparently, the deaths resulted from episodes of hyperactivity and a resulting hyperthermia, occurring at any time during the trials. Unfortunately, however, the signal from activity sensors or gas analysers could not be used to anticipate and prevent death, because many individuals showed such periods of intensive activity without any adverse effects.
V̇O2 was measured with an eight-channel respirometric system. Samples of air flowing out of an empty reference and seven animal measurement chambers were analysed sequentially, in a 13 min cycle. Oxygen and CO2 concentrations were recorded every second. V̇O2 was calculated from the values recorded in the last 20 s before switching channels. Activity of the animals and background ‘noise activity’ of the empty reference chamber were monitored continuously with MAD-1 gravimetric detectors (signal of 0–5 V range; Sable Systems, Inc., Las Vegas, NV, USA).
BMR was operationally defined as the minimum recorded V̇O2. However, if the mean activity signal in the 3 min period preceding and including the lowest readings exceeded markedly typical background noise (mean reading from the empty chamber), the entire trial was rejected. V̇O2 was not dependent on the activity signal only after setting the threshold to 0.095 V, corresponding to the upper 90% confidence limit of the noise readings. This eliminated 68 individuals and the final sample used for analyses comprised 232 individuals (C—51, A—68, P—58 and H—55). For the limited sample, the mean activity signal was similar to that of the noise signal (electronic supplementary material, Results S1). In addition, we used the signal from activity detectors as a covariate in all analyses. We also tried analyses based on BMR calculated from the mean of the two lowest readings, but in this case the sample had to be further reduced (and results were qualitatively similar to those reported here).
(c). Statistical analyses of basal metabolic rate data
For comparisons of BMR across the four selection directions, we used SAS v. 9.3 (SAS Institute, Inc., Cary, NC, USA) mixed procedure (with REML method) to estimate cross-nested mixed ANCOVA model, with selection (selected versus control) as the main, top-level fixed factor, replicated lines as random effect nested within selection and body mass as a covariate. Because BMR is known to scale allometrically with body mass, and because the distributions of both BMR and body mass were right-skewed, the analyses were performed on log-transformed values. In all the analyses, age, sex, timing, chamber type and log-transformed activity signal were included as additional fixed covariates or cofactors. The model also included a fixed selection × sex interaction and random interactions sex × line and timing × line. The above variables were a priori considered meaningful predictors either for biological or technical reasons, and therefore were retained in the model irrespective of their significance. Before estimating this final model, we tested preliminary models that included additional fixed effects of litter number, litter size and date of the measurement and a random effect of channel number (variation among the seven measurement channels), models with timing × selection, timing × sex and timing × sex × line interactions and models with interactions between body mass and the main categorical effects (selection, line, sex and timing; to check homogeneity of slopes). None of these additional effects was significant and we present here results from the final model only.
To compare body mass measured with BMR trials (log-transformed) across the selection, sex and timing groups, we applied similar mixed ANCOVA models, but not including the effects of the technical variables meaningful only for the respirometric measurements.
Note that in all the models described above, significance of the fixed effect of selection is tested by means of an F-test against variation among the replicate lines, and significance of sex, selection × sex and timing factors is tested against respective interactions with line, which protects against spurious recognition of correlated responses to selection [47]. Significance of the random effects of variation among replicate lines and respirometer channels was tested with a likelihood ratio test ([50] unlike in the main analyses, in models estimated for these tests variances were not constrained to be positive). In preliminary analyses, we tried to fit also two-level nested models with family (mother identity) as an additional random effect, nested within lines. However, because in many families only one individual was present, higher level effects could not be properly tested (because of lost degrees of freedom). In those cases where the models could be estimated, the results concerning main effects were qualitatively similar to those from the models not including the family effect. For pairwise a posteriori comparisons between groups of factors with more than two levels (selection, timing, litter number), Tukey–Kramer adjustment was applied.
Complete tables with descriptive statistics and results of the mixed ANCOVA models are presented in the electronic supplementary material, Results, and here we show adjusted least-square means with 95% confidence limits (LSM[CL]), back-transformed to original scale.
3. Results
Body mass (measured before BMR trials on all 313 individuals) increased with age (t246 = 4.21, p < 0.0001), was larger in males than in females (F1,12 = 70.5, p < 0.0001) and larger in voles from the second litters compared with those from the first (F2,30 = 5.63, p = 0.008; mass in the third litter was intermediate), but it did not differ between timing groups (F2,30 = 1.56, p = 0.23; figure 2a; electronic supplementary material, Results S1 and S2). Body mass adjusted for all the effects varied significantly among replicate lines within selection directions (LR test: χ2 = 7.44, p = 0.006) and differed among selection directions (F3,12 = 4.17, p = 0.031; figure 2a): it was larger in H than in P lines (Tukey–Kramer pairwise comparisons: t12 = 3.03, p = 0.045), whereas in A and C lines, it was intermediate and did not differ from each other or from the H or P lines (p > 0.1). The results limited to the 232 individuals for which BMR was obtained were similar, but the difference between selection directions was marginally not significant (F3,12 = 2.98, p = 0.074).
Figure 2.
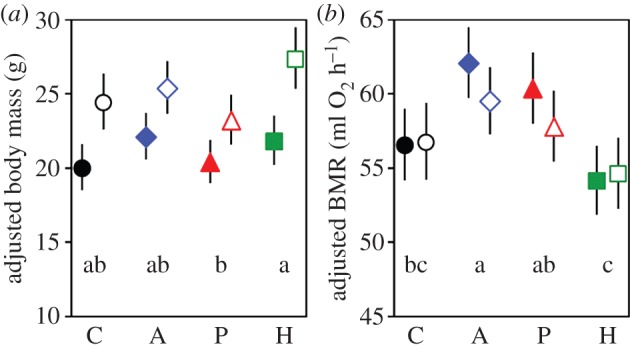
Adjusted least-square means from mixed ANCOVA models (±95% CIs) of (a) body mass measured before BMR trials (g; all individuals) and (b) basal metabolic rate (ml O2 h−1), in male and female voles from lines selected in three directions (A, Aerobic; P, Predatory and H,Herbivorous) and unselected control (C) lines. The values are back-transformed from the analyses on log-transformed data. Lowercase letters (abc) indicate selection groups not statistically different at p = 0.05 (Tukey–Kramer post hoc pairwise comparisons). Open symbols denote males, and filled symbols females. (Online version in colour.)
BMR increased with body mass (common slope ± s.e. = 0.78 ± 0.04 on log–log scale; t164 = 18.4, p < 0.0001; figure 3), and it was not significantly correlated with the activity signal (t164 = 1.57, p = 0.12). BMR adjusted for both of these effects differed among the selection directions (F3,12 = 12.87, p = 0.0005; figures 2b and 3; electronic supplementary material, Results S1 and S3). It was higher in A lines (LSM [95% CL] = 60.8 [59.1–62.5] ml O2 h−1) in comparison to both C (56.6 [54.9–58.5] ml O2 h−1; Tukey–Kramer: t12 = 3.62, p = 0.016) and H lines (54.4 [52.7–56.1] ml O2 h−1; t12 = 5.91, P = 0.0004). In P lines, BMR (59.0 [57.3–60.8] ml O2 h−1) tended to be higher, though not significantly, than in C lines (t12 = 2.09, p = 0.21), but it was significantly higher than in H lines (t12 = 4.09, p = 0.007). BMR did not differ significantly between H and C lines (t12 = 2.03, p = 0.23) or between A and P lines (t12 = 1.51, p = 0.46). The adjusted BMR did not differ between sexes (F1,12 = 1.60, p = 0.23) or, surprisingly, among the timing (night–morning–afternoon) measurement groups (F2,23 = 0.17, p = 0.85). Likelihood ratio tests showed also that none of the random effects included in the model (line, sex × line, timing × line) contributed significantly to explaining the variance of adjusted BMR (p > 0.36 for all the effects).
Figure 3.
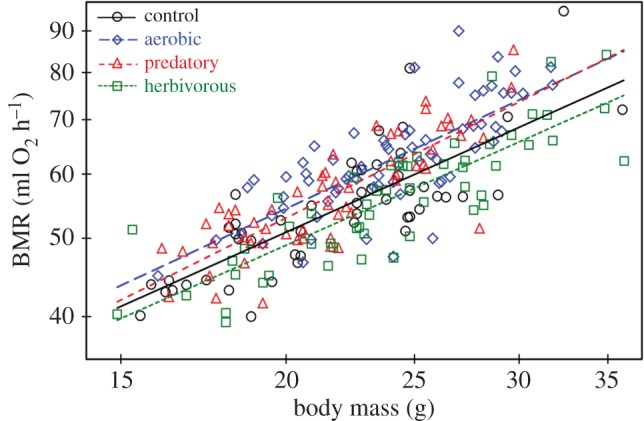
The relationship between BMR and body mass (note the log–log scale) in voles from all four selection directions. The slopes of the lines did not differ significantly (note that the values are not adjusted for any other effects used for calculation of the adjusted means presented on figure 2b; common slope from the ANCOVA model ± s.e. = 0.78 ± 0.04). (Online version in colour.)
4. Discussion
Selection was effective in all directions and resulted in substantial differences between selected and control lines in the 11th generation of bank voles (figure 1). Therefore, the selected lines provide a promising foundation for investigating both underlying molecular mechanisms responsible for differences observed at the organismal level [51,52] and a wide range of possible correlated responses [53–55].
Results of the current study confirmed the prediction that selection for high swim-induced aerobic metabolism would also result in an increase in BMR. In our previous work [32], we reported a positive genetic correlation between V̇O2swim and BMR. However, the voles in our previous study swam at 30°C and therefore V̇O2swim also comprised a thermoregulatory burden. In the current experiment, however, the voles were selected for V̇O2swim achieved at 38°C, i.e. with no thermoregulatory burden involved. Thus, the increased BMR was strictly due to selection for a locomotor-performance trait. Our analyses of complete transcriptome from heart and liver of the A-selected and C lines [52] indicated several candidate genes with differentiated proportion of single nucleotide polymorphism alleles or different levels of gene expression, which could underlie correlation between the traits. Perhaps, the most interesting in this respect are allelic differences in glycogen phosphorylase (PYGL) and glycogen-debranching enzyme (AGL), which catalyse the rate-limiting step in glycogenolysis in the liver, and thus provide fuel for the main energy-metabolism pathway [52]. Further molecular and biochemical analyses based on this unique animal model will allow us to verify if these or other candidate genes are indeed responsible for the link between aerobic exercise performance and the level of BMR.
Our result is consistent with the positive genetic correlation between BMR and the maximum forced-running V̇O2 reported in laboratory mice [17,34], but selection experiments on mice did not show the expected correlated response. In laboratory mice selected for high wheel-running activity, BMR did not increase [36], but because aerobic capacity was increased only moderately in selected lines [56,57] this result may not be very informative. BMR was also not increased in laboratory mice selected for high V̇O2swim, even though maximum forced-running V̇O2 was increased [38]. However, even if BMR were increased in that study, interpretation of the result would be unclear, because V̇O2swim was measured at 25°C and resulted in hypothermia (about 7°C [38]), so the selected trait certainly comprised a large component of thermogenesis. Finally, a recent report showed no significant increase in BMR after eight generations of selection for high maximum forced-running V̇O2, even though quantitative genetic analyses performed within the framework of the same experiment showed that both of the traits are heritable and genetically correlated [17]. The lack of change in BMR in this case could simply be due to premature termination of this selection experiment. After eight generations, the directly selected trait (maximum V̇O2) was only about 11% higher in the selected than in the control lines [17, table 2]. So, considering the large individual variation in those traits, the chance of detecting a correlated response in BMR was small. On the other hand, the quantitative genetic analyses were based on several thousand observations, which gave enough power to reliably estimate genetic correlation.
The selection experiments mentioned here, as well as most selection experiments on mammals, were performed on laboratory species. Thus, the pattern of direct and correlated responses to selection could be strongly affected by domestication (e.g. [56]) and previous selection for peculiar traits, such as high reproductive output under no food restriction. Conversely, our selection experiment has been performed on a wild rodent that had been maintained under laboratory conditions for only five to seven generations (see the electronic supplementary material for details) before the selection protocol began. Thus, it can be assumed that the standing genetic variation resembles that in a wild population. Certainly, we can expect that in addition to the intended selection, the freshly established laboratory colony was subject to an unintentional ‘laboratory natural selection’ to laboratory conditions, i.e. underwent a process of domestication [58]. However, because all our inferences are based on comparisons across the selected and control lines within one generation, rather than a comparison of the selected line with the base population, the plausible process of domestication does not undermine the inferences.
In their influential review, Hayes & Garland [3] advocated to test for a presence of an additive genetic correlation between BMR and the capacity for exercise-induced aerobic metabolism as a crucial test of the main assumption of the aerobic capacity model of the evolution of endothermy. From this perspective, our results could be treated as an elegant corroboration of the hypothesis. However, for two reasons, this conclusion should be treated with caution. First, a presence or absence of such a correlation in an extant species should not be treated as evidence concerning the state in remote ancestral species [19,20,34,42,43]. Second, we should note that the nearly 50% increase of V̇O2swim in A lines was accompanied by just a 15% increase of the absolute values of BMR (and just 7.3% of the values adjusted for all covariates; figure 2b). Thus, at least in voles, the level of aerobic capacity can evolve to a large extent with only a small change of BMR. Therefore, while the results are consistent with the idea that selection for the ability to endure high aerobic locomotor activity was a trigger for the evolution of endothermy, they also suggest that other factors must have been involved to produce a 10-fold difference in the level of resting metabolism, such as that between ectotherms and endotherms (cf. [5–9,11–16,59]).
Although BMR was not statistically significantly higher in P lines than in C lines, BMR in P lines was closer to that of A than C lines, and it was significantly higher than in the ‘lowest’ H lines (figure 2b). This pattern suggests that with the ongoing progress of selection, BMR in P lines is likely to become significantly higher in comparison to C lines. The result is consistent (with all the reservations outlined above) with the hypothesis linking the evolution of endothermy with an active predatory lifestyle (e.g. [16]) and with results of comparative analyses showing that predators have, on average, a higher BMR [25].
Another group of mammals in which comparative analyses revealed a relatively high BMR are terrestrial grazers [21,23,28]. In line with this observation, our earlier quantitative genetic analyses showed a positive genetic correlation between BMR of bank voles and their ability to cope with a low-quality, herbivorous diet [33]. However, in the current experiment, despite a significant selection progress in the ability of the voles to maintain body mass on the low-quality diet, we observed no increase of BMR (figures 2b and 3). The selection experiment was formed on the same laboratory colony that was the basis for the earlier quantitative genetic study, and therefore the discrepancy between results of the earlier quantitative genetic analysis and the selection experiment could not be due to a different genetic background. We suspect that the explanation for this discrepancy may be in a high sensitivity of the selected trait to changes of the measurement conditions. It is striking that, while the progress of selection was quite consistent in A and P lines (figure 1a,c,d), in H lines we observed large fluctuations among generations (figure 1b,d). The fluctuations observed between generations 1 and 5 were parallel for the H and C lines, so the difference between selected and control lines steadily increased (figure 1). Such among-generation fluctuations are common in selection experiments (e.g. [60]), and the actual reason is usually not identified. We suspect that in our experiment, the fluctuations were due to inevitable differences in the experimental food composition: even though the nominal composition (proportion of grass and flour; see the electronic supplementary material) was not changed, the chemical composition (e.g. of secondary plant compounds) could change. We noted that in generation 7, the experimental food was no longer challenging to H lines, therefore, in generation 8 the composition of the food was slightly changed to worse (food pellets were also harder; see the electronic supplementary material). Surprisingly, voles from H lines could not cope with the modified food better than those from C lines. In generation 9, the food was changed again, and the difference between H and C lines was again present (figure 1c,d). Thus, even the direct effect of selection turned out to be very sensitive to changes in food properties, which may also explain why a correlated response in BMR was not as predicted based on the earlier estimate of a genetic correlation.
5. Conclusion
— Results of the current selection experiment, taken together with results of our previous quantitative genetic analyses [32], provide ‘steady’ support for the assumption that selection for increased aerobic capacity should lead to increased BMR. However, the results also indicate that it is unlikely that such a selection alone could result in the roughly 10-fold difference in BMR between endotherms and ectotherms.
— On the other hand, our results suggest that even a small change in the properties of a diet may change the correlation between the ability to grow on a low-quality diet and the level of BMR. Thus, considering the complexity of the variation of natural diets, it is not surprising that wide-scale patterns of association between a general type of diet and either a high or low BMR are difficult to identify.
Supplementary Material
Supplementary Material
Acknowledgements
We are grateful to the many students and technicians who helped with animal maintenance and measurements, especially to R. Czuchnowski, K. Sęk, M. Skrzypczak, K. Ścigajło, K. Wilk and A. Zawada.
Ethics statement
All the procedures were approved by the decision of the Local Ethical Committee for Experiments on Animals in Kraków, Poland (no. 99/2006).
Data accessibility
The raw results are provided as an electronic supplementary material, appendix, and are available from the Dryad depository: http://dx.doi.org/10.5061/dryad.kh312.
Funding statement
The project was supported by grants from the Polish Ministry of Science and Higher Education N303 2752 33 and Jagiellonian University DS/BW UJ INoS 757.
References
- 1.McNab BK. 2002. The physiological ecology of vertebrates. Ithaca, NY: Cornell University Press. [Google Scholar]
- 2.Swallow JG, Hayes JP, Koteja P, Garland T., Jr 2009. Selection experiments and experimental evolution of performance and physiology. In Experimental evolution: concepts, methods, and applications of selection experiments (eds Garland T, Jr, Rose MR.), pp. 301–351. Berkeley, CA: University of California Press. [Google Scholar]
- 3.Hayes JP, Garland T., Jr 1995. The evolution of endothermy: testing the aerobic capacity model. Evolution 49, 836–847. ( 10.2307/2410407) [DOI] [PubMed] [Google Scholar]
- 4.Ruben JA. 1995. The evolution of endothermy in mammals and birds: from physiology to fossils. Annu. Rev. Physiol. 57, 69–95. ( 10.1146/annurev.ph.57.030195.000441) [DOI] [PubMed] [Google Scholar]
- 5.Farmer CG. 2000. Parental care: the key to understanding endothermy and other convergent features in birds and mammals. Am. Nat. 155, 326–334. ( 10.1086/303323) [DOI] [PubMed] [Google Scholar]
- 6.Farmer CG. 2003. Reproduction: the adaptive significance of endothermy. Am. Nat. 162, 826–840. ( 10.1086/380922) [DOI] [PubMed] [Google Scholar]
- 7.Koteja P. 2000. Energy assimilation, parental care, and the evolution of endothermy. Proc. R. Soc. Lond. B 267, 479–484. ( 10.1098/rspb.2000.1025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koteja P. 2004. The evolution of concepts on the evolution of endothermy in birds and mammals. Physiol. Biochem. Zool. 77, 1043–1050. ( 10.1086/423741) [DOI] [PubMed] [Google Scholar]
- 9.Angilletta MJ, Sears MW. 2003. Is parental care the key to understanding endothermy in birds and mammals? Am. Nat. 162, 821–825. ( 10.1086/380921) [DOI] [PubMed] [Google Scholar]
- 10.Hillenius WJ, Ruben JA. 2004. The evolution of endothermy in terrestrial vertebrates: Who? When? Why? Physiol. Biochem. Zool. 77, 1019–1042. ( 10.1086/425185) [DOI] [PubMed] [Google Scholar]
- 11.Bergman A, Casadevall A. 2010. Mammalian endothermy optimally restricts fungi and metabolic costs. mBio 1, e00212 ( 10.1128/mBio.00212-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clarke A, Pörtner HO. 2010. Temperature, metabolic power and the evolution of endothermy. Biol. Rev. 85, 703–727. ( 10.1111/j.1469-185X.2010.00122.x) [DOI] [PubMed] [Google Scholar]
- 13.Nespolo RF, Bacigalupe LD, Figueroa CC, Koteja P, Opazo JC. 2011. Using new tools to solve an old problem: the evolution of endothermy in vertebrates. Trends Ecol. Evol. 26, 414–423. ( 10.1016/j.tree.2011.04.004) [DOI] [PubMed] [Google Scholar]
- 14.Clavijo-Baque S, Bozinovic F. 2012. Testing the fitness consequences of the thermoregulatory and parental care models for the origin of endothermy. PLoS ONE 7, e37069 ( 10.1371/journal.pone.0037069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lovegrove BG. 2012. The evolution of endothermy in Cenozoic mammals: a plesiomorphic–apomorphic continuum. Biol. Rev. 87, 128–162. ( 10.1111/j.1469-185X.2011.00188.x) [DOI] [PubMed] [Google Scholar]
- 16.Gerkema MP, Davies WIL, Foster RG, Menaker M, Hut RA. 2013. The nocturnal bottleneck and the evolution of activity patterns in mammals. Proc. R. Soc. B 280, 20130508 ( 10.1098/rspb.2013.0508) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wone BWM, Madsen P, Donovan ER, Labocha MK, Sears MW, Downs CJ, Sorensen DA, Hayes JP. 2015. A strong response to selection on mass-independent maximal metabolic rate without a correlated response in basal metabolic rate. Heredity 114, 419–427. ( 10.1038/hdy.2014.122) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett AF, Ruben JA. 1979. Endothermy and activity in vertebrates. Science 206, 649–654. ( 10.1126/science.493968) [DOI] [PubMed] [Google Scholar]
- 19.Hayes JP. 2010. Metabolic rates, genetic constraints, and the evolution of endothermy. J. Evol. Biol. 23, 1868–1877. ( 10.1111/j.1420-9101.2010.02053.x) [DOI] [PubMed] [Google Scholar]
- 20.Nespolo RF, Roff DA. 2014. Testing the aerobic model for the evolution of endothermy: implications of using present correlations to infer past evolution. Am. Nat. 183, 74–83. ( 10.1086/674093) [DOI] [PubMed] [Google Scholar]
- 21.McNab BK. 1986. The influence of food habits on the energetics of eutherian mammals. Ecol. Monogr. 56, 1–19. ( 10.2307/2937268) [DOI] [Google Scholar]
- 22.Elgar MA, Harvey PH. 1987. Basal metabolic rates in mammals: allometry, phylogeny and ecology. Funct. Ecol. 1, 25–36. ( 10.2307/2389354) [DOI] [Google Scholar]
- 23.Koteja P, Weiner J. 1993. Mice, voles and hamsters: metabolic rates and adaptive strategies in muroid rodents. Oikos 66, 505–514. ( 10.2307/3544946) [DOI] [Google Scholar]
- 24.Cruz-Neto AP, Garland T, Jr, Abe AS. 2001. Diet, phylogeny, and basal metabolic rate in phyllostomid bats. Zoology 104, 49–58. ( 10.1078/0944-2006-00006) [DOI] [PubMed] [Google Scholar]
- 25.Muñoz-Garcia A, Williams JB. 2005. Basal metabolic rate in carnivores is associated with diet after controlling for phylogeny. Physiol. Biochem. Zool. 78, 1039–1056. ( 10.1086/432852) [DOI] [PubMed] [Google Scholar]
- 26.Naya DE, Spangenberg L, Naya H, Bozinovic F. 2013. How does evolutionary variation in basal metabolic rates arise? A statistical assessment and a mechanistic model. Evolution 67, 1463–1476. ( 10.1111/evo.12042) [DOI] [PubMed] [Google Scholar]
- 27.White CR, Kearney MR. 2013. Determinants of inter-specific variation in basal metabolic rate. J. Comp. Physiol. B 183, 1–26. ( 10.1007/s00360-012-0676-5) [DOI] [PubMed] [Google Scholar]
- 28.Clarke A, Rothery P, Isaac NJB. 2010. Scaling of basal metabolic rate with body mass and temperature in mammals. J. Anim. Ecol. 79, 610–619. ( 10.1111/j.1365-2656.2010.01672.x) [DOI] [PubMed] [Google Scholar]
- 29.Clarke A, O'Connor AI. 2014. Diet and body temperature in mammals and birds. Glob. Ecol. Biogeogr. 23, 1000–1008. ( 10.1111/geb.12185) [DOI] [Google Scholar]
- 30.Dohm MR, Hayes JP, Garland T., Jr 2001. The quantitative genetics of maximal and basal rates of oxygen consumption in mice. Genetics 159, 267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nespolo RF, Bacigalupe LD, Bozinovic F. 2003. Heritability of energetics in a wild mammal, the leaf-eared mouse (Phyllotis darwini). Evolution 57, 1679–1688. ( 10.1111/j.0014-3820.2003.tb00373.x) [DOI] [PubMed] [Google Scholar]
- 32.Sadowska ET, Labocha MK, Baliga K, Stanisz A, Wróblewska AK, Jagusik W, Koteja P. 2005. Genetic correlations between basal and maximum metabolic rates in a wild rodent: consequences for evolution of endothermy. Evolution 59, 672–681. ( 10.1111/j.0014-3820.2005.tb01025.x) [DOI] [PubMed] [Google Scholar]
- 33.Sadowska ET, Baliga-Klimczyk K, Labocha MK, Koteja P. 2009. Genetic correlations in a wild rodent: grass-eaters and fast-growers evolve higher basal metabolic rates. Evolution 63, 1530–1539. ( 10.1111/j.1558-5646.2009.00641.x) [DOI] [PubMed] [Google Scholar]
- 34.Wone B, Sears MW, Labocha MK, Donovan ER, Hayes JP. 2009. Genetic variances and covariances of aerobic metabolic rates in laboratory mice. Proc. R. Soc. B 276, 3695–3704. ( 10.1098/rspb.2009.0980) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Książek A, Konarzewski M, Łapo IB. 2004. Anatomic and energetic correlates of divergent selection for BMR in laboratory mice. Physiol. Biochem. Zool. 77, 890–899. ( 10.1086/425190) [DOI] [PubMed] [Google Scholar]
- 36.Kane SL, Garland T, Jr, Carter PA. 2008. Basal metabolic rate of aged mice is affected by random genetic drift but not by selective breeding for high early-age locomotor activity or chronic wheel access. Physiol. Biochem. Zool. 81, 288–300. ( 10.1086/587093) [DOI] [PubMed] [Google Scholar]
- 37.Gębczyński AK, Konarzewski M. 2009. Locomotor activity of mice divergently selected for basal metabolic rate: a test of hypotheses on the evolution of endothermy. J. Evol. Biol. 22, 1212–1220. ( 10.1111/j.1420-9101.2009.01734.x) [DOI] [PubMed] [Google Scholar]
- 38.Gębczyński AK, Konarzewski M. 2009. Metabolic correlates of selection on aerobic capacity in laboratory mice: a test of the model for the evolution of endothermy. J. Exp. Biol. 212, 2872–2878. ( 10.1242/jeb.030874) [DOI] [PubMed] [Google Scholar]
- 39.Gębczyński AK, Konarzewski M. 2011. Effects of oxygen availability on maximum aerobic performance in Mus musculus selected for basal metabolic rate or aerobic capacity. J. Exp. Biol. 214, 1714–1720. ( 10.1242/jeb.051680) [DOI] [PubMed] [Google Scholar]
- 40.Sadowska J, Gębczyński AK, Konarzewski M. 2013. Basal metabolic rate is positively correlated with parental investment in laboratory mice. Proc. R. Soc. B 280, 20122576 ( 10.1098/rspb.2012.2576) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maciak S, Bonda-Ostaszewska E, Czarnołęski M, Konarzewski M, Kozłowski J. 2014. Mice divergently selected for high and low basal metabolic rates evolved different cell size and organ mass. J. Evol. Biol. 27, 478–487. ( 10.1111/jeb.12306) [DOI] [PubMed] [Google Scholar]
- 42.Hayes JP. 2014. Present-day genetic correlations and testing the aerobic capacity model. Am. Nat. 184, 802–803. ( 10.1086/678452) [DOI] [PubMed] [Google Scholar]
- 43.Nespolo RF, Roff DA. 2014. Model falsification, quantitative genetics, and the evolution of endothermy: are we choosing the right tool? (A reply to Hayes). Am. Nat. 184, 804–805. ( 10.1086/678453) [DOI] [PubMed] [Google Scholar]
- 44.Sadowska ET, Baliga-Klimczyk K, Chrząścik KM, Koteja P. 2008. Laboratory model of adaptive radiation: a selection experiment in the bank vole. Physiol. Biochem. Zool. 81, 627–640. ( 10.1086/590164) [DOI] [PubMed] [Google Scholar]
- 45.Gammie SC, Hasen NS, Rhodes JS, Girard I, Garland T., Jr 2003. Predatory aggression, but not maternal or intermale aggression, is associated with high voluntary wheel-running behavior in mice. Horm. Behav. 44, 209–221. ( 10.1016/S0018-506X(03)00140-5) [DOI] [PubMed] [Google Scholar]
- 46.Bennett AF. 1991. The evolution of activity capacity. J. Exp. Biol. 160, 1–23. [DOI] [PubMed] [Google Scholar]
- 47.Henderson ND. 1997. Spurious associations in unreplicated selected lines. Behav. Genet. 27, 145–154. ( 10.1023/A:1025689425738) [DOI] [PubMed] [Google Scholar]
- 48.Labocha MK, Sadowska ET, Baliga K, Semer AK, Koteja P. 2004. Individual variation and repeatability of basal metabolism in the bank vole, Clethrionomys glareolus. Proc. R. Soc. Lond. B 271, 367–372. ( 10.1098/rspb.2003.2612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Górecki A. 1968. Metabolic rate and energy budget in the bank vole. Acta Theriol. 13, 341–365. ( 10.4098/AT.arch.68-20) [DOI] [Google Scholar]
- 50.Lynch M, Walsh JB. 1998. Genetics and analysis of quantitative traits. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 51.Babik W, Stuglik M, Qi W, Kuenzli M, Kuduk K, Koteja P, Radwan J. 2010. Heart transcriptome of the bank vole (Myodes glareolus): towards understanding the evolutionary variation in metabolic rate. BMC Genomics 11, 390 ( 10.1186/1471-2164-11-390) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Konczal M, Babik W, Radwan J, Sadowska ET, Koteja P. 2015. Initial molecular-level response to artificial selection for increased aerobic metabolism occurs primarily through changes in gene expression. Mol. Biol. Evol. ( 10.1093/molbev/msv038) [DOI] [PubMed] [Google Scholar]
- 53.Ołdakowski Ł, Piotrowska Ż, Chrząścik KM, Sadowska ET, Koteja P, Taylor JR. 2012. Is reproduction costly? No increase of oxidative damage in breeding bank voles. J. Exp. Biol. 215, 1799–1805. ( 10.1242/jeb.068452) [DOI] [PubMed] [Google Scholar]
- 54.Chrząścik KM, Sadowska ET, Rudolf A, Koteja P. 2014. Learning ability in bank voles selected for high aerobic metabolism, predatory behavior and herbivorous capability. Physiol. Behav. 135, 143–151. ( 10.1016/j.physbeh.2014.06.007) [DOI] [PubMed] [Google Scholar]
- 55.Stawski C, Koteja P, Sadowska ET, Jefimow M, Wojciechowski MS. 2015. Selection for high activity-related aerobic metabolism does not alter the capacity of non-shivering thermogenesis in bank voles. Comp. Biochem. Physiol. A 180, 51–56. ( 10.1016/j.cbpa.2014.11.003) [DOI] [PubMed] [Google Scholar]
- 56.Rezende EL, Chappell MA, Gomes FR, Malisch JL, Garland T., Jr 2005. Maximal metabolic rates during voluntary exercise, forced exercise, and cold exposure in house mice selectively bred for high wheel-running. J. Exp. Biol. 208, 2447–5248. ( 10.1242/jeb.01631) [DOI] [PubMed] [Google Scholar]
- 57.Rezende EL, Garland T, Jr, Chappell MA, Malisch JL, Gomes FR. 2006. Maximum aerobic performance in lines of Mus selected for high wheel-running activity: effects of selection, oxygen availability and the mini-muscle phenotype. J. Exp. Biol. 209, 115–127. ( 10.1242/jeb.01883) [DOI] [PubMed] [Google Scholar]
- 58.Garland T., Jr 2003. Selection experiments: an underutilized tool in biomechanics and organismal biology. In Vertebrate biomechanics and evolution (eds Bels VL, Gasc J-P, Casinos A.), pp. 23–56. Oxford, UK: BIOS Scientific Publishers. [Google Scholar]
- 59.Kemp TS. 2006. The origin of mammalian endothermy: a paradigm for the evolution of complex biological structure. Zool. J. Linn. Soc. Lond. 147, 473–488. ( 10.1111/j.1096-3642.2006.00226.x) [DOI] [Google Scholar]
- 60.Careau V, Wolak ME, Carter PA, Garland T. 2013. Limits to behavioral evolution: the quantitative genetics of a complex trait under directional selection. Evolution 67, 3102–3119. ( 10.1111/evo.12200) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw results are provided as an electronic supplementary material, appendix, and are available from the Dryad depository: http://dx.doi.org/10.5061/dryad.kh312.