Abstract
Invasive aspergillosis caused by Aspergillus species (Aspergillus fumigatus, A. flavus, and A. terreus) is life-threatening infections in immunocompromised patients. Understanding the innate and adaptive immune response particularly T-helper cells (TH-cells) against these Aspergillus species and how the different sub-set of TH-cells are regulated by differentiating cytokines at primary target organ site like lung, kidney and brain is of great significance to human health. This review focuses on presentation of Aspergillus through Antigen presenting cells (APCs) to the naive CD4+ T-cells in the host. The production of differentiating/effector cytokines that activate following TH-cells, e.g., TH1, TH2, TH9, and TH17 has been reported in association or alone in allergic or invasive aspergillosis. Chemokines (CXCL1, CXCL2, CCL1, and CCL20) and their receptors associated to these TH-cells have also been observed in invasive aspergillosis. Thus, further study of these TH-cells in invasive aspergillosis and other elements of adaptive immune response with Aspergillus species are required in order to have a better understanding of host response for safer and effective therapeutic outcome.
Keywords: cytokines, T-helper cells, dendritic cells, Aspergillus, antigen presenting cells, invasive aspergillosis
Introduction
Fungi are the most common microorganisms and have clinical importance. Few of them are pathogenic or opportunistic pathogen and results in morbidity and mortality to human beings. There is a rise in opportunistic fungal infections in recent years due to increased incidence of immunocompromised host (Chamilos et al., 2006; Romani, 2008). After Candida albicans, the leading causes of fungal infections in immunocompromised individuals are from Aspergillus species. Aspergillus is one of the most ubiquitous medically important opportunistic fungi (Weaver et al., 2007). The genus Aspergillus, contains about 40 species that can cause infection (Verweij and Brandt, 2007), among them A. fumigatus, A. flavus, and A. terreus are the leading cause of invasive Aspergillosis in immunocompromised individuals. These species produce conidia at a concentration of around 1–100 conidia per m3 (Barnes and Marr, 2006). Human routinely inhale hundreds of these conidia per day, despite these exposure to Aspergillus conidia, human do not develop any disease due to the clearance of conidia from lung by innate immunity especially phagocytic cells (Chamilos et al., 2006; Romani, 2008). However, due to rise in immunocompromised host, e.g., patients receiving organ transplant, immunosuppressive therapy for autoimmune or neoplastic disease and HIV patients, inhaled conidia if not cleared in these host, colonization of Aspergillus occurs (Stevens et al., 2000). The adaptive immune response in human responsible for conidia clearance is not well understood in immunocompetent host as well as where conidia colonize in immunocompromised host. It is worth to note that secondary metabolites (e.g., Gliotoxins, Aflatoxins) excreted by Aspergillus especially have been recognized to modulate immunological responses (Shankar, 2013). Thus, we reviewed recent advances made in immune responses against Aspergillus species in mice model studies and clinical aspergillosis patients. A. fumigatus is the prominent species, which cause 90% of Aspergillosis followed by A. flavus and A. terreus. Studies have showed that Aspergillus species associated with infection after hematopoietic stem cell transplantation include A. fumigatus with 56% followed by A. flavus (18.7%) and A. terreus (16%) (Steinbach et al., 2004; Morgan et al., 2005). The involvement of Aspergillus infection in pulmonary tuberculosis and in asthmatic patients has been reported by Denning et al. It has been estimated annually at least 372,385 patients developed chronic pulmonary aspergillosis worldwide following treated pulmonary tuberculosis (Denning et al., 2011). Similarly, around 4,837,000 patients develop Allergic bronchopulmonary aspergillosis out of 193 million adults with active asthma (Denning et al., 2013). However, in a recent study of Indian population by Agarwal et al. (2014) the estimated ABPA burden was 1.38 million out of 27.6 million adults with asthma. This review touches different aspect of antifungal immunity against aspergillosis that include antigen presenting cells (APCs), dendritic cells (DCs), fungal pattern recognition receptors (PRRs), TH-cells with their subsets profile during infection associated to Aspergillus species at different site of infection, e.g., lung, kidney, and brain.
Recognition of Aspergillus by the Host
Presentation of Pathogen via Soluble Receptors and Cell Bound Receptors
After the inhalation of A. fumigatus conidia, they are entrapped by the lung alveoli and if they are not efficiently cleared from lung, they germinate and establish lung infection termed invasive pulmonary aspergillosis and it may also disseminates to other organs if not treated (Park and Mehrad, 2009). The recognition of A. fumigatus conidia and hyphae occurs by PRRs those include soluble receptors and cell-bound receptors. Conidial germination starts with hydrophobic layer degradation and exposure of inner cell wall components mainly polysaccharides, which includes chitin, β-glucan, mannan, and galactomannan. These are termed as pathogen associated molecular patterns (PAMP), are recognized by PRRs (Netea et al., 2006; Inoue and Shinohara, 2014). PRRs soluble receptor such as pulmonary collectins, family of C-type lectins, pentraxin-3, pulmonary surfactant proteins-A and D have been reported in aspergillosis. Further, the cell-bound receptors in association with aspergillosis include Toll like receptor-2 (TLR), TLR-4 and TLR-9, which potentially induce the production of pro-inflammatory cytokines and reactive oxygen species through MyD88 signaling pathway (Willment and Brown, 2008).
Antigen Presenting Cells and T-cell Differentiation
Antigen Presenting Cells Triggers Cytokines Production
The activation of the innate immunity through PRRs present on the APCs that regulate the development of T-cell. APCs express wide-array of PRRs that provides the link between adaptive and innate immunity (Park and Mehrad, 2009). APCs, dominantly DCs, are responsible for antigen monitoring and then shaping T-cell response by secreting cytokines and chemokines. DCs express PRRs on their cell surface and endosomal compartments, which serve to recognize PAMPs. After interaction with DCs, naive T-cells are activated. The activation of T-cell response is regulated by the cytokines milieu predominantly framed by DCs (Akdis et al., 2011). Chemokines secreted by DCs recruit the phagocytic cells to infected areas to clear the Aspergillus components. APC cells, e.g., monocytes differentiate into distinct sub-populations of CD14+ and CD16+ cells after A. fumigatus conidia infection (Serbina et al., 2009). Monocytes interact with Aspergillus antigens resulting in maturation of monocytes into macrophages or DCs. Macrophages and DCs interact with antigens and secrete effector cytokines (Osugi et al., 2002; Ramirez-Ortiz and Means, 2012). Major sub-populations of DCs are myeloid DCs, plasmacytoid DC (pDCs) and monocyte-derived DCs (Bozza et al., 2002; Osugi et al., 2002). pDC recognize the nucleic acids from A. fumigatus via TLR-9 and lead to resistance to A. fumigatus infection in mice (Ramirez-Ortiz et al., 2008). Further, monocytes migrate toward the lung to differentiate into either DCs or alveolar macrophages during invasive aspergillosis (Cramer et al., 2011; Morton et al., 2012). Monocytes express different chemokines receptor predominantly CCR2, which help in migration of monocytes from bone marrow toward lung in response to A. fumigatus infection (Serbina et al., 2009). It has been shown that monocytes expressing CCR2 in the lung involved in conidial uptake and killing (Espinosa et al., 2014). Furthermore, alveolar macrophages induce APCs to release IL -1β in pulmonary invasive infection (Park and Mehrad, 2009). IL-18 has also been observed in lung during invasive aspergillosis mice model (Akdis et al., 2011). Recently, it has been observed that A. fumigatus pulmonary challenge induces expression of the inflammasome-dependent cytokines IL-1β and IL-18 within the first 12 h, while IL-1α expression continually increases over at least the first 48 h (Caffrey et al., 2015). Moretti et al. (2014) showed in a pulmonary invasive aspergillosis model that mice injected with IL-37 prior to A. fumigatus infection has significant reduction in IL-β production and recruitment of neutrophils and resulted in diminution in lung inflammation and damage. The anti-inflammatory activity of IL-37 has been observed as an inhibitor of the innate response. Thus, cytokines play a vital role in modulation of immune response and coordinate the innate as well as adaptive responses. APCs secrete cytokines that act on naïve T-cells leading to the differentiation of naïve T-cells. These differentiated T-cells further secrete effector cytokines and regulate the function of TH-cells. The profile of cytokine depends on the type of Aspergillus antigens, route of infection, immunological status of the host and cytokines milieu present during the interaction (Romani, 2008; Chai et al., 2010b). CD4+ T-cells can be divided into distinct subtypes according to cytokine profile, and they can differentiate to TH1, TH2 TH17, TH9, and T-follicular effector cells (Kerzerho et al., 2013; Kara et al., 2014). On the basis of the cytokine profile, these TH-cells perform distinct functions. However, it is not clear how T-follicular effector cells respond during Aspergillus infection (Wüthrich et al., 2012).
Cytokines Associated with TH1 Type of Response
Aspergillus fumigatus challenged intranasally in mice interacts with DCs and alveolar macrophages in the lung. Secretion of TH1 associated pro-inflammatory cytokines IL-12, IFN-γ, TNF-α, IL-18 has been observed after the challenge (Chotirmall et al., 2013). Among these, IL-12 is the prominent cytokine released from activated monocytes and macrophages in lung that help in shaping T-cell immune response. IL-12 is a heterodimeric cytokine composed of IL-12p35 and IL-12p40 polypeptides that form the bioactive IL-12p70. The heterodimer binds to the IL-12 receptor composed of IL-12Rβ1 and IL-12Rβ2 chains and signals through STAT-4 (Shao et al., 2005). IL-12 acts on NK cells to promote IFN-γ secretion and differentiate CD4+ T-cells into TH1-cells, once CD4+ cells differentiates to TH1-cells, they increase the secretion of IFN-γ, which suppress TH17 and TH2 response in the lung (Espinosa and Rivera, 2012; Camargo and Husain, 2014). IL-12, hence, is the most important regulator of TH1 response during lung infection. IL-12 deficient mice failed to generate a TH1 response, leading to increased secretion of IL-4 and IL-10 cytokines, which shifts the immune response toward TH2 pathway (Cenci et al., 1998). In A. fumigatus induced neutropenic aspergillosis in mice, NK cells can be the primary source of IFN-γ responsible for activating phagocytic cells and direct antifungal effectors cells against A. fumigatus (Park et al., 2009). Further, patients with invasive Candida and/or Aspergillus infections, recombinant treatment of IFN-γ in combination with antifungal drug partially restored immune function (Delsing et al., 2014). In intravenous infection of A. flavus mice model studies, lung and brain homogenate showed pro-inflammatory cytokines IL-12 and IFN-γ and relative absence of IL-4, IL-23, and IL-17 suggesting a TH1 response (Anand et al., 2013, 2015). A. terreus induced invasive aspergillosis showed the presence of IL-1β, IL-6, and reduced level of IL-10 in mice model studies. Although there is activation of TH17 type of adaptive immune response through IL-1β but the later is suppressed by TH1 cytokines particularly IFN-γ (Vyas, 2011; Lass-Florl, 2012). The TH1 response is thus also mounted by A. terreus and there is a lack of TH2 response in contrast to A. fumigatus infection where TH2 promoting cytokines are observed.
Cytokines Associated with TH17 Type Response
Aspergillus fumigatus mediated infections in lung induce TH17 and TH1-cells. These cells play an important role in protection and induction of inflammation (Chai et al., 2010b). Activation of TH17-cell depends on Dectin-1 signaling pathway. Various studies have suggested that dectin-1 deficient mice entirely activate TH1-cells. So Dectin-1 signaling not only serves as a positive factor to promote TH17 differentiation but rather act to balance TH1 versus TH17 differentiation. Activation of the APCs by Dectin-1, release the proinflammatory cytokines IL-1β, IL-6, IL-23, and IL-22 which differentiates CD4+ T-cells to TH17-cells, which further secretes IL-17A and IL-17F cytokines and maintain TH17 response (Werner et al., 2011). IL-23 is a member of IL-12 family, produced by phagocytic cells, macrophages and activated DCs in lung. IL-23 contains two subunits IL-12p40 and IL-23p19 and it binds to heterodimeric receptors IL-12Rβ1, expressed by activated T-cells (Zelante et al., 2007). IL-6 is another important cytokine involved in regulation of TH17 response. IL-6 is a multifunctional cytokine, promote TH17-cells differentiation, inflammation and acute response (Akdis et al., 2011). During TH17 differentiation, human naïve T-cells are exposed to IL-1β, IL-6, and IL-23 (Zelante et al., 2009; Gresnigt and van de Veerdonk, 2014). TH17 promoting cytokine IL-17 binds to IL-17RA and IL-17RC receptors expressed in lung cells, like fibroblast, epithelial cells and T-cells. After release of IL-17 from TH17-cells, it activates the neutrophils migration toward infected area and increases inflammation (Wilson et al., 2007). In A. flavus and A. terreus, the role of TH17-cells during lung infection is yet to be established.
Cytokines Associated with TH2 Type of Response
Aspergillus fumigatus is associated with both invasive and allergic form of aspergillosis. In case, if conidia are not cleared, they germinate to produce hyphae, which are responsible for invasion in host tissues that leads to inflammation. In a healthy human T-cells response, A. fumigatus not only evoke pro-inflammatory type of immune response via TH1 and TH17-cells but also anti-inflammatory type of immune response mediated by TH2-cells (Chaudhary et al., 2010). Immune response initiated by IL-4 and IL-10 inhibits TH1 and TH17 response and increased secretion of IL-4 and IL-10 inhibits IFN-γ and IL-12 production. TH2-cells differentiation depends on IL-4 and IL-10 and after differentiation in to TH2 cells, these cells further secretes IL-5 and IL-13 which maintain TH2 response. TH2 immune response is triggered in acute bronchopulmonary aspergillosis and also in invasive pulmonary infection during some time point of infection. IL-4 and IL-10 deficient mice show lower A. fumigatus burden and increased survival rates compared to wild type mouse in invasive pulmonary aspergillosis (Cenci et al., 2000). It has been shown that ESTs (L3 ribosomal protein, L7A ribosomal protein, Histone -H2A) have high sequence similarity with human counter parts suggesting molecular mimicry between human and pathogen protein (Shankar et al., 2004). However, role of these genes in eliciting allergic immune response needs investigations. In A. flavus mediated infection, lung homogenate showed the absence of TH2 response in a limited cytokine profile study (Anand and Tiwary, 2010). However, TH2 type response may get activated in later stages of infection in lung due to rise in IL-4 and IL-10, which suppress the TH1 response but consistent expression IFN-γ overcomes TH2 response. In addition to TH2, the role of TH9-cells has been shown during infection with a Virus, bacteria, parasites and fungi. TH9 response contributes to allergic inflammation during allergic aspergillosis due to A. fumigatus in mice model (Kerzerho et al., 2013). TH9-cells develop in the presence of IL-1α and TGF-β along with TH2-cells. However, role of TH9 and TH2 response during invasive aspergillosis remains unclear.
Is Their Co-evolution of T-helper Cells During Invasive Aspergillosis?
In a immunocompromised mice model studies, repeated exposure of A. fumigatus conidia in hosts lead to co-evolution of TH1, TH2, and TH17 response in infected lung (Murdock et al., 2011). They have observed the presence of IFN-γ and IL-17 in infected lung of mice along with TH2 response. These mixed responses might be occurring at different time points during progression of infection and leads to either protection or infection. TH1 and TH17 response probably leads to protection where as TH2 response further complicates the disease. TH2 response help in evasion of A. fumigatus from immune cells and further increase the IgE level, which leads to high inflammation at the site of infection. Mice model of ABPA demonstrated the TH2 cytokine profile consisting of IL-4, IL-10, and IL-5 (Latge, 1999).
Interplay of Cytokines; TH1 or TH2 or TH17 Type of Response
TH-cells response during invasive Aspergillus depends on differentiating cytokines. TH1 response is activated by differentiating cytokine IL-12 followed by secretion of IFN-γ. Secretion of IFN-γ further stimulates TH1 cells, if IFN-γ dominates initially it suppress the other cytokines of TH2 and TH17, i.e., IL-4 and IL-17 (Harrington et al., 2005). If IL-4 dominates during initial period of Aspergillus infection, it suppresses the protective TH1 type immune response by inhibiting differential cytokine IL-12 and IFN-γ (Harrington et al., 2005). Recognition of Aspergillus antigens by Dectin-1 signaling pathway inhibit the production of IFN-γ and IL-12 receptors suppressing TH1, which leads to differentiation of TH17-cells and production of IL-17. In this way Dectin-1 signal balances the TH1 and TH17 response through the regulation of their respective cytokines (Rivera et al., 2011; Figure 1). The development of effective CD4+ TH-cells response not only depends upon cytokines, but also on chemokines and their receptors. Chemokines help in recruitment of leukocytes, i.e., neutrophils, monocytes and NK cells toward lung during Aspergillus infection. These cells express chemokine receptors; neutrophils contain CXCR2 chemokine receptor for ligand CXCL1 and CXCL2, monocytes contain CCR2 and CCR6 receptor for CCL2 and CCL20 ligands, where as NK cells contain CCR2 receptor for CCL2 ligand. So these chemokine ligands attract monocytes, neutrophils and NK cells to clear the Aspergillus hyphae during lung infection (Park and Mehrad, 2009). These chemokines receptors are also present on DCs, regulatory T-cells (Tregs) and TH-cells and help in their trafficking (Bendall, 2005). In this way, there is an interdependent relationship between chemokines and cytokines that help in evolution of effector TH-cells response. CCL17, a chemokine, help in trafficking of DCs, Tregs and TH1-cells toward infected area during invasive aspergillosis in response to CCR4 chemokine receptor present on these cells. Further, CCR6 receptor present on DCs and TH17-cells help in migration of these cells in response to chemokine CCL20 (Bendall, 2005; Wüthrich et al., 2012).
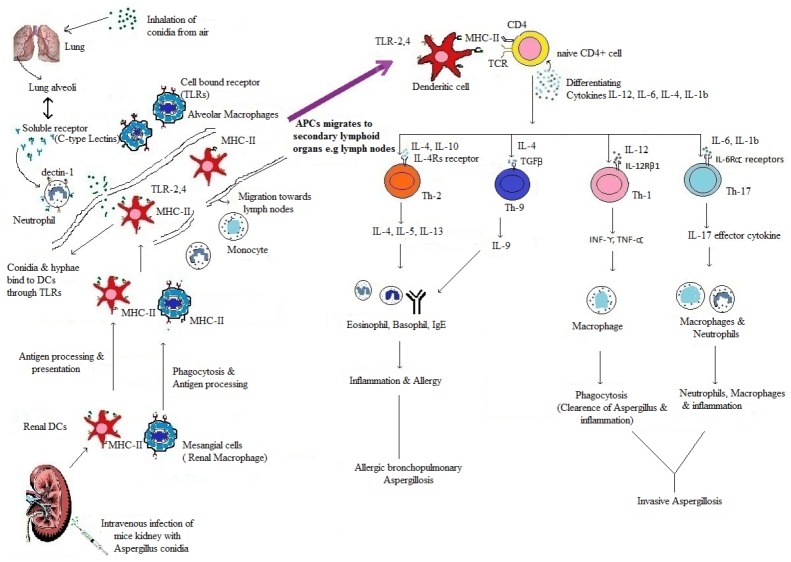
FIGURE 1.
The recognitions, processing and presentation of Aspergillus antigens to naïve CD4+ TH-cells and Production of effector TH-cells. In infected Lung and Kidney, Aspergillus antigens (conidia & germinating conidia) are recognized by PRRs, i.e., soluble (C-type lectins) and cell bound receptors; TLR2, TLR4, and TLR9 (Netea et al., 2006). They are present on antigen presenting cells (DCs and macrophages). After recognition, antigens recognized by APCs, process and present to naïve CD4+ T-cells in secondary lymphoid organs (Chai et al., 2010a). After interaction of APCs and naïve CD4+ T-cells, differential cytokines release (IL-12, IL-6, IL-4, IL-1β) (Korn et al., 2009; Chai et al., 2010a) act upon CD4+ T-cells and differentiate them into effector TH-cells. IL- 12 give rise to TH1, IL-6, IL-1β give rise to TH17 and IL-4 give rise to TH2 effector TH-cells (Murdock et al., 2011). These effector cells further secrets effector cytokines (IFN-γ, TNF-α, IL-10, IL-5, IL-17, and IL-23) (Zelante et al., 2007) which maintain effector TH-cells response. Figure shows summary of development of effector TH-cells response during Lung and Kidney infection of Aspergillus. The figure is not to the scale.
Conclusion
Cytokines are important in the development of CD4+ TH-cells. Understanding of trafficking of CD4+ TH-cells and their regulation through differentiating/effector cytokines during invasive aspergillosis will be crucial for the targeted immunotherapy. Overall, cytokines and chemokines may serve as prognostic biomarkers that could be followed to assess the effectiveness of treatment response during invasive aspergillosis. Measurement of selected cytokines in the blood samples of aspergillosis patients may be a promising tool for the monitoring of treatment responses. Also, manipulation of cytokine response e. g, IFN-γ or IFN-γ in combination with antifungal drug, IL-37, may be a future avenue for the development of better therapeutic against invasive aspergillosis.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Authors are thankful to Department of Biotechnology and Bioinformatics, Jaypee University of Information Technology, Solan, Himachal Pradesh, India, for financial support to Ph.D. students RT and ST.
References
- Agarwal R., Denning D. W., Chakrabarti A. (2014). Estimation of the burden of chronic and allergic pulmonary aspergillosis in India. PLoS ONE 9:e114745. 10.1371/journal.pone.0114745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akdis M., Burgler S., Crameri R., Eiwegger T., Fujita H., Gomez E., et al. (2011). Interleukins, from 1 to 37, and interferon-gamma: receptors, functions, and roles in diseases. J. Allergy Clin. Immunol. 127, 701–721 e701–e770. 10.1016/j.jaci.2010.11.050 [DOI] [PubMed] [Google Scholar]
- Anand R., Shankar J., Singh A. P., Tiwary B. N. (2013). Cytokine milieu in renal cavities of immunocompetent mice in response to intravenous challenge of Aspergillus flavus leading to aspergillosis. Cytokine 61, 63–70. 10.1016/j.cyto.2012.08.024 [DOI] [PubMed] [Google Scholar]
- Anand R., Shankar J., Tiwary B. N., Singh A. P. (2015). Aspergillus flavus induces granulomatous cerebral aspergillosis in mice with display of distinct cytokine profile. Cytokine 72, 166–172. 10.1016/j.cyto.2015.01.006 [DOI] [PubMed] [Google Scholar]
- Anand R., Tiwary B. N. (2010). Th1 and Th2 cytokines in a self-healing primary pulmonary Aspergillus flavus infection in BALB/c mice. Cytokine 52, 258–264. 10.1016/j.cyto.2010.07.428 [DOI] [PubMed] [Google Scholar]
- Barnes P. D., Marr K. A. (2006). Aspergillosis: spectrum of disease, diagnosis, and treatment. Infect. Dis. Clin. North Am. 20, 545–561, vi. 10.1016/j.idc.2006.06.001 [DOI] [PubMed] [Google Scholar]
- Bendall L. (2005). Chemokines and their receptors in disease. Histol. Histopathol. 20, 907–926. [DOI] [PubMed] [Google Scholar]
- Bozza S., Gaziano R., Spreca A., Bacci A., Montagnoli C., Di Francesco P., et al. (2002). Dendritic cells transport conidia and hyphae of Aspergillus fumigatus from the airways to the draining lymph nodes and initiate disparate Th responses to the fungus. J. Immunol. 168, 1362–1371. 10.4049/jimmunol.168.3.1362 [DOI] [PubMed] [Google Scholar]
- Caffrey A. K., Lehmann M. M., Zickovich J. M., Espinosa V., Shepardson K. M., Watschke C. P., et al. (2015). IL-1alpha signaling is critical for leukocyte recruitment after pulmonary Aspergillus fumigatus challenge. PLoS Pathog. 11:e1004625. 10.1371/journal.ppat.1004625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo J. F., Husain S. (2014). Immune correlates of protection in human invasive aspergillosis. Clin. Infect. Dis. 59, 569–577. 10.1093/cid/ciu337 [DOI] [PubMed] [Google Scholar]
- Cenci E., Mencacci A., Bacci A., Bistoni F., Kurup V. P., Romani L. (2000). T cell vaccination in mice with invasive pulmonary aspergillosis. J. Immunol. 165, 381–388. 10.4049/jimmunol.165.1.381 [DOI] [PubMed] [Google Scholar]
- Cenci E., Mencacci A., Fe D’ostiani C., Del Sero G., Mosci P., Montagnoli C., et al. (1998). Cytokine- and T helper-dependent lung mucosal immunity in mice with invasive pulmonary aspergillosis. J. Infect. Dis. 178, 1750–1760. 10.1086/314493 [DOI] [PubMed] [Google Scholar]
- Chai L., Netea M. G., Teerenstra S., Earnest A., Vonk A. G., Schlamm H. T., et al. (2010a). Early proinflammatory cytokines and C-reactive protein trends as predictors of outcome in invasive Aspergillosis. J. Infect. Dis. 202, 1454–1462. 10.1086/656527 [DOI] [PubMed] [Google Scholar]
- Chai L. Y., Van De Veerdonk F., Marijnissen R. J., Cheng S. C., Khoo A. L., Hectors M., et al. (2010b). Anti-Aspergillus human host defence relies on type 1 T helper (Th1), rather than type 17 T helper (Th17), cellular immunity. Immunology 130, 46–54. 10.1111/j.1365-2567.2009.03211.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamilos G., Luna M., Lewis R. E., Bodey G. P., Chemaly R., Tarrand J. J., et al. (2006). Invasive fungal infections in patients with hematologic malignancies in a tertiary care cancer center: an autopsy study over a 15-year period (1989–2003). Haematologica 91, 986–989. [PubMed] [Google Scholar]
- Chaudhary N., Staab J. F., Marr K. A. (2010). Healthy human T-Cell Responses to Aspergillus fumigatus antigens. PLoS ONE 5:e9036. 10.1371/journal.pone.0009036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotirmall S. H., Al-Alawi M., Mirkovic B., Lavelle G., Logan P. M., Greene C. M., et al. (2013). Aspergillus -associated airway disease, inflammation, and the innate immune response. Biomed. Res. Int. 2013, 723129. 10.1155/2013/723129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer R. A., Rivera A., Hohl T. M. (2011). Immune responses against Aspergillus fumigatus: what have we learned? Curr. Opin. Infect. Dis. 24, 315–322. 10.1097/QCO.0b013e328348b159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delsing C. E., Gresnigt M. S., Leentjens J., Preijers F., Frager F. A., Kox M., et al. (2014). Interferon-gamma as adjunctive immunotherapy for invasive fungal infections: a case series. BMC Infect. Dis. 14:166. 10.1186/1471-2334-14-166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning D. W., Pleuvry A., Cole D. C. (2011). Global burden of chronic pulmonary aspergillosis as a sequel to pulmonary tuberculosis. Bull. World Health Organ. 89, 864–872. 10.2471/blt.11.089441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning D. W., Pleuvry A., Cole D. C. (2013). Global burden of allergic bronchopulmonary aspergillosis with asthma and its complication chronic pulmonary aspergillosis in adults. Med. Mycol. 51, 361–370. 10.3109/13693786.2012.738312 [DOI] [PubMed] [Google Scholar]
- Espinosa V., Jhingran A., Dutta O., Kasahara S., Donnelly R., Du P., et al. (2014). Inflammatory monocytes orchestrate innate antifungal immunity in the lung. PLoS Pathog. 10:e1003940. 10.1371/journal.ppat.1003940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa V., Rivera A. (2012). Cytokines and the regulation of fungus-specific CD4 T cell differentiation. Cytokine 58, 100–106. 10.1016/j.cyto.2011.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresnigt M. S., van de Veerdonk F. L. (2014). The role of interleukin-1 family members in the host defence against Aspergillus fumigatus. Mycopathologia 178, 395–401. 10.1007/s11046-014-9776-y [DOI] [PubMed] [Google Scholar]
- Harrington L. E., Hatton R. D., Mangan P. R., Turner H., Murphy T. L., Murphy K. M., et al. (2005). Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6, 1123–1132. 10.1038/ni1254 [DOI] [PubMed] [Google Scholar]
- Inoue M., Shinohara M. L. (2014). Clustering of pattern recognition receptors for fungal detection. PLoS Pathog. 10:e1003873. 10.1371/journal.ppat.1003873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kara E. E., Comerford I., Fenix K. A., Bastow C. R., Gregor C. E., Mckenzie D. R., et al. (2014). Tailored immune responses: novel effector helper T cell subsets in protective immunity. PLoS Pathog. 10:e1003905. 10.1371/journal.ppat.1003905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerzerho J., Maazi H., Speak A. O., Szely N., Lombardi V., Khoo B., et al. (2013). Programmed cell death ligand 2 regulates TH9 differentiation and induction of chronic airway hyperreactivity. J. Allergy Clin. Immunol. 131, 1048–1057, 1057 e1041–e1042. 10.1016/j.jaci.2012.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T., Bettelli E., Oukka M., Kuchroo V. K. (2009). IL-17 and Th17 Cells. Annu. Rev. Immunol. 27, 485–517. 10.1146/annurev.immunol.021908.132710 [DOI] [PubMed] [Google Scholar]
- Lass-Florl C. (2012). Aspergillus terreus: how inoculum size and host characteristics affect its virulence. J. Infect. Dis. 205, 1192–1194. 10.1093/infdis/jis185 [DOI] [PubMed] [Google Scholar]
- Latge J. P. (1999). Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12, 310–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti S., Bozza S., Oikonomou V., Renga G., Casagrande A., Iannitti R. G., et al. (2014). IL-37 inhibits inflammasome activation and disease severity in murine aspergillosis. PLoS Pathog. 10:e1004462. 10.1371/journal.ppat.1004462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J., Wannemuehler K. A., Marr K. A., Hadley S., Kontoyiannis D. P., Walsh T. J., et al. (2005). Incidence of invasive aspergillosis following hematopoietic stem cell and solid organ transplantation: interim results of a prospective multicenter surveillance program. Med. Mycol. 43(Suppl. 1), S49–S58. 10.1080/13693780400020113 [DOI] [PubMed] [Google Scholar]
- Morton C. O., Bouzani M., Loeffler J., Rogers T. R. (2012). Direct interaction studies between Aspergillus fumigatus and human immune cells; what have we learned about pathogenicity and host immunity? Front. Microbiol. 3:413. 10.3389/fmicb.2012.00413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdock B. J., Shreiner A. B., Mcdonald R. A., Osterholzer J. J., White E. S., Toews G. B., et al. (2011). Coevolution of TH1, TH2, and TH17 responses during repeated pulmonary exposure to Aspergillus fumigatus conidia. Infect. Immun. 79, 125–135. 10.1128/iai.00508-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea M. G., Ferwerda G., Van Der Graaf C. A., Van Der Meer J. W., Kullberg B. J. (2006). Recognition of fungal pathogens by toll-like receptors. Curr. Pharm. Des. 12, 4195–4201. 10.2174/138161206778743538 [DOI] [PubMed] [Google Scholar]
- Osugi Y., Vuckovic S., Hart D. N. (2002). Myeloid blood CD11c+ dendritic cells and monocyte-derived dendritic cells differ in their ability to stimulate T lymphocytes. Blood 100, 2858–2866. 10.1182/blood.V100.8.2858 [DOI] [PubMed] [Google Scholar]
- Park S. J., Hughes M. A., Burdick M., Strieter R. M., Mehrad B. (2009). Early NK cell-derived IFN-{gamma} is essential to host defense in neutropenic invasive aspergillosis. J. Immunol. 182, 4306–4312. 10.4049/jimmunol.0803462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S. J., Mehrad B. (2009). Innate immunity to Aspergillus species. Clin. Microbiol. Rev. 22, 535–551. 10.1128/cmr.00014-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Ortiz Z. G., Means T. K. (2012). The role of dendritic cells in the innate recognition of pathogenic fungi (A. fumigatus, C. neoformans, and C. albicans). Virulence 3, 635–646. 10.4161/viru.22295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Ortiz Z. G., Specht C. A., Wang J. P., Lee C. K., Bartholomeu D. C., Gazzinelli R. T., et al. (2008). Toll-like receptor 9-dependent immune activation by unmethylated CpG motifs in Aspergillus fumigatus DNA. Infect. Immun. 76, 2123–2129. 10.1128/iai.00047-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera A., Hohl T. M., Collins N., Leiner I., Gallegos A., Saijo S., et al. (2011). Dectin-1 diversifies Aspergillus fumigatus-specific T cell responses by inhibiting T helper type 1 CD4 T cell differentiation. J. Exp. Med. 208, 369–381. 10.1084/jem.20100906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani L. (2008). Cell mediated immunity to fungi: a reassessment. Med. Mycol. 46, 515–529. 10.1080/13693780801971450 [DOI] [PubMed] [Google Scholar]
- Serbina N. V., Cherny M., Shi C., Bleau S. A., Collins N. H., Young J. W., et al. (2009). Distinct responses of human monocyte subsets to Aspergillus fumigatus conidia. J. Immunol. 183, 2678–2687. 10.4049/jimmunol.0803398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar J. (2013). An overview of toxins in Aspergillus associated with pathogenesis. Int. J. Life Sci. Biotechnol. Pharm. Res. 2, 16–31. [Google Scholar]
- Shankar J., Nigam S., Saxena S., Madan T., Sarma P. U. (2004). Identification and assignment of function to the genes of Aspergillus fumigatus expressed at 37 degrees C. J. Eukaryot. Microbiol. 51, 428–432. 10.1111/j.1550-7408.2004.tb00390.x [DOI] [PubMed] [Google Scholar]
- Shao C., Qu J., He L., Zhang Y., Wang J., Zhou H., et al. (2005). Dendritic cells transduced with an adenovirus vector encoding interleukin-12 are a potent vaccine for invasive pulmonary aspergillosis. Genes Immun. 6, 103–114. 10.1038/sj.gene.6364167 [DOI] [PubMed] [Google Scholar]
- Steinbach W. J., Benjamin D. K., Jr., Kontoyiannis D. P., Perfect J. R., Lutsar I., et al. (2004). Infections due to Aspergillus terreus: a multicenter retrospective analysis of 83 cases. Clin. Infect. Dis. 39, 192–198. 10.1086/421950 [DOI] [PubMed] [Google Scholar]
- Stevens D. A., Kan V. L., Judson M. A., Morrison V. A., Dummer S., Denning D. W., et al. (2000). Practice guidelines for diseases caused by Aspergillus. Infectious Diseases Society of America. Clin. Infect. Dis. 30, 696–709. 10.1086/313756 [DOI] [PubMed] [Google Scholar]
- Verweij P. E., Brandt M. E. (2007). Aspergillus, Fusarium and Other Opportunistic Moniliaceous Fungi. Washington, DC: ASM Press. [Google Scholar]
- Vyas J. M. (2011). The duality of Aspergillus terreus: differential immune responses to distinct conidia. Virulence 2, 181–184. 10.4161/viru.2.3.16613 [DOI] [PubMed] [Google Scholar]
- Weaver C. T., Hatton R. D., Mangan P. R., Harrington L. E. (2007). IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 25, 821–852. 10.1146/annurev.immunol.25.022106.141557 [DOI] [PubMed] [Google Scholar]
- Werner J. L., Gessner M. A., Lilly L. M., Nelson M. P., Metz A. E., Horn D., et al. (2011). Neutrophils produce interleukin 17A (IL-17A) in a dectin-1- and IL-23-dependent manner during invasive fungal infection. Infect. Immun. 79, 3966–3977. 10.1128/IAI.05493-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willment J. A., Brown G. D. (2008). C-type lectin receptors in antifungal immunity. Trends Microbiol. 16, 27–32. 10.1016/j.tim.2007.10.012 [DOI] [PubMed] [Google Scholar]
- Wilson N. J., Boniface K., Chan J. R., Mckenzie B. S., Blumenschein W. M., Mattson J. D., et al. (2007). Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat. Immunol. 8, 950–957. 10.1038/ni1497 [DOI] [PubMed] [Google Scholar]
- Wüthrich M., Deepe G. S., Jr., Klein B. (2012). Adaptive immunity to fungi. Annu. Rev. Immunol. 30, 115–148. 10.1146/annurev-immunol-020711-074958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelante T., Bozza S., De Luca A., D’angelo C., Bonifazi P., Moretti S., et al. (2009). Th17 cells in the setting of Aspergillus infection and pathology. Med. Mycol. 47(Suppl. 1), S162–S169. 10.1080/13693780802140766 [DOI] [PubMed] [Google Scholar]
- Zelante T., De Luca A., Bonifazi P., Montagnoli C., Bozza S., Moretti S., et al. (2007). IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur. J. Immunol. 37, 2695–2706. 10.1002/eji.200737409 [DOI] [PubMed] [Google Scholar]