Abstract
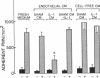
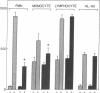
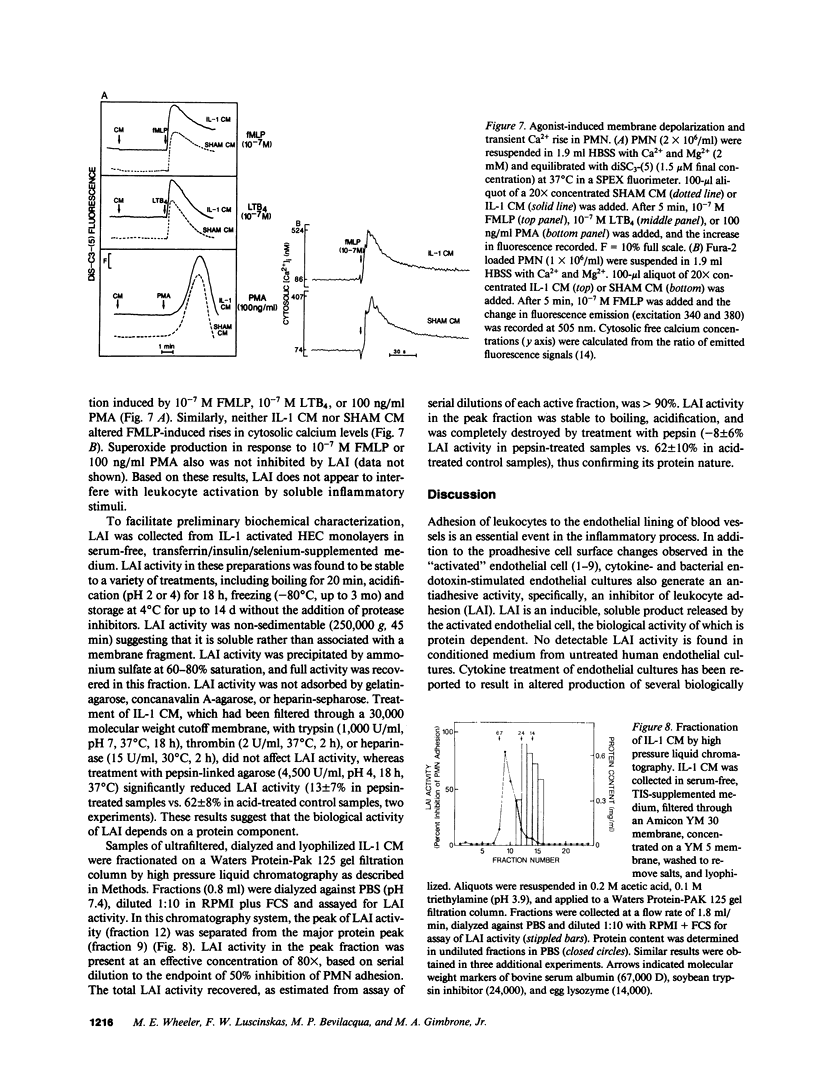
Activation of cultured human endothelial cells (HEC) by inflammatory stimuli, such as interleukin 1 (IL-1), tumor necrosis factor (TNF), and bacterial endotoxin (lipopolysaccharide, LPS), increases their surface adhesiveness for blood leukocytes and related cell lines. We now report that activated HEC also generate a soluble leukocyte adhesion inhibitor (LAI), which accumulates in conditioned media from IL-1-, TNF-, or LPS-treated, but not sham-treated, HEC cultures. LAI significantly inhibits the adhesion of PMN and monocytes to activated, but not unactivated, HEC. In contrast, LAI has no effect on the adhesion of lymphocytes, the promyelocytic cell line HL-60 or the monocyte-like cell line U937 to HEC monolayers. LAI appears to act directly on the leukocyte, but does not inhibit either agonist-induced responses in PMN (membrane depolarization, changes in cytosolic calcium concentration, superoxide production) or PMN attachment to serum-coated plastic surfaces. Endothelial generation of LAI is blocked by actinomycin D but not by aspirin or indomethacin. Preliminary biochemical characterization indicates that LAI is a soluble, protein-containing molecule that is heat- and acid-stable. Fractionation by HPLC gel filtration yields a single peak of LAI activity (14,000 less than Mr greater than 24,000). Thus, in addition to proadhesive cell surface changes, the endothelium may also actively contribute to the regulation of endothelial-leukocyte interactions at sites of inflammation in vivo through the production of soluble adhesion inhibitors such as LAI.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ager A., Gordon J. L. Differential effects of hydrogen peroxide on indices of endothelial cell function. J Exp Med. 1984 Feb 1;159(2):592–603. doi: 10.1084/jem.159.2.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrightson C. R., Baenziger N. L., Needleman P. Exaggerated human vascular cell prostaglandin biosynthesis mediated by monocytes: role of monokines and interleukin 1. J Immunol. 1985 Sep;135(3):1872–1877. [PubMed] [Google Scholar]
- Appelboom T., Pierart M., Ameryckx G., Mairesse N. Partial characterization of the soluble mediator of leukocyte adherence inhibition. J Rheumatol. 1987 Jun;14(3):420–425. [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Mendrick D. L., Cotran R. S., Gimbrone M. A., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Wheeler M. E., Cotran R. S., Gimbrone M. A., Jr Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest. 1985 Nov;76(5):2003–2011. doi: 10.1172/JCI112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Wheeler M. E., Cotran R. S., Gimbrone M. A., Jr Interleukin-1 activation of vascular endothelium. Effects on procoagulant activity and leukocyte adhesion. Am J Pathol. 1985 Dec;121(3):394–403. [PMC free article] [PubMed] [Google Scholar]
- Buchanan M. R., Haas T. A., Lagarde M., Guichardant M. 13-Hydroxyoctadecadienoic acid is the vessel wall chemorepellant factor, LOX. J Biol Chem. 1985 Dec 25;260(30):16056–16059. [PubMed] [Google Scholar]
- Buchanan M. R., Vazquez M. J., Gimbrone M. A., Jr Arachidonic acid metabolism and the adhesion of human polymorphonuclear leukocytes to cultured vascular endothelial cells. Blood. 1983 Oct;62(4):889–895. [PubMed] [Google Scholar]
- Cavender D., Haskard D., Yu C. L., Iguchi T., Miossec P., Oppenheimer-Marks N., Ziff M. Pathways to chronic inflammation in rheumatoid synovitis. Fed Proc. 1987 Jan;46(1):113–117. [PubMed] [Google Scholar]
- Cavender D., Saegusa Y., Ziff M. Stimulation of endothelial cell binding of lymphocytes by tumor necrosis factor. J Immunol. 1987 Sep 15;139(6):1855–1860. [PubMed] [Google Scholar]
- Charo I. F., Yuen C., Goldstein I. M. Adherence of human polymorphonuclear leukocytes to endothelial monolayers: effects of temperature, divalent cations, and chemotactic factors on the strength of adherence measured with a new centrifugation assay. Blood. 1985 Feb;65(2):473–479. [PubMed] [Google Scholar]
- Cohen H. J., Chovaniec M. E. Superoxide generation by digitonin-stimulated guinea pig granulocytes. A basis for a continuous assay for monitoring superoxide production and for the study of the activation of the generating system. J Clin Invest. 1978 Apr;61(4):1081–1087. doi: 10.1172/JCI109007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein B. N., Kramer S. B., Weissmann G., Hirschhorn R. Adenosine: a physiological modulator of superoxide anion generation by human neutrophils. J Exp Med. 1983 Oct 1;158(4):1160–1177. doi: 10.1084/jem.158.4.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein B. N., Levin R. I., Belanoff J., Weissmann G., Hirschhorn R. Adenosine: an endogenous inhibitor of neutrophil-mediated injury to endothelial cells. J Clin Invest. 1986 Sep;78(3):760–770. doi: 10.1172/JCI112638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E., Breviario F., Balconi G., Rossi V., Remuzzi G., de Gaetano G., Mantovani A. Stimulation of prostacyclin synthesis in vascular cells by mononuclear cell products. Blood. 1984 Dec;64(6):1280–1283. [PubMed] [Google Scholar]
- Gamble J. R., Harlan J. M., Klebanoff S. J., Vadas M. A. Stimulation of the adherence of neutrophils to umbilical vein endothelium by human recombinant tumor necrosis factor. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8667–8671. doi: 10.1073/pnas.82.24.8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Haskard D., Cavender D., Beatty P., Springer T., Ziff M. T lymphocyte adhesion to endothelial cells: mechanisms demonstrated by anti-LFA-1 monoclonal antibodies. J Immunol. 1986 Nov 1;137(9):2901–2906. [PubMed] [Google Scholar]
- Luscinskas F. W., Mark D. E., Brunkhorst B., Lionetti F. J., Cragoe E. J., Jr, Simons E. R. The role of transmembrane cationic gradients in immune complex stimulation of human polymorphonuclear leukocytes. J Cell Physiol. 1988 Feb;134(2):211–219. doi: 10.1002/jcp.1041340206. [DOI] [PubMed] [Google Scholar]
- Pearson J. D., Gordon J. L. Vascular endothelial and smooth muscle cells in culture selectively release adenine nucleotides. Nature. 1979 Oct 4;281(5730):384–386. doi: 10.1038/281384a0. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Bevilacqua M. P., Mendrick D. L., Lapierre L. A., Fiers W., Gimbrone M. A., Jr Two distinct monokines, interleukin 1 and tumor necrosis factor, each independently induce biosynthesis and transient expression of the same antigen on the surface of cultured human vascular endothelial cells. J Immunol. 1986 Mar 1;136(5):1680–1687. [PubMed] [Google Scholar]
- Pohlman T. H., Stanness K. A., Beatty P. G., Ochs H. D., Harlan J. M. An endothelial cell surface factor(s) induced in vitro by lipopolysaccharide, interleukin 1, and tumor necrosis factor-alpha increases neutrophil adherence by a CDw18-dependent mechanism. J Immunol. 1986 Jun 15;136(12):4548–4553. [PubMed] [Google Scholar]
- Recalde H. R. A simple method of obtaining monocytes in suspension. J Immunol Methods. 1984 Apr 13;69(1):71–77. doi: 10.1016/0022-1759(84)90278-3. [DOI] [PubMed] [Google Scholar]
- Roberts P. A., Newby A. C., Hallett M. B., Campbell A. K. Inhibition by adenosine of reactive oxygen metabolite production by human polymorphonuclear leucocytes. Biochem J. 1985 Apr 15;227(2):669–674. doi: 10.1042/bj2270669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleimer R. P., Rutledge B. K. Cultured human vascular endothelial cells acquire adhesiveness for neutrophils after stimulation with interleukin 1, endotoxin, and tumor-promoting phorbol diesters. J Immunol. 1986 Jan;136(2):649–654. [PubMed] [Google Scholar]
- Wallis W. J., Hickstein D. D., Schwartz B. R., June C. H., Ochs H. D., Beatty P. G., Klebanoff S. J., Harlan J. M. Monoclonal antibody-defined functional epitopes on the adhesion-promoting glycoprotein complex (CDw18) of human neutrophils. Blood. 1986 Apr;67(4):1007–1013. [PubMed] [Google Scholar]
- Whitin J. C., Chapman C. E., Simons E. R., Chovaniec M. E., Cohen H. J. Correlation between membrane potential changes and superoxide production in human granulocytes stimulated by phorbol myristate acetate. Evidence for defective activation in chronic granulomatous disease. J Biol Chem. 1980 Mar 10;255(5):1874–1878. [PubMed] [Google Scholar]
- Zimmerman G. A., Wiseman G. A., Hill H. R. Human endothelial cells modulate granulocyte adherence and chemotaxis. J Immunol. 1985 Mar;134(3):1866–1874. [PubMed] [Google Scholar]