Abstract
Polymorphic traits are central to many fundamental discoveries in evolution, yet why they are found in some species and not others remains poorly understood. We use the African genus Protea—within which more than 40% of species have co-occurring pink and white floral colour morphs—to ask whether convergent evolution and ecological similarity could explain the genus-wide pattern of polymorphism. First, we identified environmental correlates of pink morph frequency across 28 populations of four species. Second, we determined whether the same correlates could predict species-level polymorphism and monomorphism across 31 species. We found that pink morph frequency increased with elevation in Protea repens and three section Exsertae species, increased eastward in P. repens, and increased with seed predation intensity in section Exsertae. For cross-species comparisons, populations of monomorphic pink species occurred at higher elevations than populations of monomorphic white species, and 18 polymorphic species spanned broader elevational gradients than 13 monomorphic species. These results suggest that divergent selection along elevational clines has repeatedly favoured polymorphism, and that more uniform selection in altitudinally restricted species may promote colour monomorphism. Our findings are, to our knowledge, the first to link selection acting within species to the presence and absence of colour polymorphism at broader phylogenetic scales.
Keywords: cape floristic region, natural selection, elevational gradients, seed predation
1. Introduction
Phenotypic polymorphisms are compelling examples of evolution in action, inspiring many scientific discoveries over the past two centuries. The earliest studies used polymorphic traits as a tool to demonstrate patterns of inheritance ([1], reviewed in [2]) or to test models of evolutionary change [3]. More recent polymorphism research has focused on the genetic basis of polymorphisms, including the biochemical pathways and mutations involved [4–6], and how polymorphisms are maintained evolutionarily, e.g. negative frequency dependence, heterozygote advantage and temporally or spatially variable selection [7–9]. As informative as these studies are, however, they leave a fundamental question unanswered: why are some species polymorphic for a given trait, while others are not? With the exceptions of sexually dimorphic traits in animals [10] and dioecy or heterostyly in plants [11,12], there exist few empirical studies that explore the distribution of polymorphism versus monomorphism across species and landscapes.
Although the evolution of polymorphism at broader phylogenetic scales is relatively unstudied, a theoretical framework for understanding it is already in place. Several authors have pointed out, for example, that the existence of two different morphs is more likely if a species experiences divergent selection, under which different morphs are favoured at different times or places [13,14]. Monomorphism is expected when one morph consistently outperforms the other (e.g. no variation in selection), although it could also be the neutral outcome of genetic drift or founder effects, especially if populations and geographical range sizes are small. Taken more broadly, these observations suggest that the presence or the absence of polymorphism in different taxa could be the result of convergent evolution under similar ecological conditions, whether or not the underlying genetic mechanisms are similar. In this study, we test this possibility by asking whether the ecological covariates of morph frequency within species are consistent with the ecology and geography of polymorphic and monomorphic congeners.
Among all polymorphisms, few are as well-suited for such a test as those associated with flower colour. Flower colour polymorphisms are common (approx. 20% of British flora; [15]), well documented and have a long history of study. Pollinators are commonly implicated in maintenance of polymorphisms because their colour preferences may differ among guilds or individuals, over time or space, or with morph frequency [16–20]. Even so, pollinator-mediated selection on colour may be absent or consistently favouring one morph, which should act with genetic drift to eliminate polymorphism [21–23]. Alternatively, morph maintenance may involve seed predators, pathogens, or herbivores, either alone or together with pollinators [24–26]. Finally, abiotic gradients may also differentially favour colour morphs [9,27], possibly owing to pleiotropic effects of genes controlling production of red or blue pigments, which are mainly anthocyanins [28–31]. High levels of anthocyanins may be associated with increased tolerance for extreme temperatures, pests, infertile soils and ultraviolet (UV) radiation [15,32], but producing anthocyanin may also have metabolic costs [32].
Within the hyper-diverse flora of the Cape Floristic Region (CFR) of South Africa, several lineages have notably high rates of colour polymorphism. For example, approximately 40% of Protea (Proteaceae) and Erica (Ericaceae) species are polymorphic for floral colour [33,34]. In Erica, polymorphism may be associated with variation in pollinator assemblages and elevation [33,35], but it appears unrelated to avian behavioural responses within one sunbird-pollinated species [36]. Protea colour polymorphisms, by contrast, have been linked more strongly to intrinsic performance differences and pre-dispersal seed predators than to pollinators [34,37]. Using path analysis, Carlson & Holsinger [37] showed that white morphs of Protea aurea produced larger inflorescences than did pink morphs, and plants with larger inflorescences developed more ovules into seeds, but also experienced higher seed predation. Earlier work on four section Exsertae species, including P. aurea, showed that seed predators consumed more seed of white than pink morphs, but only in four of eight populations [34]. These studies suggest that variation in seed predation among sites may underlie some polymorphism in Protea, but the role of climate remains unknown.
In this study, we determine whether associations that could account for the evolutionary maintenance of polymorphism within species are consistent with patterns of polymorphism and monomorphism across a third of all Protea species. We meet this objective in two steps: (i) we perform a within-species analysis to determine which abiotic variables, biotic interactions, or traits are associated with population-level pink morph frequency in Protea repens, P. aurea, Protea lacticolor and Protea punctata; and (ii) we perform two sets of cross-species analyses to determine which abiotic variables are associated with being polymorphic versus monomorphic across 31 species, or with being monomorphic pink versus monomorphic white across 13 species. Any abiotic variables that show parallel, significant trends in both (i) and (ii) provide, to our knowledge, the first evidence that convergence on colour polymorphism across a plant genus may arise through the same environmental clines linked to morph frequency within species.
2. Material and methods
(a). Study species
The genus Protea is endemic to Africa, with over 60% of its 110–115 species restricted to the CFR of South Africa [38,39]. The CFR is characterized by nutrient-poor soils, steep elevational gradients and generally low annual rainfall (370 mm yr−1 on average; range of 20–3198 mm yr−1; [40]). Within the CFR, Protea are typically found in fire-dependent, semi-arid habitats called fynbos, and they are pollinated by sugarbirds and sunbirds or by non-flying mammals [41].
Because South African Protea species cover such diverse habitats and have such divergent pollination regimes, we restrict our analysis of trait and ecological correlates of monomorphism and polymorphism to CFR species that appear to be predominately bird pollinated (electronic supplementary material, table S1). By using only these species—nearly all being visited by the same six bird species—we are able to focus on potential agents of colour-related selection other than pollinators. Among bird-pollinated CFR species, there are at least five independent origins of pink monomorphism and at least four of white monomorphism, based on ancestral trait reconstruction using parsimony methods and a trimmed Valente et al. [39] phylogeny (J. E. Carlson & K. E. Holsinger 2014, unpublished data).
All 31 study species were deemed to be bird pollinated based primarily on observational studies. For less-studied species, we relied on Protea Atlas Project data (www.proteaatlas.org.za) or published pollination syndromes for the genus (listed in the electronic supplementary material, Appendix S1). Of the 84 excluded Protea species (electronic supplementary material, table S2), 38 were classified as CFR species not pollinated by birds, and 46 were non-cape species. The 31 included species are predominately broad-leaved, evergreen shrubs with upright or sprawling growth. They respond to fire by either resprouting or re-establishing from seeds stored above-ground in serotinous infructescences, henceforth seed heads. The seed heads, which accumulate on plants over many annual bouts of seed production, are also attacked by pre-dispersal seed predators. The larval insect communities that infest Protea seed heads belong to at least eight different families of beetles and moths [42,43].
All CFR bird-pollinated Protea share similar floral construction and patterns of floral pigmentation. Their inflorescences are typically large—up to 30 cm across—and consist of 50 to more than 500 flowers attached to a woody receptacle and subtended by showy bracts. Individual flowers are reduced, with a single ovule and long style, surrounded by a perianth tube with anthers fused inside. Individual flowers are protandrous, last at least 3–6 days, and have asynchronous development within inflorescences [44]. Seed set tends to be low in Protea, with higher rates in P. repens (27%; [45]) than in most congeners (e.g. section Exsertae approx. 14%; [34]).
Floral colour differences in Protea are driven predominately by the presence or the absence of pink anthocyanin pigment in perianths, styles or most prominently, involucral bracts. In plants lacking floral anthocyanin, bracts may be green or yellowish, but they are most often white. In plants producing floral anthocyanin, the light pink to magenta colour may be observed in some or all inflorescence parts. Although anthocyanin levels may vary continuously, our colour assessments are categorical, i.e. with or without floral anthocyanin (excluding hairs), henceforth pink or white. The long-standing horticultural use of Protea provides evidence for a heritable component to floral colour differences [38,44,46], although the genetics and pigments are as yet undescribed. In two other Proteaceae species, pink-flowered Banksia menziesii and scarlet-flowered Banksia coccinea have the same two glycosides of cyanidin but had a different glycoside of paeonidin [47].
(b). Within-species comparisons
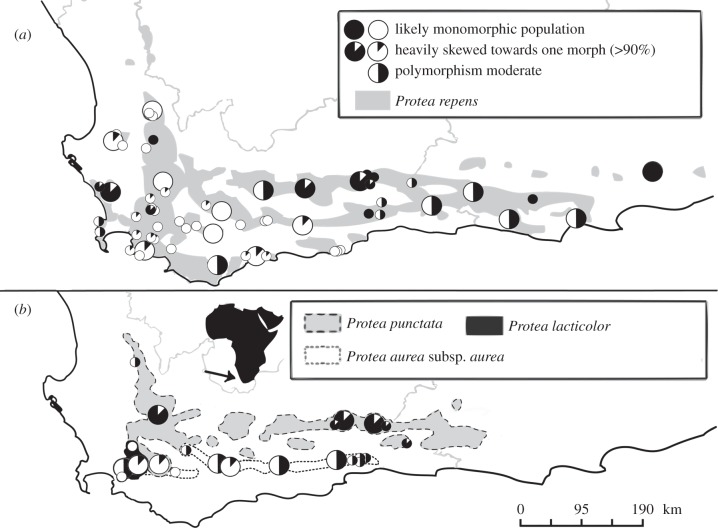
Our within-species study focuses on 28 populations of four Protea species: P. repens L., P. aurea (Burm. f.) Rourke subsp. aurea (henceforth P. aurea), P. lacticolor Salisb. and P. punctata Meisn. (figure 1). The latter three species are from section Exsertae, a monophyletic six-species clade [39]. Protea repens is from a two-species clade, but by itself has the largest geographical range of all Western Cape species. We sampled across each species' geographical range by selecting 18 populations of P. repens, four populations each of P. aurea and P. punctata, and only two of the geographically restricted P. lacticolor (figure 1). Most Exsertae populations were also used in previous studies [34,37], and sampling for this and previous studies was sometimes concurrent, spanning from 2009 to 2014.
Figure 1.
Species distributions overlaid with floral colour morph frequencies of (a) Protea repens and (b) three species in Protea section Exsertae. The large circles represent the floral colour morph frequency class for 28 populations, ranging from all plants with or without pink anthocyanin in floral parts (‘likely monomorphic’), to more than 90% belonging to the common morph (‘heavily skewed’), to a ‘moderate’ mix. The small circles represent ad hoc observations by J. Carlson and others (e.g. Protea Colour Survey participants on www.ispot.org.za; electronic supplementary material, table S3), which provide a geographical context for focal sites, but are not used in analyses.
We visited each population two times on average (range: 1–4) to collect data on floral traits, biotic interactions and colour morph frequency. For traits and biotic interactions, we sampled an average of 43 plants along transects through the population centre, with at least 1 m between each sampled plant for small populations (less than 100 plants), and 5 m for larger populations. For each study plant, we collected one to five seed heads (average 2.3) that had matured from the previous year and were at least six months old. After air-dried heads had opened, we counted and weighed all viable seeds, i.e. achenes containing seeds with endosperm. In P. repens, it was necessary to cut open three to five seeds to estimate the frequency of inviable woody seeds. The average number of viable seeds (based only on undamaged heads) was our estimate of per-head plant fecundity. As seeds were removed from each seed head, we also recorded whether any seed predation had occurred (i.e. either frass, damage or a larvae was observed). We used these data to calculate seed predation intensity, which was the number of sampled seed heads that were predated divided by the total sampled heads per population. Finally, we counted the number of flowers per inflorescence, i.e. inflorescence size, from photographs of empty seed heads.
We estimated frequency of pink and white morphs in each population using field-based surveys, which we ultimately used to classify each population into one of five morph frequency classes. We recorded frequency as classes because most populations were far too large and widespread to count all individuals, and in many sites, one colour morph was rare (less than 0.05 frequency). In these situations, estimates derived from transects or point counts could have been misleading [48], because the rare colour could be missed entirely. By scoring frequency categorically, we reduce our power to detect associations that are present, but we do not increase our chances of declaring an association present when it is not there (details in the electronic supplementary material, Appendix S2).
Contrasting colour morphs could be identified visually with relative ease, so we focused our sampling efforts on rare colour detection. We scanned each population from at least five peripheral points and along 200+ m transects through the population's centre. If all plants seen during 1–2 h of sampling were the same colour, the population was categorized as 100% pink or 100% white. If fewer than 10 contrasting morphs were seen in groups of approximately 100 plants, the population was categorized as heavily skewed white or heavily skewed pink. The remaining populations were categorized as having moderate frequencies of each.
Finally, we characterized the abiotic environment of each population, based on the site's GPS coordinates. Focal variables were longitude, elevation and mean annual precipitation (MAP). Elevation and MAP were from GIS layers by Schulze [40]. Longitude was included as a measure of distance eastward as well as a proxy for rainfall seasonality: western sites have winter-concentrated rain and eastern sites have more aseasonal rain. Electronic supplementary material, table S3 has site-specific data for dates visited, sample sizes, additional traits and environmental variables.
(c). Polymorphism definition and species-level classifications
A colour polymorphic Protea species is defined as having at least one population where both pink and white colour classes co-occur, i.e. some plants with anthocyanin in their inflorescences and some without it. We further stipulate that neither morph can be so rare across the species' entire distribution that spontaneous mutation alone could easily explain its presence (i.e. we require ‘true’ polymorphisms sensu [49]). Polymorphic Protea species often have both polymorphic and monomorphic populations (e.g. P. repens but not P. punctata in figure 1), but no Protea species, to our knowledge, has complete colour morph allopatry among monomorphic populations. Monomorphism is defined as having one of two conditions: all members of a species produce some floral anthocyanin or all members do not.
We classified each of the 31 species as polymorphic (n = 18), monomorphic pink (n = 8) or monomorphic white (n = 5) using data from several published sources [e.g. 38,46,50–52]. Variation in floral colour is well documented in Protea, owing to long-standing public, horticultural and scientific interest in the genus, including the 10 year Protea Atlas Project [53]. Published records were also confirmed in the field, with experts, and in iSpot Southern Africa, with approximately 2000 photographs of Protea since 2011 (www.ispot.org.za). Although nearly all species were easily assigned a polymorphism status, two monomorphic pink species—P. eximia and P. compacta—included extremely rare white morphs. For the former, only one white-flowered plant was recorded, and for the latter, white morphs were known at less than or equal to 1% in a few populations ([38], A. Rebelo 2011, personal communication). These rare mutant white morphs are probably not maintained by selection, so neither P. eximia nor P. compacta are classified as polymorphic.
(d). Cross-species comparisons
For our predictors of species-level polymorphism status, we selected seven variables that characterized species environmental means, breadths and geographical range sizes. We extracted the elevation, MAP and longitude for every locality record in the Protea Atlas database for each study species (76 554 total). Elevation, longitude and MAP were summarized as both the average and the data range for each species (maximum locality value minus minimum locality value). The geographical range of each species was approximated as the convex hull area around all Protea Atlas points for a given species, using the Albers Equal Area projection and R software (package alpha hull, alpha = 100; [54]). For the monomorphic species alone (8% of extracted records), we retained MAP, elevation and longitude for each record. We used the 4997 records for monomorphic pink species and 1353 records for monomorphic white species in separate monomorph-only analyses.
(e). Statistical analyses
(i). Within-species comparisons
We used path analysis on our within-species dataset to determine whether abiotic conditions, traits or biotic interactions were associated with pink morph frequency in P. repens or section Exsertae (figure 2). Although we used only abiotic effects in our cross-species comparisons, we included data on plant traits and biotic effects here to refine our understanding of within-species processes. Abiotic variables were elevation, longitude and MAP. Plant traits were per-head plant fecundity, inflorescence size and individual seed mass, each averaged across all measured individuals per population. The sole biotic variable was seed predation intensity. Each plant trait was a predictor of seed predation as well as of pink morph frequency class. Covariation between all pairs of traits was accounted for in the path model, as was covariation between pairs of abiotic variations.
Figure 2.
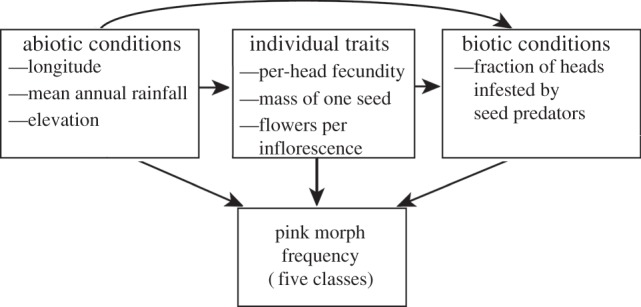
Structure of path diagram testing associations between floral colour polymorphism, traits and ecological conditions of Protea repens and three species from Protea section Exsertae.
We standardized each of the variables to a mean of zero and unit variance before fitting the path model in JAGS (http://mcmc-jags.sourceforge.net/). The regression of fecundity, inflorescence size and seed mass on abiotic variables treated the response variables as multivariate normal, with the mean determined by the regression and the covariance matrix estimated from the data. We modelled seed predation intensity as a simple multivariate regression on all preceding variables. We modelled pink morph frequency as ordered categorical variables using adjacent category logits [55]. With this approach, the logit for category i represents the log odds of that category relative to all higher categories. All regressions included a random intercept per species. We used a vague Wishart prior (5 d.f. with mean = identity matrix) for the covariance matrix of the multivariate normal, and we used independent normal priors (mean = 0, variance = 10) for each of the regression coefficients and species random effects. The Gelman–Rubin convergence diagnostic [56] was less than 1.003 for all parameters using five chains with a burn-in of 5000 iterations and a sample of 25 000 iterations (thinned by 5).
Although all regressions included separate random intercepts for all four species, separate slopes (or categorical transitions) were estimated only for P. repens and the three section Exsertae species combined. We grouped Exsertae species because they are closely related [39] and have similar pink morph correlates [34]. The selected model had an improved DIC (189), compared to one where P. repens and Exsertae species shared a common slope (314). For populations belonging to our lowest frequency category (less than 10%), the rare morph might simply reflect rare mutation or gene flow rather than polymorphism maintained by selection. To assess this possibility, we also analysed our data using a threshold of less than 25% rather than less than 10% for the lowest frequency class. Results were indistinguishable between models, so we report only the less than 10% results.
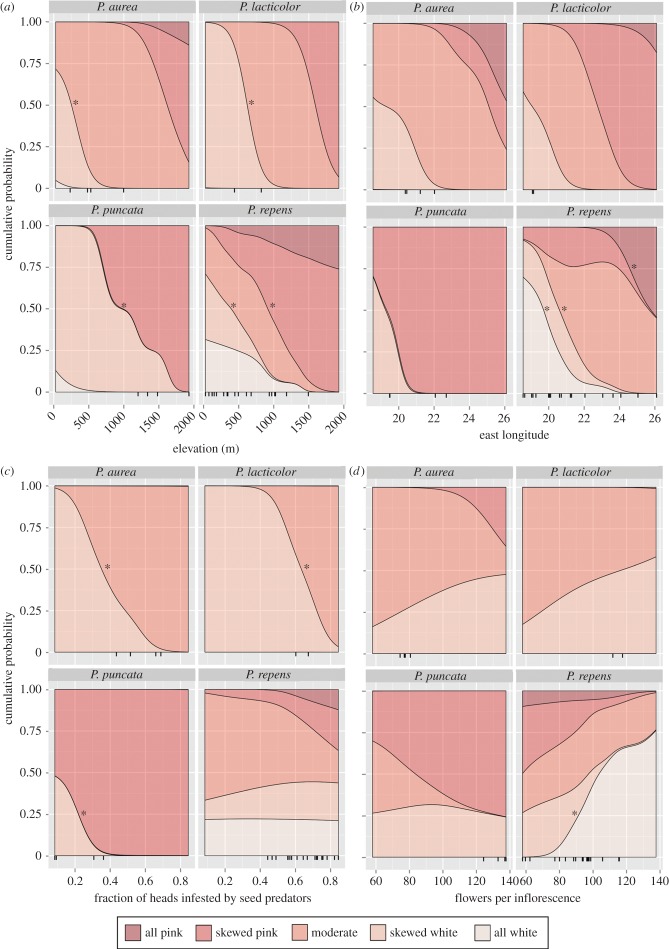
We summarize the results by plotting the probability that a population falls into one of the five pink frequency classes as a function of the three abiotic predictors, three traits and seed predation intensity. For interpreting these plots (e.g. figure 3a), the visual impression of darker shaded sections expanding from low to high elevation corresponds to an increasing posterior probability of pinker categories at high elevations. We summarize this as ‘the frequency of pink morphs increases with elevation’. Additional details on the post-stratification methodology used for creating these plots are in the electronic supplementary material, Appendix S3.
Figure 3.
The cumulative probability of each morph frequency class in four Protea species as it changes with (a) elevation, (b) east longitude, (c) fraction of seed heads infested with seed predators, and (d) the average number of flowers per inflorescence. Output is based on a path analysis with a separate slope for P. repens versus three Exsertae species and separate random intercepts for each species (see Material and methods). An asterisk represents a significant transition between all colour morph classes to the left of the asterisked line and the class to the right; asterisks for the three Exsertae species reflect a single significant result. Tick-marks underneath each subplot show the actual values for each population (n = 28). (Online version in colour.)
(ii). Cross-species comparisons: polymorphism versus monomorphism
For all 31 polymorphic and monomorphic species, we tested whether species-level polymorphism or monomorphism was associated with species range size, mean environment and environmental breadth. The seven covariates in the generalized linear model were the geographical range sizes plus means and ranges for elevation, MAP and longitude. We used a Bernoulli distribution with a logit link and accounted for variation due to evolutionary relationships among taxa using the molecular phylogeny of Valente et al. [39] and the MCMCglmm package in R (http://www.r-project.org/). Covariates were standardized to a mean of zero and unit variance before analysis. The Valente et al. [39] tree was smoothed using the default parameters for chronos() in the ape package in R. The prior for the residual variance was inverse gamma (1,1), and the prior for the variance of species random effects was inverse Wishart with 3 d.f. Covariation among abiotic variables was accounted for in a multiple-regression-type model. Again, we visually summarized our results using post-stratification. In both the within-species analysis and this analysis, we identified significant covariates as those with quantile-based 95% credible intervals (CI) that did not overlap zero.
(iii). Cross-species comparisons: monomorphic pink versus monomorphic white
In a separate pair of analyses using only the 13 monomorphic species, we tested whether population-level and species-level colour (pink versus white) could be predicted using three abiotic variables. For the first model, the binary response was whether a given population was from a monomorphic pink versus monomorphic white species, and the three predictors in the multiple regression were the population's elevation, longitude and MAP. To overcome non-independence of species and colour, we used a GEE-type marginal model with empirical variance estimates [57]. This procedure accounts for correlations within species without an explicit species effect. For the second model, the binary response was whether a monomorphic species was pink versus white, and the predictors were species-wide averages for elevation, longitude and MAP. Unlike all previous analyses, the two monomorph-only analyses were performed in PROC GLIMMIX of SAS v. 9.3, and their significance was tested with p-values based on F-statistics, as opposed to 95% CI. Finally, we qualitatively tested for extrapolation by comparing significant predictors of pink monomorphism to predictors of pink morph frequency that were significant within both P. repens and section Exsertae.
3. Results
(a). Within-species comparisons
Across the 28 study populations of four Protea species, our path analysis showed that the frequency of pink morphs increased with elevation, and for P. repens alone, with longitude east (figure 3a,b; 95% CI in the electronic supplementary material, table S4). Higher elevation populations of P. repens and section Exsertae were increasingly likely to have moderate pink morph frequencies as opposed to being heavily skewed towards or entirely white (figure 3a). The pattern was particularly striking in P. repens, for which the probability of being skewed white or all white was 70% at sea level but decreased to 10% at 1000 m elevation. Unlike elevation and longitude, the third abiotic variable (MAP) was not significantly associated with pink morph frequency (electronic supplementary material, table S4).
Pink morph frequency in the path analysis was also related to seed predation intensity in Exsertae and to inflorescence size in P. repens, but not to any other plant traits (figure 3c,d; electronic supplementary material, table S4). As predation intensity increased, Exsertae populations were more likely to be moderately pink as opposed to skewed white or all white. As the average size of inflorescences increased in P. repens, there was increasing probability that all inflorescences at that site would be white.
The three plant traits included in the path analysis were significantly related to additional variables, but only for P. repens (95% CI in electronic supplementary material, table S4). As the elevation of a population increased, plants tended to have larger inflorescences, more seeds per head, but lighter seeds. Finally, populations increasingly close to the western coast had heavier seeds, and populations that received less rainfall per year produced fewer seeds per head.
(b). Cross-species comparisons
Across the 31 bird-pollinated species, polymorphism was more likely than monomorphism in species with distributions spanning broad elevational gradients (figure 4; 95% CI = 48.21, 399.31). There was also evidence for a negative association of mean elevation with polymorphism, i.e. polymorphs occurred at lower elevations, on average, than did monomorphs (95% CI = −256.59, −17.29). Although there were five other covariates in the multiple-regression model, we detected no additional significant associations with species-level polymorphism.
Figure 4.
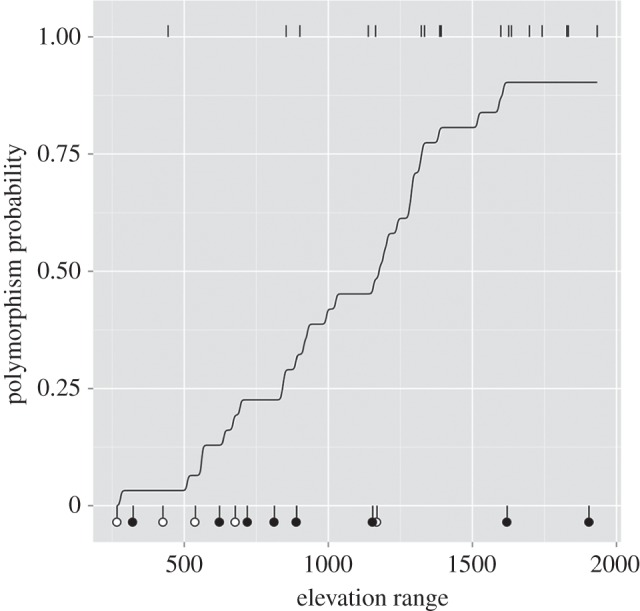
The probability that a Protea species is polymorphic versus monomorphic in relation to the range in elevation for all recorded populations of that species from the Protea Atlas Project. Tick-marks at top and bottom show the mean elevation range for 18 polymorphic species and 13 monomorphic species, respectively, with black circles for pink monomorphs and white circles for white monomorphs.
For monomorphic species alone, the population-level analysis revealed one significant abiotic predictor. The probability of a population being pink versus white was positively associated with elevation (F1,6334 = 4.3, p = 0.038). Two predictors in the population-level multiple regression were non-significant (longitude: F1,6334 = 0.782, p = 0.78; MAP: F1,6334 = 2.67, p = 0.102), as were all predictors in the species-level analysis (F1,9 < 1.74, p > 0.21).
(c). Extrapolation from within to across species
Two of the results from cross-species analyses were consistent with extrapolation of patterns detected within-species, and both involved elevation. In the within-species analysis, pink frequency increased with elevation for both P. repens and section Exsertae. If cross-species patterns were an extrapolation of those results, we would expect that: (i) monomorphic pink species should occur at higher elevations than monomorphic white species, and (ii) species spanning a wider range of elevations were more likely to be polymorphic than monomorphic. Both of these expectations were met in the cross-species analyses, although for (i), support came only at the population level and not the species level. No other abiotic variables showed significant trends within both P. repens and section Exsertae, and similarly, no other abiotic variables showed significant cross-species trends.
4. Discussion
Our study revealed that elevation was consistently associated with floral colour both within and among bird-pollinated Protea, suggesting that elevational clines have shaped the evolution of polymorphism repeatedly across the genus. Divergent selection at low versus high elevations may promote polymorphism, and directional selection on high-elevation (or low-elevation) species may maintain pink (or white) monomorphism. This study is, to our knowledge, the first to demonstrate parallels between local selection pressures and broad phylogenetic patterns in plant colour polymorphism. Our study is not the first, however, to document distribution of floral colour polymorphism and monomorphism across several plant species. Published estimates range from 22% of species from a clade within Aquilegia (from [58]) and 36% of species in Polemoniaceae [9].
Other studies of cross-species patterns take an approach different from the one used here but still point to divergent selection as favouring pigment polymorphism [e.g. 59,60]. Galeotti et al. [61] used phylogenetically independent contrasts to test for ecological correlates of plumage polymorphism in birds. Polymorphic bird species were more often associated with varied habitats and activity patterns that included both day and night, suggesting that evolutionary convergence on plumage polymorphism was promoted by within-species variation in the light conditions used (and a given pattern's crypticity under those settings). The only analogous study in plants compared collection records and pollination syndromes of 341 Erica species and concluded that polymorphic species were more strongly associated with bird pollination and large elevational gradients [33]. In Protea, we also find an association between polymorphism and elevational ranges, but in contrast to other studies, we provide more direct evidence for this pattern's underlying causes, by extrapolating from within-species patterns. The altitudinal increase in floral pigmentation shown here is rare, but not entirely unique to Protea [62]; in other species, however, morphs tend to be lighter at high elevations, which is a pattern most often attributed to pollinators [63–65].
(a). A widespread mechanism for maintaining colour polymorphism within species
The parallel elevational trends in pink morph frequency within and across Protea species could very well result from a common physiological mechanism. Such a mechanism might involve the harshness of high elevation sites, which would influence floral colour polymorphism directly or indirectly through trait pleiotropisms between floral and vegetative anthocyanins [e.g. 31,66]. Genotypes with increased vegetative anthocyanins may have an advantage when faced with abiotic stressors, because anthocyanins absorb some of the blue-green and UV wavelengths that contribute to photoinhibition, i.e. photosystem inefficiencies that are caused by high light but exacerbated by cold, drought or nutrient stress [32,67]. Indeed, several studies have found an association, albeit without direct evidence of the mechanism, between vegetative anthocyanins and increased tolerance of drought, cold, UV and nutrient deficiency [28,30,68].
If vegetative and floral anthocyanins are genetically correlated, as has been suggested for section Exsertae and several other unrelated species [31,34,69], harsh, high elevation conditions will probably favour pink floral morphs. White morphs may be favoured at milder, low elevation sites due to negative trait pleiotropisms with pigmentation or costs of anthocyanin production [32,34]. Warren & Mackenzie [15] found such a trade-off in five herbaceous polymorphic species from Britain, with anthocyanin-containing morphs having higher fitness under experimentally induced drought than those lacking anthocyanin. The fitness relationships reversed when plants were well watered. Arista et al. [27] found a similar pattern in Lysimachia arvensis, and the classic, long-term studies of Linanthus parryae suggest that environmentally variable natural selection contributes to maintenance of that blue-white polymorphism [9,68].
Elevational gradients also contribute to other aspects of phenotypic diversity in P. repens, as is evidenced by elevational trends in seed mass, fecundity and inflorescence size. These patterns could be a result of divergent selection, perhaps involving resource trade-offs, but they could also reflect environmental plasticity and differences in maternal condition along elevational clines. It is currently unknown for most floral traits other than colour whether within-species floral differences in Protea have a strong genetic component.
(b). Idiosyncratic mechanisms for maintaining colour polymorphism within species
Longitude and seed predation intensity were significantly associated with pink morph frequency, but unlike for elevation, the broad-scale implications of these relationships are less clear. In P. repens alone, pink morph frequency increased with east longitude. While rainfall seasonality varies dramatically along this axis, there is insufficient within-species evidence to suggest that this relationship should be genus-wide. Indeed, neither our analysis of environmental associations in 13 monomorphic species nor our analysis of monomorphism and polymorphism across 31 species showed a longitudinal association.
Similarly, we have evidence in Exsertae that pink morphs are more frequent in sites with heavy predation, yet such evidence is lacking for P. repens. A predation-related advantage for pink Exsertae morphs is plausible, given that in some sites, white morphs lost more seeds to pre-dispersal seed predators [34] or they had a higher proportion of seed heads infested owing to an indirect association with larger inflorescences [37]. Many other studies also find that the leaves or flowers of lighter pigmented morphs are more frequently consumed or serve as a more-beneficial food source ([24,26,70], but see [25,71]). Thus, spatial or temporal variation in insect communities [43,72] combined with intrinsic fecundity advantages of white morphs could cause pink morph abundance to increase only when predation is high, thereby favouring polymorphism within a species. Whether this explanation applies outside of Exsertae is unclear, however, and it is currently untestable due to lack of data.
(c). Extending mechanisms to cross-species patterns in colour polymorphism
Our results provide some support for convergent evolution in patterns of phenotypic polymorphism in a clade, and specifically, that species-level polymorphism could reflect an extension of altitudinally driven divergent selection within Protea species. We found that monomorphic species spanned smaller altitudinal ranges than did polymorphs, consistent with the idea that a species is more likely to be shaped by divergent pressures if it spans a broad range of environmental conditions. An association between species-level polymorphism and diversity in abiotic conditions was also predicted by Forsman et al. [14]. We also found that populations of monomorphic pink species were more frequent than populations of monomorphic white species at high elevations. A closer examination of population frequency with elevation (electronic supplementary material, figure S1), however, shows that pink monomorphs were commonly observed at both high and low elevations, whereas white monomorphs were mainly found at low elevations. The rarity of high elevation white monomorphs suggests that pigment production could be among a suite of traits that promotes population persistence and expansion in high elevation sites. When other polymorphic species are subject to harsh experimental conditions, anthocyanin-rich plants often outperform anthocyanin-deficient ones, as mentioned previously [15,27]. A powerful test of this hypothesis would require data from a wider range of polymorphic species in addition to experimental manipulations that test the relationship between pigmentation and survival.
In this study, we use parallel elevational effects observed within and across species as suggestive of a selection-driven cause for convergence, yet such correlations may also arise through other means. First, biotic and abiotic conditions currently associated with maintaining polymorphism may differ from those that a species experienced when it evolved polymorphism. Second, evolutionary transitions between pink and white may not occur at equal rates. Based on phylogenetic comparisons in a few well-studied lineages, floral anthocyanins are more likely to be lost within a lineage than to be gained [58,73]. Similarly, monomorphism should be easier to evolve from polymorphism than the inverse [12]. Even so, white monomorphs are relatively rare in Protea, and differing transition likelihoods cannot readily explain the parallel environmental associations observed both within and across Protea species.
Another caveat comes from the assumption that cross-species polymorphism patterns can have within-species origins, and that parallel pressures influence phenotypes in the same way repeatedly across smaller clades. For this to be possible, local responses to altitudinally driven selection must be fairly homogeneous across species, and when new species form, they must retain their polymorphism status and environmental associations, or shift them together when invading new sites. Although these assumptions are strengthened by parallels with ecological speciation [74], they ignore the genetic mechanisms underlying colour polymorphism, which may differ among species and constrain certain evolutionary outcomes. Different mutations can produce the same or similar pigment patterns in congeneric and even conspecific plants or animals, but each mutation may nevertheless have its own set of pleiotropic effects [29,75,76]. In plants, mutations that halt anthocyanin production may occur in structural genes for the flavonoid biochemical pathway or in transcription factor regions, with the latter presumed more tissue-specific and the former more deleterious for their plant-wide effects on the pathway by-products, including compounds that reduce physiological stress or herbivory [29]. Because we do not know whether different mutations are involved, we base our extrapolation purely on phenotypic selection. If white morphs are favoured at low altitudes and pink morphs are favoured at high altitudes, as suggested by our within-species analyses, we expect a species with a broad elevational range to include both white and pink morphs, regardless of the genetic mechanisms underlying trait differences.
In summary, the parallel biogeographic patterns in colour within several Protea species and across 31 bird-pollinated species suggest an important evolutionary role for pigmentation that varies with elevation at both narrow and broad phylogenetic scales. By extrapolating from the familiar intra-specific and species-by-species views of phenotypic polymorphisms, this study is, to our knowledge, the first to provide an explanation for why colour polymorphisms occur where they do in any plant lineage, using the charismatic genus Protea in a plant biodiversity hotspot.
Supplementary Material
Supplementary Material
Acknowledgements
We thank C. Adams for his tremendous assistance in the field. N. Mitchell, B. Tomb and A. Kowalski also contributed in the field or laboratory. Special thanks to the Protea Atlas Project (1991–2001) and to current iSpot Southern Africa contributors, especially those adding to the Protea Colour Survey. We are grateful to the South African National Biodiversity Institute at Kirstenbosch, and especially T. Rebelo and L. Nurrish for research space and logistical support. A. Wilson helped with climate data access and use. We thank the many reserve managers, municipalities and private landowners for access to and information about natural populations, especially P. Lane, P. Van Zyl, K. Shadung and G. Hill. Data were collected under Cape Nature permits AAA005–00125–0028, AAA005–00214–0028, AAA005–00224–0028 and Eastern Cape Province permit CRO 4/11 CR.
Data accessibility
Our raw data and code for analyses are publicly available at https://github.com/kholsinger/Protea-anthocyanin-polymorphsim.
Funding statement
This work was funded by the National Science Foundation (DEB-0716622 and DEB-1046328).
Author contributions
J.C. conceived of the study, coordinated the study, collected field data, researched and tabulated polymorphism status and pollinator types in Protea, participated in data analysis and drafted the manuscript; K.H. carried out most statistical analyses, participated in the studies’ conception and refinement and helped draft the manuscript. Both authors gave final approval for publication.
Competing interests
We have no competing interests.
References
- 1.Mendel JG. 1901. Experiments in hybridisation (1865). Trans. with an introduction by W. Bateson. J. R. Hortic. Soc. xxvi, 1–32. [Google Scholar]
- 2.Hoekstra HE. 2006. Genetics, development and evolution of adaptive pigmentation in vertebrates. Heredity 97, 222–234. ( 10.1038/sj.hdy.6800861) [DOI] [PubMed] [Google Scholar]
- 3.Wright S. 1943. An analysis of local variability of flower color in Linanthus parryae. Genetics 28, 139–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Streisfeld MA, Rausher MD. 2009. Altered trans-regulatory control of gene expression in multiple anthocyanin genes contributes to adaptive flower color evolution in Mimulus aurantiacus. Mol. Biol. Evol. 26, 433–444. ( 10.1093/molbev/msn268) [DOI] [PubMed] [Google Scholar]
- 5.McKinnon JS, Pierotti MER. 2010. Colour polymorphism and correlated characters: genetic mechanisms and evolution. Mol. Ecol. 19, 5101–5125. ( 10.1111/j.1365-294X.2010.04846.x) [DOI] [PubMed] [Google Scholar]
- 6.Takahashi T, Sota T, Hori M. 2013. Genetic basis of male colour dimorphism in a Lake Tanganyika cichlid fish. Mol. Ecol. 22, 3049–3060. ( 10.1111/mec.12120) [DOI] [PubMed] [Google Scholar]
- 7.Barrett JA. 1988. Frequency-dependent selection in plant fungal interactions. Phil. Trans. R. Soc. Lond. B 319, 473–483. ( 10.1098/rstb.1988.0060) [DOI] [Google Scholar]
- 8.Ågren J, Hellström F, Toräng P, Ehrlén J. 2013. Mutualists and antagonists drive among-population variation in selection and evolution of floral display in a perennial herb. Proc. Natl Acad. Sci. USA 110, 18 202–18 207. ( 10.1073/pnas.1301421110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schemske DW, Bierzychudek P. 2007. Spatial differentiation for flower color in the desert annual Linanthus parryae: was Wright right? Evolution 61, 2528–2543. ( 10.1111/j.1558-5646.2007.00219.x) [DOI] [PubMed] [Google Scholar]
- 10.Sanger TJ, Sherratt E, McGlothlin JW, Brodie ED, III, Losos JB, Abzhanhov A. 2013. Convergent evolution of sexual dimorphism in skull shape using distinct developmental strategies. Evolution 67, 2180–2193. ( 10.1111/evo.12100) [DOI] [PubMed] [Google Scholar]
- 11.Sakai AK, Weller SG, Wagner WL, Nepokroeff M, Culley TM. 2006. Adaptive radiation and evolution of breeding systems in Schiedea (Carophyllaceae), and endemic Hawaiian genus. Ann. Mo. Bot. Gard. 93, 49–63. ( 10.3417/0026-6493(2006)93[49:ARAEOB]2.0.CO;2) [DOI] [Google Scholar]
- 12.Sakai S, Wright SJ. 2008. Reproductive ecology of 21 coexisting Psychotria species (Rubiaceae): when is heterostyly lost? Biol. J. Linnean Soc. 93, 125–134. ( 10.1111/j.1095-8312.2007.00890.x) [DOI] [Google Scholar]
- 13.Gray SM, McKinnon JS. 2007. Linking color polymorphism maintenance and speciation. Trends Ecol. Evol. 22, 71–79. ( 10.1016/j.tree.2006.10.005) [DOI] [PubMed] [Google Scholar]
- 14.Forsman A, Ahnesjö J, Caesar S, Karlsson M. 2008. A model of ecological and evolutionary consequences of color polymorphism. Evolution 89, 34–40. ( 10.1890/07-0572.1) [DOI] [PubMed] [Google Scholar]
- 15.Warren J, Mackenzie S. 2001. Why are all colour combinations not equally represented as flower-colour polymorphisms? New Phytol. 151, 237–241. ( 10.1046/j.1469-8137.2001.00159.x) [DOI] [PubMed] [Google Scholar]
- 16.Levin DA, Watkins L. 1984. Assortative mating in Phlox. Heredity 53, 595–602. ( 10.1038/hdy.1984.117) [DOI] [Google Scholar]
- 17.Gigord LDB, MacNair MR, Smithson A. 2001. Negative frequency-dependent selection maintains a dramatic flower color polymorphism in the rewardless orchid Dactylorhiza sambucina (L.) Soo. Proc. Natl Acad. Sci. USA 98, 6253–6255. ( 10.1073/pnas.111162598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones KN, Reithel JS. 2001. Pollinator-mediated selection on a flower color polymorphism in experimental populations of Antirrhinum (Scrophulariaceae). Am. J. Bot. 88, 447–454. ( 10.2307/2657109) [DOI] [PubMed] [Google Scholar]
- 19.Eckhart VM, Rushing NS, Hart GM, Hansen JD. 2006. Frequency-dependent pollinator foraging in polymorphic Clarkia xantiana ssp. xantiana populations: implications for flower colour evolution and pollinator interactions. Oikos 112, 412–421. ( 10.1111/j.0030-1299.2006.14289.x) [DOI] [Google Scholar]
- 20.Newman E, Anderson B, Johnson SD. 2012. Flower colour adaptation in a mimetic orchid. Proc. R. Soc. B 279, 2309–2313. ( 10.1098/rspb.2011.2375) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waser NM, Price MV. 1981. Pollinator choice and stabilizing selection for flower color in Delphinium nelsonii . Evolution 35, 376–390. ( 10.2307/2407846) [DOI] [PubMed] [Google Scholar]
- 22.Epperson BK, Clegg MT. 1987. Frequency-dependent variation for outcrossing rate among flower-color morphs of Ipomoea purpurea. Evolution 41, 1302–1311. ( 10.2307/2409095) [DOI] [PubMed] [Google Scholar]
- 23.Campbell DR, Bischoff M, Lord JM, Robertson AW. 2012. Where have all the blue flowers gone: pollinator responses and selection on flower colour in New Zealand Wahlenbergia albomarginata. J. Evol. Biol. 25, 352–364. ( 10.1111/j.1420-9101.2011.02430.x) [DOI] [PubMed] [Google Scholar]
- 24.Frey FM. 2004. Opposing natural selection from herbivores and pathogens may maintain floral-color variation in Claytonia virginica (Portulacaceae). Evolution 58, 2426–2437. ( 10.1111/j.0014-3820.2004.tb00872.x) [DOI] [PubMed] [Google Scholar]
- 25.de Jager ML, Ellis AG. 2014. Floral polymorphism and the fitness implications of attracting pollinating and florivorous insects. Ann. Bot. 113, 213–222. ( 10.1093/aob/mct189) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCall AC, Murphy SJ, Venner C, Brown M. 2013. Florivores prefer white versus pink petal color morphs in wild radish, Raphanus sativus. Oecologia 172, 189–195. ( 10.1007/s00442-012-2480-z) [DOI] [PubMed] [Google Scholar]
- 27.Arista M, Talavera M, Berjano R, Ortiz PL. 2013. Abiotic factors may explain the geographical distribution of flower colour morphs and the maintenance of colour polymorphism in the scarlet pimpernel. J. Ecol. 101, 1613–1622. ( 10.1111/1365-2745.12151) [DOI] [Google Scholar]
- 28.Coberly LC, Rausher MD. 2003. Analysis of a chalcone synthase mutant in Ipomoea purpurea reveals a novel function for flavonoids: amelioration of heat stress. Mol. Ecol. 12, 1113–1124. ( 10.1046/j.1365-294X.2003.01786.x) [DOI] [PubMed] [Google Scholar]
- 29.Coberly LC, Rausher MD. 2008. Pleiotropic effects of an allele producing white flowers in Ipomoea purpurea. Evolution 62, 1076–1085. ( 10.1111/j.1558-5646.2008.00355.x) [DOI] [PubMed] [Google Scholar]
- 30.Dick CA, Buenrostro J, Butler T, Carlson ML, Kliebenstein DJ, Whittall JB. 2011. Arctic mustard flower color polymorphism controlled by petal-specific downregulation at the threshold of the anthocyanin biosynthetic pathway. PLoS ONE 6, e18230 ( 10.1371/journal.pone.0018230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armbruster WS. 2002. Can indirect selection and genetic context contribute to trait diversification? A transition-probability study of blossom-colour evolution in two genera. J. Evol. Biol. 15, 468–486. ( 10.1046/j.1420-9101.2002.00399.x) [DOI] [Google Scholar]
- 32.Steyn WJ, Wand SJE, Holcroft DM, Jacobs G. 2002. Anthocyanins in vegetative tissues: a proposed unified function in photoprotection. New Phytol. 155, 349–361. ( 10.1046/j.1469-8137.2002.00482.x) [DOI] [PubMed] [Google Scholar]
- 33.Rebelo AG, Siegfried WR. 1985. Colour and size of flowers in relation to pollination of Erica species. Oecologia 65, 584–590. ( 10.1007/BF00379677) [DOI] [PubMed] [Google Scholar]
- 34.Carlson JE, Holsinger KE. 2010. Natural selection on inflorescence color polymorphisms in wild Protea populations: the role of pollinators, seed predators and inter-trait correlations. Am. J. Bot. 97, 934–944. ( 10.3732/ajb.0900348) [DOI] [PubMed] [Google Scholar]
- 35.Van der Niet T, Pirie MD, Shuttleworth A, Johnson SD, Midgley JJ. 2014. Do pollinator distributions underlie the evolution of pollination ecotypes in the Cape shrub Erica plukenetii? Ann. Bot. 113, 301–316. ( 10.1093/aob/mct193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heystek A, Geerts S, Barnard P, Pauw A. 2014. Pink flower preference in sunbirds does not translate into plant fitness differences in a polymorphic Erica species. Evol. Ecol. 28, 457–470. ( 10.1007/s10682-014-9693-z) [DOI] [Google Scholar]
- 37.Carlson JE, Holsinger KE. 2013. Direct and indirect selection on floral pigmentation by pollinators and seed predators in a color polymorphic South African shrub. Oecologia 171, 905–919. ( 10.1007/s00442-012-2453-2) [DOI] [PubMed] [Google Scholar]
- 38.Rourke JP. 1980. The proteas of Southern Africa. Cape Town, South Africa: Prunell. [Google Scholar]
- 39.Valente LM, Reeves G, Schnitzler J, Mason IP, Fay MF, Rebelo AG, Chase MW, Barraclough TG. 2010. Diversification in the African genus Protea (Proteaceae) in the cape biodiversity hotspot and beyond: equal rates in different biomes. Evolution 64, 745–760. ( 10.1111/j.1558-5646.2009.00856.x) [DOI] [PubMed] [Google Scholar]
- 40.Schulze RE. 2007. South African atlas of climatology and agrohydrology: WRC Report 1489/1/06. Pretoria RSA, Water Research Commission.
- 41.Collins BG, Rebelo AG. 1987. Pollination biology of the Proteaceae in Australia and southern Africa. Aust. J. Ecol. 12, 387–421. ( 10.1111/j.1442-9993.1987.tb00958.x) [DOI] [Google Scholar]
- 42.Coetzee JH, Giliomee JH. 1987. Borers and other inhabitants of the inflorescences and infructescences of Protea repens in the Western Cape. Phytophylactica 19, 1–6. [Google Scholar]
- 43.Wright MG, Samways MJ. 1999. Plant characteristics determine insect borer assemblages on Protea species in the Cape fynbos, and importance for conservation management. Biodivers. Conserv. 8, 1089–1100. ( 10.1023/A:1008880618304) [DOI] [Google Scholar]
- 44.Coetzee JH, Littlejohn GM. 2007. Protea: a floricultural crop from the cape floristic kingdom. Scr. Horticul. 5, 77–112. [Google Scholar]
- 45.Coetzee JH, Giliomee JH. 1987. Seed predation and survival in the infructescences of Protea repens (Proteaceae). South Afr. J. Bot. 53, 61–64. [Google Scholar]
- 46.Vogts MM. 1982. South Africa's Proteaceae: know them and grow them. Melbourne, Australia: Proteaflora Enterprises Pty Ltd. [Google Scholar]
- 47.Asenstorfer RE, Morgan AL, Hayasaka Y, Sedgley M, Jones GP. 2003. Purification of anthocyanins from species of Banksia and Acacia using high-voltage paper electrophoresis. Phytochem. Anal. 14, 150–154. ( 10.1002/pca.696) [DOI] [PubMed] [Google Scholar]
- 48.Dixon PM, Ellison AM, Gotelli NJ. 2005. Improving the precision of estimates of the frequency of rare events. Ecology 86, 1114–1123. ( 10.1890/04-0601) [DOI] [Google Scholar]
- 49.Ford EB. 1945. Polymorphism. Biol. Rev. 20, 73–88. ( 10.1111/j.1469-185X.1945.tb00315.x) [DOI] [Google Scholar]
- 50.Goldblatt P, Manning JC. 2000. Cape plants: a conspectus of the vascular plants of the Cape Region of South Africa. Cape Town, South Africa: National Botanical Institute of South Africa. [Google Scholar]
- 51.Bean A, Johns A. 2005. Stellenbosch to Hermanus: South African wildflower guide 5. Cape Town, South Africa: Botanical Society of South Africa. [Google Scholar]
- 52.Rebelo AG. 2001. Proteas: a field guide to the Proteas of southern Africa, 2nd edn Vlaeberg, South Africa: Fernwood Press. [Google Scholar]
- 53.Rebelo AG. 2013. Protea Atlas Project See http://www.proteaatlas.org.za/index.htm.
- 54.Pateiro-López B, Rodríguez-Casal A. 2010. Generalizing the convex hull of a sample: the R package alphahull. J. Stat. Softw. 34, 1–28. [Google Scholar]
- 55.Agresti A. 2012. Categorical data analysis, 3rd edn New York, NY: John Wiley & Sons. [Google Scholar]
- 56.Gelman A, Rubin DB. 1992. Inference from iterative simulation using multiple sequences. Stat. Sci. 7, 457–472. ( 10.1214/ss/1177011136) [DOI] [Google Scholar]
- 57.SAS Institute Inc. 2011. The GLIMMIX procedure. In SAS/STAT 9.3 User's Guide. Cary, NC: SAS Institute Inc. [Google Scholar]
- 58.Whittall JB, Voelckel C, Kliebenstein DJ, Hodges SA. 2006. Convergence, constraint and the role of gene expression during adaptive radiation: floral anthocyanins in Aquilegia. Mol. Ecol. 15, 4645–4657. ( 10.1111/j.1365-294X.2006.03114.x) [DOI] [PubMed] [Google Scholar]
- 59.Mallet J, Joron M. 1999. Evolution of diversity in warning color and mimicry: polymorphisms, shifting balance, and speciation. Annu. Rev. Ecol. Syst. 30, 201–233. ( 10.1146/annurev.ecolsys.30.1.201) [DOI] [Google Scholar]
- 60.Seehausen O, Mayhew PJ, Van Alphen JJM. 1999. Evolution of colour patterns in East African cichlid fish. J. Evol. Biol. 12, 514–534. ( 10.1046/j.1420-9101.1999.00055.x) [DOI] [Google Scholar]
- 61.Galeotti P, Rubolini D, Dunn PO, Fasola M. 2003. Colour polymorphism in birds: causes and functions. J. Evol. Biol. 16, 635–646. ( 10.1046/j.1420-9101.2003.00569.x) [DOI] [PubMed] [Google Scholar]
- 62.Galen C, Kevan PG. 1980. Scent and color, floral polymorphisms and pollination biology in Polemonium viscosum Nutt. Am. Midland Nat. 104, 281–289. ( 10.2307/2424867) [DOI] [Google Scholar]
- 63.Mogford DJ. 1978. Pollination and flower colour polymorphism with special reference to Cirsium palustre. In The pollination of flowers by insects (ed. Richards AJ.), pp. 191–199. London, UK: Academic Press. [Google Scholar]
- 64.Shipunov A, Kosenko Y, Volkova P. 2011. Floral polymorphism in common primrose (Primula vulgaris Huds., Primulaceae) of the Northeastern Black Sea coast. Plant Syst. Evol. 296, 167–178. ( 10.1007/s00606-011-0484-5) [DOI] [Google Scholar]
- 65.Sun M, Gross K, Schiestl FP. 2014. Floral adaptation to local pollinator guilds in a terrestrial orchid. Ann. Bot. 113, 289–300. ( 10.1093/aob/mct219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Strauss SY, Whittall JB. 2007. Non-pollinating agents of selection on floral traits. In Ecology and evolution of flowers (eds Harder LD, Barrett SCH.), pp. 120–138. Oxford, USA: Oxford University Press. [Google Scholar]
- 67.Close D, Beadle C. 2003. The ecophysiology of foliar anthocyanin. Bot. Rev. 69, 149–161. ( 10.1663/0006-8101(2003)069[0149:TEOFA]2.0.CO;2) [DOI] [Google Scholar]
- 68.Schemske DW, Bierzychudek P. 2001. Evolution of flower color in the desert annual Linanthus parryae: Wright revisited. Evolution 55, 1269–1282. ( 10.1111/j.0014-3820.2001.tb00650.x) [DOI] [PubMed] [Google Scholar]
- 69.Schoen DJ, Giannasi DE, Ennos RA, Clegg MT. 1984. Stem color and pleiotropy of genes determining flower color in the common morning glory. J. Hered. 75, 113–116. [Google Scholar]
- 70.Irwin RE, Strauss SY, Storz S, Emerson A, Guibert G. 2003. The role of herbivores in the maintenance of a flower color polymorphism in wild radish. Ecology 84, 1733–1743. ( 10.1890/0012-9658(2003)084[1733:TROHIT]2.0.CO;2) [DOI] [Google Scholar]
- 71.Fineblum WL, Rausher MD. 1997. Do floral pigmentation genes also influence resistance to enemies? The W locus in Ipomoea purpurea. Ecology 78, 1646–1654. ( 10.1890/0012-9658(1997)078[1646:DFPGAI]2.0.CO;2) [DOI] [Google Scholar]
- 72.Roets F, Dreyer LL, Geertsema H, Crous PW. 2006. Arthropod communities in Proteaceae infructescences: seasonal variation and the influence of infructescence phenology. Afr. Entomol. 14, 257–265. [Google Scholar]
- 73.Rausher MD. 2008. Evolutionary transitions in floral color. Int. J. Plant. Sci. 169, 7–21. ( 10.1086/523358) [DOI] [Google Scholar]
- 74.Schluter D. 2009. Evidence for ecological speciation and its alternative. Science 323, 737–741. ( 10.1126/science.1160006) [DOI] [PubMed] [Google Scholar]
- 75.Wittkopp PJ, Williams BL, Selegue JE, Carroll SB. 2003. Drosophila pigmentation evolution: divergent genotypes underlying convergent phenotypes. Proc. Natl Acad. Sci. USA 100, 1808–1813. ( 10.1073/pnas.0336368100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith SA, Rausher MD. 2011. Gene loss and parallel evolution contribute to species differences in flower color. Mol. Biol. Evol. 28, 2799–2810. ( 10.1093/molbev/msr109) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Our raw data and code for analyses are publicly available at https://github.com/kholsinger/Protea-anthocyanin-polymorphsim.