Abstract
Cerebral arteriovenous malformations (AVMs) entail a significant risk of intracerebral hemorrhage owing to the direct shunting of arterial blood into the venous vasculature without the dissipation of the arterial blood pressure. The mechanisms involved in the growth, progression and rupture of AVMs are not clearly understood, but a number of studies point to inflammation as a major contributor to their pathogenesis. The upregulation of proinflammatory cytokines induces the overexpression of cell adhesion molecules in AVM endothelial cells, resulting in enhanced recruitment of leukocytes. The increased leukocyte-derived release of metalloproteinase-9 is known to damage AVM walls and lead to rupture. Inflammation is also involved in altering the AVM angioarchitecture via the upregulation of angiogenic factors that affect endothelial cell proliferation, migration and apoptosis. The effects of inflammation on AVM pathogenesis are potentiated by certain single-nucleotide polymorphisms in the genes of proinflammatory cytokines, increasing their protein levels in the AVM tissue. Furthermore, studies on metalloproteinase-9 inhibitors and on the involvement of Notch signaling in AVMs provide promising data for a potential basis for pharmacological treatment of AVMs. Potential therapeutic targets and areas requiring further investigation are highlighted.
Keywords: angiogenesis, arteriovenous malformations, AVM, endothelial cell, inflammation, intracerebral hemorrhage
Introduction
Cerebral arteriovenous malformations (AVMs) are comprised of vessels that directly shunt blood from the arterial to the venous system because of the absence of a capillary bed. The incidence of AVMs is 0.94 to 1.34 per 100,000 person-years with a mean age of presentation at 30 to 35 years.1, 2, 3
The prevailing theory of AVM rupture risk is the lack of intervening capillaries leading to an abnormally high-pressure blood flow through the AVM nidus. This high pressure is then transmitted to the venous vasculature, resulting in venous hypertension by impaired outflow, eventually resulting in rupture.4, 5
Arteriovenous malformation rupture is very common, making intracerebral hemorrhage (ICH) the major cause of AVM-related morbidity and mortality. The ICH risk for untreated AVMs is 2% to 6% per year.2, 6, 7, 8 However, the annual hemorrhage risk may be as high as 34% if any additional risk factors are present.9 History of hemorrhage, high blood pressure, intranidal aneurysm, venous stenosis, and deep venous drainage are the main factors that increase the likelihood of AVM rupture.4, 10, 11
Although there are several therapeutic options to eradicate AVMs, the treatment-related morbidity is significant, especially for large, deep-seated, or eloquently located nidi.6 The recently published ARUBA trial (A Randomized trial of Unruptured Brain Arteriovenous malformations) showed that medical management alone is superior to medical management with interventional therapy for the prevention of death or stroke in patients with unruptured brain AVMs.12 Thus, there is a pressing need to better understand the pathogenesis of AVMs and the molecular mechanisms that destabilize these lesions to develop novel medical therapies, which may reduce the need for invasive intervention. Studies have shown that inflammation plays a major role in vascular dysmorphogenesis by weakening the AVM vessel walls. Certain genetic polymorphisms have been shown to significantly upregulate the expression of angiogenic and proinflammatory proteins, which may, in turn, contribute to AVM destabilization and rupture. In this review, we critically analyze the existing data from in vitro, in vivo, and clinical studies implicating inflammation in the pathogenesis of cerebral AVMs.
The common pathway
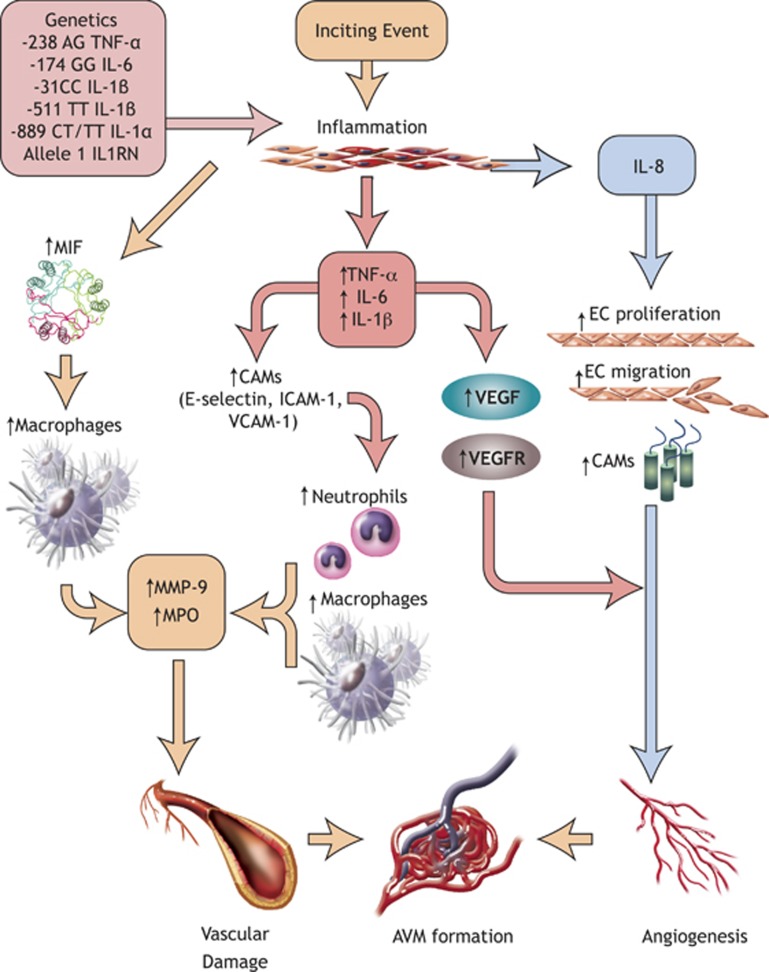
The common pathway for AVM formation and rupture appears to involve an inflammatory reaction (cytokines, neutrophils, macrophages, etc.) in the AVM walls, triggered by genetic/hemodynamic factors (Figure 1). Inflammation is then followed by increased angiogenesis and concomitant breakdown of extracellular matrix by various proteinases and cell death, all of which contribute to AVM wall weakening and rupture. These pathways will be discussed in this review.
Figure 1.
Contributors to AVM pathogenesis. The SNPs shown above upregulate the inflammatory response to inciting events and lead to the overexpression of proinflammatory cytokines, which in turn exacerbate the inflammation, recruit leukocytes and activate the endothelial cells of the AVM. As a result, increased vascular damage and angiogenesis are observed, resulting in AVM formation and expansion. AVM, arteriovenous malformation; CAMs, cell adhesion molecules; EC, endothelial cell; ICAM-1, intercellular cell adhesion molecule-1; IL1RN, interleukin-1 receptor antagonist; IL-6, interleukin-6; IL-1α, interleukin-1-alpha; IL-1β, interleukin-1-beta; IL-8, interleukin-8; MIF, macrophage migration inhibitory factor; MMP, matrix metalloproteinase; MPO, myeloperoxidase; SNPs, single-nucleotide polymorphisms; TNF-α, tumor necrosis factor-alpha; VCAM-1, vascular cell adhesion molecule-1; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Genetic predisposition
Genetics play a major role in the magnitude of the inflammatory response upon an inciting event. Several single-nucleotide polymorphisms (SNPs) have been identified in the genes of major proinflammatory cytokines that result in varying levels of inflammatory responses, thereby altering the extent of vascular dysmorphogenesis.13, 14 The cytokines, tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), interleukin-1α and 1β (ΙL-1α and IL-1β), and IL-1 receptor antagonist (IL1RN) each have several SNPs, some of which are associated with greater likelihood of pathogenesis than others (Table 1).
Table 1. Effect of gene polymorphisms on inflammation in AVMs.
| Polymorphisms | Overexpressed mediators | Outcome | Magnitude of outcome |
|---|---|---|---|
| −238 AG TNF-α | IL-6 | Increased ICH risk | 4x Increase17 |
| −174 GG IL-6 | IL-6, IL-1, TNF-α, IL-8, MMP-3, MMP-9, and MMP-12 | 3x Increase14 | |
| −31 CC IL-1β | IL-1β | 2.7x increase19 | |
| −511 TT IL-1β | IL-1β | 2.6x increase19 | |
| −889 CT or TT IL-1α | IL-1α | Increased AVM susceptibility | 2.47x Increase20 |
| Allele 1 of IL-1RN | IL-1RN | 2x Increase20 |
AVMs, arteriovenous malformations; ICH, intracranial hemorrhage; IL-6, interleukin-6; IL-1, interleukin-1; IL-8, interleukin-8; IL-1β, interleukin-1-beta; IL-1α, interleukin-1-alpha; IL-1RN, interleukin-1 receptor antagonist; MMP, matrix metalloproteinase; TNF-α, tumor necrosis factor-alpha.
Two SNPs have been identified in the IL-6 gene, −174 G>C and −572 G>C, and their effects on the rate of ICH were showed by Pawlikowska et al.14 The GG genotype of the IL-6 –174 G>C SNP, located in the promoter region, has been shown to increase the expression of IL-6 in AVMs. Increased IL-6 levels induced the expression of IL-1β, TNF-α, IL-8, matrix metalloproteinase-3 (MMP-3), MMP-9, and MMP-12, which suggests an association between IL-6 and the inflammatory and proteolytic processes in AVMs. The IL-6-induced expression of MMP-9 results in degradation of extracellular matrix in the surrounding vasculature, damage to endothelial cells (ECs), and renders the AVM more prone to rupture.15, 16 As a result, patients with the −174 GG genotype exhibit a threefold increase in hemorrhage risk, while patients with the C allele are protected (Table 1). Additional studies did not find the −572 G>C SNP of the IL-6 gene to affect the AVM rupture risk.14
Two SNPs were also identified in the gene of the proinflammatory cytokine TNF-α in AVM patients, TNF-α −238 G>A and −308 G>A. The AG genotype of the −238 G>A SNP was associated with a 6.4% rate of ICH in patients carrying this SNP presenting with a new ICH likely through overexpression of TNF-α and IL-6.17 In contrast, the TNF-α 308 G>A SNP was not found to increase the risk of new ICH.
The effect of the proinflammatory cytokine IL-1 on brain AVMs has been studied extensively as well. Three SNPs on the IL-1β gene, two SNPs on the IL-1α gene, and five alleles of the IL1RN gene have been shown to be associated with alterations in AVM hemorrhage risk.18, 19 AVM patients with the IL-1β −31 CC and the −511 TT genotypes had an increased hemorrhage risk and patients with the IL-1α −889 C>T genotype (either CT or TT) or IL1RN allele 1 had a greater AVM susceptibility (Table 1).18, 19, 20 Thus, IL-1 may be a promising therapeutic target for patients with AVMs.
Effect of increased proinflammatory cytokines on AVMs: a central role for IL-6
Various cytokines have been implicated in the pathogenesis of AVMs; most notable is the contribution of IL-6. When IL-6 is expressed in large amounts, there is a significant increase in the mRNA levels of IL-1β, TNF-α, and IL-8 in human AVM tissues.21 Through the upregulation of these cytokines, IL-6 indirectly stimulates leukocyte recruitment, endothelial activation, and vascular smooth muscle cell (SMC) proliferation (Table 2). Leukocyte recruitment occurs when the cellular adhesion molecules (CAMs) of ECs bind circulating leukocytes and affix them to the site of inflammation. Indeed, resected AVMs were found to have increased expression of CAMs: namely E-selectin, intercellular CAM-1 (ICAM-1), and vascular CAM-1 (VCAM-1). E-selectin concentration was increased to the greatest extent relative to ICAM-1 and VCAM-1.22
Table 2. Factors involved in AVM pathogenesis.
| Inflammatory mediators involved | Inflammatory pathway | |
|---|---|---|
| Leukocyte recruitment | IL-6, TNF-α, and IL-1β21 | Overexpression of E-selectin, ICAM-1, and VCAM-122 |
| Angiogenesis | IL-6, VEGF, HIF-1, NF-κB, IL-1β, and IL-821, 59, 64 | EC proliferation21, 22, 23, 43 EC migration22, 43 CAM expression22 Decreased EC apoptosis43 |
| Vascular instability | MIF, Caspase-3, MMP-9, IL-6, TNF-α, and VEGF23, 39, 40, 64 Decreased TIMP level30 | Macrophage recruitment39 ECM degradation40 Increased EC growth43 |
| Vascular permeability | VEGF68 | EC fenestrations ECM degradation65, 66, 67, 77 Sprouting angiogenesis69 |
| HCSMC proliferation | IL-623 | Upregulation of VEGF and MMP-923 |
CAM, cell adhesion molecule; EC, endothelial cell;90 ECM, extracellular matrix; HCSMC, human cerebral smooth muscle cell; HIF-1, hypoxia-inducible factor-1; ICAM-1, intercellular cell adhesion molecule-1; IL-6, interleukin-6; IL-1, interleukin-1; IL-8, interleukin-8; IL-1β, interleukin-1-beta; MIF, macrophage migration inhibitory factor; MMP, matrix metalloproteinase; NF-κB, nuclear factor-kappa B; TIMP, tissue inhibitor of metalloproteinase; TNF-α, tumor necrosis factor-alpha; VCAM-1, vascular cell adhesion molecule-1; VEGF, vascular endothelial growth factor.
In addition to leukocyte recruitment, the increased expression of IL-1β and IL-8 secondary to elevated IL-6 has a significant upregulatory effect on angiogenesis. The magnitude of angiogenesis was measured in cultured human cerebral ECs exposed to IL-6, where 100 ng/ml of IL-6 was shown to have a similar effect on human cerebral ECs as 20 ng/ml of vascular endothelial growth factor (VEGF).21 Additionally, an increase in IL-6 concentration stimulates VEGF release, VEGF receptor II activation along with human cerebral SMC proliferation and greater MMP-9 expression.23 Hence, the combination of increased angiogenesis and breakdown of extracellular matrix initiated in part by IL-6 may lead to vascular instability and AVM rupture.
Neutrophil and macrophage recruitment to AVMs leads to release of matrix metalloproteinases and vascular remodeling
The upregulation of inflammatory cytokines and the overexpression of CAMs on ECs result in the enhanced recruitment of leukocytes to the AVM tissue. Recruited leukocytes secrete myeloperoxidase, MMPs, cytokines, and other proteolytic enzymes, all of which cause damage to the AVM vessel walls, leading to rupture of the nidus.24 Chen et al25 characterized the inflammatory cells found in AVM tissue using myeloperoxidase, CD68, CD3, and CD20 as markers for neutrophils, macrophages and microglia, T-lymphocytes, and B-lymphocytes, respectively. Neutrophils were mainly found in the inner vessel walls and in AVM parenchymal tissues. Macrophages were located primarily in the outer vessel walls. In contrast, there was minimal evidence of T-cells or B cells in AVM tissues. Double immunofluorescent staining showed co-localization of myeloperoxidase and MMP-9, suggesting that neutrophils contribute a substantial portion of the MMP-9 to AVMs. It was also found that circulating MMP-9 comprised only 6% of the total MMP-9 in AVM tissues, and thus the majority of AVM-associated MMP-9 was likely derived from neutrophils. Once released upon neutrophil activation, MMP-9 breaks down the extracellular matrix, leading to vascular dilation/weakening.24, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37 Using substrate zymography, Hashimoto et al30 determined that the expression of total MMP-9, pro-MMP-9, and active MMP-9 was significantly increased in AVMs in comparison to control brain tissue. The levels of MMP inhibitors, tissue inhibitor of metalloproteinase 1 (TIMP-1), and TIMP-3, were also increased in AVMs, although to a lesser extent than MMP-9. The proportionally lower amount of TIMPs in AVMs explains the increased susceptibility to vascular breakdown and rupture.
Given the role of MMP-9 in the pathogenesis of AVMs, investigators have attempted to use MMP-9 level as a screening tool to determine the likelihood of rupture. Starke et al38 tested the plasma MMP-9 concentration in 15 AVM patients before treatment, 24 hours after embolization, 24 hours after surgical resection, and 30 days after surgery; a significant correlation was found between systemic and local MMP-9 expression. AVM patients also had significantly higher plasma pretreatment MMP-9 levels than controls, potentially providing a marker of the disease. The mean plasma MMP-9 levels rose 24 hours after endovascular embolization as well as after surgical resection before returning to pretreatment levels during the short follow-up period (30 days).38 Although normalization of MMP-9 levels could have been seen if longer postresection follow-up was pursued, it is possible that patients with AVMs have a germline disorder that is responsible for high levels of MMP-9 and as such for AVM formation and rupture.
Macrophage migration inhibitory factor (MIF) appears to be a key factor in macrophage-induced vascular degradation in AVMs. The MIF protein has been found to be elevated in AVMs—a twofold increase compared with control human brain tissue has been observed.39 Macrophage migration inhibitory factor is associated with the upregulation of MMP-2, MMP-9, MMP-12, and downregulation of TIMPs, causing dilation of the arterial wall and eventual rupture.15, 40, 41 Based on studies in abdominal aortic aneurysms, MIF has been shown to induce MMP expression and leads to increased breakdown of many types of collagen, elastin, fibrillin, fibronectin, tenascin, and osteonectin.15, 42, 43 It is noteworthy that the extracellular form of MIF was mostly found in the tunica intima and adventitia of the vasculature, while the intracellular form was found in the tunica media and activated caspase-3 was also detected in the tunica media of AVMs. This co-localization led Chen et al39 to perform a terminal deoxynucleotidyl transferase dUTP nick-end labeling assay, which showed a 10-fold increase in the apoptotic rates in AVMs. Taken together, these findings suggest that MIF promotes macrophage accumulation and weakens AVM walls through both induction of MMPs and apoptosis.
Endoglin and its role in inflammation and vascular remodeling
Endoglin is a membrane glycoprotein located on cell surfaces and is part of the transforming growth factor-β (TGF-β) receptor complex. It is involved in hereditary hemorrhagic telangiectasia, which can also result from a loss-of-function mutation in activin-like kinase receptor-1 (ALK1) or SMAD4 genes. Based on several studies, 9% to 16% of hereditary hemorrhagic telangiectasia patients had an AVM, 27% of whom had a history of ICH.44, 45, 46, 47 Homozygous loss of these genes leads to death in utero.48 ENG and ALK1 are expressed on ECs and bind TGF-β, modulating EC proliferation, migration, adhesion, and apoptosis.49 They are downregulated during adulthood, but are reactivated when the need for vascular repair arises. In addition, they are required for recruiting pericytes, producing extracellular matrix, and stabilizing blood vessels. Pericytes are closely associated with ECs and are involved in suppressing capillary development, stimulating wall growth, and upregulating cell-matrix adhesion and intercellular junction formation. Owing to the extensive involvement of ENG and ALK1 in maintaining vascular stability, missing an allele and thus expressing lower amounts of these proteins leads to vascular dysmorphogenesis and potentially hemorrhage.48
As part of the TGF-β receptor complex, ENG is also involved in the neovascularization of damaged tissue during inflammation. It binds activated circulating leukocytes, enabling them to adhere to damaged endothelium or ischemic tissues. Based on myocardial infarction studies, mice with underexpressed ENG—resembling the vasculature in patients with hereditary hemorrhagic telangiectasia—had a decreased ability to stimulate vessel repair, but this ability was restored with the injection of wild-type mononuclear cells.49 Rossi et al50 showed that leukocytes adhere and extravasate at the site of injury by binding ENG in an integrin-mediated manner in response to the inflammatory chemokine CXCL12/CXCR4. ECs that underexpress ENG therefore have two main defects: (1) they are unable to process active TGF-β, which prevents the recruitment of SMC and results in weak, unstable blood vessels; and (2) they are unable to repair the weakened vessels because of defective leukocyte adhesion.49 Matsubara et al51 compared the expression of ENG in sporadic AVMs to normal brain vessels. In normal brain, ENG was found on the endothelium of all vessels and in the adventitia of arteries and arterioles. In AVMs, ENG was expressed on both endothelium and adventitia. Arterialized veins expressed ENG-positive fibroblasts in the adventitia and perivascular connective tissue. The authors concluded that although increasing numbers of ENG-positive ECs and ENG-positive adventitial cells were seen in sporadic AVMs, ENG density was normal. Thus, the authors did not believe ENG was involved in AVM pathogenesis. However, the presence of ENG on perivascular fibroblasts suggests a possible role for this protein in the vascular remodeling process in response to increased hemodynamic stress.
Angiogenesis in AVMs and the link to inflammation: the role of nuclear factor-kappa B and hypoxia-inducible factor-1
Excessive angiogenesis and vascular remodeling likely contribute to the formation and progression of cerebral AVMs.11 Most of the cerebral vasculature is formed during embryogenesis, with minimal postnatal vasculogenesis. AVMs are generally considered congenital vascular lesions that form in the late stages of fetal development. De novo AVM formation, although rare, has also been reported.6 Spontaneous growth and regression of AVMs confirms that the formation of these lesions is not limited to the period of embryonic development.52, 53 Angiogenesis can occur later in life in response to certain physiologic conditions, such as chronic hypoxia, shear stress, exercise, or hormonal fluctuations, thereby altering AVM morphology.54, 55, 56, 57, 58, 59 Pathologic conditions such as tumor, stroke, or trauma can also drastically upregulate angiogenesis via VEGF and angiopoietins (Table 2). These factors cause the reactivation of quiescent endothelium, breakdown of vessel walls, and the fusion of perinidal capillaries with the nidus, enlarging the AVM.60 On the cellular level, AVMs are dynamic lesions, expanding or shrinking depending on the concentration of inflammatory cytokines and the expression of angiogenic factors and receptors on ECs. AVM ECs express elevated amounts of VEGF-A, -B, -C, -D, and VEGF receptor-1.61, 62 Two factors, nuclear factor κ light chain enhancer of activated B cells (NF-κB) and hypoxia-inducible factor-1 (HIF-1) play an important role in bridging inflammation and angiogenesis in AVMs (Table 2).
Inflammation stimulates lymphocytes to release several inflammatory cytokines, including IL-6, IL-1β, and ΤNF-α, all of which upregulate the expression of VEGF in a multitude of pathways. These three cytokines induce the expression of NF-κB, which binds the promoter regions of the VEGF and IL-8 genes and upregulates their expression, thereby enhancing AVM angiogenesis.64, 63 VEGF in turn increases capillary permeability and upregulates ICAM-1 and VCAM-1 on ECs, thus enhancing leukocyte accumulation, inflammation, and the degradation of the vascular walls via leukocyte-derived MMPs.65, 66, 67, 68, 69 Moreover, VEGF can create fenestrations in the endothelium previously linked by tight junctions.70 Using in vitro models, VEGF was shown to induce sprouting angiogenesis: ECs invaded the underlying matrix and formed capillary-like tubules.71 In addition, studies have shown that VEGF upregulates MMP-9 in a nitric oxide-dependent mechanism.72, 73 Together, these findings suggest that inflammation induces VEGF expression, which in turn promotes angiogenesis, vascular remodeling, and further inflammation in AVM walls.
The NF-κB-VEGF pathway in AVMs is also stimulated under hypoxic conditions by HIF-1. Owing to the shunting of blood directly from the arterial to the venous circulation, perinidal brain tissue may receive relatively lower amounts of oxygen, causing HIF-1 to accumulate and upregulate the expression of VEGF by binding to hypoxia responsive element.74, 75 The induction of VEGF by HIF-1 promotes EC proliferation and migration, while also inhibiting EC apoptosis (this is partly the result of upregulation of factors, such as Flt-1 and Flk-1)74 In fact, Wautier et al76 showed that AVM ECs resist apoptosis upon exposure to dexamethasone and TGF-β, both of which are known proapoptotic factors. Although hypoxia is a major stimulant for HIF-1 and angiogenesis, venous hypertension may be a key factor in the process, as suggested by Zhu et al77 who created a mouse model to test the effect of venous hypertension on cerebral blood flow, oxygen levels, and VEGF expression. In their experiment, venous hypertension did not alter blood flow or oxygen delivery to the AVM, but it caused a fivefold increase in HIF-1 and threefold increase in VEGF.77 These findings suggest that the presence of other factors mainly inflammatory, such as IL-1, TNF-α, and TGF-β can increase HIF-1 expression, even in the absence of hypoxia.77 In fact, it can be hypothesized that venous congestion exerts increased shear stress on the venous ECs, activating the inflammatory cascade and signaling an increased need for more vasculature, which results in upregulation of HIF-1 and VEGF.
The role of inflammatory molecules such as IL-6, MMP-9, and NF-κB, and factors such as HIF-1 and VEGF in the pathogenesis of AVMs has direct implications on AVM treatment, embolization in particular. Even though embolization is a commonly used preoperative or preradiosurgical adjunct, it induces HIF-1a, VEGF, and MMP-9 upregulation among other inflammatory mediators.38, 78, 79 In fact, many studies have shown that embolized AVMs have significantly higher amounts of HIF-1 and VEGF than nonembolized ones.78, 80 Partial AVM occlusion leads to hypoxia, which in turn upregulates VEGF, resulting in elevation of MMP-9 expression via NO- and NF-κB-dependent pathways and angiogenesis.28, 29, 38, 72, 79 These findings suggest that incomplete embolization could have a destabilizing effect on AVMs and may increase the subsequent risk of hemorrhage, warranting further investigation into what the impact of embolization is on the complication risk after surgical resection and on recovery.
The role of VEGF in AVM pathogenesis has prompted investigators to study the relationship between local and systemic VEGF levels. Although elevated local VEGF expression has been linked to an increased likelihood of ICH,33 quantifying the local amount of VEGF and thus the risk of ICH is only feasible once the AVM has been resected. To be able to predict presence of weakened vasculature from early on, Kim et al sought to determine if there was a correlation between local VEGF expression and circulating VEGF levels. They measured the plasma VEGF levels of AVM patients before treatment, 24 hours after resection, and 30 days after resection.81 The mean plasma VEGF concentration was 36.08±13.02 pg/ml on the first measurement (healthy controls had 80.52±14.02 pg/ml VEGF, P=0.028), significantly decreased to 20.09±4.54 pg/m 24 hours after resection (P=0.048), and then increased to 66.81±26.45 30 days after resection, which was not significantly different from controls.81 Even though VEGF is upregulated locally, systemic VEGF is lower in AVM patients and decreases even more immediately after AVM resection. A potential explanation for this finding is that the AVM functions as a ‘sink', since the AVM has upregulated VEGF receptors, which bind VEGF and sequester it from the circulation.62 Once the AVM is resected, the local VEGF is removed, leading to an initial decrease in its plasma concentration. However, the systemic levels increase back to normal within 1 month postoperatively. Another explanation is that the local overexpression of VEGF initiates a negative feedback loop, which downregulates systemic VEGF expression. Removal of this negative inhibition, upon AVM resection, would allow systemic VEGF levels to return to normal. In contrast, Sandalcioglu et al82 suggested that patients with AVMs have significantly elevated plasma VEGF levels in comparison to controls, contradicting the findings of Kim et al.81 Out of the 17 patients studied, however, 8 presented with hemorrhage, which may have contributed to the increased VEGF levels. Hemorrhage initiates clot formation by recruiting platelets, which contain VEGF.83, 84 Once stimulated, platelets release soluble VEGF into the circulation, increasing the systemic levels.43, 60, 85 In addition, hemorrhage-induced hypoxia may further induce VEGF expression.81 Therefore, current evidence suggests that high local VEGF expression leads to low serum VEGF levels in AVM patients.
The notch signaling pathway in cerebral AVMs
Murphy et al86 were able to induce the formation of the hallmark AVM characteristics in mice by manipulating the Notch signaling pathway. In fact, Notch signaling is involved in vascular SMC differentiation and proliferation. Inhibition of Notch has been shown to enhance arterial SMC differentiation in adults, whereas activated Notch increases human vascular SMC proliferation.87 Notch receptors and ligands are only expressed in arteries and upregulate arterial differentiation while hindering venous differentiation.86, 87 When the mammalian receptor Notch4 is expressed in the endothelium, it induces arteriovenous shunting and formation of enlarged and tangled vessels in mice.86 Increased Notch activity also inhibits sprouting angiogenesis, which decreases vessel density. Expression of Notch was sufficient to induce vascular malformations even in adult mice, signifying the importance of Notch homeostasis.88
After experiments on mice showed that Notch4 signaling is involved in AVM pathogenesis and Notch4 repression can lead to hemodynamic normalization, Murphy et al89 studied the role of Notch in human brain AVMs. Using immunofluorescent staining, they showed that the presence of activated Notch1 is significantly higher in resected AVMs in comparison to autopsy and surgical biopsy controls. They also tested for the presence of the canonical Notch downstream gene Hes1 in the AVM endothelium since Hes1 is a transcriptional target of Notch.90 Hes1 was shown to be significantly upregulated in resected human AVMs in comparison to autopsy controls, which had minimal Hes1 expression. Hes1 was also upregulated in AVMs when compared with surgical brain biopsies from non-AVM patients.89
It is not yet clear whether Notch initiates the AVM pathogenesis, or if it is an epiphenomenon of which expression is requisite for the maintenance of the AVM angioarchitecture. In contrast to data from animal studies, experiments on human AVMs do not suggest that increased Notch signaling can form AVM lesions or contribute to their development.89 It is unknown whether pharmacological inhibition of Notch activity would lead to AVM regression in humans.
Potential therapeutic targets
The degradative activity of MMP-9 is believed to be one of the primary mechanisms through which inflammation damages the AVM vasculature and often leads to hemorrhage.15, 24, 27, 36, 91 MMP-9 inhibitors have showed utility in protection from ICH.92, 93, 94 Inhibiting MMP-9 may also negate the harmful effects of AVM-related inflammatory responses. Lee et al28, 29 evaluated the effect of the MMP-9 inhibitors minocycline and pyrrolidine dithiocarbamate on an AVM mouse model and showed that MMP-9 blockade significantly reduced the extent of hemorrhage. Minocycline completely inhibited cerebral MMP-9 activity, effectively suppressing VEGF-induced ICH. Pyrrolidine dithiocarbamate also attenuated hemorrhage by suppressing ~80% of MMP-9 activity. Pyrrolidine dithiocarbamate is an inhibitor of NFκB as well, preventing it from upregulating the expression of VEGF. This is also evidence that VEGF induces MMP-9 expression and contributes to our understanding of the deleterious effect of VEGF-induced angiogenesis on AVM vasculature.28, 29, 72
Most studies of MMP-9 inhibitors for AVM stabilization have been in animal models. Hashimoto et al31 conducted a pilot study of doxycycline in 14 AVM patients to determine its effect of MMP-9 in the AVMs. Ten AVM patients received Dox 100 mg and four patients received placebo for 1 week before AVM resection. The amount of total and active MMP-9 in the resected tissue showed that Dox was effective in inhibiting MMP-9, although not to a statistically significant extent. These data were consistent with previous clinical studies of Dox, decreasing the levels of MMPs in abdominal aortic aneurysms and carotid plaques.92, 95 More studies need to be conducted with a larger sample to determine whether agents such as Dox can stabilize the AVM structurally and modify its clinical course.
A promising approach to treat cerebral AVMs medically is the use of VEGF inhibitors such as bevacizumab. In a cerebral AVM model in mice by focal Alk1 gene deletion combined with VEGF stimulation, Walker et al96 elegantly showed that bevacizumab can penetrate the blood–brain barrier in VEGF-induced angiogenic foci to inhibit angiogenesis and reduce vessel density and the number of dysplastic vessels. It remains to be seen whether VEGF inhibitors can translate into a safe and effective therapy for AVMs in humans.
The Notch pathway in AVMs may be another promising target for medical treatment. Inhibition of Notch using doxycycline treatment reversed the AVM-like lesion, preventing vessel growth and hemorrhage.28, 29, 86, 97 Murphy et al98 showed that doxycycline treatment significantly decreased the diameter of AV shunts in mice and increased the blood flow through arteries adjacent to the AVM. Nearly 70% of the AV shunts regressed without the EC loss.98 The same authors showed that a single genetic manipulation turning off Notch4 to normalize Notch signaling promptly converted large caliber, high-flow AV shunt into capillary-like vessels.98
Limitations
Many of the cell culture, animal, and human studies described in this review provide important insights into the development, progression, and rupture of AVMs; however, there are several limitations to be taken into consideration. We acknowledge that co-occurrence has been used as rationale for the cause-effect relationship both in this review as well as in the original reports. Therefore, certain assumptions may have been made regarding the role of interleukins, VEGF, and Notch in AVM pathogenesis despite insufficient scientific data.
Perhaps the most important gap in AVM research that has hindered advances in understanding of the disease is the absence of a true experimental model. Currently, there is no available true animal model for cerebral AVMs. A reliable animal model is key for studying disease mechanisms and testing new therapies. Some models have been suggested and used, yet these only resemble some but not all aspects of human cerebral AVMs.96 The first cerebral AVM model was recently developed by the distinguished University of California—San Francisco Group by combining a focal Alk1 gene deletion with VEGF stimulation (virally mediated human VEGF-A overexpression).99 This model mimics some aspects of cerebral AVMs, namely the irregular vasculature and arteriovenous shunting.
The second major limitation of AVM research is the relative rarity of the disease in the general population. For example, the recent ARUBA trial initially called for an enrollment of 800 patients but was reduced to 400 after slow trial recruitment, prompting a reassessment of the design. At the conclusion of the trial, outcome data were available for only 223 subjects.12 Further adding to the complexity of the problem is the heterogeneous nature of the disease. Specifically, there are five different groups of AVMs based on the Spetzler–Martin classification, each with a distinct natural history. Also, hemorrhagic versus nonhemorrhagic presentation, embolized versus nonembolized lesions, and time from hemorrhage to treatment are all important variables that can affect the profile of inflammatory gene expression.
The most pressing priority in AVM research is the conception of an animal model that completely resembles human brain AVMs. This will undoubtedly pave the way for development of safe and efficient medical therapies. Studying the role of Notch is crucial in the search for a treatment to achieve regression of AVM lesions solely by pharmacological means. Another avenue warranting further exploration is the use of MMP inhibitors for rupture risk reduction. A very promising strategy appears to involve VEGF blockade as well. VEGF inhibition has preliminarily proven to inhibit angiogenesis and reduce vessel density in cerebral AVMs as discussed above.96 Also, VEGF blockade could prove useful to reduce the size of the AVM making resection easier and radiosurgery safer. Furthermore, bevacizumab has been shown to have radiation-sensitizing effects.100
A promising future area in AVM research is the use of targeted imaging to assess the inflammatory status of lesions and their stability. Ferumoxytol-enhanced MRI is one such technique that is based on the critical role of inflammation and macrophages in the formation and rupture of AVMs.101 As with intracranial aneurysms, the technique may provide important information regarding the risk of AVM rupture, or may be used to monitor the effects of antiinflammatory drugs on these lesions.
Conclusions
Inflammation plays a fundamental role in the progression and rupture of cerebral AVMs via the effects of cytokines on leukocytes, ECs, and SMCs. Several genetic polymorphisms have been identified that exacerbate lesion progression and increase the risk of AVM rupture owing to increased expression of IL-6, IL-1β, IL-1α, TNF-α, and IL-8. These cytokines promote the recruitment of neutrophils and macrophages to the site of injury, and the resulting leukocyte-derived metalloproteinase release damages the vessel walls of AVMs. In addition, increased production of MIF and VEGF in AVMs may contribute to their destabilization by enhancing inflammation and angiogenesis, respectively. A multitude of therapeutic strategies have been investigated with promising results, most notably VEGF blockade and Notch inhibition. As data accrue concerning which constituents of the inflammatory response appear critical in AVM formation, progression, and/or rupture, more specific and efficacious therapeutic strategies can be devised and tested.
The authors declare no conflict of interest.
References
- Berman MF, Sciacca RR, Pile-Spellman J, Stapf C, Connolly ES, Jr., Mohr JP, et al. The epidemiology of brain arteriovenous malformations Neurosurgery 200047389–396.discussion 97. [DOI] [PubMed] [Google Scholar]
- Gross BA, Du R. Natural history of cerebral arteriovenous malformations: a meta-analysis. J Neurosurg. 2013;118:437–443. doi: 10.3171/2012.10.JNS121280. [DOI] [PubMed] [Google Scholar]
- Stapf C, Mast H, Sciacca RR, Berenstein A, Nelson PK, Gobin YP, et al. The New York Islands AVM Study: design, study progress, and initial results. Stroke. 2003;34:e29–e33. doi: 10.1161/01.STR.0000068784.36838.19. [DOI] [PubMed] [Google Scholar]
- Hademenos GJ, Massoud TF. Risk of intracranial arteriovenous malformation rupture due to venous drainage impairment. A theoretical analysis. Stroke. 1996;27:1072–1083. doi: 10.1161/01.str.27.6.1072. [DOI] [PubMed] [Google Scholar]
- Vinuela F, Nombela L, Roach MR, Fox AJ, Pelz DM. Stenotic and occlusive disease of the venous drainage system of deep brain AVM's. J Neurosurg. 1985;63:180–184. doi: 10.3171/jns.1985.63.2.0180. [DOI] [PubMed] [Google Scholar]
- Mohr JP, Parides MK, Stapf C, Moquete E, Moy CS, Overbey JR, et al. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet. 2014;383:614–621. doi: 10.1016/S0140-6736(13)62302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondra SL, Troupp H, George ED, Schwab K. The natural history of symptomatic arteriovenous malformations of the brain: a 24-year follow-up assessment. J Neurosurg. 1990;73:387–391. doi: 10.3171/jns.1990.73.3.0387. [DOI] [PubMed] [Google Scholar]
- Kim H, Sidney S, McCulloch CE, Poon KY, Singh V, Johnston SC, et al. Racial/ethnic differences in longitudinal risk of intracranial hemorrhage in brain arteriovenous malformation patients. Stroke. 2007;38:2430–2437. doi: 10.1161/STROKEAHA.107.485573. [DOI] [PubMed] [Google Scholar]
- Stapf C, Mohr JP, Choi JH, Hartmann A, Mast H. Invasive treatment of unruptured brain arteriovenous malformations is experimental therapy. Curr Opin Neurol. 2006;19:63–68. doi: 10.1097/01.wco.0000200546.14668.78. [DOI] [PubMed] [Google Scholar]
- Novakovic RL, Lazzaro MA, Castonguay AC, Zaidat OO. The diagnosis and management of brain arteriovenous malformations. Neurol Clin. 2013;31:749–763. doi: 10.1016/j.ncl.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Fleetwood IG, Steinberg GK. Arteriovenous malformations. Lancet. 2002;359:863–873. doi: 10.1016/S0140-6736(02)07946-1. [DOI] [PubMed] [Google Scholar]
- Mohr JP, Parides MK, Stapf C, Moquete E, Moy CS, Overbey JR, et al. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet. 2014;383:614–621. doi: 10.1016/S0140-6736(13)62302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Lawton MT, Wen G, Yang GY, Chaly T, Jr., Stewart CL, et al. Gene microarray analysis of human brain arteriovenous malformations Neurosurgery 200454410–423.discussion 23-5. [DOI] [PubMed] [Google Scholar]
- Pawlikowska L, Tran MN, Achrol AS, McCulloch CE, Ha C, Lind DL, et al. Polymorphisms in genes involved in inflammatory and angiogenic pathways and the risk of hemorrhagic presentation of brain arteriovenous malformations. Stroke. 2004;35:2294–2300. doi: 10.1161/01.STR.0000141932.44613.b1. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA. Matrix metalloproteinases in neuroinflammation. Glia. 2002;39:279–291. doi: 10.1002/glia.10108. [DOI] [PubMed] [Google Scholar]
- Li X, Wang R, Wang X, Xue X, Ran D, Wang S. Relevance of IL-6 and MMP-9 to cerebral arteriovenous malformation and hemorrhage. Mol Med Rep. 2013;7:1261–1266. doi: 10.3892/mmr.2013.1332. [DOI] [PubMed] [Google Scholar]
- Achrol AS, Pawlikowska L, McCulloch CE, Poon KY, Ha C, Zaroff JG, et al. Tumor necrosis factor-alpha-238G>A promoter polymorphism is associated with increased risk of new hemorrhage in the natural course of patients with brain arteriovenous malformations. Stroke. 2006;37:231–234. doi: 10.1161/01.STR.0000195133.98378.4b. [DOI] [PubMed] [Google Scholar]
- Sturiale CL, Puca A, Sebastiani P, Gatto I, Albanese A, Di Rocco C, et al. Single nucleotide polymorphisms associated with sporadic brain arteriovenous malformations: where do we stand. Brain. 2013;136:665–681. doi: 10.1093/brain/aws180. [DOI] [PubMed] [Google Scholar]
- Kim H, Hysi PG, Pawlikowska L, Poon A, Burchard EG, Zaroff JG, et al. Common variants in interleukin-1-Beta gene are associated with intracranial hemorrhage and susceptibility to brain arteriovenous malformation. Cerebrovasc Dis. 2009;27:176–182. doi: 10.1159/000185609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanella M, Rubino E, Crobeddu E, Gallone S, Gentile S, Garbossa D, et al. Brain arteriovenous malformations are associated with interleukin-1 cluster gene polymorphisms. Neurosurgery. 2012;70:12–17. doi: 10.1227/NEU.0b013e31822d9881. [DOI] [PubMed] [Google Scholar]
- Chen Y, Pawlikowska L, Yao JS, Shen F, Zhai W, Achrol AS, et al. Interleukin-6 involvement in brain arteriovenous malformations. Ann Neurol. 2006;59:72–80. doi: 10.1002/ana.20697. [DOI] [PubMed] [Google Scholar]
- Storer KP, Tu J, Karunanayaka A, Morgan MK, Stoodley MA. Inflammatory molecule expression in cerebral arteriovenous malformations. J Clin Neurosci. 2008;15:179–184. doi: 10.1016/j.jocn.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Yao JS, Zhai W, Fan Y, Lawton MT, Barbaro NM, Young WL, et al. Interleukin-6 upregulates expression of KDR and stimulates proliferation of human cerebrovascular smooth muscle cells. J Cereb Blood Flow Metab. 2007;27:510–520. doi: 10.1038/sj.jcbfm.9600365. [DOI] [PubMed] [Google Scholar]
- Chen Y, Fan Y, Poon KY, Achrol AS, Lawton MT, Zhu Y, et al. MMP-9 expression is associated with leukocytic but not endothelial markers in brain arteriovenous malformations. Front Biosci. 2006;11:3121–3128. doi: 10.2741/2037. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhu W, Bollen AW, Lawton MT, Barbaro NM, Dowd CF, et al. Evidence of inflammatory cell involvement in brain arteriovenous malformations Neurosurgery 2008621340–1349.discussion 9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Wu Y, Lawton MT, Yang GY, Barbaro NM, Young WL.Coexpression of angiogenic factors in brain arteriovenous malformations Neurosurgery 2005561058–1065.discussion 65. [PubMed] [Google Scholar]
- Rosenberg GA. Growth and bleeding in BAVM: another role for MMPs. Stroke. 2003;34:925–931. doi: 10.1161/01.STR.0000065832.67047.DD. [DOI] [PubMed] [Google Scholar]
- Lee CZ, Xu B, Hashimoto T, McCulloch CE, Yang GY, Young WL. Doxycycline suppresses cerebral matrix metalloproteinase-9 and angiogenesis induced by focal hyperstimulation of vascular endothelial growth factor in a mouse model. Stroke. 2004;35:1715–1719. doi: 10.1161/01.STR.0000129334.05181.b6. [DOI] [PubMed] [Google Scholar]
- Lee CZ, Xue Z, Zhu Y, Yang GY, Young WL. Matrix metalloproteinase-9 inhibition attenuates vascular endothelial growth factor-induced intracerebral hemorrhage. Stroke. 2007;38:2563–2568. doi: 10.1161/STROKEAHA.106.481515. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Wen G, Lawton MT, Boudreau NJ, Bollen AW, Yang GY, et al. Abnormal expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in brain arteriovenous malformations. Stroke. 2003;34:925–931. doi: 10.1161/01.STR.0000061888.71524.DF. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Matsumoto MM, Li JF, Lawton MT, Young WL. Suppression of MMP-9 by doxycycline in brain arteriovenous malformations. BMC Neurol. 2005;5:1. doi: 10.1186/1471-2377-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CZ, Yao JS, Huang Y, Zhai W, Liu W, Guglielmo BJ, et al. Dose-response effect of tetracyclines on cerebral matrix metalloproteinase-9 after vascular endothelial growth factor hyperstimulation. J Cereb Blood Flow Metab. 2006;26:1157–1164. doi: 10.1038/sj.jcbfm.9600268. [DOI] [PubMed] [Google Scholar]
- Cheng SY, Nagane M, Huang HS, Cavenee WK. Intracerebral tumor-associated hemorrhage caused by overexpression of the vascular endothelial growth factor isoforms VEGF121 and VEGF165 but not VEGF189. Proc Natl Acad Sci USA. 1997;94:12081–12087. doi: 10.1073/pnas.94.22.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, et al. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21:7724–7732. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann GF, Okada Y, del Zoppo GJ. Hemorrhagic transformation and microvascular integrity during focal cerebral ischemia/reperfusion. J Cereb Blood Flow Metab. 1996;16:1373–1378. doi: 10.1097/00004647-199611000-00036. [DOI] [PubMed] [Google Scholar]
- Montaner J, Alvarez-Sabin J, Molina CA, Angles A, Abilleira S, Arenillas J, et al. Matrix metalloproteinase expression is related to hemorrhagic transformation after cardioembolic stroke. Stroke. 2001;32:2762–2767. doi: 10.1161/hs1201.99512. [DOI] [PubMed] [Google Scholar]
- Wang X, Tsuji K, Lee SR, Ning M, Furie KL, Buchan AM, et al. Mechanisms of hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke. Stroke. 2004;35 ((Suppl 11:2726–2730. doi: 10.1161/01.STR.0000143219.16695.af. [DOI] [PubMed] [Google Scholar]
- Starke RM, Komotar RJ, Hwang BY, Hahn DK, Otten ML, Hickman ZL, et al. Systemic expression of matrix metalloproteinase-9 in patients with cerebral arteriovenous malformations Neurosurgery 201066343–348.discussion 8. [DOI] [PubMed] [Google Scholar]
- Chen G, Zheng M, Shu H, Zhan S, Wang H, Zhou D, et al. Macrophage migration inhibitory factor reduces apoptosis in cerebral arteriovenous malformations. Neurosci Lett. 2012;508:84–88. doi: 10.1016/j.neulet.2011.12.024. [DOI] [PubMed] [Google Scholar]
- Noels H, Bernhagen J, Weber C. Macrophage migration inhibitory factor: a noncanonical chemokine important in atherosclerosis. Trends Cardiovasc Med. 2009;19:76–86. doi: 10.1016/j.tcm.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Schober A, Bernhagen J, Weber C. Chemokine-like functions of MIF in atherosclerosis. J Mol Med (Berl) 2008;86:761–770. doi: 10.1007/s00109-008-0334-2. [DOI] [PubMed] [Google Scholar]
- Lukes A, Mun-Bryce S, Lukes M, Rosenberg GA. Extracellular matrix degradation by metalloproteinases and central nervous system diseases. Mol Neurobiol. 1999;19:267–284. doi: 10.1007/BF02821717. [DOI] [PubMed] [Google Scholar]
- Uría J, López-Otín C. Matrix metalloproteinases as emerging targets for cancer therapy. Revista de Oncología. 2000;2:282–293. [Google Scholar]
- Nishida T, Faughnan ME, Krings T, Chakinala M, Gossage JR, Young WL, et al. Brain arteriovenous malformations associated with hereditary hemorrhagic telangiectasia: gene-phenotype correlations. Am J Med Genet A. 2012;158A:2829–2834. doi: 10.1002/ajmg.a.35622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letteboer TG, Mager JJ, Snijder RJ, Koeleman BP, Lindhout D, Ploos van Amstel JK, et al. Genotype-phenotype relationship in hereditary haemorrhagic telangiectasia. J Med Genet. 2006;43:371–377. doi: 10.1136/jmg.2005.035451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabba C, Pasculli G, Lenato GM, Suppressa P, Lastella P, Memeo M, et al. Hereditary hemorrhagic telangiectasia: clinical features in ENG and ALK1 mutation carriers. J Thromb Haemost. 2007;5:1149–1157. doi: 10.1111/j.1538-7836.2007.02531.x. [DOI] [PubMed] [Google Scholar]
- Bayrak-Toydemir P, McDonald J, Markewitz B, Lewin S, Miller F, Chou LS, et al. Genotype-phenotype correlation in hereditary hemorrhagic telangiectasia: mutations and manifestations. Am J Med Genet A. 2006;140:463–470. doi: 10.1002/ajmg.a.31101. [DOI] [PubMed] [Google Scholar]
- Leblanc GG, Golanov E, Awad IA, Young WL. Biology of vascular malformations of the brain. Stroke. 2009;40:e694–e702. doi: 10.1161/STROKEAHA.109.563692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Laake LW, van den Driesche S, Post S, Feijen A, Jansen MA, Driessens MH, et al. Endoglin has a crucial role in blood cell-mediated vascular repair. Circulation. 2006;114:2288–2297. doi: 10.1161/CIRCULATIONAHA.106.639161. [DOI] [PubMed] [Google Scholar]
- Rossi E, Sanz-Rodriguez F, Eleno N, Duwell A, Blanco FJ, Langa C, et al. Endothelial endoglin is involved in inflammation: role in leukocyte adhesion and transmigration. Blood. 2013;121:403–415. doi: 10.1182/blood-2012-06-435347. [DOI] [PubMed] [Google Scholar]
- Matsubara S, Bourdeau A, terBrugge KG, Wallace C, Letarte M. Analysis of endoglin expression in normal brain tissue and in cerebral arteriovenous malformations. Stroke. 2000;31:2653–2660. doi: 10.1161/01.str.31.11.2653. [DOI] [PubMed] [Google Scholar]
- Du R, Hashimoto T, Tihan T, Young WL, Perry V, Lawton MT. Growth and regression of arteriovenous malformations in a patient with hereditary hemorrhagic telangiectasia. Case report. J Neurosurg. 2007;106:470–477. doi: 10.3171/jns.2007.106.3.470. [DOI] [PubMed] [Google Scholar]
- Minakawa T, Tanaka R, Koike T, Takeuchi S, Sasaki O. Angiographic follow-up study of cerebral arteriovenous malformations with reference to their enlargement and regression. Neurosurgery. 1989;24:68–74. doi: 10.1227/00006123-198901000-00011. [DOI] [PubMed] [Google Scholar]
- Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci USA. 1990;87:5568–5572. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker VR, Cui L, Miller S, Yu SP, Wei L. Whisker stimulation enhances angiogenesis in the barrel cortex following focal ischemia in mice. J Cereb Blood Flow Metab. 2007;27:57–68. doi: 10.1038/sj.jcbfm.9600318. [DOI] [PubMed] [Google Scholar]
- Xu K, Lamanna JC. Chronic hypoxia and the cerebral circulation. J Appl Physiol (1985) 2006;100:725–730. doi: 10.1152/japplphysiol.00940.2005. [DOI] [PubMed] [Google Scholar]
- Li S, Chen BP, Azuma N, Hu YL, Wu SZ, Sumpio BE, et al. Distinct roles for the small GTPases Cdc42 and Rho in endothelial responses to shear stress. J Clin Invest. 1999;103:1141–1150. doi: 10.1172/JCI5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louissaint A, Jr., Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34:945–960. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Ardelt AA, McCullough LD, Korach KS, Wang MM, Munzenmaier DH, Hurn PD. Estradiol regulates angiopoietin-1 mRNA expression through estrogen receptor-alpha in a rodent experimental stroke model. Stroke. 2005;36:337–341. doi: 10.1161/01.STR.0000153795.38388.72. [DOI] [PubMed] [Google Scholar]
- Sato S, Kodama N, Sasaki T, Matsumoto M, Ishikawa T.Perinidal dilated capillary networks in cerebral arteriovenous malformations Neurosurgery 200454163–168.discussion 8-70. [DOI] [PubMed] [Google Scholar]
- Jabbour MN, Elder JB, Samuelson CG, Khashabi S, Hofman FM, Giannotta SL, et al. Aberrant angiogenic characteristics of human brain arteriovenous malformation endothelial cells Neurosurgery 200964139–146.discussion 46-8. [DOI] [PubMed] [Google Scholar]
- Koizumi T, Shiraishi T, Hagihara N, Tabuchi K, Hayashi T, Kawano T.Expression of vascular endothelial growth factors and their receptors in and around intracranial arteriovenous malformations Neurosurgery 200250117–124.discussion 24-6. [DOI] [PubMed] [Google Scholar]
- Angelo LS, Kurzrock R. Vascular endothelial growth factor and its relationship to inflammatory mediators. Clin Cancer Res. 2007;13:2825–2830. doi: 10.1158/1078-0432.CCR-06-2416. [DOI] [PubMed] [Google Scholar]
- Li A, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003;170:3369–3376. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- Dvorak HF, Detmar M, Claffey KP, Nagy JA, van de Water L, Senger DR. Vascular permeability factor/vascular endothelial growth factor: an important mediator of angiogenesis in malignancy and inflammation. Int Arch Allergy Immunol. 1995;107:233–235. doi: 10.1159/000236988. [DOI] [PubMed] [Google Scholar]
- Dvorak HF, Harvey VS, Estrella P, Brown LF, McDonagh J, Dvorak AM. Fibrin containing gels induce angiogenesis. Implications for tumor stroma generation and wound healing. Lab Invest. 1987;57:673–686. [PubMed] [Google Scholar]
- Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- Connolly DT, Olander JV, Heuvelman D, Nelson R, Monsell R, Siegel N, et al. Human vascular permeability factor. Isolation from U937 cells. J Biol Chem. 1989;264:20017–20024. [PubMed] [Google Scholar]
- Roberts WG, Palade GE. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci. 1995;108:2369–2379. doi: 10.1242/jcs.108.6.2369. [DOI] [PubMed] [Google Scholar]
- Pepper MS, Ferrara N, Orci L, Montesano R. Potent synergism between vascular endothelial growth factor and basic fibroblast growth factor in the induction of angiogenesis in vitro. Biochem Biophys Res Commun. 1992;189:824–831. doi: 10.1016/0006-291x(92)92277-5. [DOI] [PubMed] [Google Scholar]
- Lee CZ, Xue Z, Hao Q, Yang GY, Young WL. Nitric oxide in vascular endothelial growth factor-induced focal angiogenesis and matrix metalloproteinase-9 activity in the mouse brain. Stroke. 2009;40:2879–2881. doi: 10.1161/STROKEAHA.109.552059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari V, Choo-Wing R, Chapoval SP, Lee CG, Tang C, Kim YK, et al. Essential role of nitric oxide in VEGF-induced, asthma-like angiogenic, inflammatory, mucus, and physiologic responses in the lung. Proc Natl Acad Sci USA. 2006;103:11021–11026. doi: 10.1073/pnas.0601057103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng I, Tan WL, Ng PY, Lim J. Hypoxia inducible factor-1alpha and expression of vascular endothelial growth factor and its receptors in cerebral arteriovenous malformations. J Clin Neurosci. 2005;12:794–799. doi: 10.1016/j.jocn.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Uranishi R, Baev NI, Kim JH, Awad IA.Vascular smooth muscle cell differentiation in human cerebral vascular malformations Neurosurgery 200149671–679.discussion 9-80. [DOI] [PubMed] [Google Scholar]
- Wautier MP, Boval B, Chappey O, Enjolras O, Wernert N, Merland JJ, et al. Cultured endothelial cells from human arteriovenous malformations have defective growth regulation. Blood. 1999;94:2020–2028. [PubMed] [Google Scholar]
- Zhu Y, Lawton MT, Du R, Shwe Y, Chen Y, Shen F, et al. Expression of hypoxia-inducible factor-1 and vascular endothelial growth factor in response to venous hypertension Neurosurgery 200659687–696.discussion 96. [DOI] [PubMed] [Google Scholar]
- Sure U, Battenberg E, Dempfle A, Tirakotai W, Bien S, Bertalanffy H.Hypoxia-inducible factor and vascular endothelial growth factor are expressed more frequently in embolized than in nonembolized cerebral arteriovenous malformations Neurosurgery 200455663–669.discussion 9-70. [DOI] [PubMed] [Google Scholar]
- Buell TJ, Ding D, Starke RM, Webster Crowley R, Liu KC.Embolization-induced angiogenesis in cerebral arteriovenous malformations J Clin Neurosci 2014 . http://dx.doi.org/10.1016/j.jocn.2014.04.010 . [DOI] [PubMed]
- Sure U, Butz N, Schlegel J, Siegel AM, Wakat JP, Mennel HD, et al. Endothelial proliferation, neoangiogenesis, and potential de novo generation of cerebrovascular malformations. J Neurosurg. 2001;94:972–977. doi: 10.3171/jns.2001.94.6.0972. [DOI] [PubMed] [Google Scholar]
- Kim GH, Hahn DK, Kellner CP, Hickman ZL, Komotar RJ, Starke RM, et al. Plasma levels of vascular endothelial growth factor after treatment for cerebral arteriovenous malformations. Stroke. 2008;39:2274–2279. doi: 10.1161/STROKEAHA.107.512442. [DOI] [PubMed] [Google Scholar]
- Sandalcioglu IE, Wende D, Eggert A, Muller D, Roggenbuck U, Gasser T, et al. Vascular endothelial growth factor plasma levels are significantly elevated in patients with cerebral arteriovenous malformations. Cerebrovasc Dis. 2006;21:154–158. doi: 10.1159/000090526. [DOI] [PubMed] [Google Scholar]
- Gunsilius E, Petzer A, Stockhammer G, Nussbaumer W, Schumacher P, Clausen J, et al. Thrombocytes are the major source for soluble vascular endothelial growth factor in peripheral blood. Oncology. 2000;58:169–174. doi: 10.1159/000012095. [DOI] [PubMed] [Google Scholar]
- Salgado R, Benoy I, Bogers J, Weytjens R, Vermeulen P, Dirix L, et al. Platelets and vascular endothelial growth factor (VEGF): a morphological and functional study. Angiogenesis. 2001;4:37–43. doi: 10.1023/a:1016611230747. [DOI] [PubMed] [Google Scholar]
- ZhuGe Q, Zhong M, Zheng W, Yang GY, Mao X, Xie L, et al. Notch-1 signalling is activated in brain arteriovenous malformations in humans. Brain. 2009;132:3231–3241. doi: 10.1093/brain/awp246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PA, Lam MT, Wu X, Kim TN, Vartanian SM, Bollen AW, et al. Endothelial Notch4 signaling induces hallmarks of brain arteriovenous malformations in mice. Proc Natl Acad Sci USA. 2008;105:10901–10906. doi: 10.1073/pnas.0802743105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow D, Scheller A, Birney YA, Sweeney C, Guha S, Cummins PM, et al. Notch-mediated CBF-1/RBP-J{kappa}-dependent regulation of human vascular smooth muscle cell phenotype in vitro. Am J Physiol Cell Physiol. 2005;289:C1188–C1196. doi: 10.1152/ajpcell.00198.2005. [DOI] [PubMed] [Google Scholar]
- Carlson TR, Yan Y, Wu X, Lam MT, Tang GL, Beverly LJ, et al. Endothelial expression of constitutively active Notch4 elicits reversible arteriovenous malformations in adult mice. Proc Natl Acad Sci USA. 2005;102:9884–9889. doi: 10.1073/pnas.0504391102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PA, Lu G, Shiah S, Bollen AW, Wang RA. Endothelial Notch signaling is upregulated in human brain arteriovenous malformations and a mouse model of the disease. Lab Invest. 2009;89:971–982. doi: 10.1038/labinvest.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarriault S, Brou C, Logeat F, Schroeter EH, Kopan R, Israel A. Signalling downstream of activated mammalian Notch. Nature. 1995;377:355–358. doi: 10.1038/377355a0. [DOI] [PubMed] [Google Scholar]
- Rosell A, Ortega-Aznar A, Alvarez-Sabín J, Fernández-Cadenas I, Ribó M, Molina CA, et al. Increased brain expression of matrix metalloproteinase-9 after ischemic and hemorrhagic human stroke. Stroke. 2006;37:1399–1406. doi: 10.1161/01.STR.0000223001.06264.af. [DOI] [PubMed] [Google Scholar]
- Baxter BT, Pearce WH, Waltke EA, Littooy FN, Hallett JW, Jr., Kent KC, et al. Prolonged administration of doxycycline in patients with small asymptomatic abdominal aortic aneurysms: report of a prospective (phase II) multicenter study. J Vasc Surg. 2002;36:1–12. doi: 10.1067/mva.2002.125018. [DOI] [PubMed] [Google Scholar]
- Bendeck MP, Conte M, Zhang M, Nili N, Strauss BH, Farwell SM. Doxycycline modulates smooth muscle cell growth, migration, and matrix remodeling after arterial injury. Am J Pathol. 2002;160:1089–1095. doi: 10.1016/S0002-9440(10)64929-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curci JA, Mao D, Bohner DG, Allen BT, Rubin BG, Reilly JM, et al. Preoperative treatment with doxycycline reduces aortic wall expression and activation of matrix metalloproteinases in patients with abdominal aortic aneurysms. J Vasc Surg. 2000;31:325–342. doi: 10.1016/s0741-5214(00)90163-0. [DOI] [PubMed] [Google Scholar]
- Axisa B, Loftus IM, Naylor AR, Goodall S, Jones L, Bell PR, et al. Prospective, randomized, double-blind trial investigating the effect of doxycycline on matrix metalloproteinase expression within atherosclerotic carotid plaques. Stroke. 2002;33:2858–2864. doi: 10.1161/01.str.0000038098.04291.f6. [DOI] [PubMed] [Google Scholar]
- Walker EJ, Su H, Shen F, Degos V, Amend G, Jun K, et al. Bevacizumab attenuates VEGF-induced angiogenesis and vascular malformations in the adult mouse brain. Stroke. 2012;43:1925–1930. doi: 10.1161/STROKEAHA.111.647982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning MW, Cassis LA, Daugherty A. Differential effects of doxycycline, a broad-spectrum matrix metalloproteinase inhibitor, on angiotensin II-induced atherosclerosis and abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2003;23:483–488. doi: 10.1161/01.ATV.0000058404.92759.32. [DOI] [PubMed] [Google Scholar]
- Murphy PA, Kim TN, Lu G, Bollen AW, Schaffer CB, Wang RA. Notch4 normalization reduces blood vessel size in arteriovenous malformations. Sci Transl Med. 2012;4:117ra8. doi: 10.1126/scitranslmed.3002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EJ, Su H, Shen F, Choi EJ, Oh SP, Chen G, et al. Arteriovenous malformation in the adult mouse brain resembling the human disease. Ann Neurol. 2011;69:954–962. doi: 10.1002/ana.22348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang T, Huang S, Armstrong E, Eickhoff JC, Harari PM. Enhancement of radiation response with bevacizumab. J Exp Clin Cancer Res. 2012;31:37. doi: 10.1186/1756-9966-31-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalouhi N, Jabbour P, Magnotta V, Hasan D. Molecular imaging of cerebrovascular lesions. Transl Stroke Res. 2014;5:260–268. doi: 10.1007/s12975-013-0291-0. [DOI] [PubMed] [Google Scholar]