Abstract
In phenylketonuria, elevated plasma phenylalanine concentrations may disturb blood-to-brain large neutral amino acid (LNAA) transport and cerebral protein synthesis (CPS). We investigated the associations between these processes, using data obtained by positron emission tomography with l-[1-11C]-tyrosine (11C-Tyr) as a tracer. Blood-to-brain transport of non-Phe LNAAs was modeled by the rate constant for 11C-Tyr transport from arterial plasma to brain tissue (K1), while CPS was modeled by the rate constant for 11C-Tyr incorporation into cerebral protein (k3). Brain phenylalanine concentrations were measured by magnetic resonance spectroscopy in three volumes of interest (VOIs): supraventricular brain tissue (VOI 1), ventricular brain tissue (VOI 2), and fluid-containing ventricular voxels (VOI 3). The associations between k3 and each predictor variable were analyzed by multiple linear regression. The rate constant k3 was inversely associated with brain phenylalanine concentrations in VOIs 2 and 3 (adjusted R2=0.826, F=19.936, P=0.021). Since brain phenylalanine concentrations in these VOIs highly correlated with each other, the specific associations of each predictor with k3 could not be determined. The associations between k3 and plasma phenylalanine concentration, K1, and brain phenylalanine concentrations in VOI 1 were nonsignificant. In conclusion, our study shows an inverse association between k3 and increased brain phenylalanine concentrations.
Keywords: blood–brain barrier, cerebral protein synthesis, large neutral amino acid, magnetic resonance spectroscopy, phenylketonuria, positron emission tomography
Introduction
Phenylketonuria (PKU; OMIM 261600) is an inborn error of amino acid metabolism, resulting from deficiency of hepatic phenylalanine hydroxylase (phenylalanine 4-monooxygenase, EC 1.14.16.1). This deficiency results in impaired hydroxylation of phenylalanine (Phe) to tyrosine (Tyr), manifested as elevated plasma Phe concentrations and low-to-normal plasma Tyr concentrations. Untreated, PKU is mainly characterized by severe mental retardation. Early diagnosis by neonatal screening, combined with a timely initiated Phe-restricted dietary treatment, prevents mental retardation. However, executive function deficits still occur, even in early and continuously treated patients. Although the relationship between cognitive outcome and plasma Phe concentrations has been clearly established,1 the downstream consequences of elevated plasma Phe concentrations are only partially understood.2, 3
One such consequence could be reduced cerebral protein synthesis (CPS).2, 3, 4 Cerebral protein synthesis plays a central role in cognitive development,5, 6, 7 and reduced CPS contributes to cognitive deficits in several mental retardation disorders.5, 7, 8 In recent publications, we studied CPS in PKU patients using two different approaches.9, 10, 11 First, CPS rate was estimated using positron emission tomography (PET) with 11C-Tyr as a tracer. Cerebral protein synthesis rate was calculated on a pixel-by-pixel basis, as described by Patlak and Blasberg.12 We found that CPS rate in PKU patients was strongly inversely associated with plasma Phe concentrations.9, 10 In a follow-up study, we aimed to analyze the etiology of this association in further detail. To do so, a kinetic approach was used, based on a five-compartment model described by Willemsen et al.13 CPS was modeled by the rate constant for 11C-Tyr incorporation into cerebral protein in three large volumes of interest (VOIs), which were selected for their homogenous distribution of radioactivity.10 We found that plasma Phe concentrations were strongly inversely associated with the rate constant for 11C-Tyr transport from arterial plasma to brain tissue. In turn, reduced rate constants for 11C-Tyr transport from arterial plasma to brain were strongly associated with reduced rate constants for 11C-Tyr incorporation into cerebral protein.11 We concluded that reduced blood-to-brain transport of non-Phe large neutral amino acids (LNAAs), reflected by reduced rate constants for 11C-Tyr transport from arterial plasma to brain, may explain the observed inverse association between plasma Phe concentrations and CPS rate.9, 10 The LNAAs compete for transport across the blood–brain barrier (BBB) mediated by the LNAA type-I transporter (a SLC7A5:SLC3A2 heterodimer).14, 15, 16 This facilitated transport appears to be the only physiologically significant route for LNAA transport from blood to brain, as diffusion-mediated transport of LNAAs across the BBB is hindered by the extensive presence of tight junctions between endothelial cells.17, 18, 19 Still, the contribution of diffusion-mediated LNAA transport to total LNAA BBB transport in PKU patients remains to be investigated. In decreasing order of affinity,20 the LNAAs are Phe, tryptophan, leucine, methionine, isoleucine, Tyr, histidine, valine, and threonine. Therefore, elevated plasma Phe concentrations are believed to increase Phe transport across the BBB and concomitantly decrease transport of non-Phe LNAAs across the BBB, resulting in increased brain Phe concentrations and decreased brain concentrations of non-Phe LNAAs.2, 3
These theorized effects of elevated plasma Phe concentrations on LNAA BBB transport in PKU patients are supported by several clinical studies. Using an intravenous double-indicator method, Knudsen et al14 reported that the modeled brain Phe influx was significantly higher in four PKU patients than in four age-matched non-PKU controls. In line with these data, Möller et al15 showed that in nine PKU patients, brain Phe concentrations (determined by magnetic resonance spectroscopy (MRS)) were positively associated with plasma Phe concentrations. Moreover, this relationship was consistent with a Michaelis–Menten kinetic model for transport of Phe from blood to brain.15 Furthermore, two clinical radioisotope studies support an inhibitory effect of elevated plasma Phe concentrations on blood-to-brain transport of non-Phe LNAAs in PKU patients.16, 21 Specifically, elevated plasma Phe concentrations were shown to reduce uptake of 75Se-selenomethionine21 and were associated with reduced apparent blood-to-brain transport of 6-[18F]-l-dihydroxyphenylalanine,16 a substance which, like the LNAAs, is transported across the BBB by the LNAA type-I transporter.
Considering our findings on the processes associated with CPS in PKU, we next asked ourselves to what extent the rate constant for 11C-Tyr incorporation into cerebral protein is associated with brain LNAA concentrations. As brain concentrations of non-Phe LNAAs can presently not be measured in patients in vivo, we focused on brain Phe concentrations in the current study. Using multiple linear regression analysis, the independent correlations between the rate constant for 11C-Tyr incorporation into cerebral protein and brain Phe concentrations (measured by MRS), the rate constant for 11C-Tyr transport from arterial plasma to brain, and plasma Phe concentrations were investigated in six PKU patients. We hypothesized that the rate constant for 11C-Tyr incorporation into cerebral protein is inversely associated with brain Phe concentrations, and positively associated with the rate constant for 11C-Tyr transport from arterial plasma to brain, with the latter having the strongest association.
Materials and Methods
Study Cohort and Design
Our study cohort is a subpopulation of that reported by Hoeksma et al10 and de Groot et al.11 In these studies, CPS rate and rate constants for 11C-Tyr transfer between plasma and brain compartments were investigated in 16 PKU patients by PET scanning. In our current report, 6 out of these 16 patients are described (patients 5, 8, 12, 13, 15, and 16 in the previously reported studies10, 11). In these patients, MRS was additionally performed, to measure brain Phe concentrations. After an overnight fast, MRS was performed first, followed by PET scanning 2 hours later. Three MRS measurement VOIs were identified by magnetic resonance imaging, as described in detail below. Of note, these VOIs differed from the VOIs involved in calculating the rate constant of 11C-Tyr incorporation into cerebral protein, which are defined in our previous work.10, 11 Scanning durations were 90 minutes for MRI with MRS and 50 minutes for PET. All patients were diagnosed by neonatal screening and continuously treated since, except for patients 5 and 6, who were diagnosed at age 6 and 9 years, after work-up for developmental delay. The Medical Ethical Committee of the University Medical Center Groningen approved the study. Informed consent was obtained in all subjects.
Isotope Preparation, Positron Emission Tomography Data Acquisition, and Rate Constant Modeling
Preparation of 11C-Tyr, PET data acquisition and modeling of rate constants for 11C-Tyr transfer between compartments of interest were performed as described in de Groot et al.11 In short, 11C-Tyr was produced by Bucherer–Strecker synthesis, dissolved in a 0.9% (weight:volume) NaCl solution, and purified by passage through a 0.22 μmol/l filter. This 11C-Tyr synthesis method resulted in a radiochemical purity >95%, with a specific activity >40 TBq/mmol. For PET data acquisition, 400 MBq of 11C-Tyr was injected in each subject. A whole body scanner (ECAT EXACT HR+ PET camera, Siemens/CTI, Knoxville, TN, USA) was used in two-dimensional mode. Throughout the 50 minutes measurement period, 63 two-dimensional images were obtained. The rate constants for 11C-Tyr transfer between compartments were assessed in three VOIs: two parietal volumes and one occipital volume, as defined in Hoeksma et al.10 This volume-based approach was necessary to correct for intervoxel variation, allowing compartmental analysis. Each voxel had a volume of 16.5 mm3. Additional specifications are described in Hoeksma et al10 and de Groot et al.11 The kinetic model consisted of three compartments (two of which were tissue compartments), as shown in de Groot et al.11 This three-compartment model was derived from the five-compartment model of Willemsen et al.13 The following rate constants for 11C-Tyr transfer between compartments were defined: the rate constant for 11C-Tyr transport from arterial plasma to brain (K1), the rate constant for 11C-Tyr transport from brain to plasma (k2), the rate constant for 11C-Tyr incorporation into cerebral protein (k3), and the rate constant for 11C-Tyr release from cerebral protein (k4). Of note, this rate constant nomenclature differs slightly from that in our previous publication,11 as we currently use the consensus PET terminology proposed by Innis et al.22 The present rate constant terms relate to the previous terms as follows: K1 corresponds to k2,1, k2 corresponds to k1,2, k3 corresponds to k3,2, and k4 corresponds to k2,3. Rate constants were modeled directly, using the approach described by Willemsen et al.13
The three-compartment model of our current study is based on data obtained in animal studies.23, 24, 25 These data and associated assumptions are discussed in further detail in de Groot et al.11 Specifically, this includes the assumption that the contribution of ‘recycled' 11C-Tyr (i.e., 11C-Tyr derived from proteolysis of 11C-protein) to the brain 11C-Tyr precursor pool for 11C-Tyr incorporation into cerebral protein is negligible within the time frame of this study. Therefore, k4 was excluded from our analyses. In addition to k4, k2 was not used in statistical analyses, as this rate constant was not significantly associated with k3 in our recent publication,11 and exclusion of k2 would increase the power of the statistical analyses in the current study. K1 was considered to relate to transport from plasma to brain of nonlabeled, non-Phe LNAAs, while k3 was considered to relate to net cerebral protein synthesis.
The compartment model was fitted to the 11C signal per VOI and to the arterial plasma-free 11C-Tyr signal at each time point, using the MATLAB software package (MATLAB version 5, MathWorks, Natick, MA, USA). Consequently, additional 11C-containing substances in plasma (e.g., 11C-containing proteins) were not used to calculate the rate constants for 11C-Tyr transport between compartments. The time-activity curves and graphical analyses associated with the current data are described in one of our previous publications.10
Magnetic Resonance Spectroscopy
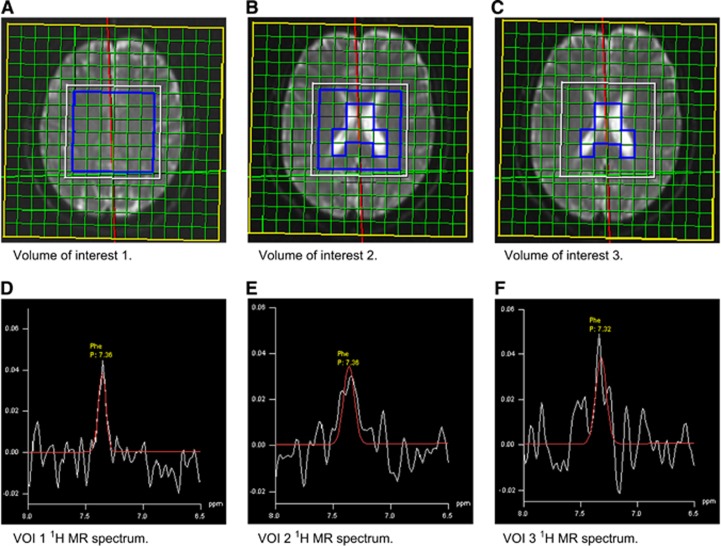
Brain Phe concentrations were measured by MRS, at 1.5 T using a standard Siemens Magnetom Vision MR scanner (Siemens AG, Erlangen, Germany). 1H chemical shift imaging was performed in a transversal area of 8 × 8 × 2 cm above the ventricles and in a transversal area of 8 × 8 × 2 cm passing through the ventricles. The following three VOIs were defined: (1) the supraventricular area, which consisted of tissue-containing voxels only (VOI 1), (2) tissue-containing voxels of the transventricular area (VOI 2), and (3) fluid-containing voxels of the transventricular area (VOI 3), which were considered to contain cerebrospinal fluid (CSF). This approach resulted in 64 voxels sized 1 × 1 × 2 cm per volume. Echo time was set to TE=135 ms, considering the median Phe T2 relaxation time of 215 ms.26 The creatine signal was used as internal reference. Since the Phe signal-to-noise ratio per voxel (2 cm3) was low, Phe signals from multiple voxels were combined to generate a Phe signal per VOI. Figure 1 shows the MRS VOIs with corresponding relevant MR spectra.
Figure 1.
Magnetic resonance spectroscopy (MRS) volumes of interest (VOIs; upper panel (A–C)) and corresponding 6.5 to 8.0 p.p.m. volumes of 1H MR spectra (lower panel (D–F)). p.p.m., parts per million.
Biochemical Analyses
Throughout the PET scanning procedure, repetitive arterial blood samples were collected for analyses of Phe, Tyr, and 11C-containing substances, according to the schedule of Willemsen et al.13 Directly after collection, samples were centrifuged at 2,000 g × 5 minutes at 4°C. Plasma aliquots were transferred to clean vials for each of the specific analyses. Samples for 11C-radioactivity determination were processed immediately. Isolation and quantification of 11C-containing substances was performed according to Willemsen et al.13 Arterial plasma 11C-radioactivity was determined with a calibrated and automated gamma well-counter. Measured values were corrected for radioactive decay since sample acquisition. Arterial plasma samples for amino acid analysis were stored at −80°C. Arterial plasma Phe concentrations were measured using the AccQ Tag method (Waters BV, Breda, The Netherlands), as described in the manufacturer's protocol. Arterial plasma Tyr concentrations were measured by high-performance liquid chromatography with postcolumn ninhydrin derivatization on a Biochrom 20 amino acid analyzer (Pharmacia, Roosendaal, The Netherlands), according to the manufacturer's protocol.
Statistical Analyses
Normality of variable distributions was tested using the Shapiro–Wilk test. Normally distributed variables are reported as mean±s.d. Nonnormally distributed variables are given as median with range in parentheses. Multiple linear regression analysis was used to analyze the associations between k3 and predictor variables of interest. These predictor variables were plasma Phe concentration, K1, brain Phe concentration in VOI 1, brain Phe concentration in VOI 2, and brain Phe concentration in VOI 3.
To obtain generalizable conclusions with multiple linear regression analysis, several assumptions must be met. These assumptions include the assumption of linearity (i.e., the assumption that predictor variables show a linear correlation with the outcome variable) and the assumption of no multicollinearity (i.e., the assumption that predictor variables do not correlate too strongly with one another). To investigate these assumptions, simple linear regression analyses between the corresponding variables were performed before the multiple linear regression analysis. A |r|>0.85 was considered to reflect collinearity.
Next, a stepwise multiple linear regression analysis was performed. In this type of multiple regression analysis, each predictor variable is subsequently entered into the regression model, while the model is constantly re-evaluated for nonsignificant predictor variables. After the multiple regression analysis, the assumptions of homoscedasticity, independence of errors, and normality of error distribution were evaluated by post hoc procedures. The presence of multicollinearity was additionally assessed by analyzing the variance inflation factors. Statistical analyses were performed with the SPSS software package (Windows version 20, SPSS, Chicago, IL, USA). A two-sided P value <0.05 was considered to be statistically significant.
Results
Cohort Characteristics
Cohort characteristics are given in Table 1. Mean patient age was 27±10 years. Mean plasma Phe concentrations at the time of study were 788±376 μmol/l, with mean plasma Tyr concentrations of 41±11 μmol/l. K1 had a mean value of 0.026±0.006 (95% confidence interval 0.020 to 0.032) ml plasma per cm3 brain tissue per minute. The rate constant k3 had a mean value of 0.388±0.110 (95% confidence interval 0.273 to 0.504) nmol/cm3 brain tissue minute. Mean Phe concentrations in brain tissue were 143±66 μmol/l, 142±78 μmol/l, and 248±114 μmol/l for VOIs 1, 2, and 3. In one subject (subject 2), no fluid-containing voxels were observed in VOI 2. Therefore, the value for brain Phe concentration in VOI 3 could not be obtained in this subject.
Table 1. Study cohort characteristics.
| Subject | Age | Plasma Phe | Plasma Tyr | K1a | k3b | Brain Phe VOI 1 | Brain Phe VOI 2 | Brain Phe VOI 3 |
|---|---|---|---|---|---|---|---|---|
| 1 | 26 | 375 | 47 | 0.031 | 0.50 | 79 | 115 | 163 |
| 2 | 21 | 477 | 31 | 0.034 | 0.42 | 132 | 77 | n.m. |
| 3 | 24 | 632 | 61 | 0.023 | 0.50 | 165 | 67 | 166 |
| 4 | 21 | 805 | 35 | 0.027 | 0.36 | 95 | 127 | 191 |
| 5 | 47 | 1078 | 33 | 0.019 | 0.21 | 125 | 273 | 429 |
| 6 | 33 | 1362 | 37 | 0.021 | 0.34 | 264 | 194 | 29 |
n.m., not measurable; Phe, phenylalanine; VOI, volume of interest; 11C-Tyr, L-[1-11C]-tyrosine. Plasma Phe concentrations shown are baseline values. Age is given in years. Plasma and brain Phe concentrations are given in μmol/l.
Rate constant for 11C-Tyr transport from arterial plasma to brain (ml plasma per cm3 brain tissue per minute).
Rate constant for 11C-Tyr incorporation into cerebral protein (nmol/cm3 brain tissue per minute).
Multiple Linear Regression Analysis
As a primary assessment of the assumptions regarding linearity and multicollinearity, simple linear regression analyses between predictor variables were performed before multiple linear regression modeling. These analyses revealed that the assumption of linearity was met, i.e., k3 showed approximately linear correlations with each predictor variable (data not shown). Table 2 shows the results of simple linear regression analyses between predictor variables. As shown in this table, the correlations between plasma Phe concentrations and the other predictor variables were below collinear values. Similarly, K1 did not show collinearity with the other predictor variables. Brain Phe concentrations of different VOIs did not show collinearity with one another, except for the brain Phe concentration in VOI 2 with the brain Phe concentration in VOI 3 (r=0.968).
Table 2. Results of simple linear regression analyses between predictor variables.
| Correlation of interest | r |
|---|---|
| Plasma Phe concentration—K1 | −0.812 |
| Plasma Phe concentration—brain Phe concentration in VOI 1 | 0.722 |
| Plasma Phe concentration—brain Phe concentration in VOI 2 | 0.752 |
| Plasma Phe concentration—brain Phe concentration in VOI 3 | 0.708 |
| K1—brain Phe VOI 1 | −0.514 |
| K1—brain Phe VOI 2 | −0.704 |
| K1—brain Phe VOI 3 | −0.783 |
| Brain Phe concentration in VOI 1—brain Phe concentration in VOI 2 | 0.210 |
| Brain Phe concentration in VOI 1—brain Phe concentration in VOI 3 | 0.244 |
| Brain Phe concentration in VOI 2—brain Phe concentration in VOI 3 | 0.968a |
Phe, phenylalanine; VOI, volume of interest. Pearson correlation coefficients (r) between each set of two predictor variables.
Indicates collinearity, defined as |r|>0.85.
Multiple linear regression analysis was performed with k3 as outcome variable and plasma Phe concentration, K1, brain Phe concentration in VOI 1, brain Phe concentration in VOI 2, and brain Phe concentration in VOI 3 as predictor variables. Since one value for brain Phe concentration in VOI 3 was missing, the analysis included five subjects. The observed model was significant (adjusted R2=0.826, F=19.936, P=0.021) and revealed the brain Phe concentration in VOI 2 as the only significant predictor variable (B=−0.001, SE B <0.001, β=−0.932). This model was described by the following equation:
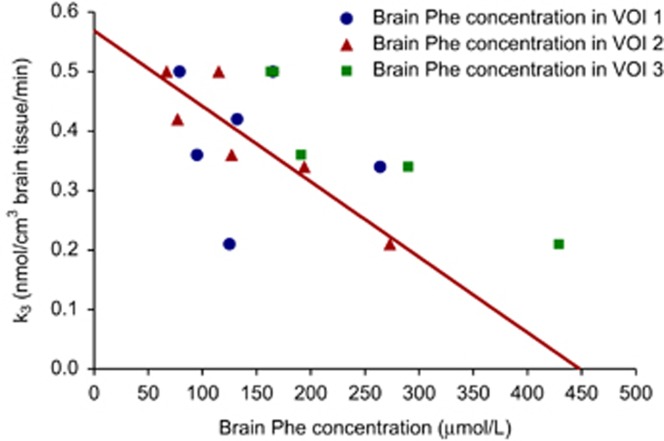
Owing to the high degree of collinearity between brain Phe concentrations in VOI 2 and VOI 3, it could not be determined to which extent each of these predictor variables was associated with k3. Figure 2 shows the relationships between k3 and the brain Phe concentration in each VOI. Post hoc analyses showed that the assumptions of homoscedasticity, independence of errors, and normality of error distribution were met. As observed in the simple linear regression analyses, brain Phe concentrations in VOI 2 and VOI 3 showed multicollinearity in the post hoc analyses, based on the variance inflation factor of brain Phe concentration in VOI 3.
Figure 2.
Relations between the rate constant for l-[1-11C]-tyrosine (11C-Tyr) incorporation into cerebral protein (k3) and brain phenylalanine (Phe) concentrations in each volume of interest (VOI). The red line represents the simple linear regression model between k3 and brain Phe concentration in VOI 2.
Discussion
This study is the first to sequentially investigate plasma Phe concentrations, K1, brain Phe concentrations, and k3 within a very short time frame on 1 day. The main finding of this study is the strong inverse association between k3 and brain Phe concentrations outside VOI 1, without K1 and plasma Phe concentration as significant predictor variables. In contrast to this association, the association between k3 and brain Phe concentrations in VOI 1 was nonsignificant. Before interpreting these findings, we discuss the strengths and limitations of this study.
A strong point of our study design is the combination of PET and MRS scanning in each subject on the same day under standardized conditions. Owing to the time needed to prepare and perform both procedures, this set-up required subjects to remain fasted for 4 to 5 hours, in addition to overnight fasting. The logistical challenge of this set-up reduced the number of subjects willing to participate. Thus, 6 out of the previously reported10, 11 16 patients underwent MRS scanning in addition to PET scanning. One may wonder whether plasma concentrations of LNAAs, in particular of Phe, remained stable throughout the study. Although these data were not obtained in the current study, in previous research, we observed that plasma Phe concentrations remained stable under fasting conditions.27 Therefore, we consider plasma Phe concentrations, and associated blood-to-brain LNAA transport, not to have differed to a clinically relevant extent during the current study.
Magnetic resonance spectroscopy is a well-established method for quantifying several free metabolites in tissue, including Phe. The currently obtained brain Phe concentrations are similar to previously published concentrations using MRS,28, 29, 30, 31, 32, 33 which validates our approach. Still, the currently reported values for brain Phe concentrations are based on multiple assumptions, including a brain tissue creatine concentration of 6.1 mmol/l, as well as similar T1 and T2 relaxation rates for Phe and creatine.26 These factors were not verified in the individual patients reported in this study, as such verification was considered to be too time-consuming, in particular if chemical exchange processes would also be taken into account. In the context of validating brain Phe concentrations obtained by MRS, one may wonder whether these concentrations are similar to those obtained by other approaches. Such approaches could include conventional biochemical analyses and prediction of brain Phe concentration using the obtained brain volume of distribution for 11C-Tyr. Regarding the first approach, previous studies in PKU patients and PKU mice yield results in the μmol/l to mmol/l range.34, 35, 36, 37, 38 Thus, the brain Phe concentrations reported in this study correspond with these values. Regarding the second approach, in our view, additional data obtained by using radioactively labeled Phe would be required, for two reasons. First, the suggested approach applies to plasma and brain concentrations of a tracer under steady-state conditions, which may not apply to the current Phe concentrations in plasma and brain. Second, as the amount of administered tracer is fixed, the plasma concentrations of a labeled amino acid do not directly relate to the plasma concentrations of its unlabeled counterpart. Thus, we consider this approach to be beyond the scope of our current manuscript.
Multiple linear regression analysis was used to investigate the associations between k3 and predictor variables of interest. This analysis can be viewed as an extension of simple regression analysis (in which one outcome variable is associated with one predictor variable). Multiple regression analysis is preferable to performing serial simple regression analyses for at least three reasons. First, it models the specific associations between the outcome variable and each predictor variable, correcting for effects that predictor variables have on one another. Second, it allows detection of associations in which predictor variables only influence the outcome variable when analyzed in combination. Third, it reduces the chance to erroneously find a significant result.
Regarding the main findings of our paper, several results deserve further discussion. We found a significant correlation between k3 and brain Phe concentrations in VOI 2 and/or VOI 3. This correlation explained ~80% of the variance of k3. Considering the small sample size, one may wonder to which extent our data may be biased by certain cases, and whether this strong correlation accurately represents the relationship between CPS and brain Phe concentrations in PKU. However, two main statistical findings support the validity of the observed association. First, the relevant assumptions for multiple linear regression modeling were met, indicating that the obtained model can be generalized to other samples. Second, post hoc statistical parameters showed that no case disproportionally influenced the observed model, further supporting the robustness of the data set. As indicated by these post hoc statistical analyses, random exclusion of a particular value barely influenced the obtained R2.
Contrary to the association between k3 and brain Phe concentrations of the VOIs other than VOI 1, the association between k3 and brain Phe concentration in VOI 1 did not reach statistical significance. This nonsignificant association could result from regional variation of k3. VOI 2 and VOI 3 for brain Phe concentration measurements by MRS were situated closer to the volumes used for 11C-Tyr kinetic modeling than MRS VOI 1. In case of regional variation of k3, k3 values in MRS VOI 1 may not relate strongly to k3 values in the volumes currently used for 11C-Tyr kinetic modeling. Alternatively, the nonsignificant association between k3 and brain Phe concentration in VOI 1 could be caused by the limited power of our study, resulting from the small sample size. This limited power may also explain why the associations of k3 with K1 and with plasma Phe concentrations did not reach statistical significance in our current study, contrary to our previous findings.10, 11 Still, simple regression analyses performed in the current study supported the main conclusions from our previous work (data not shown). The finding that brain Phe concentrations outside VOI 1 were independently associated with k3, whereas K1 was not, suggests that pathophysiologic mechanisms downstream of LNAA BBB transport influence CPS in PKU.
Since brain Phe concentrations in VOI 2 and VOI 3 correlated highly with each other, the specific associations of each of these predictor variables with k3 could not be determined. During the selection of predictor variables for multiple regression analysis, we decided to include brain Phe concentrations in VOI 3 (considered to represent CSF Phe concentrations) as a predictor variable. This inclusion was based on the consideration that both CSF Phe concentrations and brain Phe concentrations could correlate to k3 more strongly than plasma Phe concentrations, as both CSF and brain tissue are situated behind the BBB. The observed strong correlation between brain Phe concentrations in VOI 2 and VOI 3 suggests that CSF Phe concentrations and brain Phe concentrations may highly influence each other. Owing to the high degree of collinearity, based on our current data, one cannot determine whether k3 relates more to Phe concentrations in brain tissue, or to Phe concentrations in CSF. However, regardless of which predictor variable contributed most to the observed association, the conclusion that processes downstream of LNAA BBB transport are likely to influence CPS in PKU remains valid.
The findings reported in this study have both fundamental and clinical implications. From a fundamental viewpoint, the finding that k3 was more strongly associated with brain Phe concentrations than with K1, justifies the investigation of cerebral metabolic processes downstream of LNAA transport from blood to brain. Considering the central role of CPS in many processes during cognitive development,5, 6, 7 reduced CPS in PKU probably negatively impacts cognition. In non-PKU mental retardation diseases, reduced CPS has been described.5, 7, 8 From a clinical viewpoint, our data may raise the question whether brain Phe concentrations are more relevant than plasma Phe concentrations when considering treatment adjustments. This question has been addressed previously by several authors.30, 31, 32, 33, 39 Increased brain Phe concentrations showed a linear relationship with decreased IQ,32, 33 as well as with increased severity of white matter abnormalities.32, 33 In line with these associations, PKU patients with relatively normal brain Phe concentrations and normal IQ, despite high plasma Phe concentrations, have been reported.31, 39 However, the relatively low costs and easy applicability of measuring plasma Phe concentrations favor using plasma Phe concentrations rather than brain Phe concentrations.
Regarding future studies, an important direction for fundamental research is investigating how elevated brain Phe concentrations and/or associated biochemical processes interact with CPS. Future clinical studies should include functional outcome parameters (e.g., reflecting neurophysiologic, neuropsychologic, and cognitive performance), in addition to analyses of LNAA concentrations in plasma, LNAA BBB transport, Phe concentrations in brain, and CPS.
In summary, our study shows a strong inverse association between brain Phe concentrations and k3 (the rate constant for 11C-Tyr incorporation into cerebral protein), while the associations of plasma Phe concentrations and K1 (the rate constant for 11C-Tyr transport from arterial plasma to brain) with k3 were nonsignificant. These findings suggest that elevated brain Phe concentrations and/or associated biochemical processes may reduce CPS in PKU, and validate studies aiming to identify associated molecular pathways. Ultimately, results from such studies may be translated to clinical care, possibly allowing for a more targeted treatment of PKU patients than currently available and improving treatment outcome.
Acknowledgments
The authors thank P Modderman and Dr A van Waarde for analytical assistance.
FJvS is a scientific advisory board member of Nutricia and Merck Serono regarding PKU treatment and has received grants and honoraria from Nutricia, Vitaflo and Merck Serono for research purposes. MJdG has received financial support from Merck Serono to attend scientific symposia. All other authors declare no conflict of interest.
Footnotes
This study was supported by Nutricia, Liverpool, UK.
References
- Blau N, van Spronsen FJ, Levy HL. Phenylketonuria. Lancet. 2010;376:1417–1427. doi: 10.1016/S0140-6736(10)60961-0. [DOI] [PubMed] [Google Scholar]
- van Spronsen FJ, Hoeksma M, Reijngoud DJ. Brain dysfunction in phenylketonuria: is phenylalanine toxicity the only possible cause. J Inherit Metab Dis. 2009;32:46–51. doi: 10.1007/s10545-008-0946-2. [DOI] [PubMed] [Google Scholar]
- de Groot MJ, Hoeksma M, Blau N, Reijngoud DJ, van Spronsen FJ. Pathogenesis of cognitive dysfunction in phenylketonuria: review of hypotheses. Mol Genet Metab. 2010;99 (Suppl 1:S86–S89. doi: 10.1016/j.ymgme.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Surtees R, Blau N. The neurochemistry of phenylketonuria. Eur J Pediatr. 2000;159 (Suppl 2:S109–S113. doi: 10.1007/pl00014370. [DOI] [PubMed] [Google Scholar]
- Greer PL, Greenberg ME. From synapse to nucleus: calcium-dependent gene transcription in the control of synapse development and function. Neuron. 2008;59:846–860. doi: 10.1016/j.neuron.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Rao MS, Jacobson M (eds). In:Developmental Neurobiology Chapters 2, 5, 8, 9, 10Kluwer Academic/Plenum Publishers: New York, NY; 2005 [Google Scholar]
- Vaillend C, Poirier R, Laroche S. Genes, plasticity and mental retardation. Behav Brain Res. 2008;192:88–105. doi: 10.1016/j.bbr.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Johnston MV. Brain plasticity in paediatric neurology. Eur J Paediatr Neurol. 2003;7:105–113. doi: 10.1016/s1090-3798(03)00039-4. [DOI] [PubMed] [Google Scholar]
- Paans AM, Pruim J, Smit GP, Visser G, Willemsen AT, Ullrich K. Neurotransmitter positron emission tomographic-studies in adults with phenylketonuria, a pilot study. Eur J Pediatr. 1996;155 (Suppl 1:S78–S81. doi: 10.1007/pl00014257. [DOI] [PubMed] [Google Scholar]
- Hoeksma M, Reijngoud DJ, Pruim J, de Valk HW, Paans AM, van Spronsen FJ. Phenylketonuria: high plasma phenylalanine decreases cerebral protein synthesis. Mol Genet Metab. 2009;96:177–182. doi: 10.1016/j.ymgme.2008.12.019. [DOI] [PubMed] [Google Scholar]
- de Groot MJ, Hoeksma M, Reijngoud DJ, de Valk HW, Paans AM, Sauer PJ, et al. Phenylketonuria: reduced tyrosine brain influx relates to reduced cerebral protein synthesis. Orphanet J Rare Dis. 2013;8:133–141. doi: 10.1186/1750-1172-8-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak CS, Blasberg RG. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J Cereb Blood Flow Metab. 1985;5:584–590. doi: 10.1038/jcbfm.1985.87. [DOI] [PubMed] [Google Scholar]
- Willemsen AT, van Waarde A, Paans AM, Pruim J, Luurtsema G, Go KG, et al. In vivo protein synthesis rate determination in primary or recurrent brain tumors using L-[1-11C]-tyrosine and PET. J Nucl Med. 1995;36:411–419. [PubMed] [Google Scholar]
- Knudsen GM, Hasselbalch S, Toft PB, Christensen E, Paulson OB, Lou H. Blood-brain barrier transport of amino acids in healthy controls and in patients with phenylketonuria. J Inherit Metab Dis. 1995;18:653–664. doi: 10.1007/BF02436753. [DOI] [PubMed] [Google Scholar]
- Möller HE, Weglage J, Wiedemann D, Vermathen P, Bick U, Ullrich K. Kinetics of phenylalanine transport at the human blood-brain barrier investigated in vivo. Brain Res. 1997;778:329–337. doi: 10.1016/s0006-8993(97)01054-8. [DOI] [PubMed] [Google Scholar]
- Landvogt C, Mengel E, Bartenstein P, Buchholz HG, Schreckenberger M, Siessmeier T, et al. Reduced cerebral fluoro-L-dopamine uptake in adult patients suffering from phenylketonuria. J Cereb Blood Flow Metab. 2008;28:824–831. doi: 10.1038/sj.jcbfm.9600571. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Blood-brain barrier carrier-mediated transport and brain metabolism of amino acids. Neurochem Res. 1998;23:635–644. doi: 10.1023/a:1022482604276. [DOI] [PubMed] [Google Scholar]
- O'Kane RL, Hawkins RA. Na+-dependent transport of large neutral amino acids occurs at the abluminal membrane of the blood-brain barrier. Am J Physiol Endocrinol Metab. 2003;285:1167–1173. doi: 10.1152/ajpendo.00193.2003. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab. 2012;32:1959–1972. doi: 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith QR. Transport of glutamate and other amino acids at the blood-brain barrier. J Nutr. 2000;130 (Suppl:S1016–S1022. doi: 10.1093/jn/130.4.1016S. [DOI] [PubMed] [Google Scholar]
- Oldendorf WH, Sisson BW, Silverstein A. Brain uptake of selenomethionine Se 75 II. Reduced brain uptake of selenomethionine Se 75 in phenylketonuria. Arch Neurol. 1971;24:524–528. doi: 10.1001/archneur.1971.00480360058007. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Ishiwata K, Vaalburg W, Elsinga PH, Paans AM, Woldring MG. Comparison of L-[1-11C]methionine and L-methyl-[11C]methionine for measuring in vivo protein synthesis rates with PET. J Nucl Med. 1988;29:1419–1427. [PubMed] [Google Scholar]
- Hawkins RA, Huang SC, Barrio JR, Keen RE, Feng D, Mazziotta JC, et al. Estimation of local cerebral protein synthesis rates with L-[1-11C]leucine and PET: methods, model, and results in animals and humans. J Cereb Blood Flow Metab. 1989;9:446–460. doi: 10.1038/jcbfm.1989.68. [DOI] [PubMed] [Google Scholar]
- Cumming P, Ase A, Kuwabara H, Gjedde A. [3H]-DOPA formed from [3H]tyrosine in living rat brain is not committed to dopamine synthesis. J Cereb Blood Flow Metab. 1998;18:491–499. doi: 10.1097/00004647-199805000-00004. [DOI] [PubMed] [Google Scholar]
- Sijens PE, Oudkerk M, Reijngoud DJ, Leenders KL, de Valk HW, van Spronsen FJ. 1H MR chemical shift imaging detection of phenylalanine in patients suffering from phenylketonuria (PKU) Eur Radiol. 2004;14:1895–1900. doi: 10.1007/s00330-004-2388-z. [DOI] [PubMed] [Google Scholar]
- van Spronsen FJ, van Rijn M, van Dijk T, Smit GP, Reijngoud DJ, Berger R, et al. Plasma phenylalanine and tyrosine responses to different nutritional conditions (fasting/postprandial) in patients with phenylketonuria: effect of sample timing. Pediatrics. 1993;92:570–573. [PubMed] [Google Scholar]
- Möller HE, Vermathen P, Ullrich K, Weglage J, Koch HG, Peters PE. In-vivo NMR spectroscopy in patients with phenylketonuria: changes of cerebral phenylalanine levels under dietary treatment. Neuropediatrics. 1995;26:199–202. doi: 10.1055/s-2007-979753. [DOI] [PubMed] [Google Scholar]
- Weglage J, Möller HE, Wiedermann D, Cipcic-Schmidt S, Zschocke J, Ullrich K. In vivo NMR spectroscopy in patients with phenylketonuria: clinical significance of interindividual differences in brain phenylalanine concentrations. J Inherit Metab Dis. 1998;21:81–82. doi: 10.1023/a:1005327801588. [DOI] [PubMed] [Google Scholar]
- Koch R, Moats R, Guttler F, Guldberg P, Nelson M. Blood-brain phenylalanine relationships in persons with phenylketonuria. Pediatrics. 2000;106:1093–1096. doi: 10.1542/peds.106.5.1093. [DOI] [PubMed] [Google Scholar]
- Moats RA, Koch R, Moseley K, Guldberg P, Guttler F, Boles RG, et al. Brain phenylalanine concentration in the management of adults with phenylketonuria. J Inherit Metab Dis. 2000;23:7–14. doi: 10.1023/a:1005638627604. [DOI] [PubMed] [Google Scholar]
- Weglage J, Wiedermann D, Denecke J, Feldmann R, Koch HG, Ullrich K, et al. Individual blood-brain barrier phenylalanine transport determines clinical outcome in phenylketonuria. Ann Neurol. 2001;50:463–467. doi: 10.1002/ana.1226. [DOI] [PubMed] [Google Scholar]
- Rupp A, Kreis R, Zschocke J, Slotboom J, Boesch C, Rating D, et al. Variability of blood-brain ratios of phenylalanine in typical patients with phenylketonuria. J Cereb Blood Flow Metab. 2001;21:276–284. doi: 10.1097/00004647-200103000-00011. [DOI] [PubMed] [Google Scholar]
- McKean CM. The effects of high phenylalanine concentrations on serotonin and catecholamine metabolism in the human brain. Brain Res. 1972;47:469–476. doi: 10.1016/0006-8993(72)90653-1. [DOI] [PubMed] [Google Scholar]
- Smith CB, Kang J. Cerebral protein synthesis in a genetic model of phenylketonuria. Proc Natl Acad Sci USA. 2000;97:11014–11019. doi: 10.1073/pnas.97.20.11014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascucci T, Ventura R, Puglisi-Allegra S, Cabib S. Deficits in brain serotonin synthesis in a genetic mouse model of phenylketonuria. Neuroreport. 2002;13:2561–2564. doi: 10.1097/00001756-200212200-00036. [DOI] [PubMed] [Google Scholar]
- Glushakov AV, Glushakova O, Varshney M, Bajpai LK, Sumners C, Laipis PJ, et al. Long-term changes in glutamatergic synaptic transmission in phenylketonuria. Brain. 2005;128:300–307. doi: 10.1093/brain/awh354. [DOI] [PubMed] [Google Scholar]
- Pascucci T, Andolina D, Ventura R, Puglisi-Allegra S, Cabib S. Reduced availability of brain amines during critical phases of postnatal development in a genetic mouse model of cognitive delay. Brain Res. 2008;1217:232–238. doi: 10.1016/j.brainres.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Möller HE, Weglage J, Wiedermann D, Ullrich K. Blood-brain barrier phenylalanine transport and individual vulnerability in phenylketonuria. J Cereb Blood Flow Metab. 1998;18:1184–1191. doi: 10.1097/00004647-199811000-00004. [DOI] [PubMed] [Google Scholar]