Abstract
Lactate acts as a ‘buffer' between glycolysis and oxidative metabolism. In addition to being exchanged as a fuel by the monocarboxylate transporters (MCTs) between cells and tissues with different glycolytic and oxidative rates, lactate may be a ‘volume transmitter' of brain signals. According to some, lactate is a preferred fuel for brain metabolism. Immediately after brain activation, the rate of glycolysis exceeds oxidation, leading to net production of lactate. At physical rest, there is a net efflux of lactate from the brain into the blood stream. But when blood lactate levels rise, such as in physical exercise, there is net influx of lactate from blood to brain, where the lactate is used for energy production and myelin formation. Lactate binds to the lactate receptor GPR81 aka hydroxycarboxylic acid receptor (HCAR1) on brain cells and cerebral blood vessels, and regulates the levels of cAMP. The localization and function of HCAR1 and the three MCTs (MCT1, MCT2, and MCT4) expressed in brain constitute the focus of this review. They are possible targets for new therapeutic drugs and interventions. The author proposes that lactate actions in the brain through MCTs and the lactate receptor underlie part of the favorable effects on the brain resulting from physical exercise.
Keywords: blood–brain barrier, energy metabolism, exercise, lactate, receptors
Introduction
L-lactate, pyruvate, and ketone bodies like β-hydroxybutyrate and acetoacetate are monocarboxylates transported across different brain cell membranes by monocarboxylate transporters (MCTs).1, 2 L-lactate is the MCT substrate that is most abundant in the brain, and fluctuates the most in concentration. As the MCTs mediate facilitative transport, they serve to equilibrate substrate concentrations across cell membranes, the concentration gradients of substrate and cotransported ion being the driving force. This means that substrates such as L-lactate migrate from sites of production toward sites of consumption, be it between cells within an organ (e.g., between glia and neurons in the brain or between glycolytic and oxidative fibers in skeletal muscle), or among different organs (e.g., skeletal muscle, heart, and brain) via the blood stream. The equilibrating action of MCTs also provides the basis for lactate acting as a volume transmitter that can mediate metabolic signals through the nervous tissue.3 The latter concept was underpinned by the demonstration that lactate can bind to the lactate receptor GPR81 (hydroxycarboxylic acid receptor, HCAR1), on brain cells and cerebral blood vessels, resulting in inhibition of adenylyl cyclase.4 The localization and function of HCAR1 and the three MCTs (MCT1, MCT2, and MCT4) expressed in brain will be the focus of this review (Figure 1).
Figure 1.
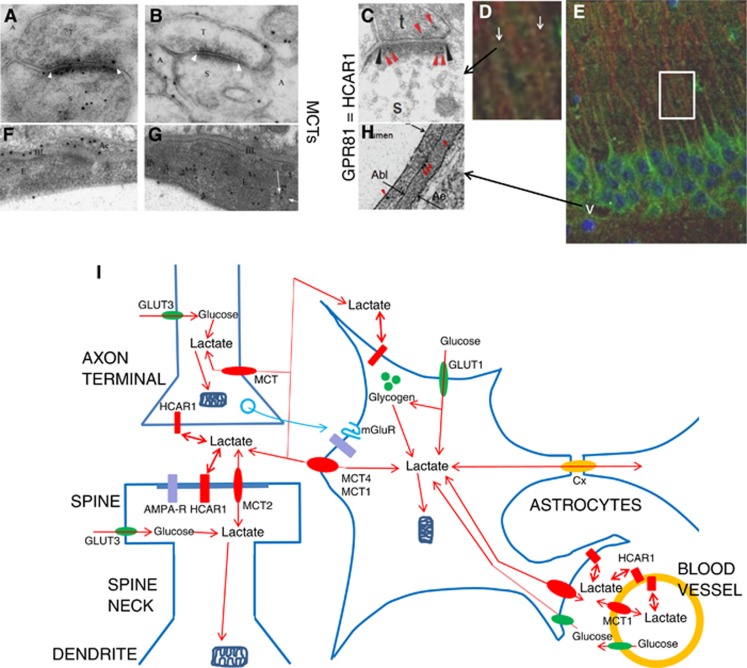
Lactate transport and signaling in the brain. (A) Electron micrograph showing double immunogold labeling at synapses between parallel fiber terminals (T) and Purkinje cell spines (S). The section was double-labeled with antibodies to GluR2/3 (15 nm gold particles) and to MCT2 (10 nm gold particles). Note colocalization of MCT2 with GluR2/3 at the synaptic membrane and intracellularly in the spine. Cerebellar cortex, molecular layer. (B) The membranes of the Bergmann glia (A) facing the parallel fiber terminals (T) and Purkinje cell spines (S) are labeled with antibody to MCT4 (10 nm gold particles). (C) The lactate receptor (10 nm gold, red arrowheads) is at the postsynaptic membrane (black arrowheads). Synapse of a nerve terminal (t) on a dendritic spine (s). Stratum radiatum, hippocampus CA1. (D, E) The lactate receptor GPR81/HCAR1 (green) is in the pyramidal cell somatodendritic compartment including spines (white arrows in D), and in microvascular endothelium (white v in E). (D) Magnification of area framed in (E) is shown. Hippocampus CA1. Neurons labeled for microtubule-associated protein 2 (MAP2) (red), nuclei with DAPI (blue). (F) The endfeet (Ae) of the Bergmann glia facing the endothelium (E) strongly express MCT4 (10 nm gold particles). (G) MCT1 (10 nm gold particles) is in the endothelium facing the lumen of the capillary, including in itracellular vesicular organelles (partly indicated by white arrows). (H) Electron micrograph showing GPR81 immunogold particles (red arrowheads) at luminal (short arrow) and abluminal (Abl) membrane of the vascular endothelium and at the perivascular astrocytic end-foot membrane (Ae) at a hippocampal blood vessel. (I) Schematic representation of L-lactate production and action at the ‘tripartite synapse' of axon, dendrite, and astrocyte, and at the glio-vascular junction (see text). Lactate, formed by glycolysis in brain cells or entering from blood, migrates down concentration gradients (of lactate and cotransported proton) between intracellular and extracellular compartments of neurons, astrocytes, and endothelial cells (orange circle), catalyzed by monocarboxylate transporters (MCT1, MCT2, and MCT4, red ovals). Lactate also migrates along the extracellular space, as well as throughout the astrocytic syncytial network via connexin gap junctions (Cx, orange oval). For simplification, pyruvate and other intermediates are not shown. HCAR1 (red rectangles), lactate receptors, the lactate binding site facing the extracellular space. GLUT1 and GLUT3 (green ovals), glucose transporters, providing glucose for brain cells. Positions of ionotropic glutamate receptors (AMPA-R, purple rectangles) and metabotropic glutamate receptors (blue serpents), influencing and influenced by lactate, are indicated. Mitochondria (dark-blue symbols) shun dendritic spines and thin astroglial processes. Astroglial processes contain glycogen particles (green spheres). (A, B, F, G modified from Bergersen1 and C, D, E, H modified from/based on Lauritzen.4)
Lactate acts as a ‘buffer' between glycolysis and oxidative metabolism. In this process, it is exchanged as a fuel between cells and tissues with different glycolytic and oxidative rates.5 According to some, lactate is a preferred fuel for brain metabolism.6 At rest, there is a net efflux of lactate from the brain into the blood stream. But when blood lactate levels rise, such as in physical exercise, there is an influx of lactate from blood to brain.7, 8 Glucose is the principal energy source of the adult brain.9 The cells can either store glucose, in the form of glycogen, or break it down to pyruvate in glycolysis. The rapid formation of adenosine triphosphate (ATP) through glycolysis may be particularly important after neuronal activation and may explain the immediate increase in glucose uptake with oxygen uptake lagging behind.10, 11 However, there is an instantaneous decrease in lactate immediately after neuronal activation,12 followed after a few seconds by a rise, indicating that the cells have a latent capacity for lactate oxidation that is rapidly exhausted. The full chemical energy in glucose is released as ATP by metabolism of pyruvate in the mitochondria through the tricarboxylic acid cycle and oxidative phosphorylation. When the rate of glycolysis exceeds that of oxidative phosphorylation, the cells convert pyruvate into lactate via lactate dehydrogenase. The lactate can exit form the cells via MCTs and be metabolized in other cells that also carry MCTs. The cotransport of protons and lactate by MCTs impacts on pH regulation, but is in line with the fact that lactic acid rather than lactate anion is the form produced in glycolysis and consumed in oxidative metabolism and anabolic reactions.13
Monocarboxylate transporters
The MCTs,2 belong to the solute carrier 16 (SLC16) gene family according to the Human Genome Organization (HUGO) nomenclature (www.genenames.org). They are classified as the monocarboxylate porter family (2.A.1.13) according to the Transporter Classification system for functional and phylogenetic classification of membrane transport proteins (http://www.tcdb.org/), approved by the International Union of Biochemistry and Molecular Biology. The MCTs are predicted to have 12 transmembrane domains with the N- and C-termini located intracellularly. Mutagenesis, chimera studies, and molecular modeling have clarified MCT structure and transport mechanism.14 Results indicate an uptake cycle in which proton binds before the lactate anion, followed by a domain rearrangement during which lactate and proton pass through the channel between the extracellular and intracellular binding site to get released to the cytosol, lactate first and then proton. A lysine residue (K38), which is thought to bind lactate after getting protonated, at the extracellular substrate binding site and an ion pair formed by aspartate (D302) and arginine (R306) at the intracellular substrate binding site are essential for transport and are not conserved in MCT family members that do not cotransport lactate and proton. The transport cycle can be repeated 12 times per second (MCT1), the rate limiting step being the reversal of the domain rearrangement to return to the extracellularly open conformation. The exchange of intracellular with extracellular monocarboxylate is faster than net transport of monocarboxylate with proton. The most widely used MCT inhibitor has been α-cyano-4-hydroxycinnamate, but this is not specific for MCTs. A novel potent inhibitor (Ki 2 nmol/L for MCT1), AR-C155858 developed by AstraZeneca (Stockholm, Sweden), blocks transport by MCT1 and MCT2, but not by MCT4.15 This inhibitor appears to bind from the cytosolic side interacting with the C-terminal part of the protein.
Among the 14 MCT isoforms that have been identified, only MCT1 to MCT4 have been shown to catalyze a proton coupled transport of lactate and other monocarboxylates, including pyruvate and ketone bodies.16 MCT1 (2.A.1.13.1, SLC16A1), MCT2 (2.A.1.13.5, SLC16A7), and MCT4 (2.A.1.13.6, SLC16A3) are widely expressed in the brain (see below), whereas MCT3 (2.A.1.13.9, SLC16A8) in the central nervous system is restricted to the basolateral membrane of cells in the retinal pigment epithelium and choroid plexus.17 Phylogenetically, as inferred from the sequence, MCT1 to MCT4 are more closely related to each other than to other SLC16 members; MCT2 is closer to MCT1 than to MCT4 and MCT3,2 which correlates with their substrate affinities.
The membrane localization and catalytic function of the transporters depend on ancillary proteins that are members of the immunoglobulin superfamily engaged in cell recognition, and, in contrast to the MCTs, are heavily glycosylated: basigin (CD147, extracellular matrix metalloproteinase inducer, 8.A.23.1.1) forms a complex with MCT1, MCT3, or MCT4, and embigin (gp70, 8.A.23.1.2) interacts with MCT2.14 The preference for ancillary protein is not absolute and in rat erythrocytes embigin interacts with MCT1. Interestingly, in human erythrocytes, where basigin is the Ok blood group antigen (and MCT1 is the lactate transporter), basigin is also the receptor for a malaria parasite surface protein and is required for invasion of the erythrocytes by Plasmodium falciparum.18 Neuroplastins, other members of the immunoglobulin superfamily adhesion molecules, which regulate the surface expression of glutamate and GABA receptor subunits, have recently been shown to be required for the surface expression of MCT2 and the associated lactate transport,19 linking MCT2 with synaptic function (see below under Neurons). Sorting signals in the C-terminal tail direct MCT3 and MCT4 to the basolateral membrane of retinal pigment epithelium cells, whereas MCT1, which lacks such basolateral sorting signal,20 requires interaction with basigin for targeting to the apical membrane.21
The MCT isoforms have different affinities for lactate and other substrates, partly correlating with their tissue distributions: high-affinity isoforms (MCT1 and MCT2) are expressed in cells that mainly import lactate, the low-affinity isoform (MCT4) in lactate-exporting cells. Consistent with this, MCT4 has a very low affinity for pyruvate, which saves pyruvate for conversion to lactate by lactate dehydrogenase concomitantly converting NADH into NAD+, which serves to maintain glycolysis. The rate of transport in either direction depends on the prevailing gradients of substrate and pH, such that net transport is the difference between efflux and influx. At equilibrium, [lactate-]i/[lactate-]o = [H+]o/[H+]i where brackets denote concentration and subscripts i and o denote intracellular and extracellular, respectively.
While MCT mediated net transport always runs in the direction of the combined concentration gradients of monocarboxylate and proton, intracellular lactate is generally higher than extracellular,22 meaning that a high affinity will favor the capture of extracellular substrate at low concentration, and a low affinity will avoid saturation by intracellular substrate at high concentration.
Whereas a high affinity indicates that the transport will be saturated and independent of the lactate concentration, the surface expression of the transporter is subject to regulation by trafficking between the plasma membrane and intracellular vesicular organelles. Electron microscopy shows such organelles to contain MCT2 in synaptic spines and MCT1 in vascular endothelium23, 24 and in cardiac muscle.25 It has been suggested that signal sequences in the C-terminus may be involved in such translocation targeting.2
Regulation of MCT expression in relation to activity was shown by neuromuscular trans-reinnervation experiments,26 regulation in response to pathologic conditions was shown in cardiac ischemia and heart failure models.25 The MCT protein contents are regulated at the transcriptional level and even more at the translational level.2 The 3′-untranslated region, which is particularly long in MCT1, may be important for translational regulation. Hypoxia causes strong upregulation of MCT4, which is consistent with the presence of potential binding sites for hypoxia inducible factor 1α in the MCT4 promoter but not in the MCT1 and MCT2 promoters.2 Upregulation of the high-capacity, low-affinity MCT4 by hypoxia, recently showed to be mediated transcriptionally by hypoxia inducible factor 1α in cultured astrocytes,27 would meet the needs of cells to export excess lactate formed in anaerobic glycolysis. Astrocytic expression of MCT4, along with glucose transporter 1 and glycolytic lactate production, is enhanced by hypoxia inducible factor 1α produced in response to nitric oxide from cocultured vascular endothelial cells.28 In a neural-derived cell line, MCT1 as well as MCT4 were found to be upregulated in hypoxia, whereas MCT2 was downregulated.29 As MCT2 has a particularly high affinity for pyruvate, downregulation of MCT2 would limit the loss of pyruvate from the cell, saving it for conversion to lactate to regenerate NAD+ from NADH. AMP-activated protein kinase, which guards against deficient ATP regeneration by upregulating catabolic pathways including glycolysis,30 likely contributes to the upregulation of MCTs in response to hypoxia and increased activity-dependent energy demand in the brain, such as observed for MCT1 and MCT4 in heart and skeletal muscle on chronic stimulation or exercise.31 The transcriptional coactivator PGC-1α (peroxisome proliferator-activated receptor-γ (PPARγ) coactivator-1α), a master regulator of reactive oxygen species scavenging enzymes and of mitochondrial biogenesis in brain,32 may also participate. Thus, PGC-1α33 is known to upregulate MCTs in other tissues, and so does PPARα,34 which mediates adaptive responses to energy restriction and induces the formation of ketone bodies. Food deprivation has been shown to upregulate MCT2, but not MCT1, in a brain stem nucleus35 (the area postrema–solitary tract nucleus).
Brain-derived neurotrophic factor (BDNF) upregulates MCT2, but not MCT1 or MCT4, through several signaling mechanisms involved in synaptic plasticity, including mitogen-activated protein kinase, and mammalian target of rapamycin, in cultured mouse cortical neurons36 and in hippocampus in vivo.37 Noradrenalin38 as well as insulin and insulin-like growth factor39 also cause MCT2 upregulation through mitogen-activated protein kinase and mammalian target of rapamycin. The upregulation of MCT2 by these mechanisms is in line with its enrichment at glutamatergic synapses and its involvement in neuronal activity, synaptic plasticity, and memory formation (see below under Neurons).
In the brain, the low-affinity and high-capacity transporter MCT4 is on astrocytes (Figures 1B, 1F, and 1I),23, 40 cells that are glycolytic and can supply lactate to other brain cells. Due to the high Km (about 30 mmol/L), MCT4 will usually not be saturated, and the transport rate will therefore increase with the lactate concentration. MCT2 is found on the cell bodies, dendrites, dendritic spines (Figures 1A and 1I), and axons of neurons,23, 24, 41 cells that are highly oxidative and are likely to mainly import lactate.42, 43, 44 The Km of less than 1 mmol/L45 suggests that MCT2 will be saturated with lactate in most conditions, and that efflux as well as uptake rates will be largely independent of concentration. MCT1 is mainly detected on endothelial cell of the blood–brain barrier (BBB) (Figures 1G and 1I), both in rodents23, 46, 47, 48 and in humans.49, 50 By being expressed on cerebral microvessels, MCT1 is part of the physiologic barrier that controls the passage of these molecules between the blood and the brain, for example, during exercise when blood lactate is high.51 MCT1 has an intermediate-to-high affinity for lactate (about 4 mmol/L).45 However, Lee et al52 found no MCT1 in endothelial cells. Still more controversial is the presence of MCT1 on mitochondria5 which is challenged by others and is difficult to reconcile with the generally large difference in NADH/NAD+ ratios between the cytosol and the mitochondria.2 Recently, MCT1 was found on oligodendrocytes.53 This isoform is expressed even on the myelin sheath.52, 53 MCT3 has an intermediate affinity (Km 6 mmol/L)54 and is predominantly expressed in retinal epithelial cells and in the choroid plexus epithelial cells (which are developmentally homologous to the latter), but not in the ependymal cells lining the cerebral ventricles.17
The high-affinity transporter MCT2 is abundantly detected on neurons and astrocytes by immunohistochemical methods,55 but shows substantial species differences in distribution. In rat brain, MCT2 immunoreactivity has primarily been found on perivascular endfeet8, 9, 56 and postsynaptic densities.23, 24 MCT4 is believed to be mainly localized on astrocytes, both on plasma membranes of perisynaptic processes and on perivascular endfeet.23, 40
In situ hybridization pictures (http://mouse.brain-map.org/experiment/show?id=72001956, The Allen Institute for Brain Science) are consistent with the immunocytochemical observations. MCT2 (Slc16a7) pictures indicate a general distribution of mRNA in neurons of the mouse brain, including in pyramidal cells in the hippocampal and cerebral cortex, as well as in blood vessels. MCT4 (Slc16a3) pictures (http://mouse.brainmap.org/experiment/show?id=69838404) show a less clear signal, compatible with localization in the astrocytic network. MCT1 (Slc16a1) pictures indicate vascular localization (http://mouse.brain-map.org/experiment/show?id=68203467).
Lactate receptor GPR81/HCAR1
In addition to its role as a metabolite and energy substrate, lactate is a signaling substance. It may signal in several ways, thus lactate modifies prostaglandin action contributing to vasomotor regulation,57 and influences the NADH/NAD+ ratio contributing to redox regulation.5 Lactate signaling through a specific receptor protein, HCAR1, was shown in the brain only in 2013,4 and the distribution of the protein (Figures 1C, 1D, 1E, 1H, and 1I) was delineated the year before.58 In a review, published in July 2013,12 the possibility that HCAR1 mediates lactate action in brain was mentioned as an outstanding question—‘Functional demonstration of HCAR1 in brain may reveal a whole new dimension to metabolic signaling in this organ'. Subsequently, HCAR1 was shown to modify electrical activity in primary neuronal cultures.59 More recently, action of lactate through a yet unidentified receptor was reported in locus coeruleus60 (without reference to the previous work). Lactate is the endogenous ligand for the HCAR161, 62 previously classified as an orphan receptor. Lactate activates HCAR1 within the physiologic concentration range (1 to 20 mmol/L) of lactate. Computational modeling and mutation analysis have shown that Arg71, Arg99, Glu166, and Arg240 of the human HCAR1 are important sites for lactate interaction.63
GPR81, a G-protein-coupled orphan receptor was previously discovered to be selectively activated by lactate, downregulating cAMP through Gi action to inhibit lipolysis in adipocytes.61, 62, 64 GPR81 is therefore subsequently named hydroxycarboxylic acid receptor 1 (HCA1, IUPHAR nomenclature; HCAR1, HUGO nomenclature), and has cogeners, HCAR2 and HCAR3, that respond to other metabolites than lactate.65, 66 The HCARs are categorized as the G-protein coupled receptor family, Class A (rhodopsin-type), 7: Hydroxy-carboxylic acid receptors (http://www.genenames.org/genefamilies/GPCR). The HCAR genes are all located on the short arm of human chromosome 12 (12q24.31), where also the gene of the neuronal MCT (MCT2) is located (12q.13).
In adipose tissue, where HCAR1 mRNA is expressed at levels that by far exceed those in other tissues, the lactate receptor appears to serve a special role, as it does not respond primarily to increases in ambient lactate supplied via the circulation, but rather to locally high-lactate concentrations released from adipocytes as an autocrine produced in response to insulin-dependent uptake of glucose.64 Could GRP81 signaling occur in the brain?
Lactate in the physiologic concentration range was shown to downregulate forskolin-stimulated cAMP levels in hippocampal slices.4 3,5-Dihydroxy benzoic acid (3,5-DHBA), which stimulates HCAR1 but not the cogeners HCAR2 and HCAR367 reproduced the effects of lactate, with concentration dependency similar to that in adipocytes. The results indicate that the lactate receptor is present and active in brain at levels high enough to regulate cAMP-dependent functions. Immunofluorescence showed that HCAR1 is highly concentrated in the principal neurons, such as hippocampal pyramidal cells and Purkinje cells, as well as in interneurons in multiple brain regions including the hippocampus and the cerebellum. In the principal cells, it is concentrated in the somatodendritic compartment, particularly in the spines. Astroglia and vascular endothelium showed some labeling. Electron microscopic immunogold quantification showed the highest immunoreactivity at the postsynaptic membrane of excitatory type synapses on dendritic spines of pyramidal cells, but also a signal in vascular endothelial cells, i.e., at the BBB, and in perivascular and perisynaptic processes of astrocytes.4
The action of lactate described in locus coeruleus60 increases rather than suppresses cAMP levels and in contrast to HCAR1 is selective for L-lactate versus D-lactate. The authors therefore dismissed the option that the effects were mediated by HCAR1 and proposed that a yet unidentified receptor is involved. However, G-protein-coupled receptors are known to signal through varying coupling mechanisms showing bias for different mechanisms among different ligands,68 which implies the possibility that HCAR1 could cause the observed effects.
While rtPCR indicated HCAR1 mRNA to be an order of magnitude less abundant in hippocampus than in adipose tissue (per protein content),4 in situ hybridization has shown a distribution of HCAR1 mRNA throughout the brain, including in principal neurons of the cerebral, hippocampal, and cerebellar cortices (The Allen Institute for Brain Science, http://www.brain-map.org; GENSTAT, http://www.ncbi.nlm.nih.gov/gensat; St Jude Children's Research Hospital, http://www.stjudebgem.org). These observations indicate functions of the lactate receptor in widespread regions of the central nervous system, mediating effects of lactate that may serve to link synaptic function, energy metabolism, and cerebral blood flow.
Neurons
The main MCT expressed in neurons is MCT2 (Figures 1A and 1I).1 Early work reported colocalization of MCT2 with microtubule associated protein 2.69 Notably, MCT2 is concentrated at the postsynaptic membranes of glutamatergic synapses on spines of Purkinje cells in cerebellum23 and pyramidal cells in the hippocampus.24 The density is lower at mossy fiber synapses, which have lower prevalent firing rate, compared with Schaffer-collateral synapses. The lack of MCT2 at inhibitory type synapses on pyramidal cell somata suggests a connection to glutamatergic neurotransmission. In agreement with this notion, quantitative immunogold double-labeling (Figure 1A) showed that MCT2 is colocalized with subunits GluR2/3 (GluA2/3) of the AMPA type glutamate receptor.24 This receptor is known to undergo trafficking between the plasma membrane and intracellular vesicular stores in the spines as part of activity-dependent synaptic plasticity.70
HCAR1, like MCT2, shows the highest concentration in neurons, including pyramidal cells (Figures 1D, 1E, and 1I). HCAR14, 58 as well as MCT224 show a dual localization on the synaptic membrane (Figures 1C and 1I) and intravesicular organelles, probably reflecting protein trafficking. The presence of the lactate receptor along with a lactate transporter on intracellular vesicular organelles brings up the interesting possibility that intravesicular lactate could contribute to lactate signaling, which would be a novel principle. In any case, the main finding of the two proteins at postsynaptic membranes of glutamatergic synapses, together with glutamate receptors, suggests an intimate relation of lactate to glutamatergic neurotransmission.
Such a relation has previously been advanced on purely metabolic grounds as part of the astrocyte-to-neuron lactate shuttle (ANLS) hypothesis, according to which supply of lactate from astrocytes, driven by glycolysis due to astrocytic uptake of transmitter glutamate released from neurons,43 is essential for neuronal functions such as memory formation.71 Although the ANLS is disputed,22 the role of lactate in synaptic plasticity is consistent with the observation that MCT2 expression is enhanced, together with postsynaptic density 95 protein and glutamate receptor subunit 2 (GluR2) protein, by BDNF,37 a central mediator of synaptic plasticity. However, the direction of flux through this MCT2 is not known. Lactate, whether from blood or produced in astrocytes, may enter dendritic spines through the MCT2 in the postsynaptic membrane to assist aerobic energy production. This would be in line with the notion that postsynaptic ion currents consume the major part of the energy production in the brain.72 However, while mitochondria are essential for spine development and function, the synaptic spines themselves rarely have mitochondria73 meaning that the lactate would have to diffuse into the dendrite branch before being oxidized to yield ATP. In line with this, the initial response to neuronal activation appears to be glycolysis for fast ATP production,11 rather than glucose oxidation. An obvious reason for this is that cytosolic glycolysis takes place close to the sites of ATP consumption while mitochondria are at a distance. In agreement, there is evidence that the glycolytic complex of enzymes is associated with plasma membranes as well as with endoplasmic reticulum membranes, and that regulation and function of ion channels and transporters, including the ATPases that pump K+, Na+ and Ca2+, depend on ATP generated by glycolysis.13 Lactate produced in the spine may either diffuse to reach mitochondria in the dendrite or exit the spine through the MCT2 of the synaptic membrane for further metabolism elsewhere, including reentering the neuron later, when the intraneuronal lactate concentration decreases. On activation of AMPA receptors by climbing fiber stimulation, cerebellar Purkinje cells produce lactate from glucose in the absence of glycogenolysis; blocking AMPA receptors by CNQX prevented the rises in extracellular lactate, postsynaptic currents, blood flow, and glucose and oxygen consumption.74 These observations indicate that the extracellular lactate produced on neuronal stimulation exits rather than enters the neurons (Figure 1I). A similar conclusion was arrived at based on computer modulations.75
The lactate leaving neurons immediately after activation may be dispersed in the extracellular space or through the astrocytic, gap-junction connected syncytial network to be flushed out into cerebrospinal fluid, blood, or lymph where the lactate concentration is low at physical rest. It may also be taken up and oxidized in neighboring neurons. The total contribution of the ANLS may account for some 10% of total glucose uptake and oxidation in brain.76 An interesting thought is that mobilization of astrocyte glycogen during brain activation may reduce utilization of blood-borne glucose by astrocytes because of hexokinase inhibition, increasing glucose availability for activated neurons.77 Regardless of the quantitative energetic importance of the ANLS, the lactate generated on neuronal activation could exert functions, perhaps partly through HCAR1, that may contribute to observations by the Magistretti group.71, 78
An additional rationale for having an MCT at the spine membrane would be to limit acidification, which is known to adversely affect glutamate receptors. Thus, NMDA receptors and kainate receptors are sensitive to pH.79, 80 In addition, intracellular acidification may regulate excitability through modifying chloride channels, as observed in skeletal muscle.81 We suggest that lactate produced through activity initiated glycolysis in the spine exits the spine through the synaptic MCT2 to activate the colocalized lactate receptor, acting as an autocrine to confine the rate of spine glycolysis.
Astrocytes
The low-affinity transporter MCT4 appears to be the main MCT in astrocytes (Figures 1B, 1F, and 1I),23, 40 at least in rodents. As low affinity is considered suitable for efflux rather than influx (see above), the expression of MCT4 in astrocytes is in line with the notion of the astrocyte as mainly glycolytic and the fact that it is the only brain cell containing appreciable quantities of glycogen.73 MCT1 was initially reported in cultured astrocytes45 and MCT250 has also been reported to be expressed to some extent in astrocytes. The discrepancies in localization may result from differences among species and in experimental conditions. MCT4 appears to be restricted to astrocytes throughout development and reaches adult levels 14 days postnatally,40 i.e., proceeding the time of synaptogenesis.82 MCT4 is primarily located on the plasma membrane, on perisynaptic as well as perivascular processes of the astrocyte, which means that the astrocyte forms a direct channel for lactate between the blood vessels and the computing machinery of the brain. As expected, because the total area of the perisynaptic membranes is larger than that of the juxtavascular area, the density of MCT4 is considerably higher on the juxtavascular face of the perivascular endfeet compared with other parts of the astrocyte.
HCAR1 is relatively sparsely expressed in astrocytes in the brain, but occurs at astrocytic endfeet (Figures 1H and 1I).4
Oligodendrocytes
Lactate is taken up and used by developing oligodendrocytes in primary culture83 and organotypic brain slices.53 During development, the need for substrates to support lipid synthesis is highly increased: at the peak of myelination, oligodendrocytes have been estimated to synthesize an amount of lipids equal to three times their cell body weight daily.84 The acetylation of histones, which is important for oligodendrocyte differentiation and for myelination, also requires carbon substrates.85 The myelinating oligodendrocytes and their precursor cells are particularly vulnerable to energy deprivation, compared with neurons and other brain cells.86 Energy deprivation leads to a reversal of glutamate transporters, which increases the extracellular glutamate concentration. Energy deprivation also triggers activation of AMPA/kainate receptors and NMDA receptors expressed on oligodendrocyte lineage cells causing damage through increased intracellular Ca2+.87, 88, 89, 90 Glutamate receptors are also expressed in axons,91, 92 which are damaged by the same mechanisms. Lack of energy inhibits myelination by limiting the accessibility of carbon substrates for the synthesis of myelin lipids.53 In this way, oligodendrocytes require alternative substrates such as lactate for survival and to satisfy their biosynthetic demand. Similarly, organotypic brain slices cultured in low-glucose media showed loss of oligodendrocytes and lack of myelination, which could be avoided by supplying lactate.53, 93 Besides lactate, ketone bodies are present at high concentrations in the blood at the time of myelination in humans and rats94 and are important for myelination84 and hence for sustaining long axons.
After myelination has taken place, the use of lactate by oligodendrocytes apparently changes. In adult mice, the myelin sheath was found to release lactate that was used by axons.52, 93, 95 This is in line with the notion that long axons are supported by lactate from glycolysis in oligodendrocytes.96 The results suggest that oligodendrocytes may depend on import of lactate for ATP production and lipid synthesis during development, followed by a switch to other metabolic pathways and lactate export after myelination comes to a steady state. Support for this developmental switch was obtained using mutant mice in which oxidative phosphorylation was disabled in the oligodendrocytes. The findings suggested that mature myelinating oligodendrocytes do not need oxidative phosphorylation and can rely solely on glycolysis for ATP production. Knockdown of MCT1 expression specifically in oligodendrocytes established that lactate released from the oligodendroglial compartment is crucial for axon health.52 The MCT1 knockdown led to abnormal axon morphology and neuronal death in organotypic cultures that could be rescued by supplying lactate.52 MCT1 knockdown caused pathology similar to that seen in patients with amyotrophic lateral sclerosis (ALS). In addition, ALS patients and SOD1 mutant mice (a mouse model for ALS) show reduced levels of MCT1 in oligodendrocytes. Failure in the supply of lactate from oligodendrocytes (or disruptions upstream of this) could therefore be involved in the pathogenesis of ALS and other neurodegenerative diseases.
Roles of lactate in oligodendrocytes and myelinated axons have been reviewed recently.93
The blood–brain barrier
MCT1 is the MCT expressed at the BBB, constituted by the endothelial cells of cerebral blood vessels (Figures 1G and 1I). This barrier represents the body–brain interface, the gateway to the brain. The transporter is localized at similar densities at the luminal and abluminal surfaces of the endothelium, as well as over cytoplasm, presumably on vesicular organelles.1, 23 This intracellular pool of MCT1 may constitute a reservoir for regulating the amount of transporter at the plasmalemma according to need.
A similar dual plasmalemmal and intracellular localization was observed for the lactate receptor HCAR1 in brain capillary endothelium, which showed receptor levels intermediate between those in neurons and in astrocytes (Figures 1H and 1I).4
Lactate—A volume transmitter
A ‘volume transmitter', as opposed to a ‘wiring transmitter' at synapses, reaches receptors distributed in a considerable volume surrounding the site of release.97 The existence of a lactate receptor in brain4 bolsters the role of lactate as a ‘volume transmitter', a role suggested in a perspectives article in 2012.3 The distribution of MCTs among brain cells (Figure 1) sets the scene for this volume transmitter action of lactate: through the equilibrating, facilitatory transport mediated by the MCTs, lactate will diffuse down its concentration gradient from sites of net production to reach lactate receptor sites via intracellular and extracellular compartments. The entry of lactate into brain tissue at the BBB to act on HCAR1 on brain cells is related to the volume transmission concept.
The activation of the lactate receptor to lower cAMP levels may serve to regulate multiple cellular processes, including curbing glycolysis and glycogenolysis to save energy and limit acidification when glucose breakdown is faster than oxidation through mitochondria. This may counteract damage in hypoxic conditions, but may even have a role in the first moments of cell activation, when glucose uptake and its conversion to lactate increase earlier than oxygen uptake.11, 75 As dealt with further below, another beneficial effect would be to limit the activity of cAMP activated potassium channels and thereby counteract excessive hyperpolarization. In addition, HCAR1 may have noncanonical activities, such as through β-arrestin. The downstream mechanisms and the physiologic importance of these and other effects of stimulating the lactate receptor remain to be explored.
Besides acting through the lactate receptor, we suggested3 that the volume transmitter action of lactate could take effect through modification of the redox state of cells. When lactate equilibrates over cellular membranes, the redox potential changes, and could act as a ‘redox switch mechanism'.98, 99 Numerous cellular processes are subjected to redox regulation, notably through modified gene expression by histone deacetylases.100 Interestingly, redox modulation of the NMDA receptor has recently been shown to mediate several of the effects of lactate on brain function.101
Targets for new therapeutic drugs and interventions
Neuroprotective Effects of Lactate in Brain Injury
Availability of lactate is neuroprotective in the reoxygenation phase after cerebral stroke or trauma.102 It reduces glutamate-induced neurotoxicity in brain cortex in vivo.103 Lactate administration increasing systemic blood lactate to 5 mmol/L has a beneficial effect in brain injury patients, with increased lactate, glucose, and pyruvate, and tendency toward reduced glutamate in the cerebral dialysate, and a reduced intracranial pressure.104 This indicates that lactate infusion serves to alleviate the persistent metabolic deficiency without ischemia common after traumatic brain injury.105 It will be interesting to investigate to what extent the receptor action of lactate contributes to these effects.
A just discovered action of lactate through HCAR1 is that it counteracts tissue damaging components of innate immunity by inflammasome blocking and can prevent death from pancreatitis and hepatitis in mice.106 This action goes through molecular interaction of HCAR1 with the intracellular adaptor protein arrestin β2 and is independent of cAMP. Several of the mediators involved (Toll-like receptor 4-mediated induction of Il1B, Nlrp3, and Casp1; activation of NFκB; release of IL1β; and cleavage of CASP1) also operate in brain and are implicated in brain pathology. It is now exceedingly important to determine whether and to what extent similar tissue protective actions of the lactate receptor occur in brain.
Can Lactate Rescue Cognitive Decline?
Excessive levels of cAMP may contribute to impairment in diverse and common conditions with cognitive decline such as normal aging107 and Alzheimer's disease.108 The multiple downstream effects include stimulation of ion channels that block neural network activity107 and stimulation of amyloid precursor protein expression and amyloidogenic processing.109 Lowering cAMP levels through stimulation of HCAR1 could counteract such effects.
In their elegant studies in aging monkeys, the Arnsten group107, 110, 111 showed that age-dependent working memory decline results from excessive levels of cAMP causing too high activity of the HCN (hyperpolarization-activated cyclic nucleotide-gated) potassium channel and ensuing functional disruption of dynamic networks of the prefrontal cortex that underlie working memory. The effect could be rescued by lowering cAMP levels through α2-adrenoceptor activation or other means. The HCN channels and α2-adrenoceptors are colocalized on dendritic spines (in layer III of the prefrontal cortex).110 The fact that also GRP81 and MCT2 are colocalized on dendritic spines (see above) underlines the possibility that lowering cAMP by lactate receptor stimulation could contribute to enhancing working memory. Similar mechanisms as for age-associated working memory loss operate in stress and schizophrenia.111 The necessity of fine tuning is illustrated by the typically bell-shaped activity-response curves of both dopamine and noradrenaline: input to the prefrontal cortex is blocked when either dopamine or noradrenaline is too high, but when either of them is too low, network dynamics become noisy and activity vanishes.112 Noradrenergic stimulation of α- and β-adrenoceptors at multiple cellular sites serves to optimize central nervous system performance.113 In a similar way as the α-adrenoceptors, the lactate receptor may prove to have a role in the fine tuning of this regulation through harnessing cAMP.
However, HCAR1, like other G-protein-coupled receptors, may activate networks of intracellular signaling pathways, which include also noncanonical, G-protein-independent signaling, such as through β-arrestin.68 HCAR1 activation may mediate neurotrophicactions, including through enhancing production and release of BDNF, possibly through noncanonical mechanisms. Lactate increases BDNF mRNA and protein in neuronal as well as in glial cells.114 The latter quoted paper did not clarify the molecular mechanisms by which lactate raised BDNF. Vascular endothelial growth factor is also involved in exercise effects on synaptic plasticity,115 augmenting memory not only through increasing/normalizing vascularization,116 but also, like BDNF, by effects on the neurons.117 It is intriguing that BDNF administration increases the expression of MCT2,36, 37 which is localized at the glutamatergic synapses on spines,24 i.e., in a position to gate lactate flux at HCAR1 sites (see above and Figure 1).
Neurotrophic effects may contribute to the plastic changes related to neuronal activity, including long-term potentiation and consolidation of memory, which involve BDNF action,118 partly through modulating BDNF processing.119, 120 Enhanced BDNF is therefore likely to rescue cognitive decline.
The spreading of lactate through the brain parenchyma, and the entry of blood-borne lactate, are dependent on the MCTs on the brain cell membranes and on the endothelium of the BBB. The MCTs are therefore possible drug targets.
Is Lactate An Endogenous Antiepileptic?
Mesial temporal lobe epilepsy (MTLE), a common form of epilepsy that includes most of the cases of medication resistant epilepsy, is characterized by sclerosis of the hippocampus including loss of hippocampal pyramidal cells.121 In MTLE, the levels of MCT1 and MCT2 are strongly downregulated at the endothelium and astocytic endfeet, respectively, at blood vessels in the area of hippocampal sclerosis, compared with the situation in temporal lobe epilepsy without hippocampal sclerosis. At the same time, MCT1 and MCT2 are upregulated on the perisynaptic astrocytic processes in the neuropil.50 Similar changes have been observed in specimens removed from patients at surgery for intractable MTLE50 and in animal models of the disease.50 A downregulation of MCT4 in human and experimental temporal lobe epilepsy was reported recently, but whether it affects the perisynaptic and perivascular astrocytic processes was not resolved.122
The presence and localization of the lactate receptor HCAR1 could help explain these observations on lactate and lactate transporters in MTLE. An intriguing possibility is that the MCT downregulation observed represents a compensatory mechanism to protect against seizures emanating from the sclerotic hippocampal tissue, presumably through pyramidal cells in tissue bordering on the sclerotic area. As lactate flux is from brain to blood at body rest,123 the lack of MCTs would be expected to lead to increased tissue levels of lactate, which agrees with the reports on extracellular lactate concentrations in MTLE, both during seizures and interictally.50 Through HCAR1, the elevated lactate would lower intracellular cAMP, which in turn would lower seizure proneness. Thus, α2-adrenoceptor agonists, reducing cAMP, inhibit seizure activity while α2-adrenoceptor antagonists make it worse.124 In contrast, β-adrenoceptor agonists, which increase cAMP levels, and activation of adenylyl cyclase, increase epileptiform activity in hippocampus while β-adrenoceptor antagonists have the opposite effect.124 On the same note, the MCT upregulation in perisynaptic astrocytic processes may serve to supply lactate from astroglia, a glycolytic cell, to the synaptic environment where the lactate receptor is concentrated, namely at the postsynaptic membranes of excitatory synapses on hippocampal pyramidal cells.4
If downregulation of MCT1 at the BBB serves to protect against seizures, then the MCT1 inhibitors might be beneficial in epilepsy. Such inhibitors are being developed to treat cancer by breaking the ‘symbiosis' between oxidative cells in the shell and glycolytic cells in the core of the tumor.125, 126 A challenge for drug development along these lines is to avoid penetration of the drug beyond the BBB, which would be expected to seriously affect brain metabolism.
Physical Exercise and ‘Life-Style' Interventions
Could lactate actions in the brain through MCTs and the lactate receptor underlie part of the favorable effects on the brain resulting from physical exercise? Physical exercise elevates lactate levels in blood and brain7, 123, 127 and could enhance BDNF through HCAR1 (see above). Physical exercise is a low-cost, low-risk intervention, which has multiple beneficial effects on the body and the brain, including on impairment in normal aging and age-related brain disease, such as cardiovascular and Alzheimer's disease,128 and several mental disorders.129 The fact that HCAR1 agonists, such as 3,5-DHBA, occur in fruits and berries67 indicates the possibility for nutritional intervention, in addition to opportunity for development of new drugs acting via the lactate receptor.
A massive body of literature implicates BDNF as a mediator of beneficial effects of physical exercise on learning and memory and problem solving,130 changes in cortical volume, neuroneogenesis, and synaptogenesis, and functional loss associated with Alzheimer's disease, other forms of dementia, Parkinson's disease, and depression.131, 132 Low BDNF in brain and in blood is associated with cognitive impairment and Alzheimer's disease.133 The major source as well as the major site of action of BDNF appears to be brain neurons, indicating that BDNF is a neuro-autocrine factor. Most brain neurons are thought to produce BDNF, and the brain releases BDNF to the blood, the release being increased by physical exercise,127, 134 including in elderly and Alzheimer's disease patients.135 However, increased brain BDNF does not necessarily lead to a measurable increase in the blood level of BDNF.136
The mechanism(s) by which physical exercise causes the level of BDNF in brain and blood to increase remain to be worked out, but there is reason to hypothesize that an action of lactate via HCAR1 contributes (see above). Lactate stimulates production of BDNF by astrocytes and neuronal cells.114 Many reports indicate that CREB (cAMP response element binding protein) is a ‘master regulator' of memory formation and long-term potentiation, partly through regulating BDNF.137 The CREB, in turn is regulated by PPARα, another transcription factor, known for regulating fatty acid catabolism in the liver and upregulated in muscle by exercise138, 139 but expressed also in hippocampal neurons.140 The PGC-1α, an important mediator of exercise-induced mitochondrial proliferation in skeletal muscle,141 is expressed also in neurons in the hippocampus, where it mediates exercise-induced elevation of BDNF through enhancing the expression of a membrane protein known as fibronectin type III domain-containing protein 5 and its cleavage product irisin, a 112 amino-acid peptide myokine.142 The cAMP-reducing effect of HCAR1 would be expected to reduce the CREB-dependent influence on memory and BDNF, but a noncanonical effect of HCAR1 would have the opposite effect on BDNF (see above) and might even regulate PPARα, PGC-1α, and irisin. Here, again a network of positive and negative effects may provide opportunity for fine tuning, as discussed above for opposing effects of catecholamines.
The potential actions of lactate receptors in the brain, and their interaction with lactate transporters, need to be explored. This is a just opened field of research that may lead to novel therapeutic strategies.
The authors declare no conflict of interest.
Footnotes
This study has been supported by grants from the University of Oslo, ‘Nasjonalforeningen for folkehelsen', and The Norwegian Research Council (including Unikard, a joint Research Council—Health Authority grant), Norway, and from the University of Copenhagen and the Lundbeck Foundation, Denmark.
References
- Bergersen LH. Is lactate food for neurons? Comparison of monocarboxylate transporter subtypes in brain and muscle. Neuroscience. 2007;145:11–19. doi: 10.1016/j.neuroscience.2006.11.062. [DOI] [PubMed] [Google Scholar]
- Halestrap AP. The SLC16 gene family—structure, role and regulation in health and disease. Mol Aspects Med. 2013;34:337–349. doi: 10.1016/j.mam.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Bergersen LH, Gjedde A. Is lactate a volume transmitter of metabolic states of the brain. Front Neuroenergetics. 2012;4:5. doi: 10.3389/fnene.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen KH, Morland C, Puchades M, Holm-Hansen S, Hagelin EM, Lauritzen F, et al. Lactate receptor sites link neurotransmission, neurovascular coupling, and brain energy metabolism. Cereb Cortex. 2013;24:2784–2795. doi: 10.1093/cercor/bht136. [DOI] [PubMed] [Google Scholar]
- Brooks GA. Cell-cell and intracellular lactate shuttles. J Physiol. 2009;587:5591–5600. doi: 10.1113/jphysiol.2009.178350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D, Pernet A, Hallett WA, Bingham E, Marsden PK, Amiel SA, et al. Lactate: a preferred fuel for human brain metabolism in vivo. J Cereb Blood Flow Metab. 2003;23:658–664. doi: 10.1097/01.WCB.0000063991.19746.11. [DOI] [PubMed] [Google Scholar]
- Rasmussen P, Nyberg N, Jaroszewski JW, Krogh-Madsen R, Secher NH, Quistorff B, et al. Brain nonoxidative carbohydrate consumption is not explained by export of an unknown carbon source: evaluation of the arterial and jugular venous metabolome. J Cereb Blood Flow Metab. 2010;30:1240–1246. doi: 10.1038/jcbfm.2010.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hall G. Lactate kinetics in human tissues at rest and during exercise. Acta Physiol (Oxf. 2010;199:499–508. doi: 10.1111/j.1748-1716.2010.02122.x. [DOI] [PubMed] [Google Scholar]
- Sokoloff L. The brain as a chemical machine. Prog Brain Res. 1992;94:19–33. doi: 10.1016/s0079-6123(08)61736-7. [DOI] [PubMed] [Google Scholar]
- Dalsgaard MK. Fuelling cerebral activity in exercising man. J Cereb Blood Flow Metab. 2006;26:731–750. doi: 10.1038/sj.jcbfm.9600256. [DOI] [PubMed] [Google Scholar]
- Dienel GA. Fueling and imaging brain activation. ASN Neuro. 2012;4 doi: 10.1042/AN20120021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros LF. Metabolic signaling by lactate in the brain. Trends Neurosci. 2013;36:396–404. doi: 10.1016/j.tins.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Dhar-Chowdhury P, Malester B, Rajacic P, Coetzee WA. The regulation of ion channels and transporters by glycolytically derived ATP. Cell Mol Life Sci. 2007;64:3069–3083. doi: 10.1007/s00018-007-7332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MC, Meredith D, Bunnun C, Sessions RB, Halestrap AP. Studies on the DIDS-binding site of monocarboxylate transporter 1 suggest a homology model of the open conformation and a plausible translocation cycle. J Biol Chem. 2009;284:20011–20021. doi: 10.1074/jbc.M109.014217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovens MJ, Davies AJ, Wilson MC, Murray CM, Halestrap AP. AR-C155858 is a potent inhibitor of monocarboxylate transporters MCT1 and MCT2 that binds to an intracellular site involving transmembrane helices 7-10. Biochem J. 2010;425:523–530. doi: 10.1042/BJ20091515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP, Price NT. The proton-linked monocarboxylate transporter (MCT family: structure, function and regulation. Biochem J. 1999;343:281–299. [PMC free article] [PubMed] [Google Scholar]
- Philp NJ, Yoon H, Grollman EF. Monocarboxylate transporter MCT1 is located in the apical membrane and MCT3 in the basal membrane of rat RPE. Am J Physiol. 1998;274:R1824–R1828. doi: 10.1152/ajpregu.1998.274.6.R1824. [DOI] [PubMed] [Google Scholar]
- Crosnier C, Bustamante LY, Bartholdson SJ, Bei AK, Theron M, Uchikawa M, et al. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature. 2011;480:534–537. doi: 10.1038/nature10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MC, Kraus M, Marzban H, Sarna JR, Wang Y, Hawkes R, et al. The neuroplastin adhesion molecules are accessory proteins that chaperone the monocarboxylate transporter MCT2 to the neuronal cell surface PLoSONE20138e78654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castorino JJ, Deborde S, Deora A, Schreiner R, Gallagher-Colombo SM, Rodriguez-Boulan E, et al. Basolateral sorting signals regulating tissue-specific polarity of heteromeric monocarboxylate transporters in epithelia. Traffic. 2011;12:483–498. doi: 10.1111/j.1600-0854.2010.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deora AA, Philp N, Hu J, Bok D, Rodriguez-Boulan E. Mechanisms regulating tissue-specific polarity of monocarboxylate transporters and their chaperone CD147 in kidney and retinal epithelia. Proc Natl Acad Sci USA. 2005;102:16245–16250. doi: 10.1073/pnas.0504419102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA. Brain lactate metabolism: the discoveries and the controversies. J Cereb Blood Flow Metab. 2012;32:1107–1138. doi: 10.1038/jcbfm.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergersen L, Waerhaug O, Helm J, Thomas M, Laake P, Davies AJ, et al. A novel postsynaptic density protein: the monocarboxylate transporter MCT2 is co-localized with delta-glutamate receptors in postsynaptic densities of parallel fiber-Purkinje cell synapses. Exp Brain Res. 2001;136:523–534. doi: 10.1007/s002210000600. [DOI] [PubMed] [Google Scholar]
- Bergersen LH, Magistretti PJ, Pellerin L. Selective postsynaptic co-localization of MCT2 with AMPA receptor GluR2/3 subunits at excitatory synapses exhibiting AMPA receptor trafficking. Cereb Cortex. 2005;15:361–370. doi: 10.1093/cercor/bhh138. [DOI] [PubMed] [Google Scholar]
- Jóhannsson E, Lunde PK, Heddle C, Sjaastad I, Thomas MJ, Bergersen L, et al. Upregulation of the cardiac monocarboxylate transporter MCT1 in a rat model of congestive heart failure. Circulation. 2001;104:729–734. doi: 10.1161/hc3201.092286. [DOI] [PubMed] [Google Scholar]
- Bergersen LH, Thomas M, Jóhannsson E, Waerhaug O, Halestrap A, Andersen K, et al. Cross-reinnervation changes the expression patterns of the monocarboxylate transporters 1 and 4: An experimental study in slow and fast rat skeletal muscle. Neuroscience. 2006;138:1105–1113. doi: 10.1016/j.neuroscience.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Rosafio K, Pellerin L. Oxygen tension controls the expression of the monocarboxylate transporter MCT4 in cultured mouse cortical astrocytes via ahypoxia-inducible factor-1α-mediated transcriptional regulation. Glia. 2014;62:477–490. doi: 10.1002/glia.22618. [DOI] [PubMed] [Google Scholar]
- Brix B, Mesters JR, Pellerin L, Jöhren O. Endothelial cell-derived nitric oxide enhances aerobic glycolysis in astrocytes via HIF-1α-mediated target gene activation. J Neurosci. 2012;32:9727–9735. doi: 10.1523/JNEUROSCI.0879-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Edin NF, Lauritzen KH, Aspmodal I, Christoffersen S, Jian L, et al. Alterations of monocarboxylate transporter densities during hypoxia in brain and breast tumour cells. Cell Oncol (Dordr. 2012;35:217–227. doi: 10.1007/s13402-012-0081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viollet B, Athea Y, Mounier R, Guigas B, Zarrinpashneh E, Horman S, et al. AMPK: Lessons from transgenic and knockout animals. Front Biosci (Landmark Ed. 2009;14:19–44. doi: 10.2741/3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto M, Takeyama M, Hamada T. Possible involvement of AMPK in acute exercise-induced expression of monocarboxylate transporters MCT1 and MCT4 mRNA in fast-twitch skeletal muscle. Metabolism. 2013;62:1633–1640. doi: 10.1016/j.metabol.2013.06.010. [DOI] [PubMed] [Google Scholar]
- Chen SD, Yang DI, Lin TK, Shaw FZ, Liou CW, Chuang YC, et al. Roles of oxidative stress, apoptosis, PGC-1α and mitochondrial biogenesis in cerebral ischemia. Int J Mol Sci. 2011;12:7199–7215. doi: 10.3390/ijms12107199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König B, Koch A, Giggel K, Dordschbal B, Eder K, Stangl GI, et al. Monocarboxylate transporter (MCT-1 is up-regulated by PPARalpha. Biochim Biophys Acta. 2008;1780:899–904. doi: 10.1016/j.bbagen.2008.03.002. [DOI] [PubMed] [Google Scholar]
- König B, Fischer S, Schlotte S, Wen G, Eder K, Stangl GI, et al. Monocarboxylate transporter 1 and CD147 are up-regulated by natural and synthetic peroxisome proliferator-activated receptor alpha agonists in livers of rodents and pigs. Mol Nutr Food Res. 2010;54:1248–1256. doi: 10.1002/mnfr.200900432. [DOI] [PubMed] [Google Scholar]
- Matsuyama S, Ohkura S, Iwata K, Uenoyama Y, Tsukamura H, Maeda K, et al. Food deprivation induces monocarboxylate transporter 2 expression in the brainstem of female rat. J Reprod Dev. 2009;55:256–261. doi: 10.1262/jrd.20214. [DOI] [PubMed] [Google Scholar]
- Robinet C, Pellerin L. Brain-derived neurotrophic factor enhances the expression of the monocarboxylate transporter 2 through translational activation in mouse cultured cortical neurons. J Cereb Blood Flow Metab. 2010;30:286–298. doi: 10.1038/jcbfm.2009.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinet C, Pellerin L. Brain-derived neurotrophic factor enhances the hippocampal expression of key postsynaptic proteins in vivo including the monocarboxylate transporter MCT2. Neuroscience. 2011;192:155–163. doi: 10.1016/j.neuroscience.2011.06.059. [DOI] [PubMed] [Google Scholar]
- Chenal J, Pellerin L. Noradrenaline enhances the expression of the neuronal monocarboxylate transporter MCT2 by translational activation via stimulation of PI3K/Akt and the mTOR/S6K pathway. J Neurochem. 2007;102:389–397. doi: 10.1111/j.1471-4159.2007.04495.x. [DOI] [PubMed] [Google Scholar]
- Chenal J, Pierre K, Pellerin L. Insulin and IGF-1 enhance the expression of the neuronal monocarboxylate transporter MCT2 by translational activation viastimulation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin pathway. Eur J Neurosci. 2008;27:53–65. doi: 10.1111/j.1460-9568.2007.05981.x. [DOI] [PubMed] [Google Scholar]
- Rafiki A, Boulland JL, Halestrap AP, Ottersen OP, Bergersen L. Highly differential expression of the monocarboxylate transporters MCT2 and MCT4 in the developing rat brain. Neuroscience. 2003;122:677–688. doi: 10.1016/j.neuroscience.2003.08.040. [DOI] [PubMed] [Google Scholar]
- Tekkok SB, Brown AM, Westenbroek R, Pellerin L, Ransom BR. Transfer of glycogen-derived lactate from astrocytes to axons via specific monocarboxylate transporters supports mouse optic nerve activity. J Neurosci Res. 2005;81:644–652. doi: 10.1002/jnr.20573. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Pellegri G, Bittar PG, Charnay Y, Bouras C, Martin JL, et al. Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle. Dev Neurosci. 1998;20:291–299. doi: 10.1159/000017324. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Sweet sixteen for ANLS. J Cereb Blood Flow Metab. 2012;32:1152–1166. doi: 10.1038/jcbfm.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AM, Tekkok SB, Ransom BR. Glycogen regulation and functional role in mouse white matter. J Physiol. 2003;549:501–512. doi: 10.1113/jphysiol.2003.042416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broer S, Broer A, Schneider HP, Stegen C, Halestrap AP, Deitmer JW, et al. Characterization of the high-affinity monocarboxylate transporter MCT2 in Xenopus laevis oocytes. Biochem J. 1999;341:529–535. doi: 10.1042/0264-6021:3410529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart DZ, Enerson BE, Zhdankina OY, Leino RL, Drewes LR. Expression of monocarboxylate transporter MCT1 by brain endothelium and glia in adult and suckling rats. Am J Physiol. 1997;273:E207–E213. doi: 10.1152/ajpendo.1997.273.1.E207. [DOI] [PubMed] [Google Scholar]
- Koehler-Stec EM, Simpson IA, Vannucci SJ, Landschulz KT, Landschulz WH. Monocarboxylate transporter expression in mouse brain. Am J Physiol. 1998;275:E516–E524. doi: 10.1152/ajpendo.1998.275.3.E516. [DOI] [PubMed] [Google Scholar]
- Leino RL, Gerhart DZ, Drewes LR. Monocarboxylate transporter (MCT1 abundance in brains of suckling and adult rats: a quantitative electron microscopic immunogold study. Brain Res Dev Brain Res. 1999;113:47–54. doi: 10.1016/s0165-3806(98)00188-6. [DOI] [PubMed] [Google Scholar]
- Froberg MK, Gerhart DZ, Enerson BE, Manivel C, Guzman-Paz M, Seacotte N, et al. Expression of monocarboxylate transporter MCT1 in normal and neoplastic human CNS tissues. NeuroReport. 2001;12:761–765. doi: 10.1097/00001756-200103260-00030. [DOI] [PubMed] [Google Scholar]
- Lauritzen F, Eid T, Bergersen LH.Monocarboxylate transporters in temporal lobe epilepsy: roles of lactate and ketogenic diet Brain Struct Funct 2013. doi: 10.1007/s00429-013-0672-xe-pub ahead of print. [DOI] [PubMed]
- Dalsgaard MK, Quistorff B, Danielsen ER, Selmer C, Vogelsang T, Secher NH, et al. A reduced cerebral metabolic ratio in exercise reflects metabolism and not accumulation of lactate within the human brain. J Physiol. 2004;554:571–578. doi: 10.1113/jphysiol.2003.055053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487:443–448. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinholm JE, Hamilton NB, Kessaris N, Richardson WD, Bergersen LH, Attwell D, et al. Regulation of oligodendrocyte development and myelination by glucose and lactate. J Neurosci. 2011;31:538–548. doi: 10.1523/JNEUROSCI.3516-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grollman EF, Philp NJ, McPhie P, Ward RD, Sauer B. Determination of transport kinetics of chick MCT3 monocarboxylate transporter from retinal pigment epithelium by expression in genetically modified yeast. Biochemistry. 2000;39:9351–9357. doi: 10.1021/bi000464+. [DOI] [PubMed] [Google Scholar]
- Chiry O, Fishbein WN, Merezhinskaya N, Clarke S, Galuske R, Magistretti PJ, et al. Distribution of the monocarboxylate transporter MCT2 in human cerebral cortex: an immunohistochemical study. Brain Res. 2008;1226:61–69. doi: 10.1016/j.brainres.2008.06.025. [DOI] [PubMed] [Google Scholar]
- Cornford EM, Hyman S. Blood–brain barrier permeability to small and large molecules. Adv Drug Deliv Rev. 1999;36:145–163. doi: 10.1016/s0169-409x(98)00082-9. [DOI] [PubMed] [Google Scholar]
- Gordon GR, Choi HB, Rungta RL, Ellis-Davies GC, MacVicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456:745–749. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergersen LH, Lauritzen KH, Lauritzen F, Puchades M, Gjedde A.Lactate receptor locations link neurotransmission, neurovascular coupling, and brain energy metabolism J Cereb Blood Flow Metab 201232S183[9th International Symposium on Functional Neuroreceptor Mapping of the Living Brain (NRM, Baltimore, MD, USA, August 09-11, 2012]. [Google Scholar]
- Bozzo L, Puyal J, Chatton JY. Lactate modulates the activity of primary cortical neurons through a receptor-mediated pathway. PLoS ONE. 2013;8:e71721. doi: 10.1371/journal.pone.0071721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F, Lane S, Korsak A, Paton JF, Gourine AV, Kasparov S, et al. Lactate-mediated glia-neuronal signalling in the mammalian brain. Nat Commun. 2014;5:3284. doi: 10.1038/ncomms4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai TQ, Ren N, Jin L, Cheng K, Kash S, Chen R, et al. Role of GPR81 in lactate-mediated reduction of adipose lipolysis. Biochem Biophys Res Commun. 2008;377:987–991. doi: 10.1016/j.bbrc.2008.10.088. [DOI] [PubMed] [Google Scholar]
- Liu C, Wu J, Zhu J, Kuei C, Yu J, Shelton J, et al. Lactate inhibits lipolysis in fat cells through activation of an orphan G-protein-coupled receptor, GPR81. J Biol Chem. 2009;284:2811–2822. doi: 10.1074/jbc.M806409200. [DOI] [PubMed] [Google Scholar]
- Kuei C, Yu J, Zhu J, Wu J, Zhang L, Shih A, et al. Study of GPR81, the lactate receptor, from distant species identifies residues and motifs critical for GPR81 functions. Mol Pharmacol. 2011;80:848–858. doi: 10.1124/mol.111.074500. [DOI] [PubMed] [Google Scholar]
- Ahmed K, Tunaru S, Tang C, Müller M, Gille A, Sassmann A, et al. An autocrine lactate loop mediates insulin-dependent inhibition of lipolysis through GPR81. Cell Metab. 2010;11:311–319. doi: 10.1016/j.cmet.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Offermanns S, Colletti SL, Lovenberg TW, Semple G, Wise A, IJzerman AP, et al. International Union of Basic and Clinical Pharmacology. LXXXII: Nomenclature and classification of hydroxy-carboxylic acid receptors (GPR81, GPR109A, and GPR109B. Pharmacol Rev. 2011;63:269–290. doi: 10.1124/pr.110.003301. [DOI] [PubMed] [Google Scholar]
- Offermanns S. Free fatty acid (FFA and hydroxy carboxylic acid (HCA receptors. Annu Rev Pharmacol Toxicol. 2014;54:407–434. doi: 10.1146/annurev-pharmtox-011613-135945. [DOI] [PubMed] [Google Scholar]
- Liu C, Kuei C, Zhu J, Yu J, Zhang L, Shih A, et al. 3,5-Dihydroxybenzoic acid, a specific agonist for hydroxycarboxylic acid 1, inhibits lipolysis in adipocytes. J Pharmacol Exp Ther. 2012;341:794–801. doi: 10.1124/jpet.112.192799. [DOI] [PubMed] [Google Scholar]
- Reiter E, Ahn S, Shukla AK, Lefkowitz RJ. Molecular mechanism of β-arrestin-biased agonism at seven-transmembrane receptors. Annu Rev Pharmacol Toxicol. 2012;52:179–197. doi: 10.1146/annurev.pharmtox.010909.105800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre K, Pellerin L, Debernardi R, Riederer BM, Magistretti PJ. Cell-specific localization of monocarboxylate transporters, MCT1 and MCT2, in the adult mouse brain revealed by double immunohistochemical labeling and confocal microscopy. Neuroscience. 2000;100:617–627. doi: 10.1016/s0306-4522(00)00294-3. [DOI] [PubMed] [Google Scholar]
- Malenka RC. Synaptic plasticity and AMPA receptor trafficking. Ann NY Acad Sci. 2003;1003:1–11. doi: 10.1196/annals.1300.001. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, et al. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144:810–823. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Gibb A. Neuroenergetics and the kinetic design of excitatory synapses. Nat Rev Neurosci. 2005;6:841–849. doi: 10.1038/nrn1784. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster H.The fine structure of the nervous system3rd edOxford University Press: Oxford; 1991 [Google Scholar]
- Caesar K, Hashemi P, Douhou A, Bonvento G, Boutelle MG, Walls AB, et al. Glutamate receptor-dependent increments in lactate, glucose and oxygen metabolism evoked in rat cerebellum in vivo. J Physiol. 2008;586:1337–1349. doi: 10.1113/jphysiol.2007.144154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab. 2007;27:1–26. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNuzzo M, Mangia S, Maraviglia B, Giove F. Changes in glucose uptake rather than lactate shuttle take center stage in subserving neuroenergetics: evidence from mathematical modeling. J Cereb Blood Flow Metab. 2010;30:586–602. doi: 10.1038/jcbfm.2009.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNuzzo M, Mangia S, Maraviglia B, Giove F. Glycogenolysis in astrocytes supports blood-borne glucose channeling not glycogen-derived lactate shuttling to neurons: evidence from mathematical modeling. J Cereb Blood Flow Metab. 2010;30:1895–1904. doi: 10.1038/jcbfm.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase M, Takahashi Y, Watabe AM, Kubo Y, Kato F. On-site energy supply at synapses through monocarboxylate transporters maintains excitatory synaptic transmission. J Neurosci. 2014;34:2605–2617. doi: 10.1523/JNEUROSCI.4687-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Cull-Candy SG. Pharmacological properties and H+ sensitivity of excitatory amino acid receptor channels in rat cerebellar granule neurones. J Physiol. 1991;433:727–763. doi: 10.1113/jphysiol.1991.sp018453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott DD, Washburn MS, Zhang S, Dingledine RJ. Subunit-dependent modulation of kainate receptors by extracellular protons and polyamines. J Neurosci. 2003;23:1179–1188. doi: 10.1523/JNEUROSCI.23-04-01179.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen TH, Nielsen OB, Lamb GD, Stephenson DG. Intracellular acidosis enhances the excitability of working muscle. Science. 2004;305:1144–1147. doi: 10.1126/science.1101141. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Bloom FE. The formation of synaptic junctions in developing rat brain: a quantitative electron microscopic study. Brain Res. 1967;6:716–727. doi: 10.1016/0006-8993(67)90128-x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Abarca LI, Tabernero A, Medina JM. Oligodendrocytes use lactate as a source of energy and as a precursor of lipids. Glia. 2001;36:321–329. doi: 10.1002/glia.1119. [DOI] [PubMed] [Google Scholar]
- Nehlig A, Pereira de Vasconcelos A. Glucose and ketone body utilization by the brain of neonatal rats. Prog Neurobiol. 1993;40:163–221. doi: 10.1016/0301-0082(93)90022-k. [DOI] [PubMed] [Google Scholar]
- Wu M, Hernandez M, Shen S, Sabo JK, Kelkar D, Wang J, et al. Differential modulation of the oligodendrocyte transcriptome by sonic hedgehog and bone morphogenetic protein 4 via opposing effects on histone acetylation. J Neurosci. 2012;32:6651–6664. doi: 10.1523/JNEUROSCI.4876-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoni L, Garcia JH, Gutierrez JA. Cerebral white matter is highly vulnerable to ischemia. Stroke. 1996;27:1641–1646. doi: 10.1161/01.str.27.9.1641. [DOI] [PubMed] [Google Scholar]
- Karadottir R, Cavelier P, Bergersen LH, Attwell D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005;438:1162–1166. doi: 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter MG, Fern R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature. 2005;438:1167–1171. doi: 10.1038/nature04301. [DOI] [PubMed] [Google Scholar]
- Micu I, Jiang Q, Coderre E, Ridsdale A, Zhang L, Woulfe J, et al. NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature. 2006;439:988–992. doi: 10.1038/nature04474. [DOI] [PubMed] [Google Scholar]
- Manning SM, Talos DM, Zhou C, Selip DB, Park HK, Park CJ, et al. NMDA receptor blockade with memantine attenuates white matter injury in a rat model of periventricular leukomalacia. J Neurosci. 2008;28:6670–6678. doi: 10.1523/JNEUROSCI.1702-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouardouz M, Coderre E, Basak A, Chen A, Zamponi GW, Hameed S, et al. Glutamate receptors on myelinated spinal cord axons: I. GluR6 kainate receptors. Ann Neurol. 2009;65:151–159. doi: 10.1002/ana.21533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouardouz M, Coderre E, Zamponi GW, Hameed S, Yin X, Trapp BD, et al. Glutamate receptors on myelinated spinal cord axons: II. AMPA and GluR5 receptors. Ann Neurol. 2009;65:160–166. doi: 10.1002/ana.21539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinholm JE, Bergersen LH. The wrap that feeds neurons. Nature. 2012;487:435–436. doi: 10.1038/487435a. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Karthigasan J, Borenshteyn NI, Flax JD, Kirschner DA. Myelination in the developing human brain: biochemical correlates. Neurochem Res. 1994;19:983–996. doi: 10.1007/BF00968708. [DOI] [PubMed] [Google Scholar]
- Funfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485:517–521. doi: 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave KA. Myelination and the trophic support of long axons. Nat Rev Neurosci. 2010;11:275–283. doi: 10.1038/nrn2797. [DOI] [PubMed] [Google Scholar]
- Agnati LF, Guidolin D, Guescini M, Genedani S, Fuxe K. Understanding wiring and volume transmission. Brain Res Rev. 2010;64:137–159. doi: 10.1016/j.brainresrev.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Cerdán S, Rodrigues TB, Sierra A, Benito M, Fonseca LL, Fonseca CP, et al. The redox switch/redox coupling hypothesis. Neurochem Int. 2006;48:523–530. doi: 10.1016/j.neuint.2005.12.036. [DOI] [PubMed] [Google Scholar]
- Ramírez BG, Rodrigues TB, Violante IR, Cruz F, Fonseca LL, Ballesteros P, et al. Kinetic properties of the redox switch/redox coupling mechanism as determined in primary cultures of cortical neurons and astrocytes from rat brain. J Neurosci Res. 2007;85:3244–3253. doi: 10.1002/jnr.21386. [DOI] [PubMed] [Google Scholar]
- Gambini J, Gomez-Cabrera MC, Borras C, Valles SL, Lopez-Grueso R, Martinez-Bello VE, et al. Free [NADH]/[NAD(+] regulates sirtuin expression. Arch Biochem Biophys. 2011;512:24–29. doi: 10.1016/j.abb.2011.04.020. [DOI] [PubMed] [Google Scholar]
- Yang J, Ruchti E, Petit JM, Jourdain P, Grenningloh G, Allaman I, et al. Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc Natl Acad Sci USA. 2014;111:12228–12233. doi: 10.1073/pnas.1322912111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cureton EL, Kwan RO, Dozier KC, Sadjadi J, Pal JD, Victorino GP, et al. A different view of lactate in trauma patients: protecting the injured brain. J Surg Res. 2010;159:468–473. doi: 10.1016/j.jss.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Ros J, Pecinska N, Alessandri B, Landolt H, Fillenz M. Lactate reduces glutamate-induced neurotoxicity in rat cortex. J Neurosci Res. 2001;66:790–794. doi: 10.1002/jnr.10043. [DOI] [PubMed] [Google Scholar]
- Bouzat P, Sala N, Suys T, Zerlauth JB, Marques-Vidal P, Feihl F, et al. Cerebral metabolic effects of exogenous lactate supplementation on the injured human brain. Intensive Care Med. 2014;40:412–421. doi: 10.1007/s00134-013-3203-6. [DOI] [PubMed] [Google Scholar]
- Vespa P, Bergsneider M, Hattori N, Wu HM, Huang SC, Martin NA, et al. Metabolic crisis without brain ischemia is common after traumatic brain injury: a combined microdialysis and positron emission tomography study. J Cereb Blood Flow Metab. 2005;25:763–774. doi: 10.1038/sj.jcbfm.9600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoque R, Farooq A, Ghani A, Gorelick F, Mehal WZ. Lactate reduces liver and pancreatic injury in Toll-like receptor- and inflammasome-mediated inflammation via GPR81-mediated suppression of innate immunity. Gastroenterology. 2014;146:1763–1774. doi: 10.1053/j.gastro.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Gamo NJ, Yang Y, Jin LE, Wang XJ, Laubach M, et al. Neuronal basis of age-related working memory decline. Nature. 2011;476:210–213. doi: 10.1038/nature10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez M, Hernández AI, Hernanz A. Increased cAMP immunostaining in cerebral vessels in Alzheimer's disease. Brain Res. 2001;922:148–152. doi: 10.1016/s0006-8993(01)03009-8. [DOI] [PubMed] [Google Scholar]
- Canepa E, Domenicotti C, Marengo B, Passalacqua M, Marinari UM, Pronzato MA, et al. Cyclic adenosine monophosphate as an endogenous modulator of the amyloid-β precursor protein metabolism. IUBMB Life. 2013;65:127–133. doi: 10.1002/iub.1109. [DOI] [PubMed] [Google Scholar]
- Wang M, Ramos BP, Paspalas CD, Shu Y, Simen A, Duque A, et al. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Prefrontal cortical network connections: key site of vulnerability in stress and schizophrenia. Int J Dev Neurosci. 2011;29:215–223. doi: 10.1016/j.ijdevneu.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery MC, Dutt N, Krichmar JL. A large-scale neural network model of the influence of neuromodulatory levels on working memory and behavior. Front Comput Neurosci. 2013;7:133. doi: 10.3389/fncom.2013.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell J, Zeppenfeld D, McConnell E, Pena S, Nedergaard M. Norepinephrine: a neuromodulator that boosts the function of multiple cell types to optimize CNS performance. Neurochem Res. 2012;37:2496–2512. doi: 10.1007/s11064-012-0818-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coco M, Caggia S, Musumeci G, Perciavalle V, Graziano AC, Pannuzzo G, et al. Sodium L-lactate differently affects brain-derived neurothrophic factor, inducible nitric oxide synthase, and heat shock protein 70 kDa production in human astrocytes and SH-SY5Y cultures. J Neurosci Res. 2013;91:313–320. doi: 10.1002/jnr.23154. [DOI] [PubMed] [Google Scholar]
- Voss MW, Erickson KI, Prakash RS, Chaddock L, Kim JS, Alves H, et al. Neurobiological markers of exercise-related brain plasticity in older adults. Brain Behav Immun. 2013;28:90–99. doi: 10.1016/j.bbi.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Religa P, Cao R, Religa D, Xue Y, Bogdanovic N, Westaway D, et al. VEGF significantly restores impaired memory behavior in Alzheimer's mice by improvement of vascular survival. Sci Rep. 2013;3:2053. doi: 10.1038/srep02053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, et al. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36:827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- Bekinschtein P, Kent BA, Oomen CA, Clemenson GD, Gage FH, Saksida LM, et al. BDNF in the dentate gyrus is required for consolidation of ‘pattern-separated' memories. Cell Rep. 2013;5:759–768. doi: 10.1016/j.celrep.2013.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR, et al. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124:71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Ding Q, Ying Z, Gómez-Pinilla F. Exercise influences hippocampal plasticity by modulating brain-derived neurotrophic factor processing. Neuroscience. 2011;192:773–780. doi: 10.1016/j.neuroscience.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lanerolle NC, Kim JH, Williamson A, Spencer SS, Zaveri HP, Eid T, et al. A retrospective analysis of hippocampal pathology in human temporal lobe epilepsy: evidence for distinctive patient subcategories. Epilepsia. 2003;44:677–687. doi: 10.1046/j.1528-1157.2003.32701.x. [DOI] [PubMed] [Google Scholar]
- Liu B, Niu L, Shen MZ, Gao L, Wang C, Li J, et al. Decreased astroglial monocarboxylate transporter 4 expression in temporal lobe epilepsy. Mol Neurobiol. 2014;50:327–338. doi: 10.1007/s12035-013-8619-z. [DOI] [PubMed] [Google Scholar]
- van Hall G, Strømstad M, Rasmussen P, Jans O, Zaar M, Gam C, et al. Blood lactate is an important energy source for the human brain. J Cereb Blood Flow Metab. 2009;29:1121–1129. doi: 10.1038/jcbfm.2009.35. [DOI] [PubMed] [Google Scholar]
- Mueller AL, Dunwiddie TV. Anticonvulsant and proconvulsant actions of alpha- and beta-noradrenergic agonists on epileptiform activity in rat hippocampus in vitro. Epilepsia. 1983;24:57–64. doi: 10.1111/j.1528-1157.1983.tb04866.x. [DOI] [PubMed] [Google Scholar]
- Draoui N, Schicke O, Fernandes A, Drozak X, Nahra F, Dumont A, et al. Synthesis and pharmacological evaluation of carboxycoumarins as a new antitumor treatment targeting lactate transport in cancer cells. Bioorg Med Chem. 2013;21:7107–7117. doi: 10.1016/j.bmc.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Parks SK, Chiche J, Pouysségur J. Disrupting proton dynamics and energy metabolism for cancer therapy. Nat Rev Cancer. 2013;13:611–623. doi: 10.1038/nrc3579. [DOI] [PubMed] [Google Scholar]
- Rasmussen P, Brassard P, Adser H, Pedersen MV, Leick L, Hart E, et al. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp Physiol. 2009;94:1062–1069. doi: 10.1113/expphysiol.2009.048512. [DOI] [PubMed] [Google Scholar]
- Foster PP, Rosenblatt KP, Kuljiš RO. Exercise-induced cognitive plasticity, implications for mild cognitive impairment and Alzheimer's disease. Front Neurol. 2011;2:28. doi: 10.3389/fneur.2011.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zschucke E, Gaudlitz K, Ströhle A. Exercise and physical activity in mental disorders: clinical and experimental evidence. J Prev Med Public Health. 2013;46:S12–S21. doi: 10.3961/jpmph.2013.46.S.S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman AS, Young DE, He X, Chen TC, Wagenaar RC, Stern CE, et al. Interaction between serum BDNF and aerobic fitness predicts recognition memory in healthy young adults. Behav Brain Res. 2014;259:302–312. doi: 10.1016/j.bbr.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adlard PA, Engesser-Cesar C, Cotman CW. Mild stress facilitates learning and exercise improves retention in aged mice. Exp Gerontol. 2011;46:53–59. doi: 10.1016/j.exger.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komulainen P, Pedersen M, Hänninen T, Bruunsgaard H, Lakka TA, Kivipelto M, et al. BDNF is a novel marker of cognitive function in ageing women: the DR's EXTRA Study. Neurobiol Learn Mem. 2008;90:596–603. doi: 10.1016/j.nlm.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Seifert T, Brassard P, Wissenberg M, Rasmussen P, Nordby P, Stallknecht B, et al. Endurance training enhances BDNF release from the human brain. Am J Physiol Regul Integr Comp Physiol. 2010;298:R372–R377. doi: 10.1152/ajpregu.00525.2009. [DOI] [PubMed] [Google Scholar]
- Coelho FG, Gobbi S, Andreatto CA, Corazza DI, Pedroso RV, Santos-Galduróz RF, et al. Physical exercise modulates peripheral levels of brain-derived neurotrophic factor (BDNF: a systematic review of experimental studies in the elderly. Arch Gerontol Geriatr. 2013;56:10–15. doi: 10.1016/j.archger.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Lanz TA, Bove SE, Pilsmaker CD, Mariga A, Drummond EM, Cadelina GW, et al. Robust changes in expression of brain-derived neurotrophic factor (BDNF mRNA and protein across the brain do not translate to detectable changes in BDNF levels in CSF or plasma. Biomarkers. 2012;17:524–531. doi: 10.3109/1354750X.2012.694476. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Mol Brain. 2012;5:14. doi: 10.1186/1756-6606-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida S. A functional role for CREB as a positive regulator of memory formation and LTP. Exp Neurobiol. 2012;21:136–140. doi: 10.5607/en.2012.21.4.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S, Yoon M. Swimming's prevention of ovariectomy-induced obesity through activation of skeletal-muscle PPARα. Int J Sport Nutr Exerc Metab. 2012;22:1–10. doi: 10.1123/ijsnem.22.1.1. [DOI] [PubMed] [Google Scholar]
- Roy A, Jana M, Corbett GT, Ramaswamy S, Kordower JH, Gonzalez FJ, et al. Regulation of cyclic AMP response element binding and hippocampal plasticity-related genes by peroxisome proliferator-activated receptor α. Cell Rep. 2013;4:724–737. doi: 10.1016/j.celrep.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell AP, Foletta VC, Snow RJ, Wadley GD. Skeletal muscle mitochondria: a major player in exercise, health and disease. Biochim Biophys Acta. 2014;1840:1276–1284. doi: 10.1016/j.bbagen.2013.11.016. [DOI] [PubMed] [Google Scholar]
- Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, et al. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 2013;18:649–659. doi: 10.1016/j.cmet.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]