Abstract
Artemisia is the most important outdoor allergen throughout China. It can cause allergic rhinitis, asthma, or both of them. Since it was verified as an allergenic pollen in 1960, it was identified two times in the Chinese National Pollen Survey (1984, 2009). The first oral immunotherapy double-blinded trial for Artemisia pollen asthma research was conducted in China in 1989 and published in 1990. 40 years since that study, there have been many published research reports on Chinese Artemisia allergy. This review summarizes the information regarding the discovery of Artemisia as an allergenic pollen, pollen account, epidemiology, allergen components, immunological changes in hay fever patients, natural course from rhinitis to asthma, diagnosis, and immunotherapies in China.
1. Introduction
Artemisia species, or mugwort, is an anemophilous genus included in the Compositae family. Mugwort plants produce high pollen grain quantities [1–4]. This plant is characterized by a huge production of small pollen grains that can be transported for several hundreds or even thousands of kilometers by large air masses. The occurrence of Artemisia species is associated with dry or slightly moist habitats and full exposure to light, which are important components of steppes [5].
Pollen from the various Artemisia species is one of the most frequent and serious pollinosis causes in many parts of the world [5–9]. The genus Artemisia includes 57 species in Europe [10] and 187 species in China [11]. In this review, we summarize Artemisia allergy research in China.
2. Discovery of Artemisia as an Allergenic Pollen
In the 1950's, Professor Ye in ENT Department of Peking Union Medical College Hospital (PUMCH) had finished his allergy practice training in Johns Hopkins Hospital in USA and learnt that ragweed was the main allergenic pollen in autumn in Europe and United States. He found that many allergy patients showed negative reaction with ragweed prick skin test. Confusingly, there were too many allergic rhinitis patients visiting his clinic every autumn. After then, Ye [12] found that there was a type of weed, Artemisia annua, that grew widely in North China. Further, they found that the most abundant pollen count dates were September 4, August 24, and September 5 in 1962, 1963, and 1972, respectively. The allergic patient numbers and their symptom severities were related to the local pollen counts. By a nasal challenge test with Artemisia annua extract, this study verified that Artemisia annua is a major outdoor allergen source in North China.
To clarify the allergenicity of the nonpollen containing components of the plant, they collected and extracted Artemisia annua leaves and stems before the pollination period in 1987 [13]. They showed that the pollen-free plant extracts did have in vivo allergenic activities.
3. Artemisia Allergic Rhinitis and Airway Hyperresponsiveness
To study the relationship between Artemisia allergic rhinitis and airway hyperresponsiveness, Ma et al. [14] chose 50 Artemisia hay fever patients and 20 normal controls. Each of these subjects was separately engaged with a skin test with Artemisia annua extract. All 50 patients had an intensely positive reaction to the Artemisia annua pollen skin test, but all of the controls were negative. Additionally, they conducted a bronchial provocation test (BPT) with a 1 : 100 (w/v) Artemisia annua pollen dilution. Before and after the BPT, they separately tested the forced expiratory volume in first second (FEV1) and serum eosinophil cationic protein (ECP) levels. They concluded that patients with Artemisia allergic rhinitis not only had chronic inflammation in their nose mucosa that resulted from an allergic reaction but also had chronic bronchial inflammation, leading to airway hyperresponsiveness (AHR). Their results also reveal that most patients with allergic rhinitis have AHR (76%).
4. Features of Pollen Prevalence
4.1. National Pollen Surveys
From 1984 to 1989, Ye [15], at PUMCH, led the first national pollen survey in mainland China. They found that Artemisia pollen could be counted in each province in mainland China (Figure 1). At the same time, they got Artemisia pollen count data from many Chinese cities in 1988 autumn (Table 1). In 2009, Yin, at the same allergy department, led the second national epidemic allergy study from 100,000 populations in 18 provinces and cities in mainland China, and that data will be published soon.
Figure 1.
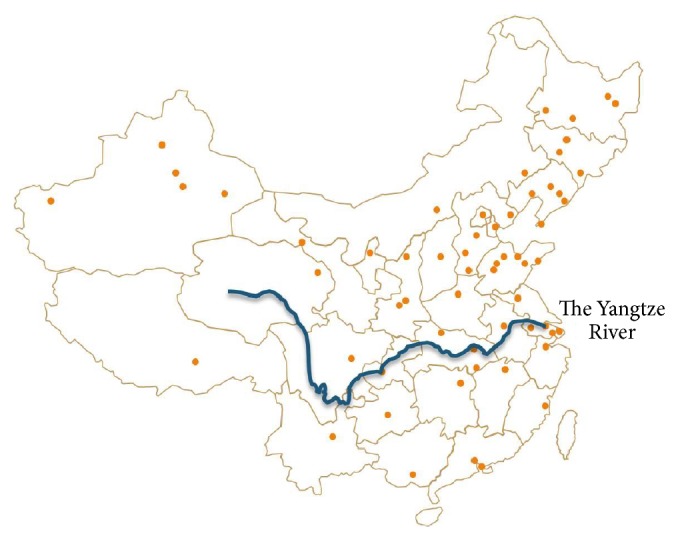
Distribution of Artemisia pollen in China.
Table 1.
Artemisia pollen season distribution in Chinese cities (July–October), unit: grain.
| Province | City | July | August | September | October |
|---|---|---|---|---|---|
| Beijing | Beijing* | 85 | 1773 | 1492 | 186 |
| Tianjin | Tianjin* | 107 | 632 | 631 | 42 |
| Hebei | Baoding* | 81 | 778 | 1852 | 185 |
| Hebei | Shijiazhuang* | 42 | 282 | 590 | 164 |
| Shanxi | Taiyuan* | 52 | 3203 | 1518 | 26 |
| Shanxi | Yuncheng* | 0 | 81 | 581 | 972 |
| Inner Mongolia | Hohhot* | 1071 | 1639 | 1088 | 152 |
| Inner Mongolia | kerqinzuoyihou* | 467 | 1156 | 896 | 25 |
| Liaoning | Shenyang* | 72 | 1330 | 327 | 39 |
| Liaoning | Dalian* | 72 | 554 | 802 | 169 |
| Jilin | Changchun* | 822 | 1620 | 210 | 5 |
| Jilin | Tonghua* | 6 | 385 | 171 | 6 |
| Heilongjiang | Harbin* | 14 | 1977 | 139 | 99 |
| Heilongjiang | Jiamusi* | 2 | 2178 | 47 | 24 |
| Heilongjiang | Qiqihar* | 18 | 1748 | 43 | 22 |
| Shandong | Ji'nan* | 31 | 421 | 1936 | 131 |
| Shandong | Qingdao* | 96 | 106 | 319 | 86 |
| The Ningxia | Yinchuan* | 377 | 2805 | 533 | 101 |
| Shaanxi | Xi'an* | 8 | 256 | 1132 | 431 |
| Gansu | Jiuquan* | 5 | 24 | 8 | 5 |
| Qinghai | Xining* | 49 | 1048 | 1673 | 84 |
| Xinjiang | Urumqi* | 10 | 711 | 1555 | 196 |
| Xinjiang | Hami* | 21 | 34 | 68 | 75 |
| Tibet | Lhasa* | 1325 | 1817 | 549 | 93 |
| Henan | Zhengzhou* | 28 | 35 | 836 | 249 |
| Hubei | Wuhan# | 23 | 37 | 221 | 61 |
| Hubei | Xiangyang# | 3 | 1150 | 1194 | 354 |
| Anhui | Hefei* | 3 | 62 | 24 | 8 |
| Sichuan | Chengdu* | 16 | 114 | 28 | 3 |
| Shanghai | Shanghai# | 8 | 7 | 207 | 64 |
| Jiangsu | Nanjing# | 2 | 71 | 643 | 35 |
| Jiangsu | Suzhou# | 0 | 0 | 58 | 13 |
| Zhejiang | Hangzhou# | 0 | 0 | 0 | 6 |
| Jiangxi | Nanchang# | 2 | 52 | 1050 | 45 |
| Fujian | Fuzhou# | 0 | 53 | 77 | 48 |
| Guizhou | Guiyang# | 0 | 1 | 1 | 0 |
| Yunnan | Kunming# | 23 | 13 | 97 | 324 |
| Hunan | Changsha# | 0 | 0 | 56 | 27 |
| Guangdong | Guangzhou# | 0 | 0 | 14 | 20 |
| Guangxi | Nanning# | 0 | 3 | 226 | 156 |
*Cities in north of the Yangtze River; #Cities in south of the Yangtze River.
4.2. Other Pollen Count Studies
During the periods that occurred between 1983 and 1986 and between 1999 and 2007, daily airborne pollen monitoring was performed by the gravitational method, using Ye's sampler at the top of the Peking Union Medical Hospital Outpatient Department Building, Beijing, China. He et al. [16] found that Artemisia was the most abundant airborne pollen and showed that it was produced over the longest period within a year, from the beginning of July until the end of September. The Artemisia levels were followed by Humulus pollen levels. Similar reports were conducted with the Durham gravity method in Xi An city (northwestern region of China) [17] in the 1980's and in Wuhan city (central region of China) in the 1990's [18]. From March 31, 2001–April 1, 2002, pollen counts were evaluated in Nanchang city (central region of China). Additionally, Xie et al. [19] found that the highest airborne presence (the percent of total yearly pollen counts) was from Ambrosia (35.73%), followed by Pinaceae and Artemisia (11.94%).
The Burkard volumetric trap was used to sample airborne pollen in Beijing city from August 1, 2007 to October 10, 2007. Yao [20] determined that (1) Artemisia and Humulus (including Cannabis sativa L.) were the main airborne pollen types observed during August and September in Beijing city, which accounted for 31% and 51% of the total pollen levels, respectively; (2) the Artemisia pollen season was from August 8th to October 8th; (3) the daily peak mugwort pollen concentration was 267 grain/m3, with an average of 71 g/m3; and (4) 88.5% of the outpatients that suffered from hay fever or asthma during the Autumn season were allergic to Artemisia. This was the first time that the Burkard volumetric sampler was employed for Artemisia and Humulus concentration monitoring in Beijing city.
In 2005, Qiao et al. [4] published a color atlas of airborne pollens and plants that are prevalent in China. In this book, the main Artemisia species on the China mainland were described, which included Artemisia argyi Levl. Et Vant., Artemisia sieversiana Willd., Artemisia annua L., Artemisia capillaris Thunb., and Artemisia lavandulaefolia DC.
5. Epidemiology of Artemisia Allergy
A cross-sectional survey was performed that included 6,304 patients who suffered from asthma and/or rhinitis in 17 cities across China [21]. These patients completed a standardized questionnaire that determined their respiratory and allergy symptoms. They also underwent skin prick tests with 13 common aeroallergens. The overall prevalence of the positive skin prick responses was 11.3% for Artemisia vulgaris, 6.5% for Ambrosia artemisiifolia, 3.5% for mixed grass pollen, and 2.2% for mixed tree pollen. The severity of rhinitis and asthma was significantly correlated with the skin reactivity index to Artemisia vulgaris and Ambrosia artemisiifolia and to D. pteronyssinus, D. farinae, and Blomia tropicalis (P < 0.001). The main pollen and spore families in Beijing are Artemisia genus, Ambrosia genus, Chenopodiaceae, and Gramineae. They can reach approximately 307,000 grains of pollen/1000 m3 of air in August [6].
A total of 215,210 tests with the ImmunoCAP system were assayed in the past three years in the PUMCH Allergy Department, which is the largest allergy department in China, and 76% were inhalant allergens. Among these allergens, the three most prevalent allergens were Dermatophagoides pteronyssinus, Dermatophagoides farinae, and Artemisia [22].
Many studies had been done about Artemisia pollen survey and its relationship with allergic diseases in China. The authors showed that Artemisia pollen was the most allergenic pollen in northern part area of Yangtze River in China (Figure 2) [23–64].
Figure 2.
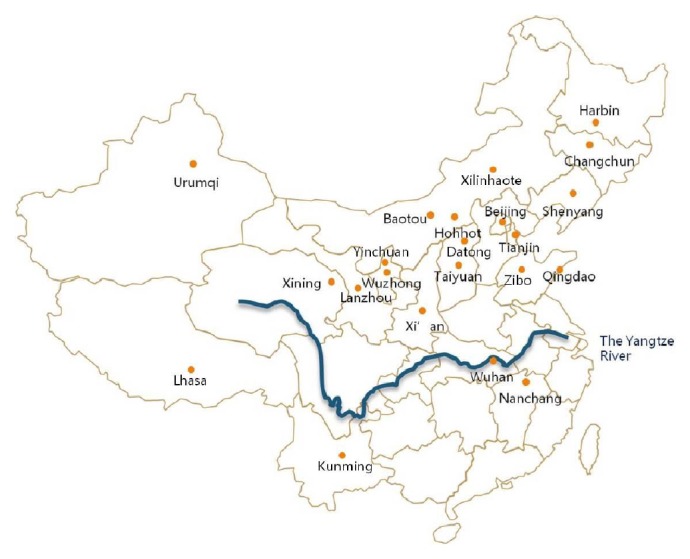
Artemisia pollen was the most allergenic pollen in north part area of Yangtze River in China (with skin test or sIgE blood test).
A total of 1,144 subjects (aged from 5 to 68) from June to October 2011 underwent intradermal testing using a panel of 25 allergen sources [65]. Of the 1,144 subjects, 170 had positive intradermal reactions to pollen and 144 donated serum for IgE testing from these 170 subjects. The positive intradermal response prevalence to Artemisia sieversiana, Artemisia annua, Ambrosia artemisiifolia, and Humulus scandens pollen was 11.0%, 10.2%, 3.7%, and 6.6%, respectively. Among the intradermal positive subjects, the specific IgE antigen prevalence to Artemisia vulgaris was 58.3%, to Ambrosia artemisiifolia was 14.7%, and to Humulus scandens was 41.0%. The specific IgE antigen prevalence to the Art v 1 allergen was 46.9% and to the Amb a 1 allergen was 11.2%. The correlation between the presence of IgE antibodies that were specific to Artemisia vulgaris and to the Art v 1 antigen was very high. Subjects with Ambrosia artemisiifolia specific IgE also had Artemisia vulgaris specific IgE but with relatively high levels of Artemisia vulgaris IgE antibodies. There were no correlations between the presence of IgE antibodies that were specific to Humulus scandens and Artemisia vulgaris. They concluded that the specific IgE antibody correlations suggest that pollen allergens from Artemisia and Humulus are independent sources for primary sensitization.
6. Artemisia Allergens
In 1992, Ou [66] purified the major allergen, A2c, from a wild Artemisia sieversiana extract. Its molecular weight was 31 kDa, and its pI was 5.3 kDa and 6.35 kDa. We verified this finding by evaluating the components of wild Artemisia sieversiana allergen extract with two-dimensional electrophoresis analyses [67].
To isolate and identify the Artemisia argyi and Artemisia apiacea pollen, Yang et al. [68] precipitated Artemisia argyi and Artemisia apiacea pollen extract with saturated ammonium sulfate and then evaluated it with SDS-polyacrylamide gel electrophoresis (SDS-PAGE). They identified the major and minor allergens by western blot analysis. Specifically, they found more than twenty protein bands and 9 allergens in the Artemisia argyi pollen extract. The major allergens were 62 kDa, 43 kDa, and 38 kDa. Similarly, in the Artemisia apiacea pollen extract, there were 11 allergens. The major allergens were 43 kDa and 38 kDa. The pollen from both species shares many allergens. Unique allergen protein bands in each of the pollen allergens were also identified.
Wu [69] analyzed Artemisia and ragweed extract antigens with SDS-PAGE and western blot analysis. They found that there were 13 bands between 18 kDa and 100 kDa in the Artemisia extract and 5 bands between 18 kDa and 63 kDa in the ragweed extract. Additionally, cross-reactivity between the Artemisia and ragweed extracts existed in the 18 kDa protein.
7. Immunological Characteristic in Artemisia Hay Fever Patients
7.1. The Th1 and Th2 Balance
Qiu et al. [70] collected tonsil lymphocytes in Artemisia pollen allergic patients and nonatopic people with a lymphocyte separation medium and incubated these cells with Artemisia pollen antigen. They found that the levels of IL-4 and IL-5 in the tonsil lymphocytes of the Artemisia pollen allergic patients were higher than those in the nonatopic controls after they were stimulated with this specific antigen, but the levels of IFN-γ were lower than those in nonatopic controls (P < 0.01). Obvious proliferation for the levels of IL-4 and IL-5 was observed in the allergic group after lymphocytes were stimulated with the specific antigen (P < 0.01), but there were no significant changes in the control group. The authors demonstrated that cytokines from Th cells of tonsil lymphocytes from Artemisia pollen-allergic people after they were specifically stimulated by Artemisia pollen antigen became imbalanced, indicating that Th1 drifts to Th2.
7.2. Basophils
With 119 hay fever patients who were allergic to Artemisia and 30 nonallergic patients, Zhang et al. [71] found that basophil numbers (mucosal mast cells) in the nasal mucosa as well as nasal eosinophil numbers increased during the pollen season and decreased during the nonpollen season. They concluded that basophils in the nasal mucosa and nasal eosinophils were related to stimulation by Artemisia in hay fever patients.
7.3. ICAM-1
Eleven patients with Artemisia allergic rhinitis were evaluated by Wang et al. [72]. Among them, 8 were studied during pollen season and 3 were studied outside of pollen season. Intercellular adhesion molecule-1 (ICAM-1) was detected on nasal epithelial cells by reverse transcription polymerase chain reaction (RT-PCR). The results showed that ICAM-1 was detectable in all of the pollen season samples. However, during the nonpollen season, 2 of the 3 samples were negative and 1 was positive (this subject was also positive to house dust). It was suggested that ICAM-1 is detectable on nasal epithelial cells during exposure to specific allergens (Artemisia).
7.4. HLA-DR
To investigate whether susceptibility or resistance to Artemisia allergic rhinitis is associated with HLA-DRB alleles or not, Xing [73] tested the frequency distribution of HLA-DRB alleles in 41 patients with Artemisia allergic rhinitis (AR) and in 41 healthy controls from Beijing, China, using PCR-SSP (sequence-specific primer polymerase chain reaction). The frequency of HLA-DRB1∗ 0301.2 and HLA-DRB4∗ 0101 was lower in the AR subjects than the controls (2.44% versus 17.07%, P < 0.05; 29.27% versus 51.22%, P < 0.05). They showed that the HLA-DRB1∗ 0301.2 and HLA-DRB4∗ 0101 alleles might confer protection against AR.
To determine whether alleles at one or more of the HLA loci were associated with Artemisia pollen hypersensitivity in allergic rhinitis patients, Xing et al. [74] also tested the frequency distribution of the HLA-DQA1 and DQB alleles in 41 patients with allergic rhinitis (AR) and in 41 healthy controls from Beijing with PCR-SSP. They demonstrated that the frequency of HLA-DQA1∗ 0201 and DQB1∗ 0602 was lower in the AR subjects than the controls (24.39% and 4.88% versus 46.34% and 26.83%, resp.) and the frequency of DQA1∗ 0302 was increased among the AR patients (58.54% versus 14.63%). They concluded that the HLA-DQA1∗ 0201 and DQB1∗ 0602 alleles might confer protection against AR and that DQA1∗ 0302 may be an Artemisia pollen hypersensitivity susceptibility factor.
8. The Natural Course from Rhinitis to Asthma
A total of 1,096 patients with autumnal pollinosis, which excluded those with typical seasonal rhinitis or asthma symptoms but with positive skin tests and serum IgE specific to dust mites and fungi, included 511 with pure allergic rhinitis and 585 with allergic rhinitis complicated with asthma. These subjects underwent inhalant allergen skin tests, evaluations for serum IgE specific to autumnal pollens, and a questionnaire survey. Yin et al. [75, 76] found that the average onset age of the allergic rhinitis patients induced by autumnal pollens was 27.9 years, and this age was significantly younger than that of the allergic asthma patients (32.6 years, P < 0.001). Out of the 1,120 patients, 1,096 (97.9%) had allergic rhinitis, 602 (53.8%) had asthma, 507 (45.3%) only had allergic rhinitis, and 10 (0.9%) only had allergic asthma. Among the 1,096 allergic rhinitis patients, 585 (53.4%) suffered from seasonal asthma. Among the 602 asthma patients, 585 (97.2%) suffered from seasonal rhinitis and 183 of the 602 patients (30.8%) needed emergency treatment. The authors showed that autumnal pollens are very important inducers of asthma during the autumn season in northern China and that almost half of the patients with autumnal pollen allergic rhinitis develop seasonal allergic asthma within 9 years [75–77].
From July 1 to October 31, 2006, Wen also observed 18 patients with only allergic rhinitis and 31 patients with allergic rhinitis and asthma [77]. The authors reported that there was a significant correlation between the Artemisia pollen count and the scores for night and daytime asthma symptoms, PEF, and diurnal variation in PEF (rs = 0.762, rs = 0.682, rs = −0.649, rs = −0.596, rs = 0.549, P < 0.001). It was also concluded that Artemisia pollen could trigger autumnal asthma in northern China.
9. Diagnostic
To evaluate the value of intradermal skin test (IDT) and serum sIgE detection in diagnosing Artemisia sensitivity, 1,150 patients with autumnal rhinitis or asthma were evaluated by experienced physicians. These subjects then underwent IDT with a 1 : 1,000 dilution of (W/V) Artemisia annua extract [78]. Then, all patients were examined for Artemisia sIgE (w6). The diagnostic standards were established based on the IDT and sIgE results. A reference standard was established according to the typical history and symptoms and a wheal with a diameter ≥ 5 mm and a sIgE level ≥ 0.35 kU(A)/L; a wheal with a diameter ≥ 10 mm alone; or a sIgE level ≥ 0.70 kUa/L alone. When using the reference standard as the criteria, the IDT had better sensitivity (96.2%), specificity (74.2%), positive predictive value (+PV, 93.5%), negative predictive value (−PV, 85.7%), and efficiency (91.6%) than using the sIgE ≥ 0.35 kUa/L alone as the IDT criteria. Additionally, the sIgE detection had better sensitivity (97.6%), specificity (94.9%), +PV (98.7%), −PV (91.1%), and efficiency (97.0%) than using wheal diameter ≥ 5 mm alone as the sIgE detection criteria. The IDT false positive and sIgE detection rates decreased from 35% and 22.7% to 25.6% and 5.1%, respectively, when using a wheal diameter ≥ 10 mm or sIgE ≥ 0.70 kUa/L as the positive criteria.
It was showed that IDT and sIgE detection were well correlated with each other in diagnosing Artemisia pollinosis, whereby both of them had the possibility of being false positive, but IDT had a higher false positive rate than sIgE detection. The IDT and sIgE detection false-positive rates can be decreased by increasing the positive criteria to a higher grading criterion.
Sera from 50 weed pollen-induced allergic rhinitis patients were tested for specific serum IgE reactivity against allergenic Artemisia extracts (Artemisia vulgaris, Art v) and single Art v 1 or Art v 3 allergens [79]. Sera from 88% of the patients demonstrated a positive specific IgE reactivity to Art v, and of these, 82% were positive to Art v 1. The authors found that specific IgE reactivity towards the major mugwort allergen, Art v 1, was a good indicator for Art v sensitization.
10. Immunotherapy
10.1. Intradermal Immunotherapy
A one-year controlled trial for an immunotherapy was conducted in 50 Artemisia sensitive hay fever patients (treatment group) [80]. From October 1985 to July 1986, all of the treatment group patients received regular Artemisia pollen allergen extract injections over one year, which totaled 30,000 protein nitrogen units (PNU). For these patients, the symptom score indices of the posttreatment 1986 pollination season were compared with those from the pretreatment 1985 season and also with the scores of a similar group of 30 Artemisia sensitive patients treated only with symptomatic medications during the 1986 season (control group). The 1986 symptom scores for the treatment group were significantly improved, and the effective rate was 78%. An immunological study with the human basophil degranulation test (HBDT) showed a significant decrease in degranulation reactions after immunotherapy. Moreover, the decline in the HBDT positive rate in the treatment group was significantly greater in the patients with improved symptoms than the patients with unchanged symptoms. No difference was observed in basophil degranulation in those patients tested with a pollen-free plant extract, which was not applied in the immunotherapy. The results suggested that immunotherapy could induce basophil desensitization and that the induction might be allergen specific. Basophil desensitization may play an important role in immunotherapy mechanisms.
10.2. Oral Immunotherapy
In 1989, eighteen asymptomatic Artemisia pollen asthma patients with normal pulmonary functions were selected for a double-blinded oral immunotherapy trial [81]. Each patient had a positive Artemisia pollen extract skin test and also had a positive bronchial challenge response to the same extract. The patients were randomly assigned to an active treatment or a placebo group and received intensive oral administration of Artemisia pollen extract over a 50-day course. The nine patients who received the active treatment ingested a cumulative dose of 396,652 PNU and showed a significant decrease in serum-specific IgE antibodies (P ≤ 0.05) and a significant reduction in bronchial sensitivity to the same extract (P ≤ 0.01). The changes in these two variables correlated well. The nine patients who received the placebo showed no significant changes in serum-specific IgE or bronchial sensitivity to the Artemisia pollen extract. Follow-ups for two cases with the same extract showed that the reductions in serum-specific IgE as well as bronchial sensitivity induced by oral immunotherapy were maintained for 3 months.
Clinically, sublingual immunotherapy (SLIT) that uses allergen extracts effectively alleviates allergic rhinitis and asthma symptoms. Ma et al. [82] hypothesized that the oral administration of a high dose of allergen extracts imitates SLIT and may prevent IgE-related responses in allergic diseases. In this study, they investigated the effects of the oral administration of mugwort (Artemisia) pollen (MP) allergen extracts on allergen-induced inflammation and airway hyperresponsiveness (AHR) in an allergic mouse model. After the administration of MP drops containing Art v 1 and Art v 4 extracts derived from MP specifically in MP-sensitized mice, the effects of the MP drops on AHR, inflammatory cell accumulation, cytokine production in the bronchoalveolar lavage fluid and lung tissue, and serum IgE and IgG levels were investigated. The results indicated that the MP drops not only prevented AHR in response to methacholine in a dose-dependent manner but also significantly reduced the total serum and allergen-specific IgE levels. All of the maximal effects were achieved at a dose of 100 μg/(kgd) and were comparable to the effects of dexamethasone at a dose of 0.5 mg/(kgd). Furthermore, the oral administration of the MP drops dose dependently elevated allergen-specific serum IgG2a levels, reduced total and allergen-specific IgE levels, and normalized the imbalance between the Th1 cytokine IL-12 and the Th2 cytokines IL-4 and IL-5. Finally, the oral administration of the MP drops significantly reduced goblet cell hyperplasia and eosinophilia in the MP-sensitized allergic mouse model. These sets of data suggest that the MP drops effectively improve specific allergen-induced inflammation and AHR in MP-sensitized and MP-challenged mice and provide the rationale for the clinical use of MP drops in specific allergen-induced asthma. Currently, a Stage I clinical SLIT trial for Artemisia annua L. was permitted by the Chinese Food and Drug Administration for one Chinese pharmaceutical company (http://www.sfda.gov.cn/WS01/CL0001/).
11. Summary
Artemisia pollen is a major important outdoor allergen in China. It has been verified as an allergen by nasal challenge and bronchial provocation tests, and these allergens have been shown to occur not only in its pollen but also in its leaves and stems. Two national allergenic pollen surveys have been conducted in China. The main allergenic Artemisia species in mainland China were recorded and described by color photos. Immunological changes from Artemisia pollen-allergic subjects were studied, including Th1 and Th2 balance, basophils, HLA-DR, and ICAM-1.
Artemisia pollen can trigger not only allergic rhinitis but also asthma alone or both of them. Almost half of the patients with autumnal pollen allergic rhinitis developed seasonal allergic asthma within 9 years. IDT and sIgE detection are well correlated with each other in Artemisia pollinosis diagnoses. Specific IgE reactivity towards the major mugwort allergen Art v 1 is a good indicator for Art v sensitization. In 1989, asymptomatic Artemisia pollen asthma patients were selected for a double-blinded oral immunotherapy trial. Recently, a Stage I clinical SLIT trial for Artemisia annua L. was permitted by the Chinese Food and Drug Administration for one Chinese pharmaceutical company. Artemisia immunotherapy could induce the desensitization of basophils, and basophil desensitization may play an important role in immunotherapy mechanisms.
Acknowledgments
The authors would like to thank Professor Peisong Gao (Division of Allergy and Clinical Immunology, Johns Hopkins Asthma and Allergy Center, Johns Hopkins University School of Medicine, USA), Dr. H. Henry Li (Institute of Allergy and Asthma, Washington DC, USA), and Yin Diao (Department of Allergy, Peking Union Medical College Hospital, Beijing, China) for helping writing the paper.
Abbreviations
- AHR:
Airway hyperresponsiveness
- ECP:
Eosinophil cationic protein
- HBDT:
Human basophil degranulation test
- LTP:
Lipid transfer protein
- ICAM-1:
Intercellular adhesion molecule-1
- IDT:
Intradermal skin test
- MP:
Mugwort pollen
- PUMCH:
Peking Union Medical College Hospital
- SDS-PAGE:
SDS-polyacrylamide gel
- SLIT:
Sublingual immunotherapy
- FEV1:
The forced expiratory volume in first second.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Subba-Reddi C., Reddi N. S. Pollen production in some anemophilous angiosperms. Grana. 1986;25:55–61. [Google Scholar]
- 2.Ramay B. Botany of artemisia. Journal of Allergy and Clinical Immunology. 1987;19(6):250–252. [PubMed] [Google Scholar]
- 3.Caramiello R., Siniscalco C., Polini V. Analises aéropalynologiques, morphométriques et phénologiques d’Artemisia . Grana. 1989;28:105–113. [Google Scholar]
- 4.Qiao B. S., Wang L. L., Yin J., Bao J. N. Color Atlas of Air-Bone Pollens and Palnts in China. Beijing, China: Peking Union Medical College Press; 2005. [Google Scholar]
- 5.Puc M., Wolski T. Forecasting of the selected features of Poaceae (R. Br.) Barnh., Artemisia L. and Ambrosia L. pollen season in Szczecin, North-Western Poland, using Gumbel's distribution. Annals of Agricultural and Environmental Medicine. 2013;20(1):36–47. [PubMed] [Google Scholar]
- 6.Li J., Lu Y., Huang K., et al. Chinese response to allergy and asthma in olympic athletes. Allergy. 2008;63(8):962–968. doi: 10.1111/j.1398-9995.2008.01816.x. [DOI] [PubMed] [Google Scholar]
- 7.Stach A., García-Mozo H., Prieto-Baena J. C., et al. Prevalence of Artemisia species pollinosis in western Poland: impact of climate change on aerobiological trends, 1995–2004. Journal of Investigational Allergology and Clinical Immunology. 2007;17(1):39–47. [PubMed] [Google Scholar]
- 8.D'Amato G., Spieksma F. T. M., Liccardi G., et al. Pollen-related allergy in Europe. Allergy. 1998;53(6):567–578. doi: 10.1111/j.1398-9995.1998.tb03932.x. [DOI] [PubMed] [Google Scholar]
- 9.Spieksma F. T. M., Charpin H., Nolard N., Stix E. City spore concentrations in the European economic community (EEC). IV. Summer weed pollen (Rumex, Plantago, Chenopodiaceae, Artemisia), 1976 and 1977. Clinical Allergy. 1980;10(3):319–329. doi: 10.1111/j.1365-2222.1980.tb02114.x. [DOI] [PubMed] [Google Scholar]
- 10.Tutin T. G., Heywood V. H., Burges N. A., Valentine D. H., Walters S. M., Webb D. A. Flora Europaea. Cambridge, UK: Cambridge University Press; 1980. [Google Scholar]
- 11.Ling Y. On the floristics of Artemisia L. in the world. Bulletin of Botanical Research. 1995;15(1):1–37. [Google Scholar]
- 12.Ye S. T. Allergology. Beijing, China: Science Press; 1998. (pp. 195–224). [Google Scholar]
- 13.Leng X., Ye S. T. An investigation on in vivo allergenicity of Artemisia annua leaves and stems. Asian Pacific Journal of Allergy and Immunology. 1987;5(2):125–128. [PubMed] [Google Scholar]
- 14.Ma R., Qu B., Liu R., Li Y. Study on the relationship between allergic rhinitis and airway hyperresponsiveness. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 2000;14(2):55–56. [PubMed] [Google Scholar]
- 15.Leadership Group of Chinese Airbone and Allergenic Survey. National Survey of Airbone and Allergenic Pollen in China. Beijing Press; 1991. [Google Scholar]
- 16.He H. J., Wang L. L., Zhang H. Y. Analysis of airbone pollens in Beijing urban area. Chinese Journal of Allergy and Clinical Immunolgy. 2008;2(3):179–183. [Google Scholar]
- 17.Sun X. Z., Li Y. F. Primary study of airborne pollen in Xi An. Journal of Environmental Health. 1994;11(2):80–81. [Google Scholar]
- 18.Li M. Z. Study of airborne pollen in Wuchang District, Wuhan. Wuhan Botanic Research. 1997;15(1):66–72. [Google Scholar]
- 19.Xie S. X., Liu J. X., Liu Z. G. The investigation on airborne pollen in urban district of Nanchang. Journal of Environmental Health. 2004;21(6):381–383. [Google Scholar]
- 20.Yao L., Zhang H. Concentration of airborne pollen in Beijing city with burkard sampler. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2009;23(20):913–916. [PubMed] [Google Scholar]
- 21.Li J., Sun B., Huang Y., et al. A multicentre study assessing the prevalence of sensitizations in patients with asthma and/or rhinitis in China. Allergy: European Journal of Allergy and Clinical Immunology. 2009;64(7):1083–1092. doi: 10.1111/j.1398-9995.2009.01967.x. [DOI] [PubMed] [Google Scholar]
- 22.Wang R., Zhang H. Analysis of two hundred thousand results of allergen specific IgE test. Chinese Journal of Allergy and Clinical Immunology. 2012;6(1):18–23. [Google Scholar]
- 23.Wu J., Song L., Liu S. M. Investigation on airborne allergenic pollen and analysis on clinical data of pollinosis in Hohhot. OccuP and Health. 2013;29(3):266–269. [Google Scholar]
- 24.Yang Y. P., Xu X. Y., Sun D. Y., et al. Pathogenesis survey of allergic pollinosis in Baotou. Chinese Journal of Asthma (Electronic Edition) 2011;5(5):325–330. [Google Scholar]
- 25.Zhu J. G., Dong Z., Li J. H. Study on air pollen survey and its relationship with allergic diseases in Changchun area. Jilin Medicine. 1989;10(1):53–55. [Google Scholar]
- 26.Bian Y. F., Li G. Q. Antigen skin test and clinical analysis of 240 cases pollinoses in Datong area. Journal of Datong Medical College. 2002;1:13–14. [Google Scholar]
- 27.Ma E., Yang F. Analysis on seasonal allergic rhinitis inhalant allergen intradermal test in Lhasa, Tibet. Tibetan Medicine. 2009;30(2):25–26. [Google Scholar]
- 28.He Y. Q., Yang S. S., Bai C. J., Li Y., Chen Z., Yang L. Distribution of urban airborne pollen and relationship between the airborne pollen and allergic diseases in Lijiang. Chinese Journal of Allergy and Clinical Immunology. 2010;4(3):176–185. [Google Scholar]
- 29.Zhang J. Y. Investigation on result of allergen test in allergic patients of Shandong province. Chinese Journal of General Practice. 2008;6(8):841–842. [Google Scholar]
- 30.Menghe B. L. G., Lin C., Han D. M., et al. Detection and analysis the allergen related to autumn allergic rhinitis in urban crowd of huhhot. Inner Mongolia Medical Journal. 2008;40(7):817–818. [Google Scholar]
- 31.Liu W. T., Wu Y. R., Zhang J., et al. Allergen test of in 260 allergic rhinitis patients from Wuzhong area in Ningxia. Ningxia Medical Journal. 2013;35(3):p. 266. [Google Scholar]
- 32.Yu X. H., Jiang X. B., Zhou Y. Q. An analysis of common allergens of children with bronchial asthma in Chonzhou city, Sichuan. Sichuan Medcine. 2013;34(5):672–674. [Google Scholar]
- 33.Gao Y. Q., Ma J., Lu T., et al. Spectrum of sensitized allergens in pediatric patients with allergic rhinitis in Kunming. Chinese Journal of Otorhinolaryngology-Skull Base Surgery. 2013;19(5):403–407. [Google Scholar]
- 34.Wu X. D., Wang C. Q., Li M. Intradermal skin test of inhalant allergen in allergic rhinitis in Xilinhot, Inner Mongolia. Inner Mongolia Medical Journal. 2011;43(4):462–463. [Google Scholar]
- 35.Yue H. H., Dong X. J., Ma X. P., et al. Epidemiological investigation of allergic rhinitis in servicemen of Chinese People's Armed Police Force stationed at Urumqi. Medical Journal of Chinese People's Liberation Army. 2011;36(7):770–772. [Google Scholar]
- 36.Song W. W., Lin X. P., Zhong H. H. Spectrum of sensitized inhalant allrgens in allergic rhinitis patients in Liaoning province. Chinese Journal of Allergy and Clinical Immunology. 2011;5(4):263–267. [Google Scholar]
- 37.Wei X., Zuo C., Liu H. Analysis on allergen test from 1296 cases in Shanxi province. Shanxi Clinical Medical Journal. 2001;10(12):900–901. [Google Scholar]
- 38.Bai Y. R., Liu Y., Pang L. Analysis of pollinosis in Tianjin. Chinese Journal of Allergy and Clinical Immunology. 2001;1(2):193–196. [Google Scholar]
- 39.Li C., Feng M. L. Survey of allergic pollen in air and treatment of asthma in Zibo City. China Tropical Medicine. 2007;7(9):1731–1732. [Google Scholar]
- 40.Li Z. H., Zhu W., Guo X. W., et al. Analysis of allergen of allergic rhinitis patients in Ningxia. China Modern Medicine. 2010;17(9):35–36. [Google Scholar]
- 41.Jiang S. X., Zhu X. M., Li Q. S., et al. Spectrum of major sensitized inhalant allergens in aestivo -autumnal allergic rhinitis patients in Shenyang. Chinese Archives of Otolaryngology-Head and Neck Surgery. 2014;21(3):144–146. [Google Scholar]
- 42.Han Y., Zhang H. Epidemiological investigation of allergic rhinitis in the primary school students in grade three of Shihezi city. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2009;23(23):1074–1078. [PubMed] [Google Scholar]
- 43.Yang Y., Zhao Y., Wang C.-S., Wang X.-D., Zhang L. Prevalence of sensitization to aeroallergens in 10 030 patients with allergic rhinitis. Chinese Journal of Otorhinolaryngology Head and Neck Surgery. 2011;46(11):914–920. [PubMed] [Google Scholar]
- 44.Ding J., Zhang J., Xu F., Xu Y., Zhu H. Analyzing of the inhaled allergens profiles of 890 allergic rhinitis patients. Journal of Clinical Otorhinolaryngology, Head, and Neck Surgery. 2012;26(4):164–166. doi: 10.13201/j.issn.1001-1781.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 45.Lü Y., Xie Z., Zhao S., et al. Prevalence of allergens for Changsha patients with allergic rhinitis. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2011;25(11):491–494. [PubMed] [Google Scholar]
- 46.Huang F., Zhao Y., He J., et al. Analyzing of the inhaled allergens profiles of allergic rhinitis patients in district of Jingmen. Journal of Clinical Otorhinolaryngology, Head, and Neck Surgery. 2010;24(8):341–343. [PubMed] [Google Scholar]
- 47.Wang C., Zhang L., Han D., Zhou B., Zhao Y., Wang X. Prevalence of sensitization to aeroallergens in Beijing patients with allergic rhinitis. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 2006;20(5):204–207. [PubMed] [Google Scholar]
- 48.Jiang C., Li L., Tan G. Aeroallergen spectrum of 387 patients with allergic rhinitis in Changsha area. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2008;22(17):794–797. [PubMed] [Google Scholar]
- 49.Li J., Huang Y., Lin X., et al. Influence of degree of specific allergic sensitivity on severity of rhinitis and asthma in Chinese allergic patients. Respiratory Research. 2011;12, article 95 doi: 10.1186/1465-9921-12-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhong Z., Wang F., Wang T., Li L., Tan G. Aeroallergen spectrum of patients with child allergic rhinitis in Changsha area of China. Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2011;25(17):774–776. [PubMed] [Google Scholar]
- 51.Liu G.-H., Zhu R.-F., Zhang W., Li W.-J., Wang Z.-X., Chen H. Survey of airborne pollen in Hubei Province of China. Chinese Medical Sciences Journal. 2008;23(4):212–217. doi: 10.1016/S1001-9294(09)60041-9. [DOI] [PubMed] [Google Scholar]
- 52.Ouyang Y.-H., Zhang D.-S., Fan E.-Z., Li Y., Zhang L. Correlation between symptoms of pollen allergic rhinitis and pollen grain spreading in summer and autumn. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2012;47(8):623–627. [PubMed] [Google Scholar]
- 53.Liu G., Lu X., Ren Y. The analysis of the allergens in 576 patients with allergic rhinitis in Qingyang of Gansu province. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2013;27(23):1307–1309. [PubMed] [Google Scholar]
- 54.Chen S., Zhong C., Teng Y., et al. Detection and analysis of common allergens in 680 allergic rhinitis patients in Hangzhou. Chinese Journal of Health Laboratory Technology. 2014;24(3):403–405. [Google Scholar]
- 55.Sun L. Y., Guo Y. S., Wang J. I., Wang Y. W., Xu Y. P. Clinical analysis of the skin prick test in Shanghai area. Modern Immunology. 2011;31(1):66–70. [Google Scholar]
- 56.Zhao H., Li W., Zhu L., et al. Inhalant allergen analysis of allergic rhinitis and asthma in Jiamusi, Xinjiang. Journal of Jiamusi Medical College. 1996;19(3):49–50. [Google Scholar]
- 57.Zhao Q., Deng H., Zheng P. Survey of distribution of snesitinogens in allergic rhinitis patients. China Tropical Medicine. 2008;8(7):p. 1185. [Google Scholar]
- 58.Ma Y., Fang P., Liu Y., et al. Allergens distribution and clinical significance with allergic rhinitis and asthma in Hefei of Anhui Province. Acta Universitatis Medicinalis Anhui. 2013;48(10):1249–1251. [Google Scholar]
- 59.Chen D., Wu S. Analysis of allergens in 1088 patients accepting skin prick tests in the border region of Guangdong and Guangxi. New Medicine. 2013;44(6):385–388. [Google Scholar]
- 60.Wang H., Xiang Y., Chen X., et al. Investigation of allergens in patients with asthma and allergic rhinitis in Xinjiang. Journal of Xinjiang Medical University. 2007;30(10):1086–1088. [Google Scholar]
- 61.Ma G., Yang C., Yin G., et al. Detection analysis of allergens in children with allergic rhinitis in Chengde district. Journal of Clinical and Experimental Medicine. 2013;12(20):1639–1641. [Google Scholar]
- 62.Liu J., Lu D. W., Guo Y. L., et al. Analysis of airborne pollens in Qingdao since 2010 to 2011. Chinese Journal of Allergy and Clinical Immunology. 2010;6(3):191–197. [Google Scholar]
- 63.Yang B., Chu Y. D. Analysis of allergenic pollen and season of hay fever in Xi Ning City, Qinghai Province. Qinghai Medicine. 2001;31(2):6–7. [Google Scholar]
- 64.Yang J., Hu S. P. Study of major allergenic airborne pollen in Wuchang district, Wuhan city. Journal of Hubei Medical University. 1998;19(1):37–39. [Google Scholar]
- 65.Hao G. D., Zheng Y. W., Gjesing B., et al. Prevalence of sensitization to weed pollens of Humulus scandens, Artemisia vulgaris, and Ambrosia artemisiifolia in northern China. Journal of Zhejiang University B. 2013;14(3):240–246. doi: 10.1631/jzus.B1200185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ou Y. Study on the purification of major allergen A2c in Artemisia extract. Chinese Journal Microbiology Immunology. 1992;12:11–14. [Google Scholar]
- 67.Sun J., Zhang H., Ying W., Qian X. Two-dimensional electrophoresis analysis of components in Artemisia allergen extract. Chinese Journal Microbiology Immunology. 2001;21, supplement:16–19. [Google Scholar]
- 68.Yang H., Liu Z., Hang Q., Hao S., Liang G. Analysis of allergen composition in Artemisia argyi and Artemisia apiacea pollen. Immunological Journal. 2004;20(2):120–123. [Google Scholar]
- 69.Wu X., Huang W. Antigen analysis for Artemisia pollen and Ragweed pollen. Guangdong Medical Journal. 2004;25(10):1136–1138. [Google Scholar]
- 70.Qiu J., Liu Z., Liu M., Lu Y. The Dynamic changes of cytokines produced by tonsil lymphocytes after stimulated by Artemisia pollen. Acta Academiae Medicinae Jiangxi. 2004;44(4):1–6. [Google Scholar]
- 71.Zhang Z., Zhang K., Qin Z., Peng J., Sun H. Seasonal change of basophils in the nasal mucosa from Artemisia hay fever patients. Journal of Beijing Medical University. 1991;23(1):73–74. [Google Scholar]
- 72.Wang L., Fan W., Yin L. Influence of intercellular adhesion molecule-1 transcription on nasal epithelial cell by airborne allergenic pollens. Zhonghua Er Bi Yan Hou Ke Za Zhi. 1997;32(3):174–176. [PubMed] [Google Scholar]
- 73.Xing Z., Yu D. Linkage of allergic rhinitis with HLA-DRB alleles polymorphism. Journal of Clinical Otorhinolaryngology. 2001;15(5):199–201. [PubMed] [Google Scholar]
- 74.Xing Z., Yu D., An S. Association of hypersensitivity to wormwood pollen in patients with allergic rhinitis with HLA alleles polymorphism. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 2002;16(12):678–680. [PubMed] [Google Scholar]
- 75.Yin J., Yue F.-M., Wang L.-L., et al. Natural course from rhinitis to asthma in the patients with autumnal pollinosis: a clinical study of 1096 patients. Zhonghua Yi Xue Za Zhi. 2006;86(23):1628–1632. [PubMed] [Google Scholar]
- 76.Yin J., Yue F.-M., Wang L.-L., et al. The clinical study of the relationship between allergic rhinitis and allergic asthma in the patients with autumnal pollinosis. Zhonghua Yi Xue Za Zhi. 2005;85(24):1683–1687. [PubMed] [Google Scholar]
- 77.Wen Z., Yin J. Correlation of the Artemisia and Humulus pollen count in the air and the severity of asthma symptoms in patients with autumnal pollinosis. Chinese Journal of Allergy and Clinical Immunology. 2012;6(1):10–17. [Google Scholar]
- 78.Yin J., He H.-J., Wang R.-Q., et al. Value of intradermal skin test and serum sIgE detection in diagnosing artimisia pollinosis. Zhonghua Yi Xue Za Zhi. 2006;86(25):1759–1763. [PubMed] [Google Scholar]
- 79.Han D., Lai X., Gjesing B., Zhong N., Zhang L., Spangfort M. D. The specific IgE reactivity pattern of weed pollen-induced allergic rhinitis patients. Acta Oto-Laryngologica. 2011;131(5):533–538. doi: 10.3109/00016489.2010.539265. [DOI] [PubMed] [Google Scholar]
- 80.Leng X., Ye S.-T. One year observation of immunotherapy for Artemisia hay fever in China: a clinical and immunological study. Asian Pacific Journal of Allergy and Immunology. 1987;5(2):167–172. [PubMed] [Google Scholar]
- 81.Leng X., Fu Y.-X., Ye S.-T., Duan S.-Q. A double-blind trial of oral immunotherapy for Artemisia pollen asthma with evaluation of bronchial response to the pollen allergen and serum-specific IgE antibody. Annals of Allergy. 1990;64(1):27–31. [PubMed] [Google Scholar]
- 82.Ma W.-J., Bao M.-J., Zhu J.-P., et al. Oral administration of allergen extracts from mugwort pollen desensitizes specific allergen-induced allergy in mice. Vaccine. 2012;30(8):1437–1444. doi: 10.1016/j.vaccine.2012.01.005. [DOI] [PubMed] [Google Scholar]


