Abstract
Patients with unilateral occlusive processes of the internal carotid artery (ICA) show subtle cognitive deficits. Decline in cerebral autoregulation and in functional and structural integrity of brain networks have previously been reported in the affected hemisphere (AH). However, the association between cerebral autoregulation, brain networks, and cognition remains to be elucidated. Fourteen neurologically asymptomatic patients (65±11 years) with either ICA occlusion or high-grade ICA stenosis and 11 age-matched healthy controls (HC) (67±6 years) received neuropsychologic testing, transcranial Doppler sonography to assess cerebral autoregulation using vasomotor reactivity (VMR), and magnetic resonance imaging to probe white matter microstructure and resting-state functional connectivity (RSFC). Patients performed worse on memory and executive tasks when compared with controls. Vasomotor reactivity, white matter microstructure, and RSFC were lower in the AH of the patients when compared with the unaffected hemisphere and with controls. Lower VMR of the AH was associated with several ipsilateral clusters of lower white matter microstructure and lower bilateral RSFC in patients. No correlations were found between VMR and cognitive scores. In sum, impaired cerebral autoregulation was associated with reduced structural and functional connectivity in cerebral networks, indicating possible mechanisms by which severe unilateral occlusive processes of the ICA lead to cognitive decline.
Keywords: carotid artery, cognition, diffusion tensor imaging, functional MRI, ultrasound
Introduction
Brain metabolism is crucially dependent on a constant supply of oxygen and glucose. To preserve this supply, cerebral blood flow is rapidly modulated based on demands of local brain functions, and changes in systemic blood pressure.1 This ability is important to maintain adequate neuronal functions and to prevent ischemia-related brain damage, and has been described as cerebral autoregulation.2 Variability of cerebral blood flow can be assessed by intracerebral hemodynamic features such as cerebral vasomotor reactivity (VMR) using transcranial Doppler sonography.2 VMR evaluates the capacity of cerebral resistance vessels to react to a given vasodilatory stimulus. In cerebrovascular diseases, reduced VMR has been shown to be associated with higher stroke risk3 and lower cognitive function.4
Advanced magnetic resonance imaging (MRI) techniques have greatly improved our understanding of the relationship between brain structure, function, and blood flow. Among others, diffusion tensor imaging and blood oxygen level-dependent functional MRI that was used to assess resting-state functional connectivity (RSFC) could help explore brain function in healthy individuals5 and in patients with cerebrovascular diseases.6 Diffusion tensor imaging measures the vectored diffusivity of water molecules (fractional anisotropy, FA) in a spatially coherent manner and thus characterizes the microstructure of white matter tracts.7 Resting-state functional connectivity assumes that temporal correlations of intrinsic low-frequency blood oxygen level-dependent signal fluctuations in widely separated brain regions reveal organized functional brain networks.8 Resting-state functional connectivity thus offers means to characterize interindividual differences in intrinsic brain activity and is regarded as a potential biomarker of neurologic and psychiatric disorders.9
Individuals with severe stenosis or occlusion of the internal carotid artery (ICA) show lower VMR on the affected side when compared with the unaffected side. Pathophysiologically, perfusion pressure distal to the stenosis or occlusion is decreased, leading to reflex dilatation of arterioles to maintain the blood supply of related brain regions.10 On a cellular level, changes in synaptic plasticity because of hypoperfusion may account for cognitive deterioration in these patients.11, 12 With regard to large-scale networks, changes in white matter microstructure and RSFC may account for cognitive decline in these patients.13
In neurodegenerative diseases, impaired cerebral hemodynamics indicate lower capacity to modulate resistance of cerebral vessels because of cerebral atrophy.14 Recently, associations between cerebral hemodynamics and white matter hyperintensities have also been reported in older adults without extracranial artery stenoses.15 Taken together, it remains unclear whether impaired cerebral hemodynamics cause white matter hyperintensities or vice versa.16 Here, studies on patients with unilateral decreased VMR offer the advantage of direct comparison of cerebral hemodynamics and structural measurements within a given subject. This could be achieved by comparing the affected and unaffected hemispheres within the limits of the holistic organization of cerebral networks. So far, associations between VMR and microstructural integrity and RSFC have not been investigated; thus, it remains speculative if altered cerebral autoregulation determines structural and functional deterioration.
Our aims for this study were therefore to explore hemispheric structural and network sequeale of unilateral severe stenosis or occlusion of the ICA to further elucidate the underlying mechanisms of cognitive deterioration in these patients. Based on previous findings, we expected that VMR, microstructure, and RSFC would be decreased in the affected hemisphere (AH) in patients when compared with the unaffected hemisphere (UH) and with healthy controls (HC), and that patients would score lower on cognitive tests when compared with controls. Moreover, we hypothesized that decreases in VMR would be associated with lower white matter microstructure, RSFC, and cognitive functions.
Materials and methods
Subjects
Patients were recruited from the database of the ultrasound laboratory of the Department of Neurology of the Charité University Hospital in Berlin, using the key words ‘Occlusion' OR ‘High grade Stenosis' AND ‘Internal carotid artery'. Healthy controls were chosen from a large database of healthy volunteers from the Department of Neurology. Fourteen patients with unilateral occlusive process of the ICA (65±11 years, range 50 to 80 years, 11 men, occlusion: n=10, high-grade stenosis (>80%, European Carotid Surgery Trial criteria17): n=4; diagnosis >1 year before enrollment in the study) and 11 HC without stenosis or occlusion of the ICA or other extracranial vessels (67±6 years, range 55 to 74 years, 7 men) were included. Patients with bilateral stenosis of the ICA (cutoff ≥50%, European Carotid Surgery Trial criteria17) were not considered. The main inclusion criterion for patients was unilateral high-grade stenosis (>80%) or occlusion of the ICA. Further inclusion criteria for patients and controls comprised the following: normal motor functions on neurologic examination; no intake of medication that act on the central nervous system; no signs of severe cognitive deficits (Mini Mental State Examination score ≥26; ref. 18); no signs of relevant depression (Beck's depression inventory ≤12; ref. 19); no severe internal or psychiatric disorders, as indicated by a standardized questionnaire; and right-handedness according to the Edinburgh Handedness Inventory.20 Healthy controls were chosen to match the patients with regard to age, sex, and years of education. Baseline characteristics of patients and HC are provided in Table 1.
Table 1. Baseline characteristics of patients and HC.
| Patients (N=14) | HC (N=11) | t value | P value | |
|---|---|---|---|---|
| Demographic parameters (mean+s.d.) | ||||
| Age (years) | 66.1±9.7 | 67±5.9 | −0.25 | 0.81 |
| Sex (male/female) | 9/5 | 7/4 | 0.97 | |
| Education (years) | 13.9±2.9 | 15.5±1.9 | −1.67 | 0.11 |
| Diagnosis (patients) | ||||
| Side of stenosis/occlusion (no. of patients) | right: 8; left: 6 | |||
| Degree of stenosis (no. of patients) | Occlusion: 10; ≥80% stenosis: 4 | |||
| Etiology (no. of patients) | Artherothrombotic: 11; dissection: 3 | |||
HC, healthy controls; s.d., standard deviation.
Study Outline
The study was approved by the ethics committee of the Charité University Hospital in Berlin and conducted in accordance with the Declaration of Helsinki. Patients were recruited from a large database of the ultrasound laboratory of the Department of Neurology, Charité University Hospital in Berlin, and HC were recruited from a database of healthy volunteers. All participants received a clinical interview and short medical examination before inclusion. Neurologic examination included modified Rankin Scale and NIH Stroke Scale. Participants then underwent neuropsychologic testing and MRI scanning to quantify global volumetric measurements, structural integrity of the white matter, and functional connectivity. For further details regarding volumetric measurements and white matter hyperintensities, see Supplementary Methods.
Ultrasound
Color-coded duplex sonography
Extracranial color-coded sonography was performed using a 7 MHz linear transducer (Toshiba Powervision 6000, Tokyo, Japan). The grade of ICA stenosis was assessed according to the standard ultrasound protocols21 and graded according to the European Carotid Surgery Trial criteria.17
Transcranial Doppler sonography and evaluation of vasomotor reactivity
Transcranial Doppler sonography was carried out in a quiet room in a lying position. Two TCD dual 2 MHz transducers were fitted on a headband and placed on the temporal bone windows. To assess VMR, mean flow velocity (MFV) was recorded over the middle cerebal artery (MCA) at rest (MFV−MCABASELINE) and after 2 minutes of carbogen inhalation (5%CO2+95% oxygen) (MFV−MCACO2). Vasomotor reactivity was then obtained using the formula:
In two patients (patients 7 and 14), VMR could not be evaluated becasue of insufficient bone window.
Neuropsychologic Testing
A comprehensive neuropsychologic test battery22 was administered to each participant. Processing speed, cognitive flexibility, and executive functions/set shifting were assessed with the Trail-Making Test (TMT) A and B and the Regensburger Verbal Fluency Test (RWT); for phonemic fluency: ‘S words', ‘G–R words' and for semantic fluency: ‘food items' and category switching for ‘clothes–flowers'. The German version of the Auditory Verbal Learning Test was used to examine verbal learning capacity across five trials and the retrieval from verbal memory by delayed recall (30 minutes) (‘Verbaler Lern- und Merkfähigkeitstest, Version A'). Forward and backward digit span (part of the revised Wechsler Memory Scale) was performed to assess working memory performance.
Magnetic Resonance Imaging Acquisition
Magnetic resonance imaging were obtained on a 3-T system (Magnetom TIM Trio, Siemens Healthcare, Erlangen, Germany) using a 12-channel head coil. Each subject underwent a three-dimensional scanning protocol using diffusion-weighted images in a spin-echo EPI sequence (TR=7,500 ms, TE=86 ms, 61 axial slices, voxel size of 2.3 × 2.3 × 2.3 mm3; 64 directions with a b-value of 1,000 s/mm2 and 1 or 10 b0). In addition, high-resolution T1-weighted MPRAGE images (TR=1,900 ms, TE=2.52 ms, 192 sagittal slices, voxel size of 1.0 × 1.0 × 1.0 mm3, flip angle=9°) and fluid attenuation inversion recovery images were acquired. The parameters for the continuous resting-state acquisition were: resolution, 3 × 3 × 4 mm3; TR/TA=2,300; TE=30; flip angle, 90° 34 transverse slices, no gap; interleaved acquisition, field of view, 192 × 192; and acquisition matrix, 64 × 64, 150 functional whole-brain volumes.
Diffusion tensor imaging
Diffusion tensor imaging data analyses were performed using the Diffusion Toolbox package and tract-based-spatial statistics from the FMRIB Software Library FSL 4.1 (http://www.fmrib.ox.ac.uk/fsl). Briefly, preprocessing included correction for eddy current distortions and head motion using affine registration, extraction of non-brain tissue using FSL's automated brain extraction tool23 and fitting of a diffusion tensor model at each voxel to obtain individual FA maps. The hemisphere ipsilateral to the carotid stenosis (AH) was set to the right side by flipping along the mid-sagittal axis. The FA images of all subjects were then aligned to every other one using nonlinear registrations, to identify the ‘most representative' image. This was carried out by calculating the (mean and median) average extent of transformation that is needed to register every other FA image to a single mean FA image. The FA image with the less extensive average transformation was chosen as the target. This target image was then registered to the 1 × 1 × 1 mm2 Montreal Neurological Institute standard space using affine registration, comprising the study-specific template. Next, all FA images were registered to the study-specific template.
Next, the so-registered images were smoothed with a Gaussian kernel of σ=2 (~4.6 mm full-width at half-maximum), to account for residual variation that may decrease the sensitivity to regional effects, and averaged to get a mean FA image. This mean FA image was thinned (‘skeletonized') and thresholded at >0.2 to create a mean FA skeleton, which serves as a representation of the centers of all tracts common to the group of subjects. Each subject's aligned FA image was then projected onto this skeleton and mean FA values of the whole-brain white matter skeleton and each hemisphere were extracted separately to test for global between-group differences. Second, the resulting data were fed into voxelwise statistics. Voxelwise correlations across the skeleton between FA values and VMR measures were performed in each group separately, to obtain clusters associated with perfusion. Gender and age were included as nuisance covariates. Permutation-based statistical analyses were conducted using the program ‘randomise'24 implemented in FSL, with 5,000 permutations, with correction for multiple comparisons using threshold-free cluster enhancement (threshold for significant voxels: P<0.05), and using the –D option and a constant event for demeaning the data. The analysis was restricted to voxels of the skeleton, which excluded the cerebellum and brain stem. For visualization, significant clusters were ‘thickened' (by convolving the thresholded statistical images by a 3 mm sphere) and overlaid onto the group mean FA skeleton.
Resting-state functional connectivity
AFNI (http://afni.nimh.nih.gov/afni) and FSL (http://www.fmrib.ox.ac.uk) were used to process functional and anatomic data using customized scripts from the 1,000 Functional Connectome Project (www.nitrc.org/projects/fcon_1000).25 Individual anatomic scans (T1-weighted images) were brain extracted, segmented into different tissue types, and bias-field corrected. Individual functional scans were corrected for motion and slice timing, spatially smoothed with a 6-mm full-width at half-maximum Gaussian kernel, temporally filtered (0.01 to 0.1 Hz), and detrended. Functional images were coregistered with the anatomic image using linear registration. Nuisance signals (motion parameters, white matter, cerebrospinal fluid, and global signal) were removed by multiple regression. For group analyses, individual images were spatially normalized to Montreal Neurological Institute space using linear and nonlinear registrations. The hemisphere ipsilateral to the carotid stenosis (AH) was set to the right side by flipping along the mid-sagittal axis. According to Cheng et al,13 14 regions of interest (ROIs) (4 mm radius) were defined as seed regions representing the default-mode network (DMN), the frontoparietal network (FPN), and the dorsal attention network (DAN). Mean time series for each seed were extracted from the data and then correlated with each voxel in the brain to produce individual-level correlation maps. These maps were then converted to z-value maps using Fisher's r-to-z transformation for subsequent analyses. First, individual inter- and intrahemispheric functional connectivity (FC) strength between each ROI pair was calculated (17 ROI pairs according to Cheng et al13). Second, voxelwise group-level analyses were carried out using a mixed-effects model (FLAME) as implemented in FSL. For each seed region, group-level analyses produced maps of voxels where FC with the seed exhibited significant variation in association with VMR. These analyses were restricted to voxels within the cortex and Bonferroni-corrected for multiple testing (number of seeds, n=6). In addition, in the patients group, a one-sample t-test was computed separately for each hemisphere to find clusters within the respective networks that were associated with VMR, adjusted for age and sex. Gaussian random field theory was used to correct for multiple comparisons at the cluster-level (cluster-wise correction Z>2.3, P<0.05).
Statistical Analyses
Statistical analyses outside FSL were carried out using SPSS Version 20.0. (IBM Corp, Armonk, NY). Shapiro–Wilk test was used to test for normality (set to P>0.05) before data analysis. Unpaired t-tests were used to compare baseline characteristics, cognitive scores, VMR, ventricle volume, cortical volume, and FA and FC between patients and HC. Paired sample t-tests were used to compare VMR and FA between the hemispheres in the patient group and in the HC. Pearson's correlation coefficients were used to probe associations between VMR and MRI measurements, as well as associations between VMR and cognitive scores in patients. All statistical tests were considered significant at a level of P<0.05. All data are expressed as mean±s.e.m. unless specified otherwise. The two patients in whom VMR could not be evaluated were included in the analyses with regard to baseline characteristics, neuropsychologic tests, and structural and functional MRI, including analyses of differences between groups and analyses of differences between the affected and unaffected hemispheres. These patients were excluded from all analyses involving VMR.
Results
Vasomotor Reactivity in Patients and HC
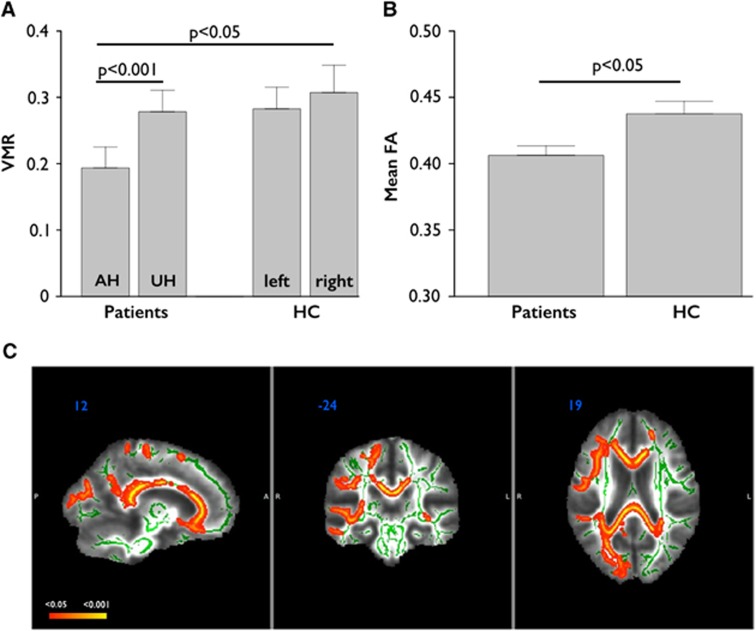
Vasomotor reactivity of the AH differed significantly from VMR of the UH in patients (t=−7.14, P<0.001). Vasomotor reactivity of the AH in patients was also significantly different from VMR of the right hemishphere (RH) in the control group (t=−2.11, P=0.047) (Figure 1A). No differences were found in the control group between the left hemisphere (LH) and RH (t=1.16, P=0.31).
Figure 1.
Differences between groups regarding vasomotor reactivity (VMR) and mean fractional anisotropy (FA). (A) Differences in VMR between hemispheres in patients and healthy controls (HC). (B) Differences in whole-brain mean FA between patients and HC. (C) Differences between groups regarding FA. The side of stenosis/occlusion in patients was set to the right. Patients showed diffuse and significant FA decreases (red-yellow; P<0.05, corrected using threshold-free cluster enhancement (TFCE); white matter skeleton shown in green) when compared with HC, particularly in the frontoparietal regions ipsilateral to the stenosis, including anterior thalamic radiation, forceps minor, forceps major, and corpus callosum. Data in (A and B) are given as mean±s.e.m. AH, affected hemisphere; FA, fractional anisotropy; LH, left hemisphere; RH, right hemisphere; UH, unaffected hemisphere.
Mini Mental State Examination, Beck's Depression Inventory, and Detailed Neuropsychologic Testing in Patients and HC
No differences in Mini Mental State Examination and Beck's depression inventory emerged between patients and HC. Compared with HC, the patients showed significantly poorer results on detailed neuropsychologic testing in several cognitive scores, most notably in verbal learning and delayed recall, and also in most scores evaluating cognitive flexibility and executive functions (all Ps<0.05, uncorrected). No differences were found in working memory scores (Table 2).
Table 2. Neuropsychologic test results in patients and HC.
| Patients (N=14) | HC (N=11) | t value | P value | |
|---|---|---|---|---|
| Verbal learning/memory | ||||
| AVLT, sum 1–5 (immediate recall) | 40.57±7.07 | 52.09±11.81 | −3.033 | 0.006a |
| AVLT 7 (delayed recall) | 7.86±2.98 | 10.55±3.11 | −2.195 | 0.038a |
| Processing speed and cognitive flexibility | ||||
| TMT A | 39.14±11.77 | 30.91±10.2 | 1.838 | 0.079 |
| TMT B | 121.43±64.23 | 74.82±31.16 | 2.204 | 0.038a |
| Executive functions | ||||
| TMT B−TMT A | 82.29±63.22 | 43.91±28.30 | 2.03 | 0.057 |
| RWT, S words | 18±6.73 | 25.09±7.94 | −2.418 | 0.024a |
| RWT, G and R words | 17.07±4.45 | 22.82±6.01 | −2.75 | 0.011a |
| RWT, food | 27.64±6.69 | 33.45±7.26 | −2.078 | 0.049a |
| RWT, clothes and flowers | 19±4.22 | 22.36±3.7 | −2.086 | 0.048a |
| Working memory | ||||
| Forward digit span | 7.21±2.12 | 7.91±1.97 | −0.822 | 0.420 |
| Backward digit span | 5.43±1.6 | 6.09±1.14 | −1.158 | 0.259 |
AVLT, Auditory Verbal Learning Test; HC, healthy controls; RWT, Regensburger Word Fluency Test; TMT, Trail-Making Test.
Data are given as mean±s.d.
P<0.05, uncorrected.
Magnetic Resonance Imaging
Radiologic examination and global volumetric measurements
Patients displayed significantly more white matter lesions when compared with HC (t=2.56, P=0.02). Magnetic resonance imaging in the patients group revealed silent infarctions in five patients (AH: n=4; UH: n=1) (see Supplementary Table 1 in Supplementary Results for MRI characteristics in patients). No previous silent strokes were found in HC. Global volumetric measurements of lateral ventricles and cortical volume to assess cerebral atrophy revealed no significant differences between patients and HC (UH versus RH, AH versus RH, UH versus LH, AH versus LH, all Ps>0.05). When comparing UH versus AH within the patients group, cortical volume was trendwise lower on the AH (P=0.061). For further results regarding volumetric measurements and white matter hyperintensities in patients see Supplementary Table 2 in Supplementary Results.
White matter microstructure
Whole-brain mean FA was significantly lower in patients than in HC (t=−2.63, P=0.016; see Figure 1B). When computing voxelwise permutation testing to assess differences between patients and HC, we found widespread clusters of decreased FA in the patients group, mainly in the frontoparietal areas of the AH (P<0.05, threshold-free cluster enhancement corrected; Figure 1C).
Resting-state functional connectivity
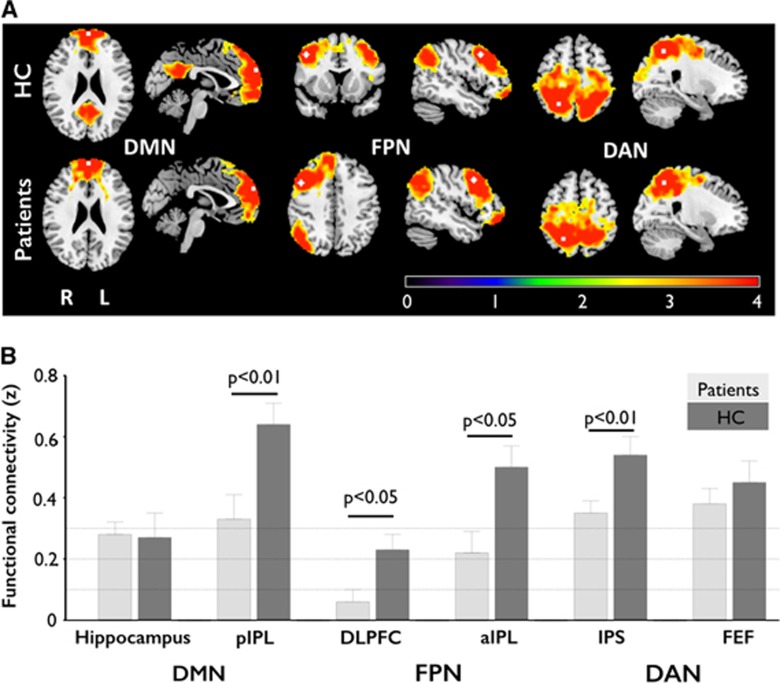
Resting-state functional connectivity was lower in patients when compared with controls in several ROI pairs, both between and within hemispheres: first, the long-distance interhemispheric RSFC between bilateral dorsolateral prefrontal cortices (DLPFCs) (t=−2.606, P=0.016), bilateral anterior inferior parietal lobules (aIPLs) (t=−2.713, P=0.012), bilateral posterior inferior parietal lobules (pIPLs) (t=−2.851, P=0.009) and bilateral intraparietal sulcus (t=−2.838, P=0.009) were significantly reduced in patients when compared with HC, thus indicating significant decreases within the bilateral DMN, FPN and DAN (Figures 2A and 2B). Second, patients showed decreased intrahemispheric RSFC, specifically detected in the DMN between the posterior cingulate cortex and the medial prefrontal cortex (t=−2.71, P=0.012) when compared with HC. When comparing AH and UH within the patients group, significant differences were found in the AH with reduced RSFC between the hippocampus and the pIPL (t=−2.56, P=0.018), as well as between the hippocampus and the posterior cingulate cortex (t=−2.10, P=0.047).
Figure 2.
Resting-state functional connectivity (RSFC) in patients and healthy controls (HC). (A) Topography of functional connectivity of three functional networks with seeds in predefined regions of interest (upper panel: HC; lower panel: patients). The seeds were located at the medial prefrontal cortex (MPFC), the dorsolateral prefrontal cortex (DLPFC), and the intraparietal sulcus (IPS) of the default-mode network (DMN), the frontoparietal network (FPN), and the dorsal attention network (DAN), respectively (see also Cheng et al13). Scale was given as z-values. The hemisphere of carotid stenosis was set to the right. P<0.05, corrected. (B) Group differences in interhemispheric RSFC. The patients showed lower interhemispheric RSFC within the DMN, the FPN, and the DAN when compared with HC. Error bars denote mean±s.e.m. aIPL, anterior inferior parietal lobule; FEF, frontal eye field; pIPL, posterior inferior parietal lobule.
Correlations Between Vasomotor Reactivity, Magnetic Resonance Imaging Measurements, and Cognitive Scores in the Patients Group
Correlations between vasomotor reactivity and white matter microstructure
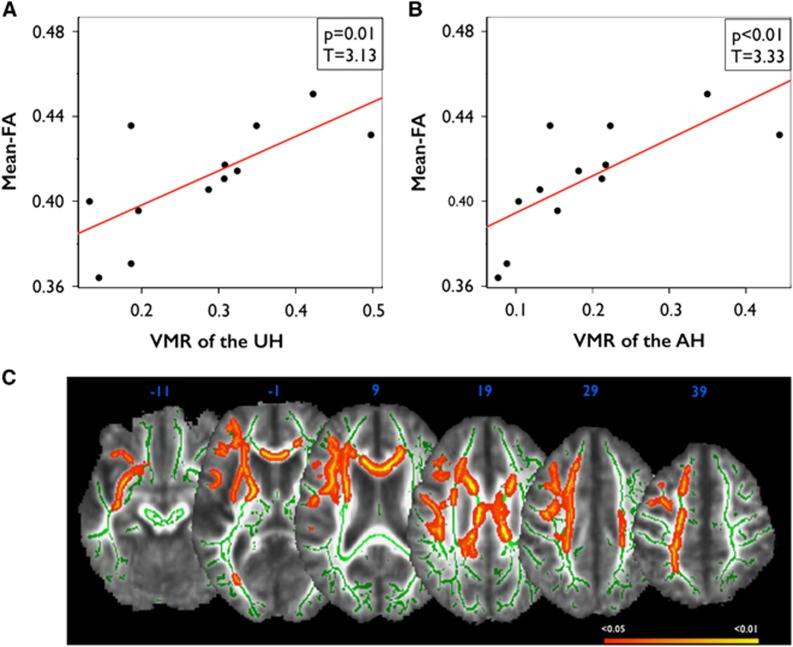
Lower VMR was correlated with lower whole-brain mean FA in patients (AH: r=0.73, P=0.008; UH: r=0.70, P=0.011). Moreover, lower VMR of the AH and lower VMR of the UH were correlated with lower mean FA in the respective hemisphere (AH: r=0.73, P=0.007; UH: r=0.7, P=0.011) (Figures 3A and 3B). Voxelwise permutation testing additionally showed that decreased VMR was significantly associated with decreased FA in specific white matter tracts of the AH (P<0.05, threshold-free cluster enhancement corrected, AH is set to the right; Figure 3C). Specifically, significant voxels were located in the cingulum, the capsula interna and externa, the anterior thalamic radiation, uncinate fasciculus, and superior longitudinal fasciculus of the AH, and bilaterally in the callosal body. Voxelwise analysis revealed no significant correlations in HC.
Figure 3.
Correlations between vasomotor reactivity (VMR ) and fractional anisotropy (FA) in patients. (A) Correlations between mean FA of the unaffected hemisphere (UH) and VMR of the UH in patients, and (B) correlations between mean FA of the affected hemisphere (AH) and VMR of the AH in patients. (C) Clusters of FA that are significantly correlated with VMR of the AH in patients (red-yellow; white matter skeleton shown in green). Colors indicate significant voxels (P<0.05, corrected using TFCE), superimposed on a study-specific FA template.
Correlations between vasomotor reactivity and resting-state functional connectivity
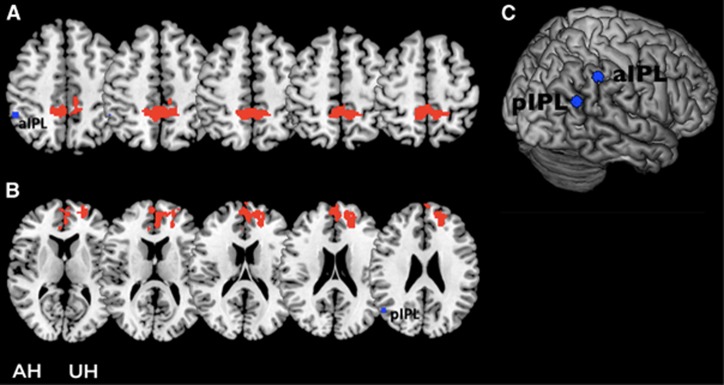
Correlations between VMR and RSFC were assessed separately for each hemisphere using seed-based analysis. Six ipsilateral seeds (DLPFC, aIPL, pIPL, hippocampus, frontal eye field, intraparietal sulcus) were defined (see Supplementary Methods for the exact location of seeds). In the AH, lower VMR was correlated with lower RSFC from aIPL to the bilateral precuneus and lower RSFC from the pIPL to the contralateral cingulate cortex (BA32) and the frontal superior medial cortex (BA10) (Figure 4). The analysis with seeds located in the UH and VMR of the UH yielded no significant results.
Figure 4.
Correlational analysis between resting-state functional connectivity (RSFC) and vasomotor reactivity (VMR) in patients. (A) Clusters of significant positive correlation between VMR of the affected hemisphere (AH) and RSFC within the frontoparietal network (FPN) (seed located in the anterior inferior parietal lobule (aIPL) of the AH). No negative correlations were found. (B) Clusters of significant positive correlation between VMR of the AH and RSFC within the default-mode network (DMN) (seed located in the posterior inferior parietal lobules (pIPLs) of the AH). No negative correlations were found. (C) Localization of the seeds on the brain surface of the AH. The hemisphere of carotid stenosis was set to the right. P<0.05, corrected. UH, unaffected hemisphere.
Correlations between vasomotor reactivity and cognitive scores and between magnetic resonance imaging measurements and cognitive scores
No correlations were detected between VMR and cognitive scores within the patients group (all Ps> 0.1; see Supplementary Table 3 in Supplementary Results for details on effect sizes), or in HC. In an exploratory approach, correlations between MRI measurements and cognitive scores revealed that the number of words correctly retrieved in the semantic verbal fluency task positively correlated with whole-brain FA (r=0.611, P<0.05, uncorrected) and FC between bilateral pIPL (r=0.645, P<0.05, uncorrected) in patients but not in HC. Moreover, the number of words correctly retrieved in the delayed recall task was also positively correlated with FC between bilateral DLPFC (r=0.586, P<0.05, uncorrected) in patients but not in HC.
Discussion
Here, we aimed to explore the association between cerebral autoregulation and white matter microstructure, functional connectivity, and cognition, in patients with stenosis or occlusion of the ICA. In line with previous studies, patients scored significantly worse on various cognitive tests when compared with HC. In addition, patients showed distinctive deteriorations in white matter microstructure and RSFC, mainly in the AH, when compared with the UH and with HC. Moreover, we showed for the first time an association between lower VMR of the AH and decreased white matter microstructure in several ipsilateral tracts, as well as a decreased connectivity in bilateral resting-state networks.
Vasomotor Reactivity, Stroke, and Cognition
Several studies reported a decrease in VMR of the AH when compared with the UH in patients with occlusive processes of the ICA.26 In line with these findings, our patients presented with markedly decreased VMR in the AH when compared with the UH and with HC, indicative of decreased cerebral autoregulation within the AH. Magnetic resonance imaging further revealed silent brain infarctions in 4 of 14 patients on the AH, although we could not detect differences between UH and AH regarding ventricle volume or cortical volume.
Additionally, patients showed distinct decreases in neuropsychologic test scores, particularly with regard to verbal fluency and memory, as well as cognitive flexibility and executive functions, in line with previous studies of our group and others.11, 27 Changes in memory performance may have been mediated by the diminished connectivity within the DMN regions found in the patients group; in fact, decreased connectivity in the DMN has previously been associated with lower scores on tasks of executive function and processing speed in cognitively normal older adults,28 as well as with lower memory functions in patients with mild cognitive impairment when compared with HC.29 In the current study, decreases in interhemispheric functional connectivity in the default mode (pIPL) and the frontoparietal (DLPFC) networks were correlated with lower scores on verbal fluency and delayed recall, respectively. For microstructural integrity, we found that lower whole-brain values were associated with lower scores in semantic verbal fluency in patients, in line with reports on associations between lower whole-brain microstructural integrity and lower cognitive flexibility and executive function in patients with extensive white matter damage.30 The heterogeneity of pathologic changes because of unilateral stenosis/occlusion renders the definition of related structural and cognitive changes difficult. However, our findings suggest that deterioration in networks related to memory and attention as a result of occlusive processes are associated with lower performance in verbal learning and verbal fluency tasks, possibly mediated by low VMR.
Vasomotor Reactivity and White Matter Microstructure
A recent study highlighted associations between cerebral hemodynamics and whole-brain load of white matter lesions on fluid attenuation inversion recovery-weighted images.14 In line with these findings, patients in our study displayed significantly more white matter lesions than controls across the entire brain. Additionally we showed that reduced white matter microstructure in patients was specific to the AH when compared with the UH and with HC, see also Cheng et al.13 These findings were further expanded by showing that reduced VMR of the AH was associated with lower whole-brain FA and lower FA of the AH. In an additional voxelwise approach, lower VMR of the AH was associated with clusters of lower white matter microstructure within the frontal lobe of the AH in the patients group. This part of the brain is mainly supplied by the ICA.31 In stenosis/occlusion of the ICA, downstream ipsilateral middle cerebral artery and anterior cerebral artery regularly reveal decreased blood flow, characteristically associated with decreased ipsilateral VMR.32 These patients are specifically vulnerable to infarcts within the watershed area of the anterior cerebral artery/middle cerebral artery territory in the frontal lobe.33 Even in patients without infarctions, perfusion MRI revealed a hemispheric imbalance in quantitative perfusion measures such as cerebral blood flow within watershed white matter regions mainly within the frontal lobe.34 Our data support the hypothesis that decreased VMR and subsequent chronic hypoperfusion10 result in decreased FA mainly within the frontal lobe. Several white matter tracts, important for memory encoding and retrieval, as well as executive functions, such as arcuate fasciculus, uncinate fasciculus, and anterior thalamic radiation, pass through the frontal lobe.35, 36 Decreased integrity of white matter microstructure in these tracts may therefore be responsible for lower functions in the respective cognitive domains.
Vasomotor Reactivity and Resting-State Functional Connectivity
In line with Cheng et al,13 we found alterations of RSFC within the DMN37 when comparing patients with severe stenosis/occlusion of the ICA and HC, and within unilateral DMN and the FPN, when comparing UH and AH of the patients. Additionally, we showed that lower unilateral VMR of the AH was associated with lower connectivity within bilateral DMN and the FPN. Specifically, we could show that seeds in IPL as part of the FPN and the DMN were less strongly connected to precuneus and anterior cingulate in the AH versus UH of patients. The DMN is implicated in episodic memory processing, and connectivity within this network decreases in the course of mild cognitive impairment and even more so in Alzheimer dementia.38 The FPN has been associated with executive task performance39 and its connectivity decreases, for example, in patients with multiple sclerosis who frequently present with impaired executive functions.40 Corresponding to lower RSFC in these networks, both episodic memory and executive scores were lower in our patients group compared with controls. In sum, we provide evidence that unilateral decreases of VMR are characterized by both ipsilateral and bilateral alterations in connectivity between major nodes of the DMN and the FPN. Therefore, unilateral changes of cerebral autoregulation may drive changes in RSFC not only in the AH but also throughout the entire brain, thus leading to bilateral changes in connectivity, similar to what has been reported for RSFC in patients with cognitive complaints and with mild cognitive impairment.29
Limitations and Strengths
First, number of subjects was small. However, we included a comprehensive assessment of patients and controls for changes in VMR, white matter microstructure and RSFC in each hemisphere. Thus, we were able to directly compare hemispheres within groups, rendering age, sex, educational level, or general atherosclerosis unlikely to interact with these findings. Note that patients with stenosis/occlusion due to artherosclerosis and patients with stenosis/occlusion due to carotid artery dissection were included in the study, and white matter hyperintensity load and cerebrovascular risk may differ between these patients. However, the present design allowed us to analyze the effects of VMR on FA and RSFC within each patient, thus controlling for possible differences in white matter hyperintensity through the direct comparison of AH and UH. Second, although the cross-sectional data cannot determine causality regarding VMR and changes in white matter microstructure, it is unlikely that changes in white matter microstructure lead to extracranial artery occlusion. The differential findings of white matter microstructural integrity in AH versus UH rather suggest that impaired cerebral hemodynamics lead to changes in white matter structure. Third, we could not observe associations of VMR and cognitive scores, even though we found overall lower scores for memory and executive functions in patients compared with HC. Here, small sample size rendered a separate evaluation of cognitive sequelae of ICA stenosis/occlusion in dependence of the affected (left versus right) hemisphere4 inappropriate, possibly contributing to the lack of direct association between VMR and cognitive scores. However, as we could show that unilateral decreases in cerebral VMR modulated functional networks in both the AH and UH, a more general effect of decreased VMR on cognition, irrespective of the AH, seems plausible, and direct correlations between VMR and cognitive scores may emerge in larger patient populations.
Conclusion
Decreased cerebral VMR due to high-grade stenosis or occlusion of the ICA is associated with decreases in microstructural and functional connectivity of brain networks, suggesting a mechanism by which severe unilateral occlusive processes of the ICA may lead to decline in cognitive scores. Strategies to improve VMR such as physical activity or statins may help to prevent further structural and functional deterioration, a hypothesis to be tested in future interventional trials.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This study was funded by DAAD (KA: A/09/98515), Deutsche Forschungsgemeinschaft (AF: Fl-379-8/1; 379-10/1; 379-11/1; DFG-Exc-257) and Bundesministerium für Bildung und Forschung (AF: FKZ0315673A; 01EO0801; 01GY1144). JL is participant in the Clinical Scientist Program funded by the Charité Universitätsmedizin Berlin
Supplementary Material
References
- Girouard HH, Iadecola CC. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol. 2005;100:328–335. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- Aaslid R. Cerebral autoregulation and vasomotor reactivity. Front Neurol Neurosci. 2006;21:216–228. doi: 10.1159/000092434. [DOI] [PubMed] [Google Scholar]
- Ringelstein EB, Sievers C, Ecker S, Schneider PA, Otis SM. Noninvasive assessment of CO2-induced cerebral vasomotor response in normal individuals and patients with internal carotid artery occlusions. Stroke. 1988;19:963–969. doi: 10.1161/01.str.19.8.963. [DOI] [PubMed] [Google Scholar]
- Silvestrini M, Paolino I, Vernieri F, Pedone C, Baruffaldi R, Gobbi B,, et al. Cerebral hemodynamics and cognitive performance in patients with asymptomatic carotid stenosis. Neurology. 2009;72:1062–1068. doi: 10.1212/01.wnl.0000345015.35520.52. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Buchler NG, White LE, Madden DJ, Cabeza R. Assessing the effects of age on long white matter tracts using diffusion tensor tractography. Neuroimage. 2009;46:530–541. doi: 10.1016/j.neuroimage.2009.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehme AK, Grefkes C. Cerebral network disorders after stroke—evidence from imaging-based connectivity analyses of active and resting brain states in humans. J Physiol (Lond) 2013;591:17–31. doi: 10.1113/jphysiol.2012.243469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N,, et al. Diffusion tensor imaging—concepts and applications. J Magn Reson Imaging. 2001;13:534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21:424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- Powers WJ. Cerebral hemodynamics in ischemic cerebrovascular disease. Ann Neurol. 1991;29:231–240. doi: 10.1002/ana.410290302. [DOI] [PubMed] [Google Scholar]
- List J, Albers J, Kürten J, Schwindt A, Wilbers E, Flöel A. Reperfusion does not improve impaired rapid-onset cortical plasticity in patients with severe stenosis of the internal carotid artery. PLoS One. 2012;7:e41004. doi: 10.1371/journal.pone.0041004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- List J, Hertel-Zens S, Kübke JC, Lesemann A, Schreiber SJ, Flöel A. Cortical reorganization due to impaired cerebral autoregulation in individuals with occlusive processes of the internal carotid artery. Brain Stimul. 2014;7:381–387. doi: 10.1016/j.brs.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Cheng HL, Lin CJ, Soong BW, Wang PN, Chang FC, Wu YT,, et al. Impairments in cognitive function and brain connectivity in severe asymptomatic carotid stenosis. Stroke. 2012;43:2567–2573. doi: 10.1161/STROKEAHA.111.645614. [DOI] [PubMed] [Google Scholar]
- Doepp F, Valdueza JM, Schreiber SJ. Transcranial and extracranial ultrasound assessment of cerebral hemodynamics in vascular and Alzheimer's dementia. Neurol Res. 2006;28:645–649. doi: 10.1179/016164106X130380. [DOI] [PubMed] [Google Scholar]
- Purkayastha S, Fadar O, Mehregan A, Salat DH, Moscufo N, Meier DS,, et al. Impaired cerebrovascular hemodynamics are associated with cerebral white matter damage. J Cerebr Blood Flow Metab. 2014;34:228–234. doi: 10.1038/jcbfm.2013.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok V, Ding D, Fu J, Xiong Y, Chu WWC, Wang D,, et al. Transcranial Doppler ultrasound for screening cerebral small vessel disease—a community study. Stroke. 2012;43:2791–2793. doi: 10.1161/STROKEAHA.112.665711. [DOI] [PubMed] [Google Scholar]
- Warlow C. MRC European Carotid Surgery Trial: interim results for symptomatic patients with severe (70–99%) or with mild (0–29%) carotid stenosis. Lancet. 1991;337:1235–1243. [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state'. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Hautzinger M, Bailer M, Worall H, Keller F. Beck-Depressions-Inventar (BDI) Beck-Depressions-Inventar Bern: Huber, Germany; 1994. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness—the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Valdueza JM, Schreiber SJ, Roehl JE, Klingebiel R.Neurosonology and neuroImaging Stroke. ThiemeStuttgart, Germany2008
- Lezak MD, Howieson DB, Loring DW.Neuropsychological Assessment4th edn. New York, NY, USA; Oxford University Press; 2004(hardcover). [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE,, et al. Tract-based spatial statistics—voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo X-N, Gohel S, Kelly C, Smith SM,, et al. Toward discovery science of human brain function. Proc Natl Acad Sci USA. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panerai RB. Transcranial Doppler for evaluation of cerebral autoregulation. Clin Auton Res. 2009;19:197–211. doi: 10.1007/s10286-009-0011-8. [DOI] [PubMed] [Google Scholar]
- Mathiesen EB, Waterloo K, Joakimsen O, Bakke SJ, Jacobsen EA, Bønaa KH. Reduced neuropsychological test performance in asymptomatic carotid stenosis—The Tromsø Study. Neurology. 2004;62:695–701. doi: 10.1212/01.wnl.0000113759.80877.1f. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita ES, Barkhof F, Scheltens P, Stam CJ,, et al. Reduced resting-state brain activity in the ‘default network' in normal aging. Cerebral Cortex. 2008;18:1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- Wang Y, Risacher SL, West JD, McDonald BC, Magee TR, Farlow MR,, et al. Altered default mode network connectivity in older adults with cognitive complaints and amnestic mild cognitive impairment. J Alzheimers Dis. 2013;35:751–760. doi: 10.3233/JAD-130080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters N, Opherk C, Danek A, Ballard C, Herzog J, Dichgans M. The pattern of cognitive performance in CADASIL—a monogenic condition leading to subcortical ischemic vascular dementia. Am J Psychiatry. 2005;162:2078–2085. doi: 10.1176/appi.ajp.162.11.2078. [DOI] [PubMed] [Google Scholar]
- Krejza J, Baumgartner RW. Clinical applications of transcranial color-coded duplex sonography. J Neuroimag. 2004;14:215–225. doi: 10.1177/1051228403259274. [DOI] [PubMed] [Google Scholar]
- List J, Röhl JE, Doepp F, Valdueza JM, Schreiber SJ. Transcranial ultrasound analysis of cerebral blood flow during induced hypertension in acute ischemic stroke—a case series. Crit Ultrasound J. 2013;5:4. doi: 10.1186/2036-7902-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringelstein EB, Dittrich R, Stögbauer F.Borderzone infarctsIn: Caplan LR, van Gijn J (eds). Stroke Syndromes Cambridge University Press: Cambridge, MA, USA; 2012480–500. [Google Scholar]
- Soinne L, Helenius J, Tatlisumak T, Saimanen E, Salonen O, Lindsberg PJ. Cerebral hemodynamics in asymptomatic and symptomatic patients with high-grade carotid stenosis undergoing carotid endarterectomy. Stroke. 2003;34:1655–1661. doi: 10.1161/01.STR.0000075605.36068.D9. [DOI] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain—aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heide Von Der RJ, Skipper LM, Klobusicky E, Olson IR. Dissecting the uncinate fasciculus—disorders, controversies and a hypothesis. Brain. 2013;136:1692–1707. doi: 10.1093/brain/awt094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging—evidence from functional MRI. Proc Natl Acad Sci USA. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H,, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Gómez AJ, Ventura-Campos N, Belenguer A, Avila C, Forn C. The link between resting-state functional connectivity and cognition in MS patients. Mult Scler. 2013;20:338–348. doi: 10.1177/1352458513495584. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.