Abstract
Epidemiologic studies have shown sex differences in ischemic stroke. The four core genotype (FCG) mouse model, in which the testes determining gene, Sry, has been moved from Y chromosome to an autosome, was used to dissociate the effects of sex hormones from sex chromosome in ischemic stroke outcome. Middle cerebral artery occlusion (MCAO) in gonad intact FCG mice revealed that gonadal males (XXM and XYM) had significantly higher infarct volumes as compared with gonadal females (XXF and XYF). Serum testosterone levels were equivalent in adult XXM and XYM, as was serum estrogen in XXF and XYF mice. To remove the effects of gonadal hormones, gonadectomized FCG mice were subjected to MCAO. Gonadectomy significantly increased infarct volumes in females, while no change was seen in gonadectomized males, indicating that estrogen loss increases ischemic sensitivity. Estradiol supplementation in gonadectomized FCG mice rescued this phenotype. Interestingly, FCG male mice were less sensitive to effects of hormones. This may be due to enhanced expression of the transgene Sry in brains of FCG male mice. Sex differences in ischemic stroke sensitivity appear to be shaped by organizational and activational effects of sex hormones, rather than sex chromosomal complement.
Keywords: cerebrovascular disease, brain ischemia, genetics, reperfusion
Introduction
Ischemic stroke is recognized as a sexually dimorphic disease with women enjoying a lower stroke incidence relative to men until an advanced age.1 Preclinical studies in animal models confirm and replicate this clinical epidemiology.2, 3 This sexual dichotomy has largely been attributed to the activational effects of gonadal hormones—predominantly androgens and estrogens.4 The male gonadal hormone, testosterone has been shown to contribute to the male ‘ischemic sensitivity' phenotype both clinically5 and in animal models.6, 7 However, the role of testosterone in ischemic stroke remains controversial, as other studies have seen an age and dose-dependent protection with testosterone supplementation.8 In contrast, estrogens have been consistently shown to be neuroprotective in the majority of preclinical studies.9 Ovariectomized females have increased histologic injury compared with ovary intact females, and this is reversed with estradiol supplementation. Exogenous estradiol administration also reduces injury in males, suggesting that estrogen exercises its beneficial effects independently of the gonadal sex.
Although hormonal effects on stroke can be reversed by gonadectomy in most models, recent experimental and clinical studies suggest that not all the sex differences in stroke can be attributed to the activational effects of sex hormones. The most convincing evidence comes from pediatric literature which shows that even in prepubertal children (with equivalent levels of sex hormones), boys have a higher risk of ischemic stroke and higher mortality than girls.10 In vitro experiments have also shown sex differences in ischemic sensitivity even in the absence of sex steroids in the medium.11 These studies suggest that the differences in ischemic sensitivity may be innate. Since the sex chromosome complement is intrinsically different in males (XY) and females (XX), we hypothesized that this may contribute to sex differences.
The objective of this study was to investigate the underlying etiology of sex differences in ischemic stroke sensitivity. As it is extremely difficult to specifically dissociate the organizational/activational effects of hormones from sex chromosome effects, we used the ‘four core genotype' (FCG) mouse model in our studies. In this model, Sry, the testes determining gene is deleted from Y chromosome12 and inserted on an autosome giving a XY Male (XYM) genotype.13 In this XYM (gonadal and chromosomal male), the autosome becomes male determining instead of the Y chromosome. A cross between this XYM and a wild-type XXF gives the FCGs, XXM (XX male, a gonadal male with XX chromosomal complement), XYM (XY male, a gonadal male with XY chromosomal complement), XXF (XX female, a gonadal female with XX chromosomal complement), and XYF (XY female, a gonadal female with XY chromosomal complement).14 Outcomes after an induced middle cerebral artery occlusion (MCAO) in the FCG mice were compared in the intact gonadal state, after gonadectomies and after estradiol supplementation to study the influence of sex chromosome (XX/XY) versus sex hormones on ischemic response.
Materials and methods
Experimental Animals
C57BL/6J XYM mice were shipped from University of California to University of Connecticut Health Center, and bred to C57BL/6J wild-type females producing the FCGs. The mice tested were littermates so that litter effects are distributed across groups. We also included a cohort of wild-type (WTM) C57BL/6J males (with Sry present on the Y chromosome rather than an autosome) to compare the effects of Sry transgene with endogenous Sry. All animals (breeding pairs and progeny) were maintained on a 12:12-hour day-night cycle in the animal facility at University of Connecticut Health Center. Food and water was available ad libitum. The progeny of the breeding pairs was subjected to sham/MCAO surgery in the intact gonadal state at 2 to 3 months of age (weight 20 to 25 g). All animals were fed with wet mash after surgery until sacrifice.
Mice were genotyped by PCR using tail DNA as described previously. The sequence of PCR primers used was Myo F: TTA CGT CCA TCG TGG ACA GCA T; Myo R: TGG GCT GGG TGT TAG TCT TAT; YMTFP1: CTG GAG CTC TAC AGT GAT GA; YMTRP1: CAG TTA CCA ATC AAC ACA TCA C; Sry FP: AGC CCT ACA GCC ACA TGA TA; and Sry RP: GTC TTG CCT GTA TGT GAT GG. The Myogenin primer (used as an amplification control) gives a band of ~250 bp, Ymt detects the Y chromosome and gives a band of ~350 bp, and Sry primer gives a band of ~420 bp. Accordingly, the XXF mice have one band of myo; XYF mice have two bands Myo and Ymt; XXM mice have two bands Myo and Sry; and XYM mice have three bands Myo, Ymt, and Sry.
The present study was conducted in accordance with the National Institute of Health guidelines for the care and use of animals in research and under protocols approved by the Center for Lab Animal Care at the University of Connecticut Health Center. All studies were performed blinded to genotype.
Ischemic Model
Focal transient cerebral ischemia was induced by 90 minutes of MCAO using a 0.21-mm monofilament (for all groups) under isoflurane anesthesia, followed by reperfusion, as previously described.15 A mono-therm system was used to monitor and maintain rectal temperature at around 37°C during surgery through an automated temperature control feedback system (Table 1). Cerebral blood flow was measured by Laser Doppler flowmetry (Moor Instruments Ltd, Devon, England) in all animals. There was no difference in the intraischemic and postreperfusion cerebral blood flow among groups, P>0.05 (Table 1).
Table 1. Mean body temperature, Laser Doppler cerebral blood flow values, intraischemic (MCAO) and postreperfusion in FCG mice (gonad intact).
| Genotypes | Temperature (°C) | Ischemia (% of baseline) | Reperfusion (% of baseline) |
|---|---|---|---|
| XXF | 36.8±0.6 | 12.4±0.9 | 90.2±1.1 |
| XYF | 37.0±0.3 | 11.5±0.5 | 89.4±1.0 |
| XXM | 37.2±0.2 | 11.9±1.2 | 89.8±1.5 |
| XYM | 36.9±0.4 | 12.2±1.3 | 88.0±1.4 |
| WTM | 37.1±0.4 | 10.1±1.7 | 87.5±1.5 |
FCG, four core genotype; MCAO, middle cerebral artery occlusion; XXF, XX female; XXM, XX male; XYF, XY female; XYM, XY male; WTM, wild-type male.
Gonadectomy and Estradiol/Oil Supplementation
Gonadectomies were performed between 35 and 45 days of age as previously described.16 Briefly, skin and muscle incisions were made under isoflurane anesthesia and the gonads (ovaries or testes) were removed by cauterizing the vascular supply. The muscle layer was sutured and the skin incisions were stapled.
In a separate cohort of animals, silastic capsules (0.062 inch inner diameter; 0.125 inch outer diameter) containing either 0.035 mL of 17β estradiol (180 μg/mL; Sigma, St Louis, MO, USA) or vehicle (sesame oil) were implanted subcutaneously at the time of gonadectomies by an investigator blinded to genotype or treatment group, as described previously.16 The MCAO surgery was performed within 21 days of pellet implantation.
Testosterone and Estradiol Enzyme-Linked Immunoassay
A cohort of naïve male (XXM, n=8; XYM, n=5; and WTM n=7) and female (XXF, n=7; XYF, n=6) mice (age 2 to 3 months; mice of similar gonadal sex were housed together) was sacrificed by Avertin overdose (intraperitoneal) for blood sample collections (during the light cycle) from the right ventricle of the heart using heparinized syringes. Blood was also collected from all the genotypes subjected to 24 hours of MCAO. The blood sample was centrifuged at 3 824 g for 10 minutes at 4°C to yield serum for hormone detection. Serum was stored at −80°C until use. Enzyme-linked immunoassay for testosterone and estradiol was performed using kits for testosterone (Calbiotech, Spring Valley, CA, USA) and 17β-estradiol (BQ, San Diego, CA, USA), following the manufacturers protocol. Gonadal weights (testis and uterus) were also measured from the cohort of naïve mice of all genotypes. All the weights were taken by a single observer blinded to genotype.
Vascular Anatomy
Naïve FCG mice (n=4 to 8/genotype) were anesthetized by avertin intraperitoneal injections and perfused via the left ventricle with cold phosphate-buffered saline having 1% heparin and then with 4% paraformaldehyde. This was followed by injection of 2 mL of India ink (Sigma) and elemental iron (1% FeSO4, 20% India ink in phosphate-buffered saline). The mice were decapitated and the brains were harvested with the circle of Willis intact. The brains were placed in 10% formalin overnight at 4°C and the large vessel anatomy was examined. Large vessel anatomy was examined by inspection and the posterior communicating artery (PcomA) plasticity was quantified as described previously.17 Briefly, the PcomA was evaluated by a blinded investigator under a dissecting microscope and scored on scale of 0 to 3 with 0=no anastomosis between posterior cerebral artery and superior cerebellar artery, 1=anastomosis between posterior cerebral artery and superior cerebellar artery in capillary phase, 2=small truncal PcomA, and 3=truncal PcomA. The average scores of left and right PcomA were used for statistical analysis.
RNA Extraction and Sry RT-PCR
Brain hemispheres from naïve FCG mice were flash frozen in 2-methyl butane. Following the TRIzol Reagent protocol, total RNA was isolated from the ipsilateral hemisphere of the brain (Invitrogen, Carlsbad, CA, USA). RNA sample concentration and purity was determined using a Nano 2000 spectrometer (Thermo Scientific, Wilmington, DE, USA). Sample purity was determined by a 260/280 ratio of absorbance. Only samples with a total RNA purity of higher than 1.8 were included in the experiment. RNA was reverse transcribed into cDNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). Primer sequences amplify the linear transcripts for the Sry gene were 5′-AAG CGC CCC ATG AAT GCA TTT ATG GT-3′ and 5′-ACA CTT TAG CCC TCC GAT GAG GCT GA-3′ (Integrated DNA Technologies, Coralville, IA, USA). The housekeeping gene, GAPDH, had primer sequences of 5′-CAG CAA CAG GGT GGT GGAC-3′ and 5′-GGA TGG AAA TTG TGA GGG AGA TG-3′ (Integrated DNA Technologies). The PCR conditions were 95°C for 10 minutes (one cycle) followed by 95°C for 15 seconds and 60°C for 60 seconds (40 cycles). RT-PCR was performed using the 7500 Real Time qPCR System (Applied Biosystems). The delta-delta-Ct method was used to determine the relative concentration of mRNA levels from the RT-PCR results.
Western Blot Analysis
Gonadectomized FCG and WTM mice were randomly distributed to sham or MCAO groups. Brains were flash frozen at 24 hours end point. Subcellular fractionation was performed into cytosolic fraction, protein concentrations determined by BCA assay, equal amounts of protein loaded on 4% to 15% SDS/PAGE (Bio-Rad, Hercules, CA, USA) gel, probed with αII spectrin (1:500, Millipore, Billerica, MA, USA), and signal was detected as previously described.18 Quantification of digital images was performed using the Image J software (National Institute of Health, Bethesda, MD, USA).
Terminal Histopathology
Mice subjected to MCAO surgery were sacrificed at either a 24- or 72-hour end point with Avertin overdose (intraperitoneal). There was no mortality at 24 hours in gonadally intact mice. There was a total mortality of 3% and 2% at 72 hours end point in gonadectomized and pellet groups after MCAO, respectively. There was no significant difference in mortality across genotypes and sex. Transcardial perfusion was performed with cold phosphate-buffered saline followed by 4% paraformaldehyde; the brain was fixed for 24 hours and placed in cyroprotectant (30% sucrose) as described previously.19 The brains were cut into 30-μm free-floating sections on a freezing microtome and every eighth slice was stained by cresyl violet stain to evaluate ischemic cell damage. The images were digitalized and infarct volumes were measured using the computer software (Sigma scan Pro5, Chicago, IL, USA) as previously described. The infarct volumes were calculated as percentage of contralateral hemispheric structure to correct for edema using Swanson's method, 20 by an investigator blinded to genotype.
Statistical Analysis
All data are expressed as mean±s.e.m. For infarct volumes in the intact gonadal state, data were analyzed using two-way ANOVA with factors of sex chromosomal complement (XX/XY) and sex (male/ female). Infarct volumes of intact and gonadectomized FCG mice were analyzed using three-way ANOVA with factors of gonadectomy (intact/ gonadectomized), sex and sex chromosomal complement. Similarly, the estradiol supplementation trial was analyzed using three-way ANOVA with factors of treatment (oil/estrogen), sex and sex chromosomal complement. The effects of Sry were compared in WTM and XYM by a two-way ANOVA with factors of gonadectomy (intact/ gonadectomized) and Sry (exogenous/endogenous). Posterior communicating artery plasticity scores were analyzed using the Kruskal–Wallis test with factors of sex and sex chromosome complement. Serum estradiol concentrations were evaluated using two-way ANOVA with factors of sex chromosomal complement (XX/XY) and stroke (MCAO/naïve). A three-way ANOVA with factors of sex chromosomal complement (XX/XY), stroke (MCAO/ naïve), and Sry (endogenous/ transgene) was performed for analysis of serum testosterone levels. Two sample independent Student's t-test (two-tailed) with unequal variance was performed to compare the infarct volumes and PcomA scores between XYM and WTM mice; and also for comparing uterine and testicular weights. P<0.05 was considered as statistically significant using the SPSS 19 software (Chicago, IL, USA).
Results
Vascular Anatomy Was Similar in Four Core Genotype Mice
Large cerebral vessel anatomy was assessed in the FCG mice. No cerebral large vessel anatomic abnormality was seen on gross inspection in the FCG mice (Figure 1). The mean PcomA plasticity scores were XYM, 1±0.25; XXM, 0.75±0.28; XXF, 0.67±0.31; XYF, 0.83±0.3; WTM, 1.1±0.51, n=4 to 8/genotype. There was no significant effect of sex (X2(2)=4.325, P=0.11), or sex chromosomal complement (X2(2) =0.248, P=0.88) in the PcomA plasticity score. No significant difference in the mean PcomA plasticity score was seen between XYM and WTM.
Figure 1.
Vascular anatomy of large cerebral vessels in four core genotype (FCG) and wild-type male (WTM) control mice. No large vessel abnormalities are seen on gross inspection. There was no significant difference in PcomA plasticity score across the genotypes.
Infarct Volumes (24 and 72 Hours) Show a Sex Effect in the Intact Gonadal State
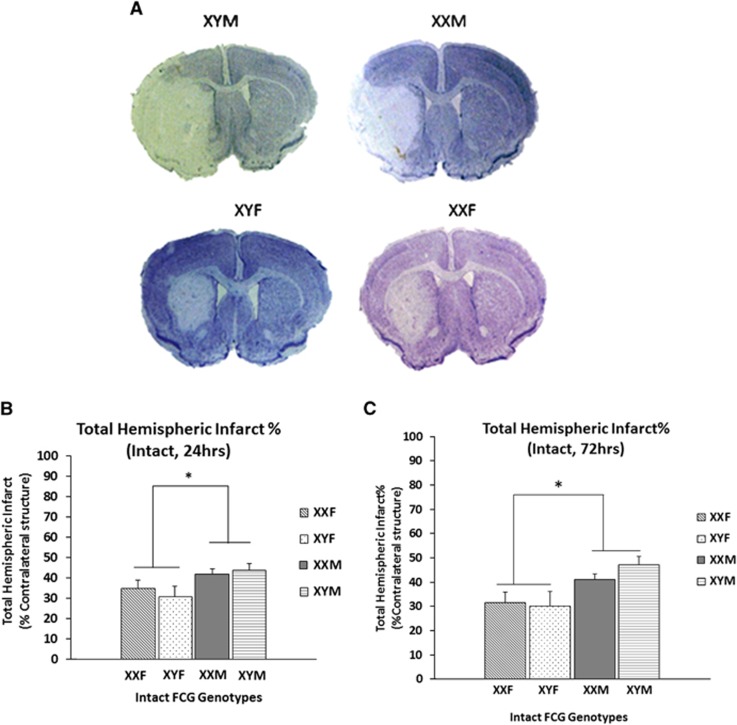
Infarct volumes were measured in the FCG mice at 24 and 72 hours after 90 minutes of MCAO (Figures 2A and 2B). The mean total hemispheric infarct (% contralateral hemisphere) in XYM (43.9±3.1%) and XXM mice (41.9±2.57%) was significantly higher than XYF (30.8±5.09%) and XXF (34.9±3.9%), n=9 to 14/genotype (Figure 2B). There was a significant main effect of sex (F (1, 42)=6.2; P =0.01) but no main effect of sex chromosome, suggesting that gonadal sex (but not sex chromosomal complement) influences stroke outcomes in gonadally intact mice.
Figure 2.
(A) Representative cresyl violet stained sections of the brains (90 minutes middle cerebral artery occlusion (MCAO), 24 hours end point) in the FCG mice. Significantly higher injury is seen in males as compared with females. (B) Total hemispheric infarct percentage at 24 hours end point after 90 minutes of MCAO. *Significant main effect of sex was seen. (C) Total hemispheric infarct percentage at 72 hours end point after 90 minutes of MCAO. *Significant main effect of sex was seen. FCG, four core genotype.
The inflammatory response to ischemic stroke peaks at approximately 72 hours in this model. To ensure the assessment of injury was accurate and the infarct was complete,21 we also assessed the infarct volumes at a 72-hour end point (Figure 2C). Similar to the 24-hour assessment, a significant main effect of sex (F (1, 32)=10.35; P=0.001), but no main effect of sex chromosome or interaction was seen. The mean total hemispheric infarct volume (% contralateral hemisphere) in XYM (47.16±3.54%) and XXM mice (40.9±2.39%) was higher than XYF (30.16±5.65) and XXF mice (31.48±4.31%), n=7 to 12/genotype.
As the gonadal sex effect observed could be secondary to either direct detrimental effects of the Sry gene (as mice with Sry had significantly higher infarct volumes) or beneficial effects of female sex hormones (with ovarian females (XXF and XYF) secreting estrogen), or detrimental effects of testosterone (in mice (XYM and XXM) with testes), we assessed the levels of testosterone and estradiol at baseline and after stroke in these mice.
Serum Testosterone Levels Were Similar in Adult Male Four Core Genotype Mice and Decrease Dramatically after Stroke
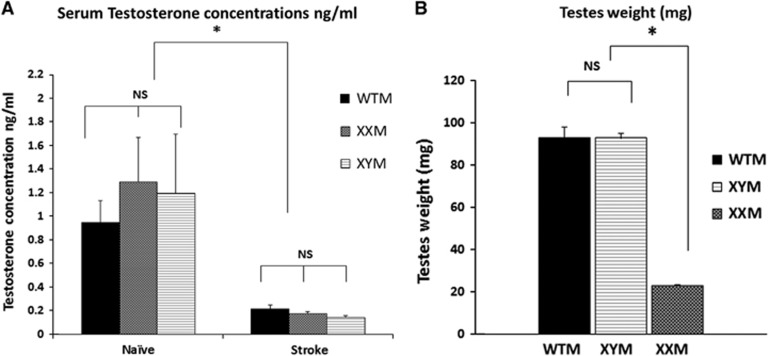
Previous studies have showed that testosterone levels are correlated with the degree of ischemic injury. Therefore, we analyzed the serum testosterone concentrations in naïve and stroke (24 hours end point), 8- to 12-week-old XXM and XYM mice to determine whether differences in sex chromosome complement led to differences in testosterone levels in males. A cohort of age and weight matched WTM (with Sry endogenously present on the Y chromosome) was used to compare the effects of Sry transgene with endogenous Sry. Figure 3A shows the serum concentrations of naïve XYM (1.19±0.5 ng/mL; n=5), naïve XXM (1.28±0.38 ng/mL; n=8), naïve WTM (0.94±0.18 ng/mL; n=7); stroke XYM (0.14±0.016 ng/mL; n=5), stroke XXM (0.17±0.01 ng/mL; n=5), and stroke WTM mice (0.21±0.03 ng/mL; n=5). There was a significant main effect of stroke (F (1, 28)=15.15; P=0.001), as serum testosterone concentrations decreased dramatically after stroke in all three gonadal male groups. No main effect of Sry (endogenous/ transgene) (F (1, 28)=0.08; P>0.05) or sex chromosomal complement (XX/XY) (F (1, 28)=0.044; P>0.05) was seen. This suggests that neither the position of Sry (autosome versus Y chromosome) nor sex chromosomal complement influences adult levels of circulating testosterone in gonadal males.
Figure 3.
(A) Serum testosterone concentrations (ng/mL) as measured by enzyme-linked immunoassay (ELISA). There was no significant difference in the serum testosterone concentrations in XY male (XYM), XX male (XXM), and wild-type male (WTM) mice. Testosterone levels decreased significantly after middle cerebral artery occlusion (MCAO) (*significant stroke effect). There was no significant effect of the location of Sry (endogenous in WTM versus autosomal in XXM and XYM) or sex chromosome complement (XX/XY) on the serum testosterone levels. (B) Testicular weights (mg) in male FCG mice. There was no significant difference in the testicular weights of WTM's and XYM's (indicating that Sry transgene has no effect in testis weight). *Testicular weights of XXM's and XYM's were significantly different, P<0.05.
Testes Weight Is Significantly Lower in XX Male
Figure 3B shows the testicular weights (combined average of right and left testis) of naïve male mice. There was no significant difference in the testicular weights of XYM (n=6) and WTM mice (n=6), P>0.05. However, there was a significant difference in the testes weight of XXM (n=6) and XYM mice, P=0.001. XXM mice are azoospermic due to the lack of other Y chromosome genes responsible for sperm formation, but as noted above this did not influence adult serum testosterone levels.22
Serum Estradiol Levels Are Similar in Adult Female Four Core Genotype Mice
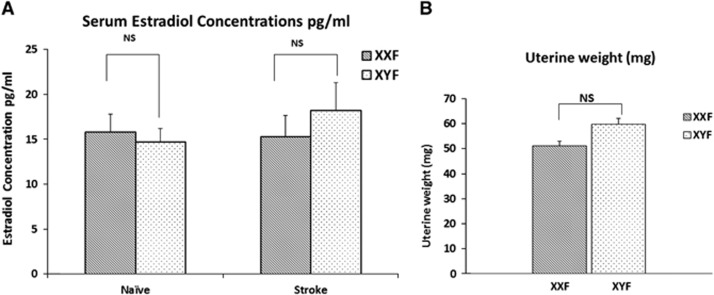
To determine whether differences in estradiol levels exist in the FCG mice at the time of ischemic injury, we evaluated serum estradiol concentrations (pg/mL) in naïve and stroke (24 hours end point) XXF and XYF mice (Figure 4A). There was no significant main effect of stroke (F (1, 22)=0.416; P>0.05) or sex chromosomal complement (XX/XY) (F (1, 22)=0.158 P>0.05). This suggests that there was no difference in the serum estradiol concentration in naïve XXF (15.28±2.01 pg/mL; n=7), naïve XYF (14.75±1.45 pg/mL; n=6), stroke XXF (15.32±2.36 pg/mL; n=8), and stroke XYF mice (18.2±3.09 pg/mL; n=5).
Figure 4.
(A) Serum estradiol concentrations (pg/mL) as measured by enzyme-linked immunoassay (ELISA). There was no significant difference in the estradiol concentrations of XY female (XYF) and XX female (XXF) in the naïve or middle cerebral artery occlusion (MCAO) group. No stroke (MCAO) or sex chromosome complement (XX/XY) effect was seen. (B) Uterine weights (mg) in female FCG mice. There was no significant difference in the uterine weights of XYF and XXF mice.
Similar Uterine Weights in Adult Female Four Core Genotype Mice
The uterus is a target end organ of estrogen secretion. Figure 4B shows the uterine weights of naïve female mice of each genotype. Consistent with the serum estradiol concentrations, there was no significant difference in the uterine weights of XXF and XYF mice (P>0.05).
The Sex Effect Disappears in Gonadectomized Four Core Genotype Mice
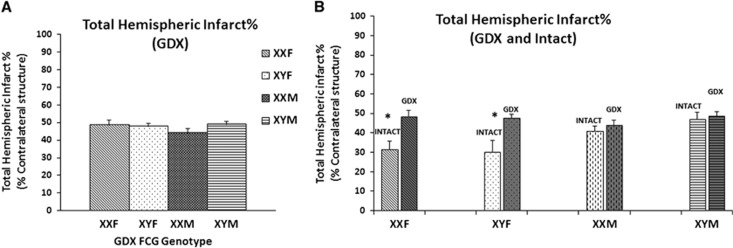
As the sex effect seen could be due to presence of sex hormones, stroke outcomes were also assessed in gonadectomized FCG mice (Figure 5A). At the 72-hour end point, we saw no significant effect of sex or sex chromosome complement in gonadectomized animals, P>0.05 (Figure 5A). The sex effect seen in the intact gonadal state was no longer present and infarct volumes in female mice after ovariectomy were significantly increased to that seen in males, XXF (48.48±2.9%) and XYF (47.7±1.6%). In male mice, injury was not different from that seen in the intact gonadal state, XXM (44.09±2.3%), XYM (48.8±1.8%), n=6 to 9/genotype.
Figure 5.
(A) Total hemispheric infarct % in gonadectomized (GDX) four core genotype (FCG) mice. Infarct volumes at 72 hours after middle cerebral artery occlusion (MCAO) in the gonadectomized state. No significant effect of sex or sex chromosome complement was seen. (B) Total hemispheric infarct % in GDX versus Intact FCG mice. *Significant increase in infarct volumes after gonadectomy in female FCG mice.
Figure 5B shows infarct volumes in both the gonadectomized and intact gonadal state. There was a significant main effect of gonadectomy (F (1, 58)=15.6; P=0.0001), sex (F (1, 58)=5.39; P=0.024) and a significant gonadectomy by sex interaction (F (1, 58)=8.85; P=0.04). These effects were primarily driven by the enhancement of injury after ovariectomy in females, suggesting that the female ‘ischemia protected' phenotype is likely due to protection by ovarian hormones, most likely estrogen. To confirm this, we then supplemented gonadectomized FCG mice with estradiol.
Estradiol Supplementation Protects Gonadectomized Female Four Core Genotype Mice, But Not Males
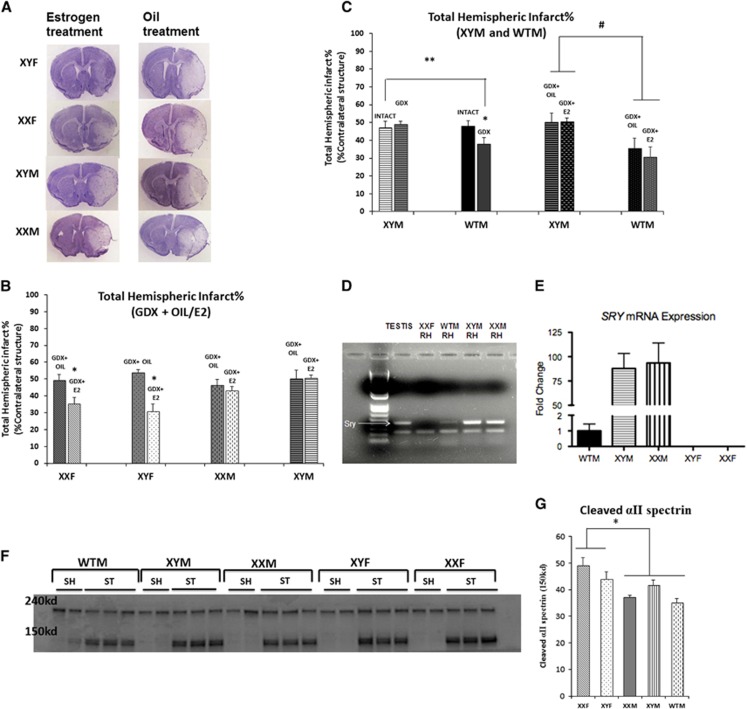
The FCG mice were supplemented with either an oil or an estradiol pellet at the time of gonadectomy and were subjected to stroke within 21 days. Figures 6A and 6B show the infarct volumes. There was a significant main effect of estrogen (F (1, 32)=13.7; P=0.001) as estradiol supplementation protected gonadal females (XXF and XYF). XXF, 35.42±3.7% in estrogen versus 49.42±3.3% in oil group and XYF, 30.9±4.4% in estrogen versus 53.7±1.6% in oil group; n=6 to 8/genotype for estrogen, n=4 to 6/genotype oil group. A significant sex effect (F (1, 32)=4.19; P=0.049) and an estrogen-by-sex interaction (F (1, 32)=10.9; P=0.002) was also seen as the male FCG mice were unresponsive to the neuroprotective effects of estrogen treatment (XXM, 43.2±2.1% estrogen versus 46.3±3.3% oil group, and XYM, 50.47±1.9% estrogen versus 50.26±4.9% oil group, n=7 to 9/genotype estrogen, n=4 to 5/genotype oil group).
Figure 6.
(A) Cresyl violet sections of four core genotype (FCG) mice supplemented with oil/estrogen (E2) and subjected to middle cerebral artery occlusion (MCAO). (B) Total hemispheric infarct % in gonadectomized FCG mice supplemented with oil/estrogen (E2). *Female FCG mice have significantly lower infarct volumes in estrogen group as compared with oil group. (C) Total hemispheric infarct % in wild-type male (WTM) and XY male (XYM). *Significant decrease in infarct volumes was seen in GDX WTM mice versus Intact WTM. **Significant decrease in infarct volumes was seen in GDX WTM mice versus GDX/Intact XYM mice. # significant effect of Sry. (D) PCR showing Sry expression in the brain (RH, right hemisphere) of FCG and WTM mice. First two on the left are controls—testis sample from XYM mice (positive control), RH from female (XXF) (negative control). Higher expression of Sry is seen in the brains of FCG males—XYM and XX male (XXM) versus WTM. (E) Sry mRNA expression in naïve FCG and WTM mice. *Significant 90-fold increase in mRNA expression of Sry in the phenotypes with the exogenous Sry (XYM and XXM) compared with WTM (P<0.05). *No expression was seen in phenotypically female mice (XY female (XYF) and XX female (XXF)). (F, G) Western blot showing caspase mediated αII spectrin cleavage (150 kd) increased in gonadectomized females (XXF and XYF). The 240- kd band is the total αII spectrin. ST—stroke (MCAO); SH—sham mice. Graph in (G) shows a significant sex effect in cleaved αII spectrin expression, n=2 shams, n=3 strokes in each group. GDX, gonadectomy.
Gonadectomy Protects Wild-Type XY Male Mice
The male FCG mice have the sex determining gene, Sry at an exogenous location on an autosome, that is different from its normal endogenous location on the Y chromosome. In contrast to the results in FCG mice, previous studies have established that estradiol is protective in both WT male and female mice.23 Therefore, we wanted to compare the sex effects seen in the XYM mice (exogenous Sry) with the WTM mice (endogenous Sry). There was no significant difference in the infarct volumes of XYM and WTM mice in the intact gonadal state, P>0.05. The infarct volumes decreased significantly in gonadectomized WTM mice, 37.8±3.8% versus 49.42±3.3% in the intact gonadal state, n=7 to 8/group (Figure 6C). However, infarct volumes did not change in the XYM after gonadectomy, leading to a significant gonadectomy versus Sry interaction (F (1, 27)=4.95; P=0.034).
Furthermore, estrogen was supplemented in the gonadectomized WTM mice and these mice were compared with the XYM FCG mice. A significant Sry effect was again seen (F (1, 20)=11.38; P=0.003) as gonadectomized WTM supplemented with estrogen (30.7±5.6%) or oil (35.5±5.6%), (n=7 to 8/genotype) had smaller infarct volumes as compared with XYM estrogen supplemented cohorts. Overall, loss of the testes or the male sex hormone, testosterone, is protective in stroked WT males with endogenous Sry but has no effect in males with the exogenous Sry. This suggests that the location of the Sry within the genome contributes to the response to sex hormones after an ischemic challenge.
Difference in Sry Transgene Expression
Sry expression was studied in the brains of the FCG and WTM mice. Figure 6D shows that FCG males (with the Sry transgene) have a higher expression of Sry in brain, compared with wild-type mice (with Sry endogenously present on the Y chromosome). Figure 6E shows approximately a 90-fold increase in Sry mRNA expression for XYM and XXM mice compared with WTM through RT-PCR analysis.
Sex Effect in Caspase 3 Activation
We assessed caspase activation using cleaved αII spectrin levels as a measure of caspase 3 activity using western blot analysis on flash frozen fractionated cytosolic samples from gonadectomized MCAO FCG mice. αII spectrin has a specific caspase 3 cleavage site, and the 150-kd cleavage product was examined to assess caspase 3 activity. A significantly greater increase in αII cleaved spectrin was seen in gonadectomized XXF and XYF mice as compared with XYM, XXM, and WTM mice (Figures 6E and 6F), suggesting that caspase activation was independent of activational effects of gonadal steroids (F (1, 12)=10; P=0.013). No sex chromosome effect was seen.
Discussion
This is the first study to systematically investigate the contribution of sex chromosomes (XX and XY) and gonadal hormones to ischemic stroke sensitivity. This novel study answers several important questions regarding the etiology of sex differences in stroke. First and foremost, our results suggest that the sex chromosomal complement (XX or XY) does not independently influence outcome after experimental ischemic stroke, either in the presence or in the absence of gonads. We initially hypothesized that the presence of the second X chromosome in females contributed to intrinsic female protection, or conversely that detrimental effects of genes on the Y chromosome led to an ischemic sensitive phenotype in males. Our earlier preliminary studies in two strains of XO mice have shown no increase in ischemic sensitivity in XO mice as compared with XX wild-type females. Microarray studies have shown that genes on the X chromosome escape inactivation and that genetic balancing by random X inactivation is far from complete. Males not only have just one copy of X chromosome genes, but also have additional male-specific expression of genes found only on the Y chromosome.24 Interestingly, a recent report did show differences in the pattern of X-chromosome gene expression between men and women after an acute ischemic stroke,25 suggesting that differences in gene expression in males and females could influence stroke outcome. To further explore the Y chromosomal effects and to dissect out the chromosomal and hormonal contributions to ischemic sensitivity in males and females, we turned to the FCG model.
In the present study, infarct volume in animals with intact gonads (XXM≈XYM>XXF≈XYF) initially suggested that the presence of Sry (which is seen in XXM and XYM) is detrimental. If there was a ‘protective' factor on the X chromosome that is expressed differently, then the infarct volumes in XXM and XXF mice would have been similar, and smaller from those of XY groups. Instead, we found no difference in injury between XX and XY mice of the same gonadal sex implying that there is no effect of the additional X chromosomes on ischemic injury.
Our study also suggests that the additional Y chromosome genes are not responsible for the male ‘ischemic sensitivity' phenotype. If the Y chromosome (without Sry) is contributing to the detrimental ischemic response, then the injury would have been equivalent in XY mice (XYM and XYF). Instead, XYF mice (with ovaries) had less injury than that of XYM mice (with testis) showing a sex effect in the intact gonadal state. All gonadal male mice (with testes), XXM, XYM, and WTM had significantly higher infarct volumes compared with gonadal females (with ovaries), XXF and XYF.
We reasoned that these findings could be due to either (1) detrimental effects of testosterone in males or (2) protective effects of estradiol in females. The major sex hormones have both organizational and activational effects.9 Organizational effects of hormones are permanent and long lasting, often occurring in utero. In contrast, activational effects of hormones are dependent on acute circulating hormone levels and, therefore, can be reversed by gonadectomy. To remove the activational effects of hormones, we gonadectomized FCG mice before stroke, removing circulating estrogen and testosterone. After gonadectomy, gonadal females (XXF and XYF) lost the ‘ischemia protective' phenotype, however FCG males (XYM and XXM) had the same degree of infarct damage even in the absence of the testes and loss of testosterone. Estradiol supplementation at the time of gonadectomy rescued gonadal females (XXF and XYF) regardless of chromosome complement. This result strongly supports the hypothesis that estrogen is the primary contributing factor to the improved histologic outcomes seen in females relative to males. 17-β Estradiol is known to be a robust neuroprotective agent in most animal models of stroke. Estrogen contributes to the sexual dimorphism in incidence and prevalence of ischemic stroke, as girls and young women have lower incidence of stroke as compared with men.9 This epidemiology reverses with advancing age, and postmenopausal women bear the brunt of this disease as incidence grows. Similarly, in animal models estradiol-mediated neuroprotection is ameliorated by ovariectomy, and has been thought to be solely an activational (reversible) effect of hormone exposure. Estradiol supplementation decreases infarct volumes in ovariectomized females as well as in gonadally intact males,26, 27 suggesting that its protective effects are independent of sex, at least when administered acutely after injury. However, estrogen supplementation in postmenopausal women lead to a higher incidence of stroke in the WHI (Women's Health Initiative) clinical trial.28 Advanced age of women in the trial, use of Premarin, timing of dose, etc. may have had a role in this translational failure as 4 years of hormone replacement early in menopause did not affect progression of atherosclerosis or increase risk.29
We found that serum testosterone levels decrease dramatically in all males after stroke, consistent with both previous clinical and animal studies.30 This could be due to decreased production of testosterone (by the testes) or increased aromatizaton to estradiol in the brain, or both. Lower testicular expression of steroidogenesis-related genes has been reported after experimental stroke in rats. Brain aromatase (which converts testosterone to estrogen) expression31, 32, 33 and production of estrogen in the brain34 are known to increase after stroke. It appears that the brain itself may trigger endogenous protective mechanisms after injury by decreasing the de novo synthesis of testosterone and/or aromatizing androgens to estrogens—the protective steroid. Exogenous testosterone administration early in life to male rats' upregulates testicular aromatase expression and increases serum estradiol levels in males. When these males were subjected to stroke in adulthood, they had significantly smaller infarct volumes compared with untreated males, recapitulating the ischemia-resistant phenotype seen in females. Local brain estradiol production also contributes to ischemic sensitivity, as female mice deficient in aromatase had significantly larger infarcts than wild-type ovariectomized females implying its contribution to extra-gonadal estrogen production. Cell death pathways differ between the two sexes, caspase-independent (nitric oxide/poly[ADP-ribose] polymerase) cell death pathway predominates in males, while caspases are mediators of cell death in females.9 A significant sex effect was seen in caspase activation. Higher αII cleaved spectin was seen in gonadectomized XXF and XYF mice as compared with XYM, XXM, and WTM mice. This suggests that the sex differences seen in caspase activation are independent of activational hormone levels (as they were gonadectomized) and are not a sex chromosome effect. Organizational effects of hormones may be responsible for these differences.18 Further studies are needed to reconcile how sex influences the balance between systemic and local levels of sex steroids, which interact or sum to influence sensitivity to ischemia. It is also important to recognize that even the early embryonic effects of hormones have a profound effect on response to brain injury.
The male gonadal hormone, testosterone has been associated with an increased risk of stroke in young boys. Although testosterone does not seem to worsen cardiometabolic risk factors, exogenous testosterone replacement increased cardiovascular risk and adverse events in men aged 65 years and older.35 Animal studies have shown that testosterone supplementation increases injury in young castrated male rats. In the present study, gonadally intact male mice had equivalent testosterone levels regardless of chromosome complement or transgenic versus endogenous expression of Sry (XXM, XYM, and WTM), consistent with other reports.36 Therefore, the higher testosterone levels could explain the enhanced ischemic sensitivity in males. Somewhat surprisingly, gonadectomy did not reverse the ‘ischemia-sensitive' phenotype in the FCG males (XXM and XYM mice) that have the Sry transgene expressed on an autosome, despite the loss of testosterone. As others have shown, that castration is protective in males, the possibility existed that transgene insertion somehow altered the response to testosterone. We first confirmed that WTM mice (with Sry endogenously on the Y chromosome) were protected by castration, consistent with other studies. We then determined whether the male FCG mice (XYM and XXM) responded to estrogen supplementation, which they did not. We hypothesized that the hormone-insensitive phenotype of the GDX FCG males (XYM and XXM) versus gonadectomy (GDX) WTM is due to either the direct effects of Sry deletion (from the Y chromosome) or positional effects of Sry transgene insertion on the autosome. The development of testes is secondary to the action of the Sry gene. Sry encodes a transcription factor of the high mobility group-box family of DNA-binding proteins. This is expressed in the undifferentiated testis at embryonic day 10.5 to 12.5, and upregulates Sox9 gene expression prompting Sertoli cell differentiation. A fetus that does not produce functional Sry protein develops into a female (with ovaries) despite the presence of the Y chromosome. As the expression of Sry gene directs the bipotential gonad to mature into testes which secrete testosterone; testes and testosterone mediate the indirect effects of Sry gene. Prenatal and early postnatal secretion of testosterone organizes the male brain.37 Differential expression of Sry (exogenous versus endogenous) may cause differences in gonadal maturation, the neonatal testosterone surge and thus change brain organization pattern.
To directly assess Sry gene expression, we examined brain Sry levels. We saw a dramatic increase in Sry transgene expression in brains of adult FCG males (XYM and XXM) versus WTM (which have the endogenous Sry gene). Higher or temporally shifted expression of Sry in the embryonic testes in the FCG mice is probable, raising the possibility that the brain sex steroid milieu may not have been equivalent early in life, leading to the differential responsiveness to sex steroids during injury in adulthood. Differences in the FCG XYM and WTM have been reported in mounting behavior, social exploration, and hypothalamic astrocytic estradiol-facilitated progesterone synthesis,38 suggesting that early organizational effects of Sry occur and manifest as changes in adult behavior and brain metabolism. Our results suggest that there are developmental effects, either patterned in utero or during the juvenile period that determine the response to estrogen supplementation or testosterone loss in the FCG model. This highlights one of the long supported concepts that the effects of hormones in adulthood may be influenced or patterned by prenatal or neonatal sex steroid exposure that have permanent effects on a phenotype.39 The magnitude and even the direction of activational effects could be conditioned by earlier organizational effects, as appears to be the case in our study.
One important limitation of this study is that murine models may not be completely representative of the complex dynamics of chromosomal inactivation seen in humans, as X inactivation is lower in mice as compared with humans.40 Nonetheless, the FCG model is a powerful tool that can be used to dissociate chromosomal and hormonal effects. We conclude that ischemic stroke sensitivity is shaped by both organizational and activational effects of sex hormones. This is the first study to evaluate the hormonal contribution to ischemic sensitivity in the brain independently of the sex chromosome complement.
Acknowledgments
The authors thank Rebecca McClusky for assistance.
The authors declare no conflict of interest.
Footnotes
This work is funded by 11PRE7440068 (to BM) and R01-NS055215-06A1 (to LDM).
References
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics—2013 update: A report from the american heart association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurn PD, Macrae IM. Estrogen as a neuroprotectant in stroke. J Cereb Blood Flow Metab. 2000;20:631–652. doi: 10.1097/00004647-200004000-00001. [DOI] [PubMed] [Google Scholar]
- Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD.Gender-linked brain injury in experimental stroke Stroke 199829159–165.discussion 166. [DOI] [PubMed] [Google Scholar]
- Yamori Y, Horie R, Sato M, Ohta K. Proceedings: Prophylactic trials for stroke in stroke-prone shr: Effect of sex hormones. Jpn Heart J. 1976;17:404–406. doi: 10.1536/ihj.17.404. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Hurn PD. Gender differences in pediatric stroke: is elevated testosterone a risk factor for boys. Ann Neurol. 2009;66:713–714. doi: 10.1002/ana.21925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawk T, Zhang YQ, Rajakumar G, Day AL, Simpkins JW. Testosterone increases and estradiol decreases middle cerebral artery occlusion lesion size in male rats. Brain Res. 1998;796:296–298. doi: 10.1016/s0006-8993(98)00327-8. [DOI] [PubMed] [Google Scholar]
- Cheng J, Alkayed NJ, Hurn PD. Deleterious effects of dihydrotestosterone on cerebral ischemic injury. J Cereb Blood Flow Metab. 2007;27:1553–1562. doi: 10.1038/sj.jcbfm.9600457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida M, Palmateer JM, Herson PS, DeVries AC, Cheng J, Hurn PD. Dose-dependent effects of androgens on outcome after focal cerebral ischemia in adult male mice. J Cereb Blood Flow Metab. 2009;29:1454–1462. doi: 10.1038/jcbfm.2009.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manwani B, McCullough LD. Sexual dimorphism in ischemic stroke: lessons from the laboratory. Women's Health. 2011;7:319–339. doi: 10.2217/whe.11.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb MR, Fullerton HJ, Nowak-Gottl U, Deveber G. Male predominance in childhood ischemic stroke: Findings from the international pediatric stroke study. Stroke. 2009;40:52–57. doi: 10.1161/STROKEAHA.108.521203. [DOI] [PubMed] [Google Scholar]
- Li H, Pin S, Zeng Z, Wang MM, Andreasson KA, McCullough LD. Sex differences in cell death. Ann Neurol. 2005;58:317–321. doi: 10.1002/ana.20538. [DOI] [PubMed] [Google Scholar]
- Lovell-Badge R, Robertson E. Xy female mice resulting from a heritable mutation in the primary testis-determining gene, tdy. Development. 1990;109:635–646. doi: 10.1242/dev.109.3.635. [DOI] [PubMed] [Google Scholar]
- Mahadevaiah SK, Odorisio T, Elliott DJ, Rattigan A, Szot M, Laval SH, et al. Mouse homologues of the human azf candidate gene rbm are expressed in spermatogonia and spermatids, and map to a y chromosome deletion interval associated with a high incidence of sperm abnormalities. Hum Mol Genet. 1998;7:715–727. doi: 10.1093/hmg/7.4.715. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Chen X. What does the "four core genotypes" mouse model tell us about sex differences in the brain and other tissues. Front Neuroendocrinol. 2009;30:1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough LD, Zeng Z, Li H, Landree LE, McFadden J, Ronnett GV. Pharmacological inhibition of amp-activated protein kinase provides neuroprotection in stroke. J Biol Chem. 2005;280:20493–20502. doi: 10.1074/jbc.M409985200. [DOI] [PubMed] [Google Scholar]
- McCullough LD, Blizzard K, Simpson ER, Oz OK, Hurn PD. Aromatase cytochrome p450 and extragonadal estrogen play a role in ischemic neuroprotection. J Neurosci. 2003;23:8701–8705. doi: 10.1523/JNEUROSCI.23-25-08701.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K, Kondo T, Kawase M, Chan PH. The development of a new mouse model of global ischemia: Focus on the relationships between ischemia duration, anesthesia, cerebral vasculature, and neuronal injury following global ischemia in mice. Brain Res. 1998;780:304–310. doi: 10.1016/s0006-8993(97)01217-1. [DOI] [PubMed] [Google Scholar]
- Siegel C, Li J, Liu F, Benashski SE, McCullough LD. Mir-23a regulation of x-linked inhibitor of apoptosis (xiap) contributes to sex differences in the response to cerebral ischemia. Proc Natl Acad Sci USA. 2011;108:11662–11667. doi: 10.1073/pnas.1102635108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manwani B, Liu F, Xu Y, Persky R, Li J, McCullough LD. Functional recovery in aging mice after experimental stroke. Brain Behav Immun. 2011;25:1689–1700. doi: 10.1016/j.bbi.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- Liu F, Schafer DP, McCullough LD. Ttc, fluoro-jade b and neun staining confirm evolving phases of infarction induced by middle cerebral artery occlusion. J Neurosci Methods. 2009;179:1–8. doi: 10.1016/j.jneumeth.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox KH, Rissman EF. Sex differences in juvenile mouse social behavior are influenced by sex chromosomes and social context. Genes Brain Behav. 2011;10:465–472. doi: 10.1111/j.1601-183X.2011.00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toung TJ, Traystman RJ, Hurn PD. Estrogen-mediated neuroprotection after experimental stroke in male rats. Stroke. 1998;29:1666–1670. doi: 10.1161/01.str.29.8.1666. [DOI] [PubMed] [Google Scholar]
- Ngun TC, Ghahramani N, Sanchez FJ, Bocklandt S, Vilain E. The genetics of sex differences in brain and behavior. Front Neuroendocrinol. 2011;32:227–246. doi: 10.1016/j.yfrne.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamova B, Tian Y, Jickling G, Bushnell C, Zhan X, Liu D, et al. The x-chromosome has a different pattern of gene expression in women compared with men with ischemic stroke. Stroke. 2012;43:326–334. doi: 10.1161/STROKEAHA.111.629337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough LD, Zeng Z, Blizzard KK, Debchoudhury I, Hurn PD. Ischemic nitric oxide and poly (adp-ribose) polymerase-1 in cerebral ischemia: Male toxicity, female protection. J Cereb Blood Flow Metab. 2005;25:502–512. doi: 10.1038/sj.jcbfm.9600059. [DOI] [PubMed] [Google Scholar]
- McCullough LD, Alkayed NJ, Traystman RJ, Williams MJ, Hurn PD. Postischemic estrogen reduces hypoperfusion and secondary ischemia after experimental stroke. Stroke. 2001;32:796–802. doi: 10.1161/01.str.32.3.796. [DOI] [PubMed] [Google Scholar]
- Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, et al. Effect of estrogen plus progestin on stroke in postmenopausal women: The women's health initiative: A randomized trial. JAMA. 2003;289:2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- Harman SM, Black DM, Naftolin F, Brinton EA, Budoff MJ, Cedars MI, et al. Arterial imaging outcomes and cardiovascular risk factors in recently menopausal women: A randomized trial. Ann Intern Med. 2014;161:249–260. doi: 10.7326/M14-0353. [DOI] [PubMed] [Google Scholar]
- Jeppesen LL, Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS, Winther K. Decreased serum testosterone in men with acute ischemic stroke. Arterioscler Thromb Vasc Biol. 1996;16:749–754. doi: 10.1161/01.atv.16.6.749. [DOI] [PubMed] [Google Scholar]
- Carswell HV, Dominiczak AF, Garcia-Segura LM, Harada N, Hutchison JB, Macrae IM. Brain aromatase expression after experimental stroke: Topography and time course. J Steroid Biochem Mol Biol. 2005;96:89–91. doi: 10.1016/j.jsbmb.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Liu M, Hurn PD, Alkayed NJ. Cytochrome p450 in neurological disease. Curr Drug Metab. 2004;5:225–234. doi: 10.2174/1389200043335540. [DOI] [PubMed] [Google Scholar]
- Yague JG, Munoz A, de Monasterio-Schrader P, Defelipe J, Garcia-Segura LM, Azcoitia I. Aromatase expression in the human temporal cortex. Neuroscience. 2006;138:389–401. doi: 10.1016/j.neuroscience.2005.11.054. [DOI] [PubMed] [Google Scholar]
- Carswell HV, Dominiczak AF, Macrae IM. Estrogen status affects sensitivity to focal cerebral ischemia in stroke-prone spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2000;278:H290–H294. doi: 10.1152/ajpheart.2000.278.1.H290. [DOI] [PubMed] [Google Scholar]
- Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–122. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatewood JD, Wills A, Shetty S, Xu J, Arnold AP, Burgoyne PS, et al. Sex chromosome complement and gonadal sex influence aggressive and parental behaviors in mice. J Neurosci. 2006;26:2335–2342. doi: 10.1523/JNEUROSCI.3743-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Bendahan CC, van de Beek C, Berenbaum SA. Prenatal sex hormone effects on child and adult sex-typed behavior: Methods and findings. Neurosci Biobehav Rev. 2005;29:353–384. doi: 10.1016/j.neubiorev.2004.11.004. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, et al. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci. 2002;22:9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Breedlove SM. Organizational and activational effects of sex steroids on brain and behavior: A reanalysis. Horm Behav. 1985;19:469–498. doi: 10.1016/0018-506x(85)90042-x. [DOI] [PubMed] [Google Scholar]
- Berletch JB, Yang F, Disteche CM. Escape from x inactivation in mice and humans. Genome Biol. 2010;11:213. doi: 10.1186/gb-2010-11-6-213. [DOI] [PMC free article] [PubMed] [Google Scholar]