Abstract
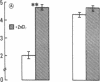
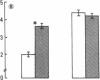
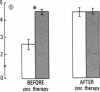
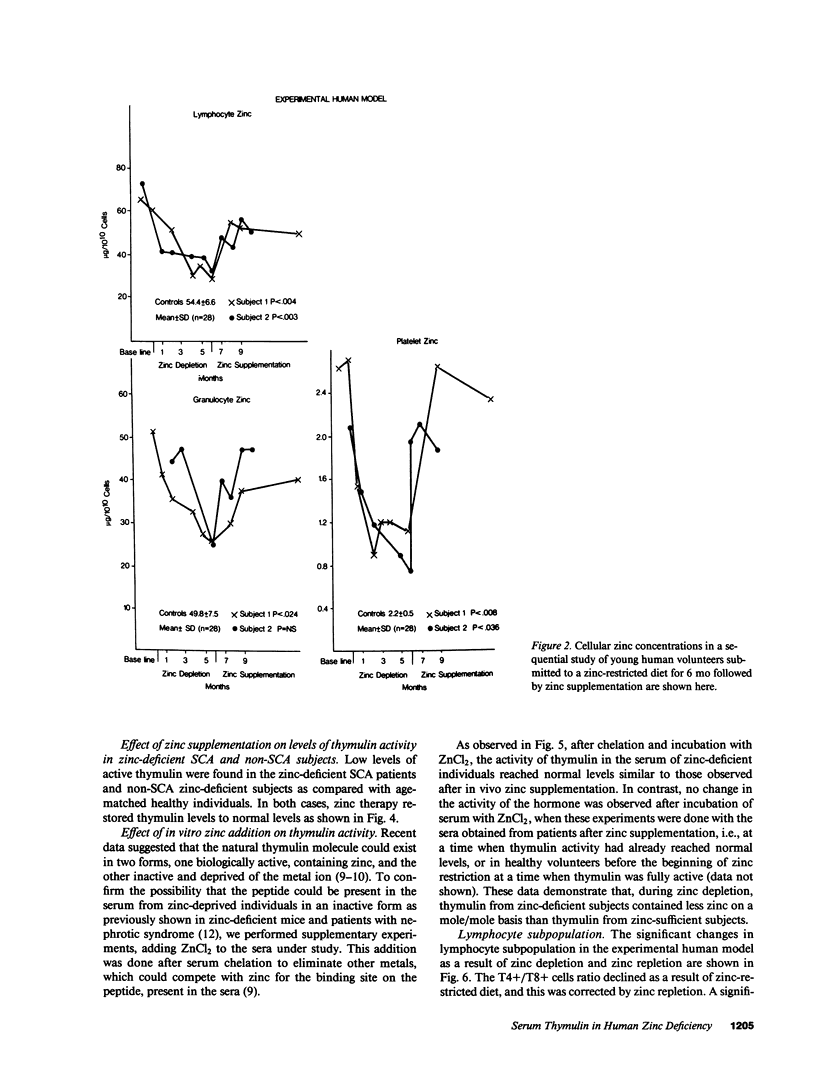
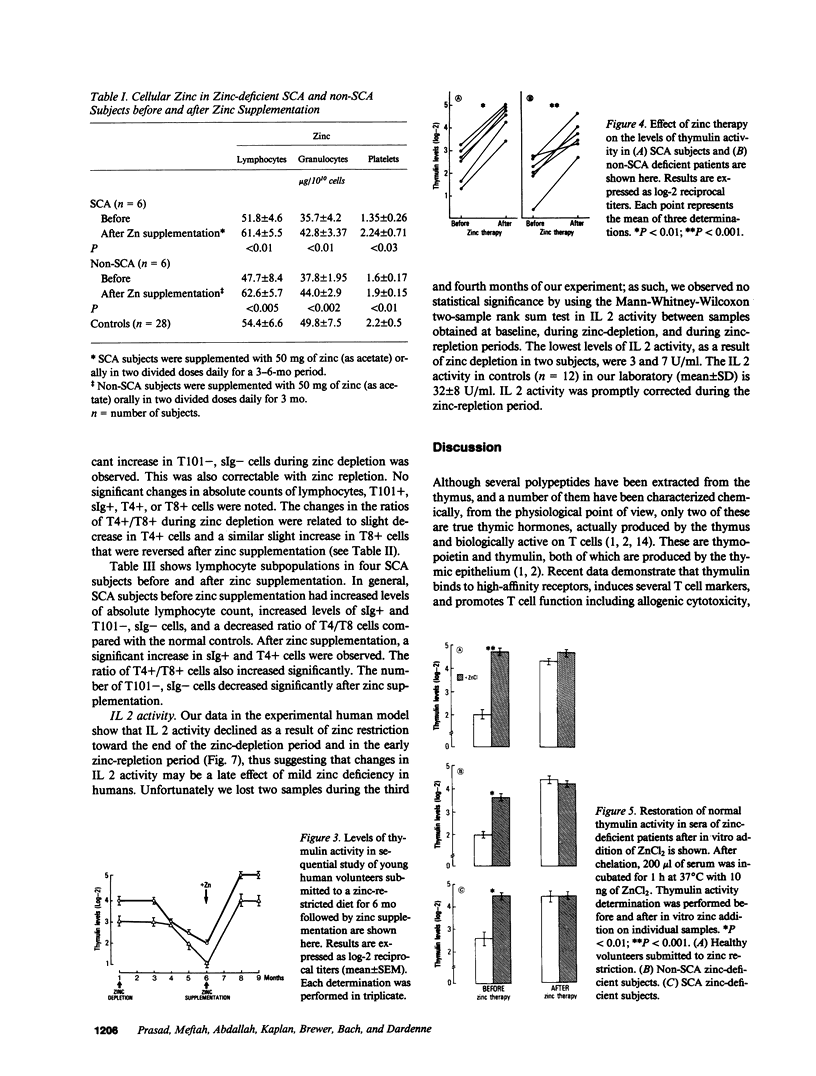
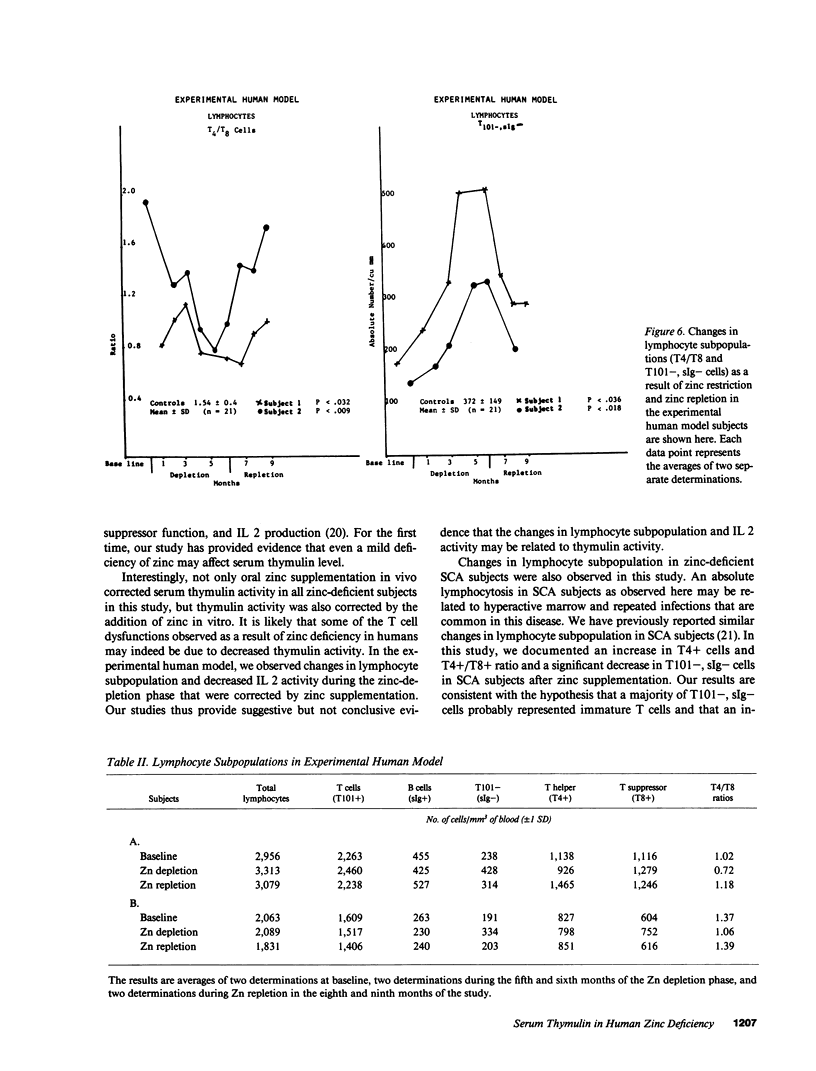
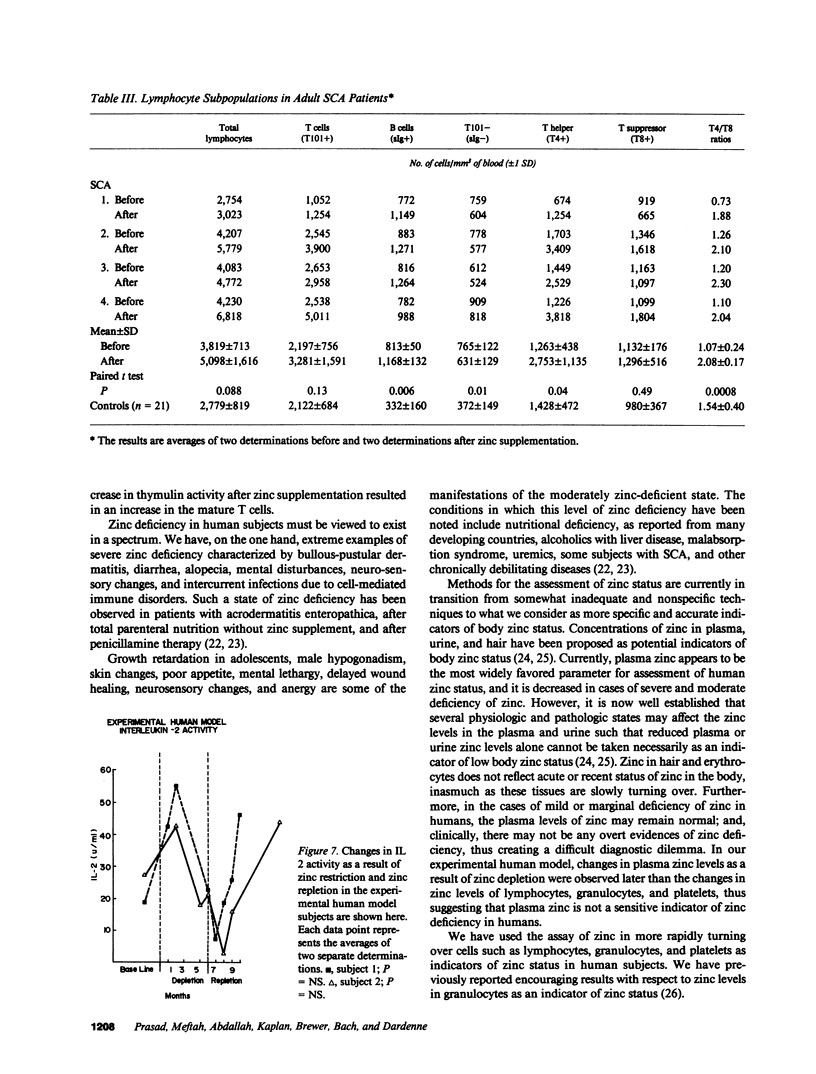
The activity of thymulin (a thymic hormone) is dependent on the presence of zinc in the molecule. We assayed serum thymulin activity in three models of mildly zinc-deficient (ZD) human subjects before and after zinc supplementation: (a) two human volunteers in whom a specific and mild zinc deficiency was induced by dietary means; (b) six mildly ZD adult sickle cell anemia (SCA) subjects; and (c) six mildly ZD adult non-SCA subjects. Their plasma zinc levels were normal and they showed no overt clinical manifestations of zinc deficiency. The diagnosis of mild zinc deficiency was based on the assay of zinc in lymphocytes, granulocytes, and platelets. Serum thymulin activity was decreased as a result of mild zinc deficiency and was corrected by in vivo and in vitro zinc supplementation, suggesting that this parameter was a sensitive indicator of zinc deficiency in humans. An increase in T101-, sIg-cells, decrease in T4+/T8+ ratio, and decreased IL 2 activity were observed in the experimental human model during the zinc depletion phase, all of which were corrected after repletion with zinc. Similar changes in lymphocyte subpopulation, correctable with zinc supplementation, were also observed in mildly ZD SCA subjects. Inasmuch as thymulin is known to induce intra- and extrathymic T cell differentiation, our studies provide a possible mechanism for the role of zinc on T cell functions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbasi A. A., Prasad A. S., Rabbani P., DuMouchelle E. Experimental zinc deficiency in man. Effect on testicular function. J Lab Clin Med. 1980 Sep;96(3):544–550. [PubMed] [Google Scholar]
- Allen J. I., Perri R. T., McClain C. J., Kay N. E. Alterations in human natural killer cell activity and monocyte cytotoxicity induced by zinc deficiency. J Lab Clin Med. 1983 Oct;102(4):577–589. [PubMed] [Google Scholar]
- Amor B., Dougados M., Mery C., de Gery A., Choay J., Dardenne M., Bach J. F. Thymuline (FTS) in rheumatoid arthritis. Arthritis Rheum. 1984 Jan;27(1):117–118. doi: 10.1002/art.1780270124. [DOI] [PubMed] [Google Scholar]
- Ballester O. F., Abdallah J. M., Prasad A. S. Lymphocyte subpopulation abnormalities in sickle cell anemia: a distinctive pattern from that of AIDS. Am J Hematol. 1986 Jan;21(1):23–27. doi: 10.1002/ajh.2830210104. [DOI] [PubMed] [Google Scholar]
- Bensman A., Dardenne M., Murnaghan K., Vasmant D., Bach J. F. Decreased biological activity of serum thymic hormone (thymulin) in children with nephrotic syndrome. Int J Pediatr Nephrol. 1984 Dec;5(4):201–204. [PubMed] [Google Scholar]
- Bordigoni P., Faure G., Bene M. C., Dardenne M., Bach J. F., Duheille J., Olive D. Improvement of cellular immunity and IgA production in immunodeficient children after treatment with synthetic serum thymic factor (FTS). Lancet. 1982 Aug 7;2(8293):293–297. doi: 10.1016/s0140-6736(82)90271-9. [DOI] [PubMed] [Google Scholar]
- Dardenne M., Pléau J. M., Nabarra B., Lefrancier P., Derrien M., Choay J., Bach J. F. Contribution of zinc and other metals to the biological activity of the serum thymic factor. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5370–5373. doi: 10.1073/pnas.79.17.5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardenne M., Savino W., Berrih S., Bach J. F. A zinc-dependent epitope on the molecule of thymulin, a thymic hormone. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7035–7038. doi: 10.1073/pnas.82.20.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardenne M., Savino W., Wade S., Kaiserlian D., Lemonnier D., Bach J. F. In vivo and in vitro studies of thymulin in marginally zinc-deficient mice. Eur J Immunol. 1984 May;14(5):454–458. doi: 10.1002/eji.1830140513. [DOI] [PubMed] [Google Scholar]
- Fabris N., Mocchegiani E. Endocrine control of thymic serum factor production in young-adult and old mice. Cell Immunol. 1985 Apr 1;91(2):325–335. doi: 10.1016/0008-8749(85)90230-8. [DOI] [PubMed] [Google Scholar]
- Foster D. M., Aamodt R. L., Henkin R. I., Berman M. Zinc metabolism in humans: a kinetic model. Am J Physiol. 1979 Nov;237(5):R340–R349. doi: 10.1152/ajpregu.1979.237.5.R340. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Gershwin M. E., Good R. A., Prasad A. Interrelationships between zinc and immune function. Fed Proc. 1986 Apr;45(5):1474–1479. [PubMed] [Google Scholar]
- Fraker P. J., Haas S. M., Luecke R. W. Effect of zinc deficiency on the immune response of the young adult A/J mouse. J Nutr. 1977 Oct;107(10):1889–1895. doi: 10.1093/jn/107.10.1889. [DOI] [PubMed] [Google Scholar]
- Gastinel L. N., Dardenne M., Pleau J. M., Bach J. F. Studies on the zinc binding site to the serum thymic factor. Biochim Biophys Acta. 1984 Feb 14;797(2):147–155. doi: 10.1016/0304-4165(84)90116-8. [DOI] [PubMed] [Google Scholar]
- Gillis S., Kozak R., Durante M., Weksler M. E. Immunological studies of aging. Decreased production of and response to T cell growth factor by lymphocytes from aged humans. J Clin Invest. 1981 Apr;67(4):937–942. doi: 10.1172/JCI110143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabarra B., Halpern S., Kaiserlian D., Dardenne M. Localization of zinc in the thymic reticulum of mice by electron-probe microanalysis. Cell Tissue Res. 1984;238(1):209–212. doi: 10.1007/BF00215165. [DOI] [PubMed] [Google Scholar]
- Palacios R., Alarcón-Segovia D., Llorente L., Ruíz-Arguelles A., Díaz-Jouanen E. Human postthymic precursor cells in health and disease. II. Their loss and dysfunction in systemic lupus erythematosus and their partial correction with serum thymic factor. J Clin Lab Immunol. 1981 Mar;5(2):71–80. [PubMed] [Google Scholar]
- Pléau J. M., Fuentes V., Morgat J. L., Bach J. F. Specific receptors for the serum thymic factor (FTS) in lymphoblastoid cultured cell lines. Proc Natl Acad Sci U S A. 1980 May;77(5):2861–2865. doi: 10.1073/pnas.77.5.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad A. S., Cossack Z. T. Neutrophil zinc: an indicator of zinc status in man. Trans Assoc Am Physicians. 1982;95:165–176. [PubMed] [Google Scholar]
- Prasad A. S. Discovery and importance of zinc in human nutrition. Fed Proc. 1984 Oct;43(13):2829–2834. [PubMed] [Google Scholar]
- Prasad A. S., Oberleas D. Binding of zinc to amino acids and serum proteins in vitro. J Lab Clin Med. 1970 Sep;76(3):416–425. [PubMed] [Google Scholar]
- Prasad A. S., Oberleas D. Changes in activities of zinc-dependent enzymes in zinc-deficient tissues of rats. J Appl Physiol. 1971 Dec;31(6):842–846. doi: 10.1152/jappl.1971.31.6.842. [DOI] [PubMed] [Google Scholar]
- Prasad A. S., Rabbani P., Abbasii A., Bowersox E., Fox M. R. Experimental zinc deficiency in humans. Ann Intern Med. 1978 Oct;89(4):483–490. doi: 10.7326/0003-4819-89-4-483. [DOI] [PubMed] [Google Scholar]
- Rabbani P. I., Prasad A. S., Tsai R., Harland B. F., Fox M. R. Dietary model for production of experimental zinc deficiency in man. Am J Clin Nutr. 1987 Jun;45(6):1514–1525. doi: 10.1093/ajcn/45.6.1514. [DOI] [PubMed] [Google Scholar]
- Savino W., Dardenne M., Papiernik M., Bach J. F. Thymic hormone-containing cells. Characterization and localization of serum thymic factor in young mouse thymus studied by monoclonal antibodies. J Exp Med. 1982 Aug 1;156(2):628–633. doi: 10.1084/jem.156.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapazoglou E., Prasad A. S., Hill G., Brewer G. J., Kaplan J. Decreased natural killer cell activity in patients with zinc deficiency with sickle cell disease. J Lab Clin Med. 1985 Jan;105(1):19–22. [PubMed] [Google Scholar]
- Whitehouse R. C., Prasad A. S., Rabbani P. I., Cossack Z. T. Zinc in plasma, neutrophils, lymphocytes, and erythrocytes as determined by flameless atomic absorption spectrophotometry. Clin Chem. 1982 Mar;28(3):475–480. [PubMed] [Google Scholar]