Abstract
Beta-glucans are a heterologous group of fibrous glucose polymers that are a major constituent of cell walls in Ascomycetes and Basidiomycetes fungi. Synthesis of β (1,3)- and (1,6)-glucans is coordinated with fungal cell growth and development, thus, is under tight genetic regulation. Here, we report that β-glucan synthesis in both asexual and sexual spores is turned off by the NF-kB like fungal regulators VosA and VelB in Aspergillus nidulans. Our genetic and genomic analyses have revealed that both VosA and VelB are necessary for proper down-regulation of cell wall biosynthetic genes including those associated with β-glucan synthesis in both types of spores. The deletion of vosA or velB results in elevated accumulation of β-glucan in asexual spores. Double mutant analyses indicate that VosA and VelB play an inter-dependent role in repressing β-glucan synthesis in asexual spores. In vivo chromatin immuno-precipitation analysis shows that both VelB and VosA bind to the promoter region of the β-glucan synthase gene fksA in asexual spores. Similarly, VosA is required for proper repression of β-glucan synthesis in sexual spores. In summary, the VosA-VelB hetero-complex is a key regulatory unit tightly controlling proper levels of β-glucan synthesis in asexual and sexual spores.
The cell wall is crucial for many aspects of fungal biology including growth, development, morphogenesis and pathogenicity1,2,3,4. It maintains the shape of the fugal cells and provides a protection against internal turgor pressure and various environmental factors5. The major components of the fungal cell wall are glycoproteins, chitin, α- glucans, β-glucans, mannans and melanin6,7. These components are cross-linked and form the fungal specific polysaccharide-based network4,5. Since the structure and components of cell wall are fungi-specific, the cell wall has been considered an attractive target for anti-fungal drug development3,8,9. In the model filamentous fungus Aspergillus nidulans, α-1,3-glucan, β-1,3-glucan and chitin are the main polysaccharides in the cell wall and associated genes include agsA, agsB (encoding α-1,3-glucan synthases), fksA (encoding β-1,3-glucan synthase) and chsA (encoding a chitin synthase)10,11,12,13,14.
Filamentous fungi produce asexual spores (conidia in higher fungi) as the main propagules and infection particles15,16. Conidia of A. nidulans are formed on the multicellular asexual reproductive structure conidiophore15,17. After conidia are formed from the phialide apex, it subsequently undergoes the maturation process consisting of three stages18,19,20. Stage I conidia contain walls consisting of C1 (outer) and C2 (inner) layers. At stage II, C1 becomes crenulated and C2 condenses. During stage III, two new wall layers, C3 (between C1 and C2) and C4 (the innermost wall) form and these two layers are required for conidial dormancy19. A key gene for conidia maturation in A. nidulans is wetA19. The wetA6 mutant conidia are defective in the formation of the C3 and C4 wall layers and condensation of the C2 wall layer, resulting in the lack of spore pigmentation and lysis of conidia. Our studies further revealed that two velvet genes vosA and velB are also important for conidia maturation21,22,23.
The velvet proteins (VeA, VelB, VelC and VosA) are fungi-specific transcription factors and contain the velvet domain with the NF-kB-like DNA binding motif21,24,25,26. These proteins form different complexes, which play differential roles in regulating developmental processes and synthesis of secondary metabolites in many filamentous fungi24,27. Among them, the VosA-VelB complex acts as a functional unit controlling maturation (trehalose biogenesis), dormancy and germination of conidia21,22,23. In addition, the vosA deletion mutant conidia are defective in the formation of the electron-light layer of the conidial cell wall21, suggesting that the VosA protein may regulate proper spore wall formation.
To gain further insights into their role in the formation of spore wall, we have performed a transcriptome analysis using the wild type (WT) and ΔvosA mutant conidia. We have found that several genes involved in the synthesis of major cell wall polysaccharides are up-regulated by the lack of VosA in conidia. Further targeted studies have revealed that the absence of vosA or velB results in elevated mRNA accumulation of fksA, gelA and gelB involved in the biosynthesis of β-1,3-glucan. Indeed, levels of β-glucan in the mutant conidia were much higher than those in WT. In addition, in vivo protein-DNA interaction studies employing chromatin-immuno-precipitation (ChIP) using the VosA and VelB proteins tagged with the epitope FLAG specifically enriched an fksA promoter region which contains the consensus DNA sequence predicted to be recognized by VosA25. Moreover, the deletion of vosA causes increased levels of fksA mRNA and total β-glucan amount in sexual spores (ascospores), and VosA-FLAG-ChIP and VelB-FLAG-ChIP occupy an fksA promoter region. Taken together, we propose that the VosA-VelB complex represses β-glucan synthesis in fungal spores by directly binding to the promoter regions of fksA and other cell wall biosynthetic genes, thereby conferring proper spore wall formation during sporogenesis in A. nidulans.
Results
Genome-wide expression studies reveal that VosA is necessary for proper repression of cell wall-related genes in conidia
To gain further insights into the complex regulatory roles of VosA during asexual sporogenesis, we carried out comparative transcriptome analysis of the WT and ∆vosA conidia. A total of 3,483 genes showed statistically significant differential expression between the ∆vosA and WT conidia (Fig. 1A, P < 0.05, T-test). A total of 2,117 genes exhibited more than two-fold reduction (1,104 genes), or induction (1,013 genes) in the ∆vosA conidia (Table S1). KOG (eukaryotic orthologous groups) analysis of genes up-regulated by VosA showed that the following categories of genes were overrepresented: cell wall/membrane/envelope biogenesis, cytoskeleton, lipid transport/metabolism, and secondary metabolites biogenesis/transport/catabolism (Fig. 1B). Further GO analyses demonstrated that a large number of the cell wall-related genes (the MIPS PEDANT 3 database, http://pedant.gsf.de/genomes.jsp?category=fungal) is differentially up-regulated in the ∆vosA conidia (Fig. 1C). Several genes involved in β-1,3-glucan synthesis including fksA, gelA and gelB (β-1,3-glucan-transglucosylase genes), and chitin synthesis including chsA (class II chitin synthase gene)28, chsB (class III chitin synthase gene)29,30, csmA (class V chitin synthase gene)31 and gfaA (glutamine-fructose 6-phosphate transaminase gene) were shown to be highly induced in the ∆vosA conidia compared to the WT conidia (Table 1). These indicate that VosA is involved in repression of cell wall-related genes in spores, and may govern the spore wall polysaccharides composition and structure.
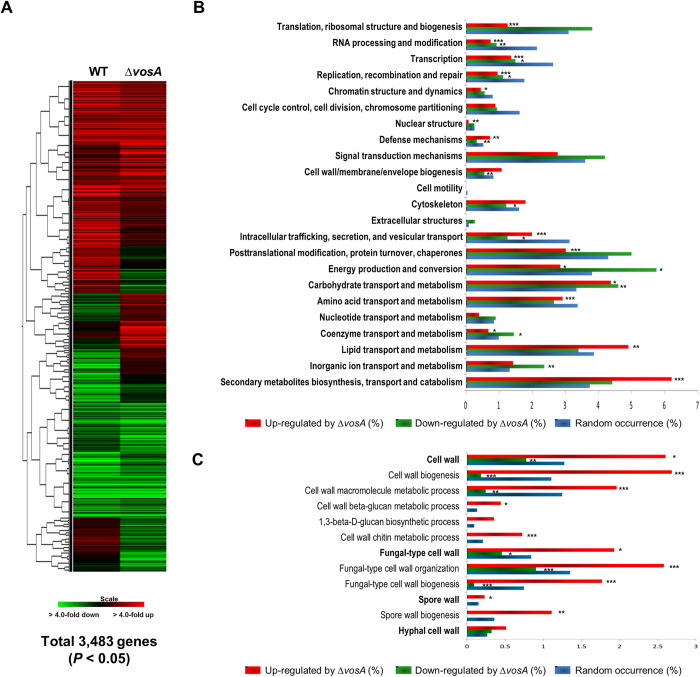
Figure 1. Genome wide analyses of A. nidulans genes whose expression is influenced by VosA in conidia.
(A) Hierarchical clustering analysis of 3,483 genes which showed significant differential expression between the WT and ∆vosA conidia (P < 0.05, T-test) is presented. (B) Functional categories of up-, or down-regulated genes in the ∆vosA conidia compared to WT conidia are shown. Each group of genes was functionally categorized by KOG description (* P < 0.05; ** P < 0.01; *** P < 0.001). (C) Functional categories and expression responses of cell wall-, or sporulation-related genes in the ∆vosA conidia compared to WT conidia are presented. Each group of genes was functionally categorized by GO description (* P < 0.05; ** P < 0.01; *** P < 0.001).
Table 1. Relative expression of cell wall-related genes in ΔvosA and WT conidia.
| Systematic name | Gene name | Descriptiona | Expression ratio (∆vosA/WT) | Occupancy of VosA-ChIPb |
|---|---|---|---|---|
| Genes involved in 1,3-β-glucan synthesis and processing | ||||
| AN3729.3 | fksA | 1,3-beta-glucan synthase | 5.01 | O |
| AN7657.3 | gelA | Putative 1,3-beta-transglycosidase | 2.34 | |
| AN0558.3 | gelB | Putative 1,3-beta-transglycosidase | 2.20 | |
| AN3730.3 | gelC | Putative 1,3-beta-transglycosidase | 2.73 | O |
| AN10150.3 | btgA | Putative beta-transglucosylase | 4.17 | |
| AN1551.3 | btgE | Putative beta-glucosidase | 3.17 | |
| AN3053.3 | crhD | Putative transglycosidase | 2.91 | O |
| AN0550.3 | Putative glucan 1,3-beta-glucosidase | 0.22 | ||
| AN0779.3 | Putative glucan 1,3-beta-glucosidase | 5.97 | ||
| AN4825.3 | Putative glucan 1,3-beta-glucosidase | 2.43 | ||
| AN4852.3 | Putative glucan 1,3-beta-glucosidase | 2.67 | ||
| AN6697.3 | sunA | Putative Sun-family protein | 4.59 | O |
| Genes involved in 1,3-α-glucan synthesis and processing | ||||
| AN5885.3 | agsA | alpha-1,3 glucan synthase | 6.11 | |
| AN1604.3 | agnE | Putative alpha-1,3-glucanase | 2.22 | |
| AN6324.3 | amyE | Putative alpha-amylase | 2.76 | |
| Genes involved in chitin synthesis and processing | ||||
| AN7032.3 | chsA | Class II chitin synthase | 2.13 | |
| AN2523.3 | chsB | Class III chitin synthase | 2.16 | |
| AN6318.3 | csmA | Class V chitin synthase | 3.37 | O |
| AN10709.3 | gfaA | Glutamine-fructose-6-phosphate transaminase | 2.43 | O |
| AN4871.3 | chiB | Class V chitinase | 3.23 | |
| AN9390.3 | chiC | Putative endochitinase | 11.33 | |
| AN11059.3 | Putative chitinase | 2.30 | ||
| AN0221.3 | Putative chitinase | 0.43 | ||
| AN8481.3 | Putative chitinase | 0.31 | ||
| AN1502.3 | nagA | Extracellular N-acetyl-beta-glucosaminidase | 2.26 | O |
| Genes involved in other enzymatic function in cell wall biosynthesis | ||||
| AN8444.3 | celA | Protein with similarity to cellulose synthase | 2.02 | |
| AN3883.3 | Putative glucanase | 2.36 | O | |
| AN6472.3 | dfgF | Putative endo-mannanase | 4.07 | |
| AN10345.3 | dfgA | Putative endo-mannanase | 2.00 | |
| AN4390.3 | ecmA | GPI-anchored cell wall organization protein | 2.50 | O |
aASPGD and de Groot et al., 2009.
bO represent genes bound by VosA and having VosA-responsive element (Ahmed et al., 2013).
Both VelB and VosA are required for repression of spore wall-related genes
Previously we showed that VosA interacts with VelB, and forms the VosA-VelB complex which is a functional unit for spore maturation including trehalose biogenesis22,23. To test whether VelB plays a role in proper spore wall formation, we first examined 8 days old conidia of WT, ∆velB and ∆vosA strains using transmission electron microscopy (TEM) (Fig. 2A). We found that, similar to ∆vosA, the ∆velB conidia appear to lack cytoplasm and clear organelle structures. In addition, the ∆velB conidia electron light layer was darker than that of WT. These results indicated that VelB, similar to VosA, plays a pivotal role in conidial wall formation and structure.
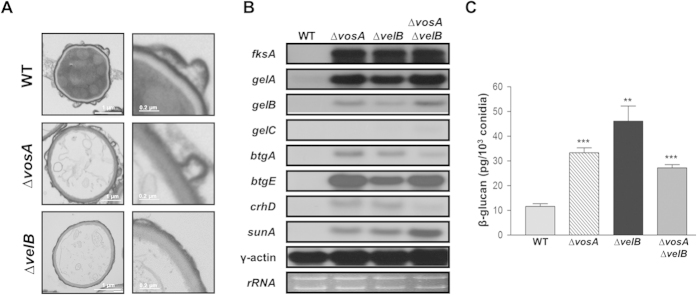
Figure 2. Requirement of vosA and velB in proper formation of conidial wall.
(A) Transmission electron micrographs of WT (FGSC4), ΔvosA (THS15.1), ΔvelB (THS16.1) mutant conidia are shown. All photomicrograph images were cropped (indicated by the red lines). (B) Levels of fksA, gelA, gelB, gelC, btgA, btgE, crhD and sunA mRNAs in conidia of WT (FGSC4), ΔvosA (THS15.1), ΔvelB (THS16.1) and ΔvelB ∆vosA (THS14.1) strains. Equal loading of total RNA was confirmed by ethidium bromide staining of rRNA and hybridization with the γ-actin gene. All Northern blot and rRNA gel images were cropped (indicated by the red lines) to show the relevant data only. For each probe hybridization, equal amounts of total conidial RNA of four strains were separated in the same agarose gel, transferred to the one nylon membrane, and hybridized in the same bag. Thus, mRNA levels of each indicated gene can be directly compared among the four strains. (C) Amount of β-glucan (pg) per 103 conidia in 2 day old conidia of WT (FGSC4), ΔvosA (THS15.1), ΔvelB (THS16.1) and ΔvelB ∆vosA (THS14.1) strains (measured in triplicates) (** P < 0.01; *** P < 0.001).
We then examined whether the absence of velB or vosA affected mRNA levels of key genes associated with synthesis and processing of β-1,3-glucan in conidia. We found that, while mRNA of 1,3-β-glucan synthetic genes including fksA, gelA and gelB was not detectable in WT conidia, mRNA levels of those genes were very high in the ∆velB and ∆vosA conidia (Fig. 2B). In accordance with the increased mRNA levels of β-1,3-glucan biosynthetic genes, β-glucan levels in the ∆velB and ∆vosA conidia were significantly higher than those of WT conidia (Fig. 2C). Moreover, the ∆vosA ∆velB double mutant conidia exhibited the essentially identical enhancement of mRNA accumulation patterns and increased β-glucan content in conidia (Fig. 2B,C). Taken together, these results suggest that both VelB and VosA are needed for proper repression of β-glucan synthesis in conidia, and they play an inter-dependent role.
VosA and VelB bind to the fksA promoter
As mentioned, a key and common property of these multi-functional velvet regulators is that they are DNA-binding proteins and function as transcription factors. Their DNA-binding velvet domain is similar to NF-kB DNA binding motif25. Previous chromatin immune-precipitation followed by microarray (ChIP-chip) studies have revealed promoter regions of a high number of genes were enriched by VosA, including several cell-wall related genes (Table 1)25. One consensus DNA sequence predicted to be recognized by VosA is located at the promoter region of fksA (Fig. 3A). To test our hypothesis that the VelB and VosA hetero-complex is a functional unit for repression of β-glucan synthesis in conidia, we checked whether both VosA-ChIP and VelB-ChIP enriched the promoter regions of fksA in vivo. As shown Fig. 3B, PCR-amplification of the fksA promoter region using DNA fragments derived from VosA-FLAG-ChIP and VelB-FLAG-ChIP gave rise clear amplicons, whereas DNA from ChIP of THS30 lacking FLAG-tagged VosA or VelB failed to do so. Collectively, these results indicate that the VosA-VelB hetero-complex binds to the promoter region of fksA and negatively regulates expression of fksA, and many other conidial wall biosynthetic genes.
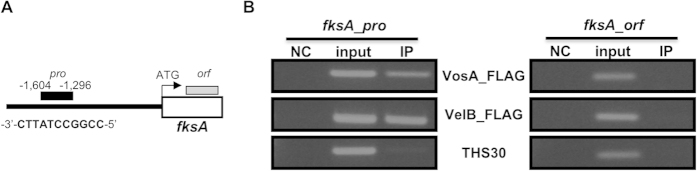
Figure 3. Both VosA and VelB bind to the fksA promoter in conidia.
(A) Schematic presentation of the promoter region of fksA. The black box (the −1,296 ~ −1,604 region of the fksA promoter) and the gray box (a region of the fksA ORF) represents the regions subject to PCR (shown in B). The 11-nucleotide sequence predicted to be recognized by VosA is shown. (B) Results of VosA-ChIP-PCR: the PCR amplicons separated on a 2% agarose gel are shown in the bottom panel. The input DNA before immuno-precipitation (IP) was used as a positive control (input). The chromatin extract being incubated with bead only (without anti-FLAG antibody) was used as a negative control (NC). The samples of THS30 lacking FLAG-tagged VosA or VelB were used as negative controls, too. Representative results are shown (measured in duplicates). All gel images were cropped (indicated by the red lines) to show the relevant data only. The ChIP-PCR amplified samples were separated in the same agarose gel. The full-length gels are presented in Supplementary Fig. S1A online.
VosA is required for β-glucan synthesis in ascospores
While asexual sporulation is the primary reproductive mode, A. nidulans can form sexual spores called ascospores. Our previous studies showed that, similar to conidia, the deletion of vosA caused the formation of defective ascospores with dis-jointed cell wall lacking long-term viability21. We envisioned that if VosA is generally required for proper conidial wall formation and structure, it would function similarly during sexual sporulation. The ∆velB mutant could not produce sexual fruiting bodies, and was excluded in ascospore analyses. To further test the roles of VosA in sexual fruiting, we examined mRNA levels of fksA and total content of β-1,3-glucan in WT and ∆vosA ascospores. As shown Fig. 4A, mRNA of fksA accumulated at high level in ∆vosA, but not in WT, ascospores. In addition, amount of β-glucan in ∆vosA ascospores was significantly higher than that of WT (Fig. 4B). Likewise in conidia, VosA-ChIP-PCR results show that VosA-ChIP specifically enriches the promoter regions of fksA in ascospores (Fig. 4C). Collectively, VosA represses expression of fksA and controls levels of β-1,3-glucan synthesis in ascospores.
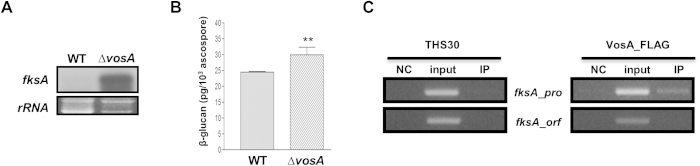
Figure 4. VosA represses β-glucan biogenesis in ascospores.
(A) Levels of fksA transcript in ascospores of WT (FGSC4) and ΔvosA (THS15.1) strains. Equal loading of total RNA was confirmed by ethidium bromide staining of rRNA. (B) Amount of β-glucan (pg per 103 ascospores) in 8 day old ascospores of WT (FGSC4) and ΔvosA (THS15.1) strains (measured in triplicates) (** P < 0.01). The Northern blot and rRNA gel images were cropped (indicated by the red lines). For fksA probe hybridization, equal amounts of total RNA of two strains’ ascospores were separated in the same agarose gel, transferred to the one nylon membrane, and hybridized in the same bag. (C) Results of VosA-ChIP-PCR: the PCR amplicons separated on a 2% agarose gel are shown in the bottom panel. The input DNA before immuno-precipitation (IP) was used as a positive control (input). The chromatin extract being incubated with bead only (without anti-FLAG antibody) was used as a negative control (NC). The samples of THS30 lacking FLAG-tagged VosA were also used as negative controls. Representative results are shown (measured in duplicates). All four gel images were cropped (indicated by the red lines) to show the relevant data only. The ChIP-PCR amplified samples were separated in the same agarose gel. The full-length gels are presented in Supplementary Fig. S1B online.
Discussion
The VosA and VelB regulators are fungi-specific transcription factors that play multifaceted roles in various biological processes including spore maturation, trehalose biogenesis, conidial germination and conidial wall integrity21,22,25. In this study, we have newly revealed following additional key roles of the VosA and VelB regulators during fungal sporogenesis. First, VosA negatively regulates expression of various genes including cell wall-related genes in conidia (Fig. 1, Table 1). Second, VosA and VelB directly bind to a promoter region of fksA, and thereby negatively control fksA expression and β-glucan synthesis in conidia (Figs. 2 and 3). Third, VosA plays a similar repressive role during sexual sporulation (Fig. 4). These results support that the VosA-VelB complex represses β-glucan synthesis in conidia and ascospores.
TEM results showed that 8 day old conidial wall of the ∆vosA and ∆velB mutant is different from that of WT. Our Northern blot analysis showed that fksA mRNA is detectable at relatively constant levels throughout the lifecycle of A. nidulans except in conidia (data not shown), suggesting that FksA may play a crucial role during fungal growth and development, but not in sporogenesis. While β-1,3-glucan is important for A. nidulans growth and development, the regulatory mechanism of β-1,3-glucan synthesis is largely unknown. Unlike Saccharomyces cerevisiae, transcription of fksA is regulated by non MpkA-RlmA pathway in A. nidulans32,33. We have revealed a new regulatory mechanism of fksA in A. nidulans that the VosA-VelB complex directly represses fksA expression in spores, thereby β-1,3-glucan synthesis.
Our discovery demonstrates that VosA plays a crucial role in both asexual and sexual spores. However, the role of VelB in fksA transcription and β-1,3-glucan synthesis could not be examined in sexual spores, because the ∆velB mutant fails to form any sexual fruiting bodies and sexual spores23,34. To predict the VelB’s role in β-1,3-glucan synthesis in sexual fruiting, we tested whether VelB-ChIP also enriches the promoter regions of fksA in ascospores. Our ChIP-PCR result showed that VelB-FLAG-ChIP enriched the fksA promoter region in ascospores (see Supplementary Fig. S1B online). Based on these results, we propose that the VosA-VelB hetero-complex directly regulates expression of fksA in both conidia and ascospores.
Beta-glucan is a fungal pathogen associated molecular pattern (PAMP) recognized by the pattern recognition receptors (PRRs) including Dectin-1 and Toll-like receptors (TLRs)4,35,36,37. This cell wall component triggers innate immune responses to fungal pathogens including Aspergillus fumigatus38,39,40. In A. fumigatus, the glucan synthase complex forms the catalytic and regulatory subunits41, where the fks1 gene encodes the catalytic subunits. Previously, we have reported the conserved and distinct roles of the velvet genes in A. fumigatus42. To test whether VosA and VelB are involved in β-glucan synthesis in A. fumigatus, we briefly examined mRNA level of fks1 A. fumigatus conidia. Our preliminary Northern blot data indicated that deletion of vosA or velB caused increased fks1 expression in A. fumigatus conidia (data not shown), suggesting that VosA and VelB may play a conserved repressive role in β-glucan synthesis in A. fumigatus spores.
Our microarray data also showed that expression levels genes associated with chitin synthesis, including chsA, chsB, csmA and gfaA, are up-regulated in the ∆vosA conidia compared to WT (Table 1). These genes are required for normal hyphal tip growth, septum formation, cell wall integrity and conidiophore development31,43,44,45,46. Previously we found that VosA-ChIP-chip enriched the promoter regions of csmA and gfaA25. In addition, expression of csmA, gfaA, and other chitin biosynthetic genes was ~2-3 fold elevated in the ∆vosA conidia compared to the WT (Table 1; see Supplementary Fig. S2 online). Based on these results, one can speculate that the VosA-VelB complex may also directly represses chitin biosynthetic genes and chitin levels in conidia. However, as some chitinase encoding genes (chiB and chiC) were also up-regulated in the ∆vosA conidia (Table 1), additional studies of the cell wall polysaccharides composition and structure in the ∆vosA and ∆velB conidia need to be carried out.
An additional discovery of our transcriptome analysis is that the deletion of vosA causes increased expression of genes associated with secondary metabolism in conidia. Particularly, the members of the sterigmatocystin (ST) biosynthesis gene cluster, including stcW, stcV, stcT, stcQ, stcI, stcF, stcE, stcA, and stcL, were up-regulated by mutation of vosA. It has been proposed that the dynamic formation of the velvet complexes may govern the determination of fungal cellular processes47. We speculate that the deletion of vosA results in increased formation of the VelB-VeA-LaeA trimeric complex, which is required for ST production.
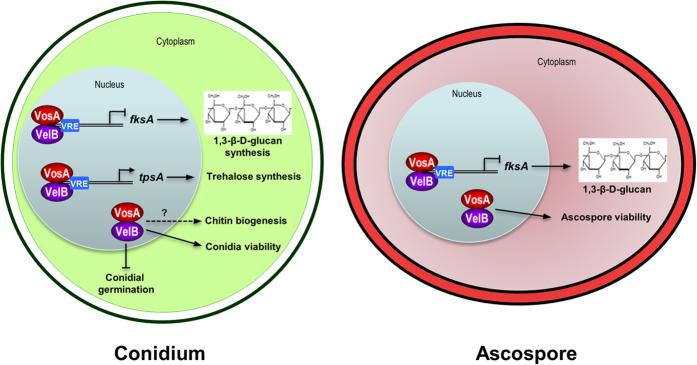
Taken together, we propose a model for the proposed roles of the VosA-VelB complex in both asexual and sexual spores (Fig. 5). Previous studies demonstrated that the VosA-VelB complex directly and/or indirectly controls developmental regulatory genes or sporogenesis-related genes, thereby regulating spore maturation, spore-wall integrity, and trehalose biogenesis in fungal spores. In addition, this hetero-complex directly binds to the fksA promoter region and negatively regulates fksA expression in conidia and ascospores. Overall, our data further corroborate the idea that the VosA-VelB complex is a master regulatory unit for structure, metabolism and physiology in both asexual and sexual spores in A. nidulans.
Figure 5. The roles of VosA and VelB in conidia and ascospores.
A model depicting the regulatory roles of VosA and VelB in integrating morphological and chemical development during conidiogenesis and ascosporogenesis is shown (see Discussion).
Methods
Strains, media and culture conditions
A. nidulans strains used in this study are listed in Table 2. Strains were grown on solid minimal medium (MM), or sexual media (SM) with supplements as described previously47,48 at 37 oC.
Table 2. Aspergillus strains used in this study.
| Strain name | Relevant genotype | References |
|---|---|---|
| FGSC4 | A. nidulans wild type, veA+ | FGSCa |
| RNI14.1 | biA1; ΔvosA::argB+; veA+ | Ni and Yu, 2007 |
| THS14.1 | pyroA4; ΔvosA::pyroA+; ΔvelB::AfupyrG+; veA+ | Park et al., 2012a |
| THS15.1 | pyrG89; pyroA4; ΔvosA::AfupyrG+; veA+ | Park et al., 2012a |
| THS16.1 | pyrG89; pyroA4; ΔvelB::AfupyrG+; veA | Park et al., 2012a |
| THS20.1 | pyrG89; pyroA::velB(p)::velB::FLAG3x::pyroAb; ΔvelB::AfupyrG+; veA+ | Park et al., 2012a |
| THS28.1 | pyrG89; pyroA::vosA(p)::vosA::FLAG3x::pyroAb; ΔvosA::AfupyrG+; veA+ | Park et al., 2012a |
| THS30.1 | pyrG89; AfupyrG+; veA+ | This study |
aFungal Genetic Stock Center.
bThe 3/4 pyroA marker causes targeted integration at the pyroA locus.
Nucleic acid manipulation
The oligonucleotides used in this study are listed in Table 3. Total RNA isolation and Northern blot analyses were carried out as previously described23. The DNA probes were prepared by PCR-amplification of the coding regions of individual genes with appropriate oligonucleotide pairs using FGSC4 genomic DNA as a template.
Table 3. Oligonucleotides used in this study.
| Name | Sequence (5′ → 3′) | Purpose |
|---|---|---|
| OMN143 | ACTTATGCCAACGTTCTGCG | 5′ fksA probe |
| OMN144 | AAAGAGCGGGCAGCATAATG | 3′ fksA probe |
| OHS518 | GCTGCAAGCGTGACATTGCGAAG | 5′ gelA probe |
| OHS519 | GTACCGGCGTTCTGGCTACCAG | 3′ gelA probe |
| OHS520 | GTAAGTTCACCGAGGTTCAGGCC | 5′ gelB probe |
| OHS521 | AGGCAGGTAACCAGCACCGTTTG | 3′ gelB probe |
| OHS972 | ATGAAGTACTCTTTCGCCCTCACC | 5′ sunA probe |
| OHS973 | CTAGTAGAAGACGAAGGTAGCGTC | 3′ sunA probe |
| OHS974 | ATGACCCTTTTTCGAAACCTGGC | 5′ crhD probe |
| OHS975 | TTAGCGCACAGGCTGGTAGCC | 3′ crhD probe |
| OHS976 | ATGAGGGGAGCTATCCTGGCC | 5′ btgE probe |
| OHS977 | GTGCTGCAAGCAGCTCCAAC | 3′ btgE probe |
| OHS978 | ATGCGCGTCGCCGGGATTAT | 5′ btgA probe |
| OHS979 | CTAGTTCGGGCACGACAAATCG | 3′ btgA probe |
| OHS980 | ATGAAGTTCTCCAGCATCCTTGC | 5′ gelC probe |
| OHS981 | GATGCAGATGTGCCAGAGGGAC | 3′ gelC probe |
| OJA174 | CATTGAGCACGGTGTTGT | 5′ γ-actin probe |
| OJA175 | ATCCCTTGATCTCGTTTG | 3′ γ-actin probe |
| OHS622 | AGCCGAGCGATCAACTCTTG | 5′ fksA_pro_ChIP-PCR |
| OHS623 | CGATGTCTGGAACTGGTATGCAG | 3′ fksA_pro_ChIP-PCR |
| OHS624 | CTCGGTTGTGGATTCCTAATCTC | 5′ fksA_orf_ChIP-PCR |
| OHS625 | AATAGGCTCCATGCCCTTGG | 3′ fksA_orf_ChIP-PCR |
Microarray and data analysis
Two-day old conidia of ∆vosA (RNI14.1) and WT (FGSC4) strains were collected and ground in liquid N2 with a mortar and pestle. Total RNA was extracted by the acid-phenol method and harvested using CsCl density gradient ultracentrifugation. The fluorescent-labeled target cDNA probes were synthesized using the EZ start RNA linear amplification kit following manufacturer’s instructions (GenomicTree, Korea). Briefly, total RNA (1 μg) was reverse transcribed in the presence of 2 μg of 5′-CTACGCTGGGCCGACCGGGCGCGGGAC-3′-dT24 primer (Bioneer, Korea) at 42 °C for 2 h to generate single stranded cDNA. The reaction mixtures were further treated with RNase H (Invitrogen), and double stranded cDNAs were synthesized in the presence of Escherichia coli DNA ligase and DNA polymerase I (Invitrogen). Double stranded cDNAs were then purified by the Qiaquick PCR purification kit (Qiagen, USA), and further labeled with Aminoallyl-dUTP (Sigma, USA) in the presence of 5′-CTACGCTGGGCCGACCGGGCGCGGGAC-3′ primer (Bioneer) and Taq polymerase (Solgent). The amplified products were purified using a Qiaquick PCR purification kit (Qiagen), and further coupled with monofunctional cyanine dye as recommended by manufacturer (Amersham; Buckinghamshire, UK). The cDNAs synthesized from reference RNA were labeled with Cy3-dUTP, and cDNAs synthesized from total RNA taken from indicated time points were labeled with Cy5-dUTP. The labeled cDNA targets were purified using a Qiaquick purification kit (Qiagen), and dissolved in 100 μl of hybridization solution containing 5 × SSC, 0.1% SDS, 30% formamide, and 20 μg of salmon sperm DNA (Invitrogen). The hybridization mixtures were heated at 100 °C for 3 min and directly applied onto the 70-mer glass slide DNA microarrays (Tiger-PFGRC, USA). Two biological replicates were performed using cultures grown in parallel.
After hybridization, microarray slides were imaged using the laser scanner (Axon 4000B; Axon, USA). The signal and background fluorescence intensities were calculated for each probe spot by averaging the intensities of every pixel inside the target region using GenePix Pro 4.0 software (Axon). The signal intensity for each spot is the difference between the median pixel signal intensity and the median local background intensity. Spots exhibiting obvious abnormalities were excluded from analysis. All data normalization, statistical analyses and cluster analyses were performed using the GeneSpring 7.3.1 (Agilent, USA). Genes were further filtered according to their intensity in both channels, and thus the spots with average intensity of both channels ≥10 after normalization were considered as reliable ones.
The information of each A. nidulans gene that was downloaded from AspGD (http://www.aspergillusgenome.org/), the chromosomal feature of A. nidulans FGSC A4 was updated on 2 March, 2014. The functional categories of A. nidulans FGSC A4 were assigned using the MIPS PEDANT 3 database (http://pedant.gsf.de/genomes.jsp?category=fungal)49, KOGs database50 was from the version p3_p130_Asp_nidul (calculation date 11 August, 2007), GO database was from the version p3_r40559_Asp_nidul (calculation date 19 September, 2013).
Transmission electron microscopy (TEM)
Samples were prepared following the methods as described21. Briefly, conidia were collected from the 8 day cultures on solid media. Samples were fixed in Karnovsky’s fixative, post –fixed in 2% osmium tetroxide, dehydrated in graded ethanol series. Polymerized samples were sectioned on a Leica UC6 ultramicrotome (80 nm) and stained with uranyl acetate and lead citrate. The stained sections were viewed on a JEOL 100CX transmission electron microscope, and documented with a SIS (Soft Imaging Systems, Lakewood, CO) MegaView III digital side mount camera.
β-glucan analysis
The β-1,3-glucan amounts in spores were determined by the Glucatell® assay (Associates of Cape Cod, USA) following the manufacturer’s instructions51. Briefly, two-day old conidia, or eight-day old ascospores of WT and mutants were collected in ddH2O. Samples of spores suspension (102 ~ 105) were prepared and resuspended in 25 μl of ddH2O. Each spores sample was mixed with 25 μl of Glucatell® reagent and incubated at 37 oC for 30 minutes. After incubation, diazo-reagents were added to stop the reaction, and optical density at 540 nm was determined. This test was performed in triplicate.
Chromatin Immuno-precipitation followed by PCR (ChIP-PCR)
Samples were prepared following the methods as described25. Briefly, ChIP-PCR analysis was performed according to the manufacturer’s instructions with a minor modification using MAGnify Chromatin Immuno-precipitation System (Invitrogen). Two-day-old conidia or eight-day-old ascospores (1 × 109) of each strain were cross-linked, washed and homogenized by a mini-bead beater. The cell lysates were sonicated for four cycles (30 s on, 60 s off) with a sonifier. The sonicated cell lysates were cleared by centrifugation. The diluted chromatin extracts were incubated with 2 μg of anti-FLAG antibody-Dynabeads complex and washed with the IP buffer. The input control and chromatin samples were eluted from the beads at 55 °C for 15 min with a reverse crosslinking buffer with Proteinase K. Enriched DNA was purified by DNA purification Magnetic Beads (Invitrogen). For amplification by PCR, the GO Taq DNA polymerase (Promega) was used. As negative controls, the chromatin extract being incubated with bead only (without anti-FLAG antibody) and samples of the fungal strain THS30 without FLAG-tagged VosA or VelB were used. Individual input DNA samples before immune-precipitation (IP) were used as positive controls. Two biological replicates have provided the essentially identical ChIP-PCR results. The primer sets used for PCR are shown in Table 3.
Additional Information
How to cite this article: Park, H.-S. et al. Velvet-mediated repression of ß-glucan synthesis in Aspergillus nidulans spores. Sci. Rep. 5, 10199; doi: 10.1038/srep10199 (2015).
Supplementary Material
Acknowledgments
We thank our lab members for helpful discussion. The work at UW-Madison was supported by USDA CSREES Hatch (WIS01195) and the Intelligent Synthetic Biology Center of Global Frontier Project (2011-0031955) funded by the Ministry of Education, Science and Technology grants to JHY. The work at CNU (PJM) was supported by Grant 2009-0070380 from the National Research Foundation (NRF) of Korea.
Footnotes
The authors declare no competing financial interests.
Author Contributions H.S.P., Y.M.Y. and M.K.L. performed the experiments. H.S.P., Y.M.Y., P.J.M., S.C.K. and J.H.Y. designed the experiments and analyzed the data. H.S.P.. Y.M.Y., M.K.L. and J.H.Y. write the main manuscript text. H.S.P. and Y.M.Y. prepared all Figures and Tables. All authors reviewed the manuscript.
References
- Klis F. M., de Jong M., Brul S. & de Groot P. W. Extraction of cell surface-associated proteins from living yeast cells. Yeast 24, 253–8 (2007). [DOI] [PubMed] [Google Scholar]
- Osherov N. & Yarden O. [The Cell Wall of Filamentous Fungi]. Cellular and Molecular Biology of Filamentous Fungi [224–237] ASM Press, Washington, 2010). [Google Scholar]
- Latge J. P. The cell wall: a carbohydrate armour for the fungal cell. Mol Microbiol 66, 279–90 (2007). [DOI] [PubMed] [Google Scholar]
- Latge J. P. Tasting the fungal cell wall. Cell Microbiol. 12, 863–72 (2010). [DOI] [PubMed] [Google Scholar]
- Free S. J. Fungal cell wall organization and biosynthesis. Adv. Genet. 81, 33–82 (2013). [DOI] [PubMed] [Google Scholar]
- Bowman S. M. & Free S.J. The structure and synthesis of the fungal cell wall. Bioessays 28, 799–808 (2006). [DOI] [PubMed] [Google Scholar]
- Klis F. M., Mol P., Hellingwerf K. & Brul S. Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol Rev 26, 239–56 (2002). [DOI] [PubMed] [Google Scholar]
- Butts A. & Krysan D. J. Antifungal drug discovery: something old and something new. PLoS Pathog. 8, e1002870 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada R., Latge J. P. & Aimanianda V. Undressing the fungal cell wall/cell membrane--the antifungal drug targets. Curr. Pharm. Des. 19, 3738–47 (2013). [DOI] [PubMed] [Google Scholar]
- Yoshimi A. et al. Functional analysis of the alpha-1,3-glucan synthase genes agsA and agsB in Aspergillus nidulans: agsB is the major alpha-1,3-glucan synthase in this fungus. PLoS One 8, e54893 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Li S. & Kaminskyj S. G. Characterization of Aspergillus nidulans alpha-glucan synthesis: roles for two synthases and two amylases. Mol. Microbiol. 91, 579–95 (2013). [DOI] [PubMed] [Google Scholar]
- Kelly R., Register E., Hsu M. J., Kurtz M. & Nielsen J. Isolation of a gene involved in 1,3-beta-glucan synthesis in Aspergillus nidulans and purification of the corresponding protein. J. Bacteriol. 178, 4381–91 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi H. Functional diversity of chitin synthases of Aspergillus nidulans in hyphal growth, conidiophore development and septum formation. Med. Mycol. 47 Suppl 1, S47–52 (2009). [DOI] [PubMed] [Google Scholar]
- de Groot P. W. et al. Comprehensive genomic analysis of cell wall genes in Aspergillus nidulans. Fungal Genet Biol. 46 Suppl 1, S72–81 (2009). [DOI] [PubMed] [Google Scholar]
- Adams T. H., Wieser J. K. & Yu J.-H. Asexual sporulation in Aspergillus nidulans. Microbiol. Mol. Biol. Rev. 62, 35–54 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbole D. J. [The Conidium]. Cellular and Molecular Biology of Filamentous Fungi. [577–590] ASM Press, Washington, 2010). [Google Scholar]
- Timberlake W. E. Molecular genetics of Aspergillus development. Annu. Rev. Genet. 24, 5–36 (1990). [DOI] [PubMed] [Google Scholar]
- Mims C. W., Richardson E. A. & Timberlake W. E. Ultrastructural analysis of conidiophore development in the fungus Aspergillus nidulans using freeze-substitution. Protoplasma 144, 132–141 (1988). [Google Scholar]
- Sewall T. C., Mims C. W. & Timberlake W. E. Conidium differentiation in Aspergillus nidulans wild-type and wet-white (wetA) mutant strains. Dev. Biol. 138, 499–508 (1990). [DOI] [PubMed] [Google Scholar]
- Ni M., Gao N., Kwon N.-J., Shin K.-S. & Yu J.-H. [Regulation of Aspergillus Conidiation]. Cellular and Molecular Biology of Filamentous Fungi [559–576] ASM Press, Washington, 2010). [Google Scholar]
- Ni M. & Yu J.-H. A novel regulator couples sporogenesis and trehalose biogenesis in Aspergillus nidulans. PLoS One 2, e970 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarikaya Bayram O. et al. LaeA control of velvet family regulatory proteins for light-dependent development and fungal cell-type specificity. Plos Genetics 6, e1001226 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.-S., Ni M., Jeong K. C., Kim Y. H. & Yu J.-H. The role, interaction and regulation of the velvet regulator VelB in Aspergillus nidulans. PLoS One 7, e45935 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayram O. & Braus G. H. Coordination of secondary metabolism and development in fungi: the velvet family of regulatory proteins. FEMS Microbiol Rev. 36, 1–24 (2012). [DOI] [PubMed] [Google Scholar]
- Ahmed Y. L. et al. The velvet family of fungal regulators contains a DNA-binding domain structurally similar to NF-κB. PLoS Biol 11, e1001750 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyhan S., Gutierrez M., Voorhies M. & Sil A. A temperature-responsive network links cell shape and virulence traits in a primary fungal pathogen. PLoS Biol. 11, e1001614 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.-S. & Yu J.-H. Genetic control of asexual sporulation in filamentous fungi. Curr Opin Microbiol 15, 669–77 (2012). [DOI] [PubMed] [Google Scholar]
- Fujiwara M. et al. Evidence that the Aspergillus nidulans class I and class II chitin synthase genes, chsC and chsA, share critical roles in hyphal wall integrity and conidiophore development. J Biochem. 127, 359–66 (2000). [DOI] [PubMed] [Google Scholar]
- Yanai K. et al. Isolation and characterization of two chitin synthase genes from Aspergillus nidulans. Biosci. Biotechnol. Biochem. 58, 1828–35 (1994). [DOI] [PubMed] [Google Scholar]
- Fukuda K. et al. Class III chitin synthase ChsB of Aspergillus nidulans localizes at the sites of polarized cell wall synthesis and is required for conidial development. Eukaryot Cell 8, 945–56 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motoyama T. et al. The Aspergillus nidulans genes chsA and chsD encode chitin synthases which have redundant functions in conidia formation Mol. Gen. Genet. 253, 520–8 (1997). [DOI] [PubMed] [Google Scholar]
- Jung U. S. & Levin D. E. Genome-wide analysis of gene expression regulated by the yeast cell wall integrity signalling pathway. Mol. Microbiol. 34, 1049–57 (1999). [DOI] [PubMed] [Google Scholar]
- Fujioka T. et al. MpkA-Dependent and -independent cell wall integrity signaling in Aspergillus nidulans. Eukaryot Cell 6, 1497–510 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayram O. et al. VelB/VeA/LaeA complex coordinates light signal with fungal development and secondary metabolism. Science 320, 1504–6 (2008). [DOI] [PubMed] [Google Scholar]
- Brown G. D. & Gordon S. Fungal beta-glucans and mammalian immunity. Immunity 19, 311–5 (2003). [DOI] [PubMed] [Google Scholar]
- Tsoni S. V. & Brown G. D. beta-Glucans and dectin-1. Ann. N. Y. Acad. Sci. 1143, 45–60 (2008). [DOI] [PubMed] [Google Scholar]
- Romani L. Immunity to fungal infections. Nat. Rev. Immunol. 11, 275–88 (2011). [DOI] [PubMed] [Google Scholar]
- Steele C. et al. The beta-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog. 1, e42 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohl T. M. et al. Aspergillus fumigatus triggers inflammatory responses by stage-specific beta-glucan display. PLoS Pathog. 1, e30 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey L. K., Moeller J. B., Schlosser A., Sorensen G. L. & Holmskov U. Induction of innate immunity by Aspergillus fumigatus cell wall polysaccharides is enhanced by the composite presentation of chitin and beta-glucan. Immunobiology 219, 179–88 (2014). [DOI] [PubMed] [Google Scholar]
- Beauvais A. et al. Glucan synthase complex of Aspergillus fumigatus. J. Bacteriol. 183, 2273–9 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.-S., Bayram O., Braus G. H., Kim S. C. & Yu J.-H. Characterization of the velvet regulators in Aspergillus fumigatus. Mol. Microbiol. 86, 937–53 (2012). [DOI] [PubMed] [Google Scholar]
- Horiuchi H., Fujiwara M., Yamashita S., Ohta A. & Takagi M. Proliferation of intrahyphal hyphae caused by disruption of csmA, which encodes a class V chitin synthase with a myosin motor-like domain in Aspergillus nidulans. J. Bacteriol. 181, 3721–9 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culp D. W. et al. The chsA gene from Aspergillus nidulans is necessary for maximal conidiation. FEMS Microbiol Lett 182, 349–53 (2000). [DOI] [PubMed] [Google Scholar]
- Ichinomiya M., Yamada E., Yamashita S., Ohta A. & Horiuchi H. Class I and class II chitin synthases are involved in septum formation in the filamentous fungus Aspergillus nidulans. Eukaryot Cell 4, 1125–36 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita N., Yamashita S., Ohta A. & Horiuchi H. Aspergillus nidulans class V and VI chitin synthases CsmA and CsmB, each with a myosin motor-like domain, perform compensatory functions that are essential for hyphal tip growth. Mol. Microbiol. 59, 1380–94 (2006). [DOI] [PubMed] [Google Scholar]
- Park H.-S., Nam T. Y., Han K.-H., Kim S. C. & Yu J.-H. VelC positively controls sexual development in Aspergillus nidulans. PLoS One 9, e89883 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafer E. Meiotic and mitotic recombination in Aspergillus and its chromosomal aberrations. Adv Genet 19, 33–131 (1977). [DOI] [PubMed] [Google Scholar]
- Walter M. C. et al. PEDANT covers all complete RefSeq genomes. Nucleic Acids Res 37, D408–11 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatusov R. L. et al. The COG database: an updated version includes eukaryotes. BMC Bioinformatics 4, 41 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odabasi Z. et al. Beta-D-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clin. Infect. Dis. 39, 199–205 (2004). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.