Abstract
Cerebral blood flow dysregulation caused by oxidative stress contributes to adverse neurologic outcome of seizures. A carbon monoxide (CO) donor CORM-A1 has antioxidant and cytoprotective properties. We investigated whether enteral supplements of CORM-A1 can improve cerebrovascular outcome of bicuculline-induced seizures in newborn piglets. CORM-A1 (2 mg/kg) was given to piglets via an oral gastric tube 10 minutes before or 20 minutes after seizure onset. Enteral CORM-A1 elevated CO in periarachnoid cerebrospinal fluid and produced a dilation of pial arterioles. Postictal cerebral vascular responses to endothelium-, astrocyte-, and vascular smooth muscle-dependent vasodilators were tested 48 hours after seizures by intravital microscopy. The postictal responses of pial arterioles to bradykinin, glutamate, the AMPA receptor agonist quisqualic acid, ADP, and heme were greatly reduced, suggesting that seizures cause injury to endothelial and astrocyte components of the neurovascular unit. In contrast, in the two groups of piglets receiving enteral CORM-A1, the postictal cerebral vascular responsiveness to these dilators was improved. Overall, enteral supplements of CORM-A1 before or during seizures offer a novel effective therapeutic option to deliver cytoprotective mediator CO to the brain, reduce injury to endothelial and astrocyte components of cerebral blood flow regulation and to improve the cerebrovascular outcome of neonatal seizures.
Keywords: antioxidants, astrocytes, cerebrovascular disease, cranial windows, epilepsy, endothelium, neurovascular unit
Introduction
Carbon monoxide (CO) is produced in various organs and tissues, including brain and cerebral vessels, by the degradation of heme in a reaction catalyzed by heme oxygenase (HO).1 Endogenously produced CO has cytoprotective functions in the cardiovascular system that include antiapoptotic, antioxidant, and antiinflammatory influences.1, 2, 3, 4, 5 Gaseous CO, administered at low concentrations, provides an effective treatment for diseases characterized by oxidative stress and inflammation.1, 5 The effects of gaseous CO can be reproduced by CO donors, termed CO-releasing molecules (CORMs).5
In the cerebral circulation, endogenously produced CO is a physiologically relevant dilator regulator of cerebral blood flow,6 and a potent antioxidant and antiapoptotic compound.3, 7, 8 Previously, we have showed that CORM-A1 [Na2(H3BCO2)], a water-soluble compound that slowly releases gaseous CO in physiologic solutions, is a convenient tool to investigate the biologic effects of CO in in vivo and in vitro studies related to the mechanism of cerebral vascular survival during oxidative stress and inflammation.3, 8, 9 CORM-A1 reduces formation of reactive oxygen species, prevents apoptosis, and promotes survival of cerebral vascular endothelial cells exposed to the excitotoxic neurotransmitter glutamate and a proinflammatory cytokine TNF-α.3, 4
Oxidative stress-related apoptosis of cerebral endothelial cells leading to endothelial injury and cerebral vascular dysfunction is a devastating consequence of neonatal epileptic seizures.3, 4, 8, 10 Adverse cerebral vascular outcome of seizures may cause or aggravate neuronal damage. Prevention of cerebral vascular dysfunction may translate into prevention of the neonatal encephalopathy caused by seizures. We have found that pretreatment with CORM-A1 administered by intravenous or intraperitoneal injections reduces oxidative stress during seizures and improves long-term postictal cerebrovascular endothelial dilator functions.7, 8, 9
Astrocytes are key cell components of the cerebral blood flow regulation.11, 12, 13, 14, 15 However, until now, the functional consequences of neonatal seizures on the astrocyte component of the neurovascular unit remain unknown. We also wanted to investigate whether the therapeutically preferable enteral route of CORM-A1 administration can deliver gaseous CO to the brain to provide long-term cerebrovascular protection.
Therefore, the present study addressed three major hypotheses: (1) neonatal seizures cause adverse long-term cerebral vascular outcomes in both astrocyte- and endothelium-dependent components of cerebral blood flow regulation; (2) enteral supplements of CORM-A1 deliver the cytoprotective gaseous mediator CO to the brain; and (3) enteral route of CORM-A1 administration during a therapeutic time window can protect the endothelial and astrocyte components of cerebral blood flow regulation and improve the long-term cerebral vascular outcome of seizures.
Materials and methods
Chemicals
Bicuculline and L-quisqualic acid were from Tocris (R&D Systems, Minneapolis, MN, USA). Pancuronium bromide was from Astra Pharmaceutical Products (Westborough, MA, USA). CORM-A1 was from Dalton Pharma Services (Toronto, Canada). All other reagents were from Sigma (St Louis, MO, USA).
Animals
Newborn piglets (1 to 5 days old, 1.5 to 2.5 kg, either sex) were purchased from a commercial breeder. Piglets were housed in a pen with warmed floor and given continual access to pig milk substitute. Veterinary care was provided by the Department of Comparative Medicine, whose staff includes four full-time veterinarians in an AAALAC accredited program. All experimental protocols using animals were approved by the Animal Care and Use Committee of the University of Tennessee Health Science Center in accordance with the National Institutes of Health guidelines for the care and use of animals in research. The experiments were performed in accordance with the ARRIVE guidelines.
Animal Model of Neonatal Seizures
Bicuculline, a GABAA receptor blocker, induces epileptic seizures by disrupting the normal balance between excitatory and inhibitory neurotransmitters. In newborn piglets, bicuculline induces epileptic seizures that last for over 2 hours.16 Bicuculline (3 mg/kg intraperitoneally) was administered to piglets anesthetized with ketamine/acepromazine (33/3.3 mg/kg intramuscularly), paralyzed with pancuronium bromide (0.2 mg/kg intravenously), orotracheally intubated and ventilated with room air.7, 9, 10 The heart rate (HR) as a reliable indicator of the seizure activity was continuously recorded. The body temperature was maintained at 37°C to 38°C by a servo-controlled heating pad. We investigated the effects of enteral supplements of CORM-A1 on long-term (2 days) cerebral vascular outcome of neonatal seizures. The intact control Group I (no seizures) and five seizure survival groups were studied: (1) saline control Group II (saline, 10 mL); (2) CORM-A1–preictal treatment Group III (2 mg/kg in 10 mL saline, 10 minutes before bicuculline); (3) CORM-A1–ictal treatment Group IV (2 mg/kg in 10 mL saline, 20 minutes after bicuculline); (4) inactivated CORM-A1–preictal treatment Group V (2 mg/kg in 10 mL saline, 10 minutes before bicuculline); and (5) bilirubin–ictal treatment Group VI (5 mg/kg, iv, 20 minutes after bicuculline). Piglets were kept on the ventilator for 2 to 3 hours until the seizure activity subsided. When fully conscious, piglets were transferred to the animal care facility and kept in warmed cages with food and water ad libitum to recover for 2 days.
Enteral Administration of CORM-A1
Anesthetized piglets were equipped with oral gastric feeding tube inserted through the mouth, down the esophagus, and into the stomach. Water-soluble CORM-A1 spontaneously releases gaseous CO in physiologic environment with half-life about 3.5 hours.9 CORM-A1 was dissolved in saline immediately before the feeding to avoid the loss of released gaseous CO. Inactivated CORM-A1 was prepared by exposing the CORM-A1 solution in saline to open air for 20 hours at room temperature to fully decompose the parent compound.5, 9 All drugs dissolved in 10 mL saline were filtered through a 0.22-μm Millipore syringe filter and placed into the stomach via an oral feeding tube.
Intravital Cranial Window Microscopy for Detection of Cerebral Vascular Function
Cerebral vascular function was tested in control and postictal piglets. Pial arterioles are important resistance cerebral vessels that can be observed using the cranial window technique as we previously described.7 Control and postictal piglets were preanesthetized with ketamine/acepromazine (33/3.3 mg/kg intramuscularly) and maintained on α-chloralose (50 mg/kg intravenously). Catheters were placed into the femoral vein and artery for recording mean arterial blood pressure (MABP) and HR, and for drug delivery and blood sampling for detection of blood gases. Body temperature was maintained at 37°C to 38°C with a servo-controlled heating pad. A closed cranial window was implanted and filled with artificial cerebrospinal fluid for intravital microscopy of pial arterioles as we previously described.7, 10 Pial arteriolar diameter was measured with a videomicrometer coupled to a television camera. Four to seven pial arterioles (20 to 80 μm) were observed in each animal. To test cerebral vascular functions, we used a variety of physiologically relevant vasodilators, including endothelium-dependent bradykinin (10−6 mol/L), astrocyte-dependent ADP (10−4 mol/L), and both endothelium and astrocyte-dependent vasodilators glutamate (10−4 mol/L), the AMPA receptor agonist L-quisqualic acid (10−4 mol/L), and the HO substrate heme (as hemin, 10−5 mol/L). Sodium nitroprusside (10−6 mol/L) was used to test the vascular smooth muscle-based responses of pial arterioles that are independent of endothelial and astrocyte influences. The doses of vasodilators that elicited near-maximum cerebral vascular responses in control intact animals were selected on the basis of our previous studies7, 9, 10, 14, 17 and preliminary experiments. All dilators were directly applied to the cerebral surface through the ports of the window via a 0.22-μm Millipore syringe filter. The stable pial arteriolar diameter achieved between 5 and 10 minutes was taken as the vascular response.
Detection of Carbon Monoxide Production by Gas Chromatography/Mass Spectrometry
To detect CO in cerebral circulation in CORM-A1-treated piglets, the samples of cortical periarachnoid cerebrospinal fluid (pCSF) were collected from the brain surface underneath the cranial window during basal conditions and during a 60-minute period after intake of CORM-A1. Periarachnoid cerebrospinal fluid (0.4 mL) was collected from under the cranial window in 10-minute intervals through a spout directly into the sealed vials containing 1.3 mL Krebs buffer. The external standard 13CO (1 mmol/L) was added to all samples for the purpose of CO quantification.8, 9 The concentration of CO in the headspace gas was detected using an Agilent 5975 GC/MSD ChemStation (Agilent Technologies Inc., Santa Clara, CA, USA).
Statistical Analysis
Values are presented as means±s.e. of absolute values or percentage of control. The proper sample sizes were calculated for a power of 0.8 in all tests. Data were analyzed by repeated measures ANOVA. A level of P<0.05 was considered as significant.
Results
Acute Effects of Enteral CORM-A1 in Systemic and Cerebral Circulation
The effects of enteral supplements of CORM-A1 (2 mg/kg in 10 mL saline) administered via oral gastric tube were tested in intact newborn piglets. Enteral CORM-A1 did not cause any significant changes in systemic circulatory parameters, including MABP, HR, PaCO2, and body temperature (Table 1). A slight reduction in PaO2 was observed 30 to 80 minutes after CORM-A1 intake, but the arterial blood oxygenation remained at a physiologic level (PaO2, 98±5 and 85±3 mm Hg, before and after CORM-A1, respectively; P=0.05).
Table 1. Systemic circulatory parameters before and after enteral supplement of CORM-A1 (2 mg/kg).
| Experiment | MABP (mm Hg) | HR (beats/min) | Arterial PCO2 (mm Hg) | Arterial PO2 (mm Hg) | pH | Body temperature (°C) |
|---|---|---|---|---|---|---|
| Baseline | 76±5 | 135±6 | 33±2 | 98±5 | 7.42±0.03 | 37.7±0.2 |
| Time after CORM-A1 | ||||||
| 10–20 minutes | 76±6 | 139±9 | 35±3 | 90±9 | 7.39±0.04 | 37.5±0.2 |
| 30–40 minutes | 75±4 | 146±11 | 37±3 | 85±3a | 7.41±0.02 | 37.9±0.1 |
| 50–80 minutes | 73±5 | 149±11 | 35±2 | 85±4a | 7.41±0.03 | 37.8±0.3 |
HR, heart rate; MABP, mean arterial blood pressure. n=6 animals.
P<0.05, compared with the baseline value.
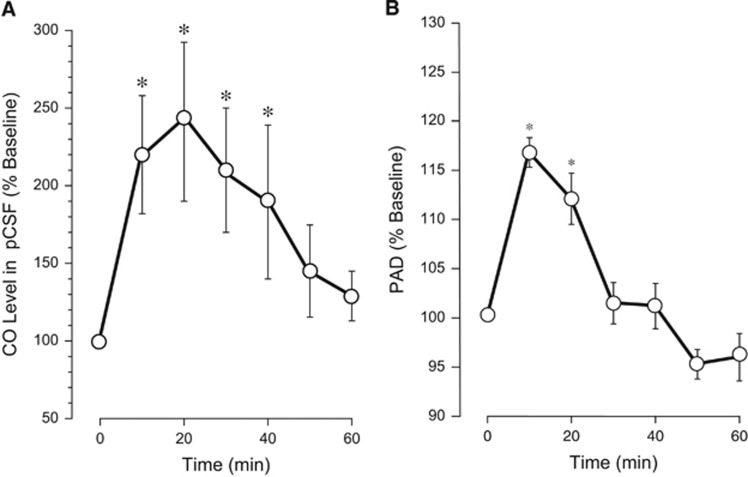
We investigated whether enteral CORM-A1 can produce an increase in the level of gaseous CO in the cerebral circulation. The samples of pCSF were collected from underneath the cranial window during the 10- to 80-minute period after intake of CORM-A1, and CO concentrations were detected by gas chromatography/mass spectrometry. The baseline level of CO in pCSF was 119±16 pmol/mL (N=5), confirming our previous reports.8, 10 Enteral CORM-A1 produced an increase in CO in pCSF (2.0- to 2.5-fold over the baseline) that was sustained during a 40-minute period (Figure 1A). Gaseous CO is a dilator of pial arterioles in newborn piglets.6 After feeding piglets with CORM-A1, we observed a rapid dilation of pial arterioles ~15% above the baseline sustained during a 20-minute period (Figure 1B). These data suggest that CO gas accumulated in the cerebral circulation after enteral intake of CORM-A1 is functionally active as a vasodilator of pial arterioles.
Figure 1.
Enteral supplement of CORM-A1 increases carbon monoxide (CO) level in cortical periarachnoid cerebrospinal fluid (pCSF) (A) and causes dilation of cerebral pial arterioles (B). CORM-A1 (2 mg/kg in 10 mL saline) was administered via an oral gastric feeding tube. pCSF samples were collected from under the closed cranial window 10 to 60 minutes after the administration of CORM-A1. (A) CO concentration in pCSF was detected by gas chromatography/mass spectrometry (GC/MS) and expressed as the percentage of the baseline values. (B) Diameters of pial arterioles (20 to 80 μm) were measured using intravital microscopy 10 to 60 minutes after the administration of CORM-A1 and expressed as the percentage of the baseline values. N=5 animals. *P<0.05 compared with the baseline values.
Postictal Cerebral Vascular Dysfunction
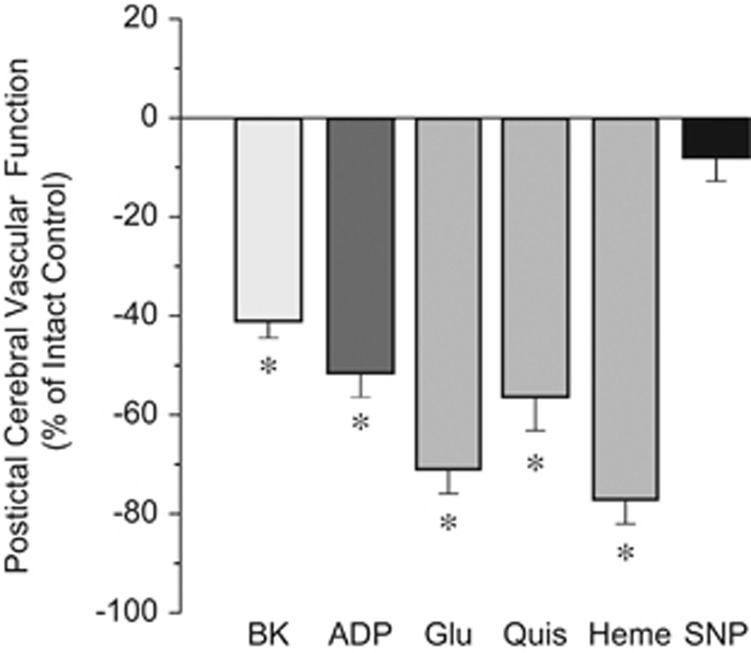
Our study is focused on long-term cerebral vascular outcome of epileptic seizures. We determined the long-term effects of seizures on cerebral vasodilator functions to endothelium-, astrocyte-, and vascular smooth muscle-dependent stimuli (Figure 2). Forty-eight hours after seizures, the postictal responses of pial arterioles to endothelium-dependent vasodilator bradykinin (10−6 mol/L) were greatly reduced, whereas the responses to a vascular smooth muscle-targeting dilator sodium nitroprusside (10−6 mol/L) were not affected (Figure 2). We investigated whether the astrocyte component of the neurovascular unit is also adversely affected by seizures. Pial arteriolar dilation to ADP requires the presence of intact and functionally active glia limitans astrocytes.14, 15 Postictal cerebral vascular responses to ADP (10−4 mol/L) were greatly reduced compared with the intact control group (Figure 2).
Figure 2.
Long-term adverse outcome of neonatal seizures on endothelium- and astrocyte-dependent components of the cerebral blood flow regulation. Postictal cerebral vascular responses to topical endothelium-dependent dilator bradykinin (BK, 10−6 mol/L), astrocyte-dependent dilator ADP (10−4 mol/L), endothelium/astrocyte-dependent dilators glutamate (Glu, 10−4 mol/L), quisqualic acid (Quis, 10−4 mol/L), heme (10−5 mol/L), and vascular smooth muscle-dependent dilator sodium nitroprusside (SNP, 10−6 mol/L) were tested in newborn piglets 48 hours after the seizure. Postictal cerebral vascular function is expressed as the percent reduction of the intact control cerebral vascular responses. N=6 animals, n=30 arterioles in each group. *P<0.05 compared with intact control values.
Pial arteriolar dilation to glutamate mediated via ionotropic glutamate receptors requires the contribution of both endothelial and astrocyte components of the neurovascular unit.4, 8, 18, 19 Postictal cerebral vascular responses to glutamate (10−4 mol/L) and the AMPA receptor agonist quisqualic acid (10−4 mol/L) were reduced by 60% to 70% compared with the intact control group (Figure 2). Heme oxygenase expressed in cerebral vascular endothelium and cortical astrocytes is an important contributor to cerebrovascular dilator function.3, 4, 6 The pial arteriolar responses to heme (10−5 mol/L), the HO substrate, were dramatically reduced 48 hours after seizures (Figure 2).
Overall, these data indicate that cerebral vascular responsiveness to endothelium- and astrocyte-dependent vasodilators is severely reduced during delayed postictal period, suggesting that seizures cause sustained dysregulation of cerebral blood flow.
Enteral CORM-A1 and the Dynamics of Heart Rate and Body Temperature During Seizures
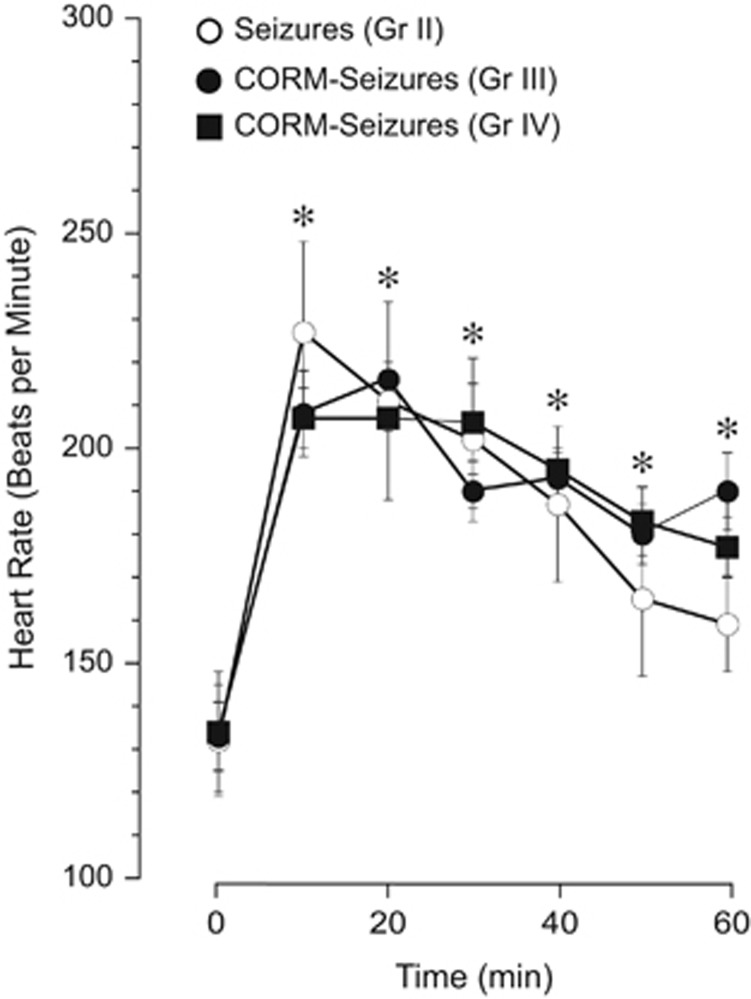
In newborn piglets, bicuculline (3 mg/kg intravenously) elicits epileptic seizures characterized by repetitive neuronal discharges and a robust elevation in EEG amplitude and spectral power in the alpha (8 to 13 Hz), beta (>13 Hz), delta (<4 Hz), and theta (4 to 8 Hz) bands.16 Severe tachycardia, a common feature of seizures in human patients, correlates with neuronal discharges and is a sensitive method for early detection of seizures in newborns and adult patients.20, 21, 22, 23, 24 In newborn piglets, we observed a robust increase in HR concomitant with vasodilation of pial arterioles for the duration of the ictal episode.7, 8, 9, 10 Therefore, we compared the bicuculline-induced dynamics of HR as a reliable indicator of seizure activity in control and CORM-A1-treated piglets (Figure 3). In all the saline control piglets (Group II), bicuculline caused an immediate robust increase in HR from 130–140 to 200–230 bpm that was sustained for over 80 minutes (Figure 3). CORM-A1 (2 mg/kg), enterally administered before or during seizures, did not alter the intensity or the duration of tachycardia in piglets (Figure 3). These observations suggest that CORM-A1 does not alter the course of neuronal activation associated with seizures.
Figure 3.
Enteral supplements of CORM-A1 before or during seizures do not affect the ictal tachycardia. The dynamics of heart rate during epileptic seizures were recorded in the saline control piglets (Group II) and in piglets supplemented with enteral CORM-A1 given via an oral gastric feeding tube 10 minutes before (Group III) or 20 minutes after (Group IV) induction of seizures by bicuculline. N=6 animals in each group. *P<0.05 compared with baseline values.
We investigated whether CORM-A1 affected body temperature during status epilepticus. In all groups, core body temperature was maintained in physiologic range (37.8±0.3). In the saline control group II, the core body temperature remained unchanged (37.8°C to 38.1°C) during the 10 to 80 minutes of the ictal period. Enteral supplements of CORM-A1 before (Group III) or during (Group IV) seizures also did not affect the core body temperature during the ictal period (38.0±0.3°C and 37.8±0.3°C, respectively).
Effects of Enteral Supplements of CORM-A1 on Systemic Circulatory Parameters During Delayed Postictal Period
We found no changes in systemic circulatory parameters in postictal newborn piglets, untreated or supplemented with enteral CORM-A1 or systemic bilirubin compared with the intact control group (Table 2). Forty-eight hours after epileptic seizures, MABP, HR, blood gases, pH, and body temperature were within the physiologic range for newborn piglets, and no differences were observed among the experimental groups. These data indicate that epileptic seizures in newborn piglets, untreated or treated with enteral CORM-A1 or parenteral bilirubin had no long-term effects on systemic circulatory parameters.
Table 2. Systemic circulatory parameters in intact control (Group I) and postictal newborn piglets (Groups II to VI).
| Group | MABP (mm Hg) | HR (beats/min) | Arterial PCO2 (mm Hg) | Arterial PO2 (mm Hg) | pH | Body temperature (°C) |
|---|---|---|---|---|---|---|
| I | 76±8 | 128±18 | 36±1 | 99±8 | 7.38±0.05 | 37.3±0.7 |
| II | 77±3 | 135±14 | 33±1 | 88±3 | 7.39±0.02 | 37.1±0.3 |
| III | 72±3 | 129±8 | 33±1 | 97±4 | 7.35±0.08 | 37.2±0.4 |
| IV | 67±7 | 112±7 | 37±2 | 89±8 | 7.36±0.03 | 37.2±0.3 |
| V | 72±5 | 120±20 | 35±2 | 81±10 | 7.40±0.01 | 37.4±0.4 |
| VI | 73±1 | 122±17 | 33±2 | 97±3 | 7.42±0.02 | 37.5±0.1 |
HR, heart rate; MABP, mean arterial blood pressure.
Group I, No-seizures intact control. Seizure Groups II to VI, 48 hours postictal period: Group II, seizure saline control; Group III, enteral CORM-A1 (2 mg/kg) before seizures; Group IV, enteral CORM-A1 (2 mg/kg) during seizures; Group V, inactivated enteral CORM-A1 (2 mg/kg) before seizures; Group VI, bilirubin (5 mg/kg intravenously) during seizures. n=4 animals in each group.
Effects of Enteral Supplements of CORM-A1 on Postictal Cerebral Vascular Functions
We investigated whether enteral supplements of CORM-A1 taken before or during status epilepticus can prevent the loss of cerebral vasodilator functions observed during delayed postictal period. We treated seizing animals with: (1) saline (Control Group II); (2) CORM-A1 (preventive Group III and therapeutic Group IV), (3) inactivated CORM-A1 (Group V, negative control), and (4) bilirubin (Group VI, positive control).
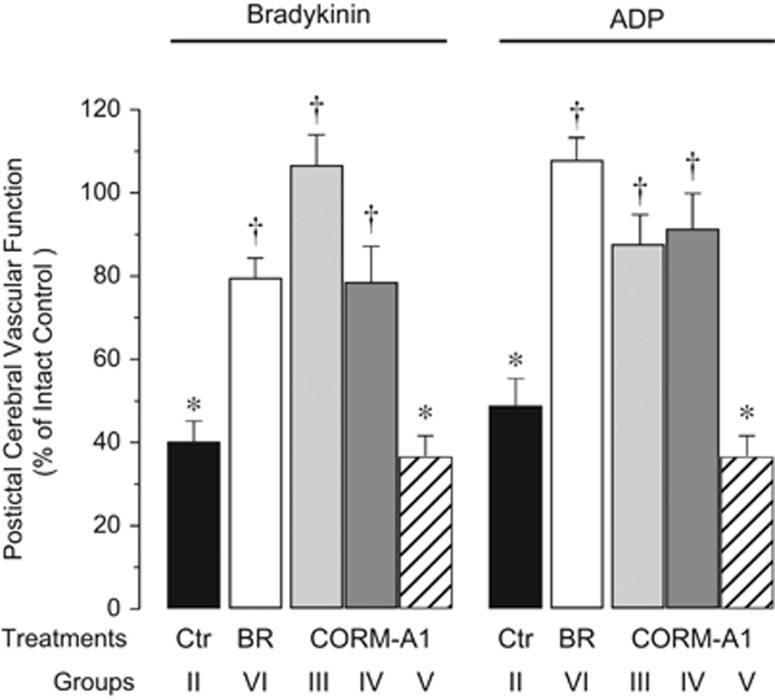
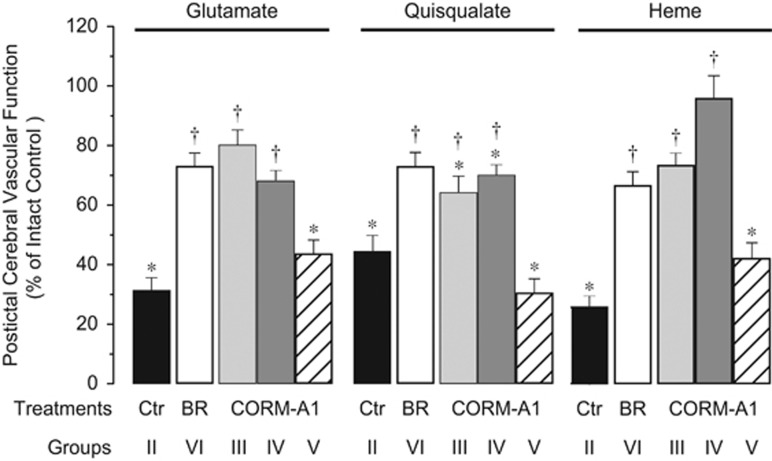
Postictal cerebral vascular responses to endothelium- and/and astrocyte-dependent vasodilators were tested 48 hours after seizures. Enteral CORM-A1 (2 mg/kg), administered 10 minutes before seizures, prevented loss of postictal cerebral vascular responses to bradykinin (Figure 4), ADP (Figure 4), glutamate (Figure 5), and heme (Figure 5). The vasodilator responses to quisqualate were also improved but remained slightly below the intact control level (Figure 5). When enteral CORM-A1 was administered during the advanced ictal period (20 minutes after bicuculline injection), we also observed that postictal cerebral vascular responses to bradykinin (Figure 4), ADP (Figure 4), glutamate (Figure 5), and heme (Figure 5) were completely preserved, and the responses to quisqualic acid were greatly improved (Figure 5).
Figure 4.
Enteral supplements of CORM-A1 improve postictal cerebral vascular responses to endothelium-dependent dilator bradykinin and astrocyte-dependent dilator ADP. Cerebral vascular responses to topical bradykinin (10−6 mol/L) and ADP 10−4 mol/L were tested 48 hours after seizures in: seizures saline control piglets (Ctr Group II); piglets treated with enteral CORM-A1 (2 mg/kg) administered 10 minutes before (CORM-A1 Group III) or 20 minutes after bicuculline (CORM-A1 Group IV); inactivated CORM-A1 (CORM-A1 Group V); and piglets treated with bilirubin administered 20 minutes after bicuculline (5 mg/kg intravenously, BR Group VI). Control responses to the vasodilators were tested in no-seizure intact control group (Group I). Postictal cerebral vascular function is expressed as % of control responses in no-seizure intact control group (Group I). N=4 animals, n=25 arterioles in each group. *P<0.05 compared with the intact control. †P<0.05 compared with the seizure saline control.
Figure 5.
Enteral supplements of CORM-A1 improve postictal cerebral vascular responses to endothelium/astrocyte-dependent dilators glutamate, quisqualate, and heme. Cerebral vascular responses to topical glutamate (10−4 mol/L), quisqualate (10−4 mol/L), and heme (hemin, 10−5 mol/L) were tested 48 hours after seizures in: seizures saline control piglets (Ctr Group II); piglets treated with enteral CORM-A1 (2 mg/kg) administered 10 minutes before (CORM-A1 Group III) or 20 minutes after bicuculline (CORM-A1 Group IV); inactivated CORM-A1 (CORM-A1 Group V); and piglets treated with bilirubin 20 minutes after bicuculline (5 mg/kg intravenously, BR Group VI). Control responses to the vasodilators were tested in no-seizure intact control group (Group I). Postictal cerebral vascular function is expressed as % of control responses in no-seizure intact control group (Group I). N=4 animals, n=25 arterioles in each group. *P<0.05 compared with the intact control. †P<0.05 compared with the seizure saline control.
We used negative and positive controls to evaluate the effectiveness of cerebroprotection by enteral supplements of CORM-A1. As a negative control, we used a fully CO-decomposed CORM-A1. Inactive CORM-A1 (2 mg/kg) enterally administered before seizures did not improve the cerebral vascular outcome of seizures to any of the tested stimuli (Figures 4 and 5). As a positive control for the effectiveness of CORM-A1 cytoprotection, we used systemic parenteral bilirubin, a byproduct of HO-catalyzed heme degradation and a potent antioxidant compound.8, 25, 26 Bilirubin (5 mg/kg), injected into the ear vein during the ictal period (20 minutes after bicuculline administration), prevented the loss of postictal cerebral vascular reactivity to all tested endothelium- and/or astrocyte-dependent vasodilators (Figures 4 and 5).
Overall, these data strongly suggest that the adverse functional cerebral vascular outcome of seizures was ameliorated after enteral supplementation with CORM-A1 given to piglets before or during seizures. Cerebroprotective properties of enteral CORM-A1 are fully comparable to the properties of parenterally administered potent antioxidant bilirubin.
Discussion
A unique focus of our study is on improving long-term adverse cerebral vascular outcomes of epileptic seizures. We have accumulated strong evidence that seizures cause sustained cerebral vascular dysfunction7, 9, 10, 14, 17 that may produce or aggravate neuronal damage. Therefore, treatments that could prevent cerebral vascular dysfunction may improve the long-term neurologic outcome of seizures. Our new findings in the newborn brain are (1) the antioxidant cytoprotective compound CORM-A1 (2 mg/kg) administered via an oral feeding tube provides a novel effective therapeutic option to deliver the cytoprotective mediator CO to normal and seizing brain; (2) enteral CORM-A1 at the dose used does not reduce blood oxygenation and does not cause acute or delayed changes in the systemic circulation in normal or seizing piglets; (3) epileptic seizures cause injury to both endothelial and astrocyte components of the neurovascular unit thus leading to dysregulation of postictal cerebral blood flow; (4) enteral CORM-A1 administered before or during seizures effectively reduces endothelial and astrocyte dysfunction and improves cerebral vascular outcome of neonatal seizures. Overall, these data suggest that the enteral route of CORM-A1 administration, either preventive or within the 20-minute therapeutic window, provides a safe alternative to the parenteral route for effective protection of endothelial and astrocyte components of the neurovascular unit during neonatal seizures.
Epileptic seizures cause prolonged neurologic complications indicated by the symptoms known as the postictal state.27 In newborn babies, seizures produce sustained neuronal injury and frequently lead to life-long neurologic and developmental disabilities.27, 28, 29, 30, 31, 32, 33 Alteration in cerebral blood flow regulation contributes to the postictal state.27 The incidence of cerebral vascular injury during postictal state has been described in adult patients and in newborns.22, 27 Insufficient cerebral blood flow is especially damaging to the neonatal brain because of the rapid development of neurons, reduced antioxidant defenses, and high susceptibility to inflammation.29, 33 Therefore, preventing postictal cerebral vascular dysfunction is a potentially valuable treatment approach to improve the outcome of seizures in patients.
Astoundingly, very little information is available on cerebral vascular effects of seizures. Our studies in a large animal model of neonatal seizures are filling this gap. Previously, we have showed that seizures cause sustained injury to cerebral vascular endothelium and loss of cerebral vascular endothelium-mediated dilator functions.2, 3, 4, 7, 9 In the model of bicuculline-induced neonatal seizures in newborn piglets, we have described the loss of responsiveness of cerebral resistance arterioles to endothelium-dependent vasodilators, including bradykinin, during delayed postictal period as the evidence of cerebral blood flow dysregulation.7, 8, 9, 10 Endothelial dysfunction caused by seizures is concomitant with endothelial injury detected by the numerous apoptotic cells in pial arterioles and by the appearance of circulating endothelial cells of brain origin in peripheral blood during the postictal period.34
We now report that seizures cause long-term dysfunction of the astrocyte component of neurovascular unit. The importance of astrocytes in the regulation of cerebral blood flow regulation cannot be overlooked.11, 12, 13, 14, 15 Cortical glia limitans astrocytes at the brain surface are critical for selected dilator responses of pial arterioles, in particular, to ADP.14, 15 In many cases, vasodilator stimuli require contribution of both endothelial and astrocyte components of the neurovascular unit. Cortical astrocytes and cerebral vascular endothelial cells express functional ionotropic and metabotropic glutamate receptors that may contribute to dilation of pial arterioles to glutamate.2, 15, 17, 19, 35 Pial arteriolar dilation to heme, the HO substrate, also involves both endothelial and astrocyte components that express HO-2 and produce vasodilator CO upon stimulation.2, 4, 14, 17, 35 During the delayed postictal period, we observed a dramatic loss of cerebral vascular dilation to astrocyte-dependent vasodilator ADP, and endothelium-astrocyte-dependent stimuli, including glutamate, quisqualate, and heme. These findings, for the first time, show that neonatal seizures cause astrocyte injury leading to loss of astrocyte regulation of cerebral blood flow.
Preventing adverse cerebral vascular events and restoring cerebral blood flow regulation is a promising approach to improve the neurologic outcome of neonatal seizures. We have found that pretreatment with the CO donor molecule CORM-A1, administered by intravenous or intraperitoneal injections, reduced reactive oxygen species formation during seizures, prevented endothelial apoptosis, and greatly improved postictal endothelial vasodilator functions.8, 9 In patients, noninvasive enteral route is by far the most convenient and preferred route of systemic drug administration. Our current findings show that enteral supplements of CORM-A1 effectively deliver gaseous cytoprotective mediator CO to the brain, and prevent cerebral vascular dysfunction by protecting both endothelial and astrocyte components of the neurovascular unit.
Importantly, the cerebroprotective effect can be achieved when enteral CORM-A1 is administered not only preventively but also therapeutically within the 20 minutes of the seizure onset. This window of opportunity for preventing cerebral vascular damage correlates with our previous observations that parenteral CORM-A1 (2 mg/kg), administered before or 20 minutes after seizure onset, reduced cerebrovascular oxidative stress, whereas increasing the window to 30 minutes was ineffective.8, 9 The effectiveness of enteral supplements of CORM-A1 in preventing endothelial- and astrocyte-mediated dysregulation of cerebral blood flow is fully comparable to the cerebroprotection achieved by parenterally administered potent oxidant bilirubin.
The mechanism of cerebroprotection by CORM-A1 is based on antioxidant properties of gaseous CO that is time dependently released by the drug. CORM-A1 systemically administered to newborn piglets reduced reactive oxygen species formation in cerebral microvessels and the astrocyte-enriched brain cortex parenchyma during status epilepticus.8 Alternatively, one may hypothesize that CO-derived CORM-A1 has anticonvulsant effects. However, CORM-A1 did not affect the dynamics of ictal tachycardia, a reliable and sensitive indicator of status epilepticus in patients,20, 21, 22, 23, 24 suggesting that the drug did not reduce neuronal activation. Furthermore, quantitative detection of neuronal activation during bicuculline-induced seizures based on spectral analysis of the EEG suggested that CO has proconvulsant, rather than anticonvulsant action.16
Importantly, CORM-A1 (2 mg/kg), administered via enteral or parenteral routes, provides a safe and effective pharmacological option for prevention of oxidative stress-induced cerebral vascular disease. We did not detect any adverse systemic or cardiovascular effects of enteral and parenteral CORM-A1, administered to healthy or seizing animals. CORM-A1 at the therapeutically effective concentration did not reduce blood oxygenation suggesting that CO binding to hemoglobin is negligible at the dose used. According to our observations, the treatment with CORM-A1 did not complicate postictal recovery and behavior of newborn pigs.
Overall, seizures cause long-term postictal dysregulation of cerebral blood flow by producing oxidative stress-related damage to both endothelial and astrocyte components of the neurovascular unit. Adverse cerebrovascular effects of seizures can be prevented by enteral supplements of CORM-A1 shortly before seizures or during a 20-minute extended treatment window. Prolonged cerebroprotection by enteral supplements of CORM-A1 plus the extended treatment window may be envisaged to provide a noninvasive pathway of the systemic drug delivery especially relevant to improve the outcome of seizures in neonates. Preventing cerebral vascular dysfunction can be translated into prevention of the neonatal encephalopathy caused by seizures.
The authors declare no conflict of interest.
References
- Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharm Rev. 2008;60:79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- Basuroy S, Leffler CW, Parfenova H. CORM-A1 prevents blood-brain barrier dysfunction caused by ionotropic glutamate receptor-mediated endothelial oxidative stress and apoptosis. Am J Physiol Cell Physiol. 2013;304:C1105–C1115. doi: 10.1152/ajpcell.00023.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfenova H, Leffler CW. Cerebroprotective functions of HO-2. Curr Pharm Des. 2008;14:443–453. doi: 10.2174/138161208783597380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffler CW, Parfenova H, Jaggar J. Carbon monoxide as an endogenous cerebrovascular modulator. Am J Physiol Heart Circ Physiol. 2011;301:H1–H11. doi: 10.1152/ajpheart.00230.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motterlini R, Otterbein LE. The therapeutic potential of carbon monoxide. Nat Rev Drug Discov. 2010;9:728–743. doi: 10.1038/nrd3228. [DOI] [PubMed] [Google Scholar]
- Leffler CW, Nasjletti A, Yu C, Johnson RA, Fedinec AL, Walker N. Carbon monoxide and cerebral microvascular tone in newborn pigs. Am J Physiol Heart Circ Physiol. 1999;276:H1641–H1646. doi: 10.1152/ajpheart.1999.276.5.H1641. [DOI] [PubMed] [Google Scholar]
- Parfenova H, Carratu P, Tcheranova D, Fedinec A, Pourcyrous M, Leffler CW. Epileptic seizures cause extended postictal cerebral vascular dysfunction that is prevented by HO-1 overexpression. Am J Physiol Heart Circ Physiol. 2005;288:H2843–H2850. doi: 10.1152/ajpheart.01274.2004. [DOI] [PubMed] [Google Scholar]
- Parfenova H, Leffler CW, Basuroy S, Liu J, Fedinec AL. Antioxidant roles of heme oxygenase, carbon monoxide, and bilirubin in cerebral circulation during seizures. J Cereb Blood Flow Metab. 2012;32:1024–1034. doi: 10.1038/jcbfm.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann A, Leffler CW, Tcheranova D, Fedinec AL, Parfenova H. Cerebroprotective effects of the CO-releasing molecule CORM-A1 against seizure-induced neonatal vascular injury. Am J Physiol Heart Circ Physiol. 2007;293:H2501–H2507. doi: 10.1152/ajpheart.00354.2007. [DOI] [PubMed] [Google Scholar]
- Carratu P, Pourcyrous M, Fedinec A, Leffler CW, Parfenova H. Endogenous heme oxygenase prevents impairment of cerebral vascular functions caused by seizures. Am J Physiol Heart Circ Physiol. 2003;285:H1148–H1157. doi: 10.1152/ajpheart.00091.2003. [DOI] [PubMed] [Google Scholar]
- Filosa JA, Iddings JA. Astrocyte regulation of cerebral vascular tone. Am J Physiol Heart Circ Physiol. 2013;305:H609–H619. doi: 10.1152/ajpheart.00359.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- Koehler RC, Roman RJ, Harder DR. Astrocytes and the regulation of cerebral blood flow. Trends Neurosci. 2009;32:160–169. doi: 10.1016/j.tins.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Leffler CW, Parfenova H, Fedinec AL, Basuroy S, Tcheranova D. Contributions of astrocytes and CO to pial arteriolar dilation to glutamate in newborn pigs. Am J Physiol Heart Circ Physiol. 2006;291:H2897–H2904. doi: 10.1152/ajpheart.00722.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HL, Ye S, Baughman VL, Feinstein DL, Pelligrino DA. The role of the glia limitans in ADP-induced pial arteriolar relaxation in intact and ovariectomized female rats. Am J Physiol Heart Circ Physiol. 2005;288:H382–H388. doi: 10.1152/ajpheart.00727.2004. [DOI] [PubMed] [Google Scholar]
- Parfenova H, Daley ML, Carratu P, Leffler CW. Heme oxygenase inhibition reduces neuronal activation evoked by bicuculline in newborn pigs. Brain Res. 2004;1014:87–96. doi: 10.1016/j.brainres.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Parfenova H, Tcheranova D, Basuroy S, Fedinec AL, Liu J, Leffler CW. Functional role of astrocyte glutamate receptors and carbon monoxide in cerebral vasodilation response to glutamate. Am J Physiol Heart Circ Physiol. 2012;302:H2257–H2266. doi: 10.1152/ajpheart.01011.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Q, Tcheranova D, Basuroy S, Parfenova H, Jaggar J, Leffler CW. Role of calcium signaling in glutamate-stimulated CO production in newborn piglet astrocytes. Am J Physiol Heart Circ Physiol. 2011;301:H428–H433. doi: 10.1152/ajpheart.01277.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiumana E, Parfenova H, Jaggar JH, Leffler CW. Carbon monoxide mediates vasodilator effects of glutamate in isolated pressurized cerebral arterioles of newborn pigs. Am J Physiol Heart Circ Physiol. 2003;284:H1073–H1079. doi: 10.1152/ajpheart.00881.2002. [DOI] [PubMed] [Google Scholar]
- Greene BR, de Chazal P, Boylan G, Reilly RB, O'Brien C, Connolly S. Heart and respiration rate changes in the neonate during electroencephalographic seizure. Med Biol Eng Comput. 2006;44:27–34. doi: 10.1007/s11517-005-0001-5. [DOI] [PubMed] [Google Scholar]
- Opherk C, Coromilas J, Hirsch LJ. Heart rate and EKG changes in 102 seizures: analysis of influencing factors. Epilepsy Res. 2002;52:117–127. doi: 10.1016/s0920-1211(02)00215-2. [DOI] [PubMed] [Google Scholar]
- Shah DK, Zempel J, Barton T, Lukas K, Inder TE. Electrographic seizures in preterm infants during the first week of life are associated with cerebral injury. Pediatr Res. 2010;67:102–106. doi: 10.1203/PDR.0b013e3181bf5914. [DOI] [PubMed] [Google Scholar]
- van Elmpt WJ, Nijsen TM, Griep PA, Arends JB. A model of heart rate changes to detect seizures in severe epilepsy. Seizure. 2006;15:366–375. doi: 10.1016/j.seizure.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Zijlmans M, Flanagan D, Gotman J. Heart rate changes and ECG abnormalities during epileptic seizures: prevalence and definition of an objective clinical sign. Epilepsia. 2002;43:847–854. doi: 10.1046/j.1528-1157.2002.37801.x. [DOI] [PubMed] [Google Scholar]
- Hegyi T, Goldie E, Hiatt M. The protective role of bilirubin in oxygen-radical diseases of the preterm infant. J Perinatol. 1994;14:296–300. [PubMed] [Google Scholar]
- Stocker R. Antioxidant activities of bile pigments. Antioxid Redox Signal. 2004;6:841–849. doi: 10.1089/ars.2004.6.841. [DOI] [PubMed] [Google Scholar]
- Fisher RS, Schachter SC. The postictal state: a neglected entity in the management of epilepsy. Epilepsy Behav. 2000;1:52–59. doi: 10.1006/ebeh.2000.0023. [DOI] [PubMed] [Google Scholar]
- Agarwal M, Fox SM.Pediatric seizures Emerg Med Clin North Am 201331733–754.2013 [DOI] [PubMed] [Google Scholar]
- Chapman KE, Raol YH, Brooks-Kayal A. Neonatal seizures: controversies and challenges in translating new therapies from the lab to the isolette. Eur J Neurosci. 2001;35:1857–1865. doi: 10.1111/j.1460-9568.2012.08140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy RR. Summary proceedings from the neurology group on neonatal seizures. Pediatrics. 2006;117:S23–S27. doi: 10.1542/peds.2005-0620D. [DOI] [PubMed] [Google Scholar]
- Ferriero DM. Neonatal brain injury. N Engl J Med. 2004;351:1985–1995. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- Glass HC. Neonatal seizures: advances in mechanisms and management. Clin Perinatol. 2014;41:177–190. doi: 10.1016/j.clp.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes GL. The long-term effects of neonatal seizures. Clin Perinatol. 2009;36:901–914. doi: 10.1016/j.clp.2009.07.012. [DOI] [PubMed] [Google Scholar]
- Parfenova H, Leffler CW, Tcheranova D, Basuroy S, Zimmermann A. Epileptic seizures increase circulating endothelial cells in peripheral blood as early indicators of cerebral vascular damage. Am J Physiol Heart Circ Physiol. 2010;298:H1687–H1698. doi: 10.1152/ajpheart.00032.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfenova H, Fedinec A, Leffler CW. Ionotropic glutamate receptors in cerebral microvascular endothelium are functionally linked to heme oxygenase. J Cereb Blood Flow Metab. 2002;23:190–197. doi: 10.1097/01.WCB.000004823561824.C4. [DOI] [PubMed] [Google Scholar]