Abstract
Treatment with tissue plasminogen activator (tPA) beyond the therapeutic time window (>4.5 hours post stroke) may produce hemorrhagic transformation (HT). Strategies that could extend the narrow time window of tPA will benefit a significant number of stroke patients. Male Sprague–Dawley rats underwent middle cerebral artery occlusion (MCAo) and given vehicle, tPA (10 mg/kg), or tPA and granulocyte colony-stimulating factor (G-CSF, 300 μg/kg), at 6 hours after MCAo. Twenty-four hours post treatment, G-CSF+tPA-treated stroke rats displayed 25% improvement in neurological functions and 38.9% reduction of hemorrhage, with Western blots showing 1.9- and 1.2-fold increments in Ang-2 expression in the ischemic cortex and striatum, respectively, and 3-fold increase in phosphorylated endothelial nitric oxide synthase expression in the ipsilateral cortex relative to tPA-treated rats. Immunohistochemistry also showed 2- and 2.8-fold increase in von-Willebrand expression, 3.2- and 2.2-fold increased CD34+ expression, and 4- and 13-fold upregulation of VEGFR-2 expression in the ischemic cortex and striatum, respectively, in G-CSF+tPA-treated stroke rats relative to tPA-treated subjects. Altogether, these findings indicate that G-CSF attenuated delayed tPA-induced HT likely via the enhancement of angiogenesis and vasculogenesis. The use of G-CSF to protect the vasculature may improve the clinical outcome of tPA even outside the currently indicated therapeutic window for ischemic stroke.
Keywords: angiogenesis, EPCs, G-CSF, hemorrhagic transformation, tPA, vasculogenesis
Introduction
Acute cerebral ischemia is a major cause of mortality and disability worldwide.1 The only FDA-approved treatment for this condition is thrombolytic therapy with tissue plasminogen activator (tPA)2, 3 which is effective when given within 4.5 hours of ischemic onset, but beyond this therapeutic window could produce deleterious side effects in the ischemic brain, notably, hemorrhagic transformation (HT).4 The HT that ensues after delayed tPA treatment is likely owing to tPA's effect on the ‘neurovascular unit,' characterized by a breakdown of the blood brain barrier (BBB), a restrictive and protective barrier between blood and the neuronal parenchyma composed mainly of brain endothelial cells.5 Therefore, preventing endothelial cell injury may lead to protection from BBB breakdown, afford better neuroprotection, and ultimately, protect against HT owing to delayed tPA reperfusion therapy post ischemia.
The granulocyte colony-stimulating factor (G-CSF), an essential member of the hematopoietic growth factor family, controls the survival, proliferation, and differentiation of hematopoietic stem cells/ hematopoietic progenitor cells.6 Aside from its effects on the hematopoietic system, G-CSF has important roles in the CNS. For instance, G-CSF has been shown to exert neuroprotection in vitro as well as in animal models of stroke in different species.7, 8 Multi-pronged neuroprotective actions of G-CSF, which have been implicated in the drug's robust reduction of cerebral infarcts, include suppressed glutamate-induced neurotoxicity,9 blockade of the apoptotic cell death,10 attenuated edema formation and interleukin-1 beta expression,11 and engagement of the cerebral G-CSF receptor.12 Furthermore, G-CSF has been shown to activate endothelial cell proliferation and migration in vitro, as well as to protect endothelial cells.13 It also stimulated endogenous neurogenesis and vascularization, and exerted angiogenic activity in the corneal angiogenesis assay.14, 15 Moreover, G-CSF has been reported to mobilize CD34+ bone marrow stem cells into the peripheral blood, which contain endothelial progenitor cells (EPCs).16 That the beneficial effects of G-CSF in stroke involve enhanced angiogenesis and vasculogenesis, which are reparative mechanisms responsible for the development of blood vessels and improvement in tissue microperfusion around the ischemic boundary zone, implicate the putative activation of endothelial cells or mobilization of bone marrow-derived EPCs as G-CSF's primary mechanism of action.13, 17
In this study, we investigated the efficacy of G-CSF to reduce HT after delayed tPA treatment in ischemic stroke rats. We hypothesized that a combination therapy of G-CSF and tPA after the therapeutic time window will reduce HT, in view of the beneficial effects of G-CSF in preclinical models of ischemia,7, 8 protective effects of G-CSF on endothelial cells, and reparative or regenerative activities of G-CSF-mobilized EPCs.13, 15, 16, 17 Furthermore, because enhancement of angiogenesis and vasculogenesis has been shown to promote neurological functional improvement after stroke,17, 18 we hypothesized that G-CSF will not only protect against delayed tPA-induced HT but also improve neurological deficits post stroke by virtue of its angiogenic and vasculogenic effects. Accordingly, we advance the notion that enhanced angiogenic and vasculogenic activities of G-CSF may be attributed to amplified activation of endothelial cells, or via G-CSF-mobilized EPCs, in view of findings from the above studies.7, 11, 13, 15, 16, 17
Materials and Methods
Animals
Male adult Sprague–Dawley rats (~9–10 weeks old; Harlan Sprague Dawley, Indianapolis, IN, USA), weighing 200 –250 g at the beginning of experiments were used for this study. All experiments have been performed according to the Public Health Service Policy on Humane Care and Use of Laboratory Animals, the Guide for the Care and Use of Laboratory Animals, and other approved guidelines of the University of South Florida System Institutional Animal Care and Use Committee (protocol #4181). Rats were housed in pairs in an AAALAC-approved Research Animal Facility with a temperature- and humidity-controlled room maintained on 12-h light/dark cycles, with free access to food and water. All surgical procedures were conducted under aseptic conditions and every effort was made to minimize animal suffering and reduce the number of animals used in the study. All experiments stipulated in this manuscript are in accordance with the ARRIVE guidelines.
Stroke Surgery
Stroke surgery was performed using the middle cerebral artery occlusion (MCAo) technique as described in our previous studies.18 Animals were anesthetized with a mixture of 1% to 2% isoflurane in NO/oxygen (69%/30%) via a face mask, and body temperature was maintained at 37 °C±0.3 °C during the surgical procedures. A midline skin incision was made in the neck with subsequent exploration of the left common carotid artery, the external carotid artery, and internal carotid artery. Thereafter, a 4-0 monofilament nylon suture (27.0 mm–28.0 mm) was advanced from the common carotid artery bifurcation until it blocked the origin of the MCA. Animals were allowed to recover from anesthesia during MCAo. Hours after MCAo, animals were re-anesthetized and reperfused by withdrawal of the nylon thread. We have standardized the MCAo model, with stroke animals showing ⩾80% reduction in regional cerebral blood flow during the occlusion period as determined by laser Doppler (Perimed, Ardmore, PA, USA). We also found no significant differences in physiological parameters, including PaO2, PaCO2, and plasma pH measurements, in our stroke animals indicating similar degree of stroke insults. Rats that reached the 80% cerebral blood flow reduction during occlusion were used for further studies. Sham animals were also included in this study. Rats were anesthetized and an incision was made in the neck to expose and isolate the common carotid and internal arteries. Thereafter, incisions were closed and animals were allowed to recover from anesthesia.
Experimental Groups and in vivo Drug Treatment
Rats underwent sham surgery or MCAo and were assigned randomly to one of the treatments (n=8–10 animals per group) with either vehicle (5% dextrose), tPA (a generous gift from Genentech, San Francisco, CA, USA) or combined G-CSF (Amgen, Thousand Oaks, CA, USA)+tPA administered immediately before reperfusion, i.e., 6 hours after MCAo.19, 20 Tissue plasminogen activator was given at 10 mg/kg dose based on previous studies,20, 21 while G-CSF was administered at 300 μg/kg dose, a dosage which reduced neuroinflammation and ameliorated traumatic brain injury (TBI)-induced impairments as reported in our previous study.22 All drugs were given via the intravenous route, i.e., via the jugular vein. Twenty-four hours after drug treatment, and after behavioral tests (see below), rats were killed and brains were harvested for subsequent analyses. A power analysis determined the starting sample size to be at least six animals per group to reject the null hypothesis at P<0.05 with a power of 0.80.
Modified Neurological Testing
Animals were observed for changes in neurological functions using a modified neurological examination described in our previous studies.18 Neurological score for each rat was obtained using three tests: (1) forelimb akinesia which measures the ability of the animal to replace the forelimb after it is displaced laterally by 2 to 3 cm, graded from 0 (immediate replacement) to 3 (replacement after several seconds or no replacement); (2) beam walking ability, graded 0 for a rat that readily traversed a 2.4-cm wide, 80-cm long beam to 3 for a rat unable to stay on the beam for 10 seconds; and (3) paw grasp which measures the ability of the rat to hold onto a 2-mm-diameter steel rod, graded 0 for a rat with normal forepaw grasping behavior to 3 for a rat unable to grasp with the forepaws. The scores from this battery of three neurological tests were pooled to obtain the mean neurological score for each treatment group. A ⩾2.5 mean neurological score is used as a criterion for stroke-induced neurological impairment.19 All behavioral tests were conducted by a single trained rater blinded to all experimental conditions.
Triphenyltetrazolium Chloride Staining
Seven coronal brain sections (2 mm thick) were stained with 2% 2,3,5-triphenyltetrazolium chloride (Sigma Aldrich, St Louis, MO, USA), and fixed with 4% paraformaldehyde to determine size of infarct. Infarct size, which is devoid of red staining, was determined on the digital images using ImageJ software (open source ImageJ software available at http://rsb.info.nih.gov/ij/). An observer blinded to experimental group assignments calculated the ratio of infarct with that of the whole brain.
Hemoglobin Assay
Hemorrhagic transformation was quantified using the spectrophotometric assay of brain hemoglobin content reported in a previous study.23 After triphenyltetrazolium chloride staining and scanning, the hemispheric brain tissues were homogenized with phosphate buffered saline and centrifuged for 30 minutes (13,000 g). Thereafter, 200 μL of reagent (QuantiChrom Hemoglobin Assay Kit, Bioassay systems, Hayward, CA, USA) was added to 50 μL of supernatant. Fifteen minutes later, optical density was measured at 400 nM with a spectrophotometer.
Western Blot
Western blotting was performed with angiogenesis markers angiopoietin-1 (1:1000; Millipore, Billerica, MA, USA) and Ang-2 (1:1000; Abcam, Cambridge, MA, USA), endothelial nitric oxide synthase (eNOS) (1:1000; Cell Signaling, Beverly, MA, USA) and phosphorylated-eNOS (Cell Signaling; 1:1000) as described previously.24 The anti-β-actin antibody (1:10,000; Sigma) was used as control. Homogenates of the entire ischemic as well as contralateral (non-ischemic) hemispheres were obtained. Equal amounts of protein (80 μg) for each group were assayed and proteins were separated by SDS–polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membrane. Membranes were blocked with 5% (w/v) bovine serum albumin in Tris-Buffered Saline with Tween 20 for 1 hour. After immunoblotting with specific antibodies, membranes were incubated with HRP-conjugated IgG (1:1000; GE Healthcare, Pittsburgh, PA, USA), and labeled proteins were detected using the electrochemiluminescence (ECL) Plus kit (Thermo Scientific, West Palm Beach, FL, USA). The band intensities obtained by western blot analysis were determined using the ImageJ program. All samples were analyzed at least in triplicates.
Histology and Immunohistochemistry
Rats were anesthetized and then perfused transcardially with 4% paraformaldehyde in phosphate buffered saline. Brains were removed, post fixed in the same fixative for 24 hours followed by 30% sucrose in phosphate buffered saline for 1 week. Frozen sections were then sliced into 40-μm sections in a cryostat and stored at −20 °C. Slide-mounted sections were incubated overnight at 4 °C with vasculogenesis marker von-Willebrand factor 19 (von-Willebrand (vWF); 1:100; Abcam); or with EPC markers CD34+ (15 μg/mL; R&D Systems, Minneapolis, MN, USA) and vascular endothelial growth receptor-2 (VEGFR-2)16, 25, 26 (1:100, Cell Signaling) in phosphate buffered saline. After washing and incubation in corresponding secondary antibodies, sections were washed and incubated for 30 minutes with Hoechst at 37 °C. After washing, slides were cover-slipped using Fluoromount mounting medium (Sigma Aldrich).
Statistical Analysis
All data are expressed as mean±s.e.m. Results were analyzed statistically using one-way analysis of variance and subsequent post-hoc Bonferroni's tests, or unpaired t-tests when comparing means of two groups. Statistical significance was set at P<0.05. All statistical analyses were conducted using GraphPad Prism 5 (San Diego, CA, USA).
Results
G-CSF Reduced Delayed tPA-Induced Hemorrhage and Neurological Deficits after MCAo in Rats
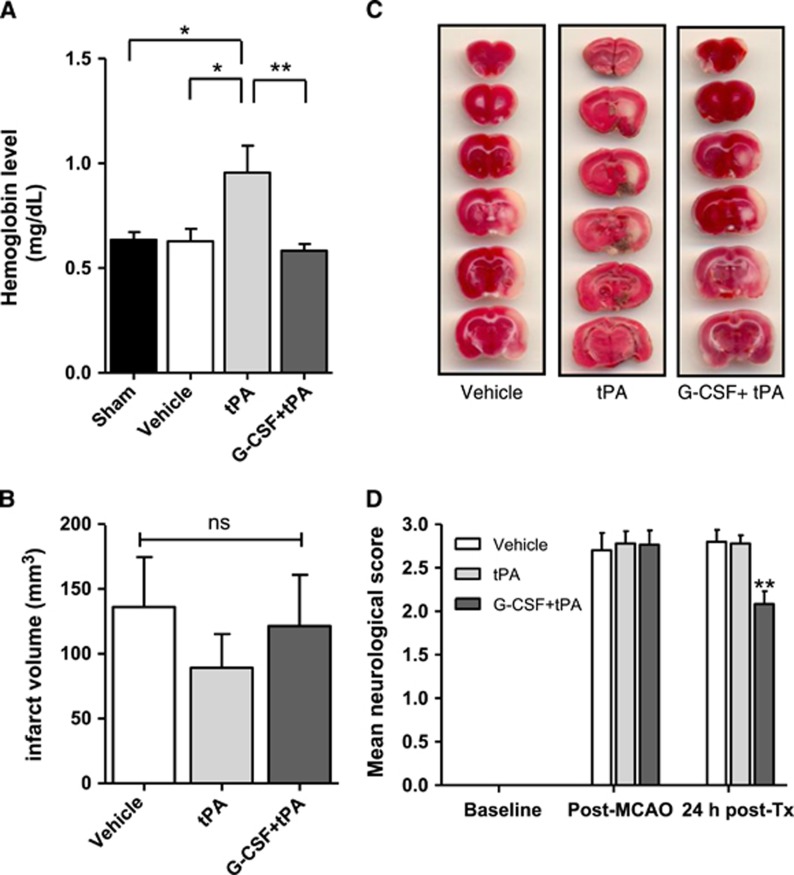
We examined whether combination treatment of G-CSF+tPA at a delayed time point (i.e., 6 hours post MCAo) influenced severity and incidence of cerebral hemorrhage in ischemic stroke rats. Quantitative analysis of cerebral hemorrhage volume using spectrophotometric hemoglobin assays, which measured the amount of hemoglobin in the brain, showed incidence of HT in tPA-treated stroke rats (compared with sham; t (9)=2.21; P<0.05), which was not observed in vehicle-treated rats (compared with tPA-treated rats; t (10)=2.33; P<0.05; Figure 1A). Moreover, treatment with G-CSF reduced delayed tPA-induced hemorrhage in MCAo rats (t (10)=2.83; P<0.01; Figure 1A). However, combination treatment of G-CSF+ tPA did not improve the infarct volumes after delayed tPA therapy (Figures 1B and 1C), albeit coinciding with the reported lack of effects of other neuroprotective agents (e.g., fasudil and cilostazol)20, 21 in the same stroke model. After MCAo, rats displayed significant neurological deficits as evidenced by neurological scores⩾2.5. Twenty-four hours after MCAo, rats given vehicle as well as those administered with tPA still showed neurological deficits. However, neurological scores varied significantly among groups (F (2,26)=9.93; P<0.001) and subjects injected with G-CSF+tPA displayed improvement of stroke- (and also probably delayed tPA-) induced neurological impairments (pairwise comparisons between groups; P<0.01; Figure 1D).
Figure 1.
Effects of granulocyte colony-stimulating factor (G-CSF) on delayed tissue plasminogen activator (tPA)-induced hemorrhage, cerebral infarction, and neurological deficits in MCAo rats. (A) Quantitative analysis of cerebral hemorrhage volume with spectrophotometric assay revealed incidence of hemorrhage (shown as increased levels of hemoglobin levels in the brain) in rats subjected to delayed tPA treatment. G-CSF treatment caused a 38% reduction of delayed tPA-induced hemorrhage (n=5–6 animals per group). (B) Quantitative analysis of infarct volume in vehicle- (n=9), tPA- (n=7) and G-CSF+tPA- (n=8) treated groups. (C) Photographs are representative coronal brain sections stained with triphenyltetrazolium chloride 24 hours after MCAo, showing infarct area (white) and intact areas (red). G-CSF had no effect on infarct volume. (D) Effects of G-CSF on delayed tPA-induced neurological deficits in MCAo rats. Twenty-four hours after drug treatment (Tx), rats injected with G-CSF displayed improvement of delayed tPA-induced neurological deficits (25% and 24.8%, relative to control and tPA-treated stroke rats, respectively). (n=8–10 animals per group), *P<0.05, **P<0.01; NS, not significant. Data are expressed as mean±s.e.m.
Enhanced Ang-2, peNOS Expression in Ischemic Hemisphere of MCAO Rats Treated with G-CSF+ tPA
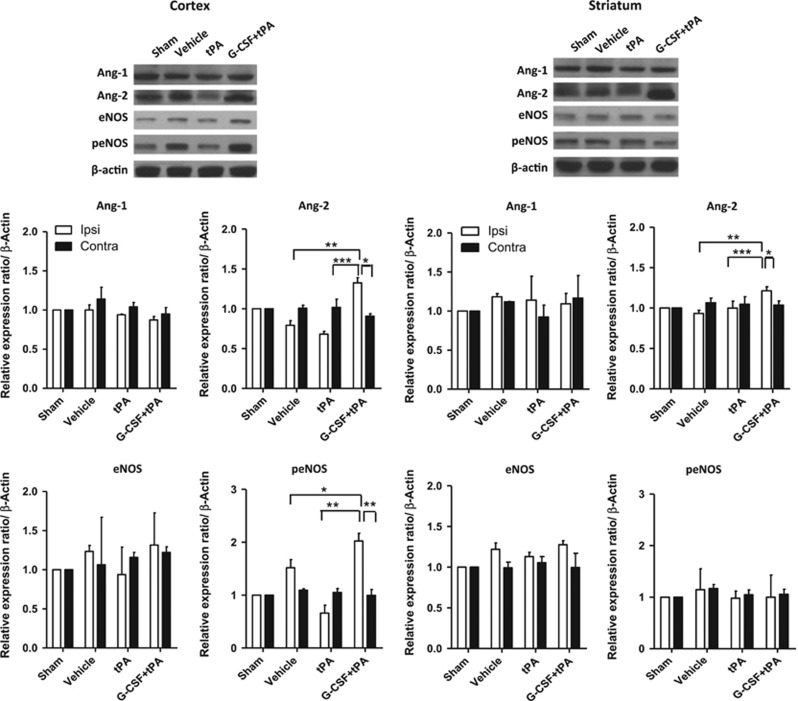
At 24 hours after drug administration (i.e., after MCAo induction), western blot analyses were conducted to measure expression levels of angiopoietins (Ang-1 and Ang-2), angiogenesis markers, as well as eNOS and peNOS, putative marker of EPCs,26, 27, 28, 29 in cerebral hemispheres ipsilateral and contralateral to MCAo. Expression of these proteins were compared against their expression profiles in the brains of sham rats and normalized to β-actin. Figure 2 shows that Ang-2, but not Ang-1 expression was upregulated in the ischemic hemisphere (including cortex and striatum) of stroke rats given G-CSF+tPA. Specifically, Ang-2 expression was higher in the ipsilateral hemisphere of G-CSF+tPA-treated rats compared with those given tPA alone (cortex: t (10)=7.73; P<0.001; striatum: t (10)=4.76; P<0.001), or with vehicle (cortex: t (10)=5.24; P<0.001; striatum: t (10)=9.81; P<0.01). G-CSF+tPA therapy also increased peNOS expression in cortex (t (8)=6.58; P<0.01 versus tPA-treated group; t (8)=2.37; P<0.05 versus vehicle-treated group), but not in the striatum of the cerebral hemisphere ipsilateral to MCAo (Figure 2). Total eNOS expression in the ischemic cortex and striatum was similar in all treatment groups although there was a trend of increased eNOS expression in the cortex of G-CSF+tPA-treated rats compared with subjects given tPA only.
Figure 2.
Western blotting for angiopoetins (Ang-1 and Ang-2), endothelial nitric oxide synthase (eNOS) and phosphorylated-eNOS. As shown in representative bands, treatment with granulocyte colony-stimulating factor+tissue plasminogen activator (G-CSF+tPA) upregulated Ang-2 (marker of angiogenesis) expression in the ischemic hemisphere. Quantitative analyses showed 1.9- and 1.2-fold increment in Ang-2 expression in the ischemic cortex and striatum, respectively, in G-CSF+tPA-treated rats relative to subjects treated with tPA only. Ang-1 expression in both ipsilateral and contralateral hemispheres was similar in all groups. Relative to tPA-treated rats, G-CSF+tPA-treated stroke rats also showed threefold increase in peNOS expression in the ischemic cortex, but not striatum, in cerebral hemisphere ipsilateral to MCAo. Total eNOS expression in the ipsilateral and contralateral hemispheres was similar in all treatment groups although there was a trend of increased eNOS expression in the cortex of G-CSF+tPA-treated versus tPA-treated rats. *P<0.05, **P<0.01, ***P<0.001 n=5–6. Data are expressed as mean±s.e.m. relative to sham group and normalized to β-actin.
Parallel in vitro antibody neutralizing experiments and quantitative reverse transcription-PCR studies were conducted to determine whether G-CSF directly modulates angiogenesis (see Supplementary File). Our studies showed that the VEGF and angiogenesis inhibitor sunitinib reduced G-CSF-induced upregulation of angiogenesis marker genes (VEGFR-2, VE-cadherin, and vWF), and dose-dependently blocked G-CSF-induced upregulation of VEGF expression in cultured endothelial cell line, indicating direct influence of G-CSF on angiogenesis rather than coincidental occurrence of angiogenesis independent of G-CSF treatment.
Enhanced vWF Expression in MCAo Rats Treated with G-CSF+tPA
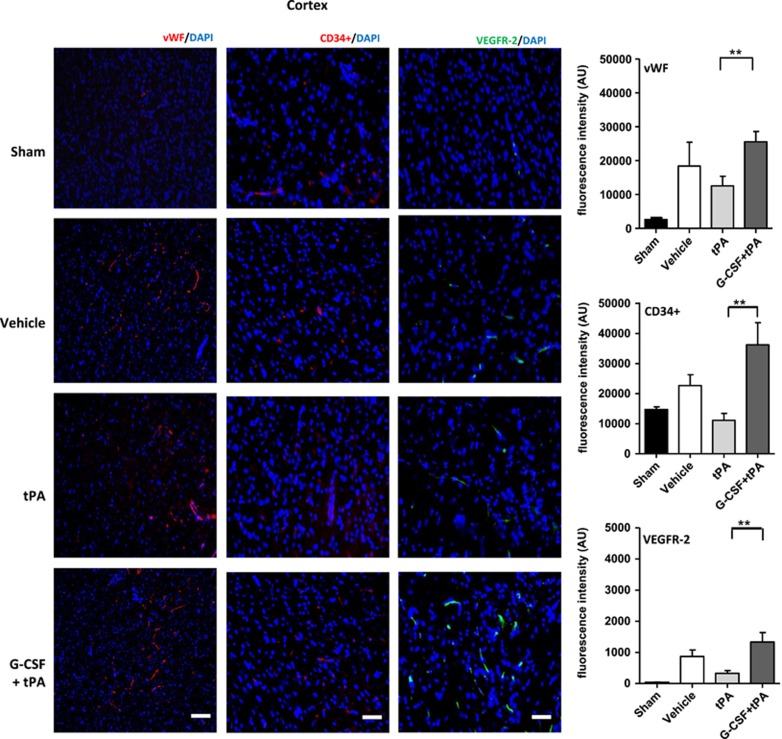
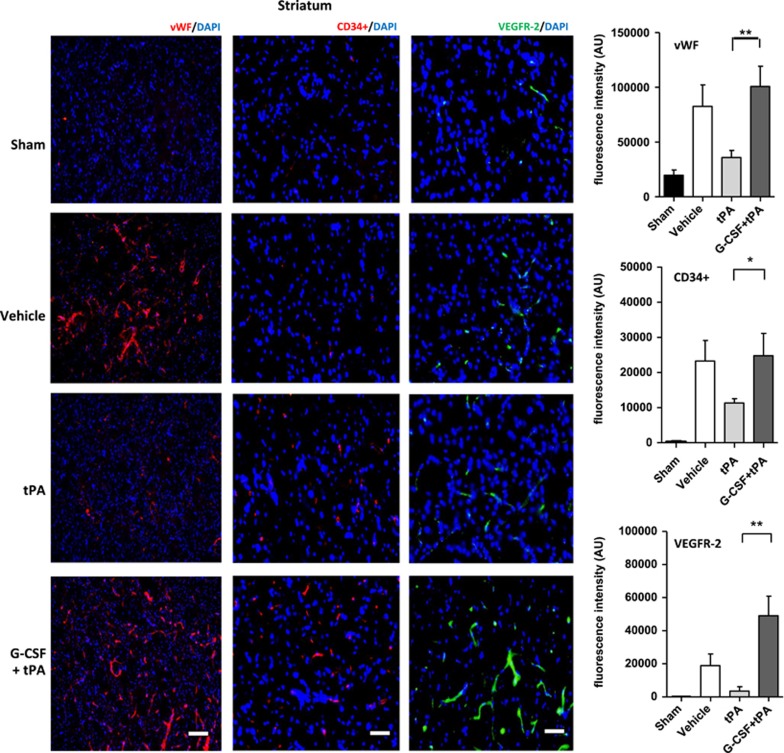
The expression of vWF is a distinctive marker for vasculogenesis as reported in previous studies.18, 27 Using immunofluorescence, we measured expression levels of vWF in the ischemic hemisphere (cortex and striatum) in all subjects at 24 hours after drug administration. Levels of vWF expression were also compared against its expression profile in the brains of sham rats. Quantification of vWF fluorescent intensities showed higher vWF expression in ipsilateral cortex (t (10)=3.12; P<0.01) and striatum (t (10)=3.30; P<0.01) of G-CSF+tPA-treated rats compared with subjects administered with tPA (Figure 4). vWF levels in the contralateral side of all rats (stroke and sham) did not vary significantly (data not shown).
Enhanced CD34+ and VEGFR-2 Expression in MCAo Rats Treated with G-CSF+tPA
EPCs are a heterogeneous group of cells that can be characterized by the expression of surface markers, such as CD34+ and VEGFR-216, 25, 26, 27, 28. We measured expression levels of these markers in the ischemic hemisphere (cortex and striatum) in all subjects at 24 hours after drug administration. CD34+ and VEGFR-2 expressions were also compared against their expression profiles in the brains of sham rats. Quantification of fluorescent intensities showed higher CD34+ in ipsilateral cortex (t (8)=3.25; P<0.01; Figure 3) and striatum (t (8)=2.08; P<0.05; Figure 4) of G-CSF+tPA-treated rats compared with subjects administered with tPA alone (Figure 4). The same holds true for VEGFR-2 expression in the ipsilateral cortex (t (8)=3.19; P<0.01; Figure 3) and striatum (t (8)=3.73; P<0.01; Figure 4) of G-CSF+tPA-treated rats compared with rats administered with tPA only. Expression levels of these surface markers in the contralateral side of all rats (stroke and sham) did not vary significantly (data not shown).
Figure 3.
Immunohistochemical analyses of von-Willebrand (vWF), CD34+ and vascular endothelial growth factor (VEGRF)-2 expression levels in the ischemic cortex. Representative merged images showing co-localization of vWF, CD34+, or VEGFR-2 with 4′,6-diamidino-2-phenylindole (DAPI; blue filter, nuclear staining). Analyses of fluorescence intensities showed 2-fold increase in the expression of vascular marker vWF, as well as 3.2- and 4-fold increment in endothelial progenitor cell markers CD34+ and VEGFR-2, respectively, in the ischemic cortex of G-CSF+tPA-treated rats compared with subjects administered with tPA only (at 6 hours post MCAO). **P<0.01, n=5–6. Data are expressed as mean±s.e.m. Horizontal bar indicates 100 μM.
Figure 4.
Immunohistochemical analyses of von-Willebrand (vWF), CD34+ and vascular endothelial growth factor (VEGRF)-2 expression levels in the ischemic striatum. Representative merged images showing co-localization of vWF, CD34+, or VEGFR-2 with 4′,6-diamidino-2-phenylindole (DAPI; blue filter, nuclear staining). Analysis of fluorescence intensities showed 2.8-fold increase in the expression of vascular marker vWF, as well as 2.2- and 13-fold increment in endothelial progenitor cell markers CD34+ and VEGFR-2, respectively, in the ischemic cortex of G-CSF+tPA-treated rats compared with subjects administered with tPA only (at 6 hours post MCAo). *P<0.05, **P<0.01, n=5–6. Data are expressed as mean±s.e.m. Horizontal bar indicates 100 μM.
Discussion
The main finding of this study was that treatment with G-CSF prevented HT associated with delayed (6 hours post MCAo) tPA therapy as evidenced by reduced hemoglobin content in the brains of G-CSF+tPA-treated rats compared with rats administered with tPA only. Concomitant with the reduction in HT, increased levels of angiogenesis marker Ang-2, vasculogenesis marker vWF, phosphorylated-eNOS, as well as EPC markers CD34+ and VEGFR-2 were observed in the ischemic hemispheres of G-CSF+tPA-treated stroke rats compared with those given tPA alone. Moreover, G-CSF+tPA-treated subjects relative to rats given tPA only, showed improvement in neurological outcomes at 24 hours post-drug treatment. Together, these findings support our hypothesis that G-CSF reduces delayed tPA-induced HT and consequently improves neurological improvement post stroke via angiogenic and vasculogenic activities of G-CSF and/or proliferative or regenerative actions of G-CSF-recruited EPCs.
Reperfusion by thrombolysis, achieved via treatment with tPA, is the only available therapeutic strategy for stroke, a procedure which in itself can also be detrimental and induce hemorrhage and reperfusion injury.2, 3, 4, 5 Therefore, equally important as discovering new drugs for acute ischemic stroke is finding interventions that will reduce detrimental effects associated with delayed tPA treatment and/or extend the thrombolytic efficacy of tPA. Indeed, identifying these types of drugs will benefit a significant number of stroke patients who may have missed the opportunity for tPA treatment. In this study, we found that G-CSF given in tandem with tPA at a delayed time point (i.e., 6 hours post MCAo) significantly reduced the risk of HT and improved post-stroke neurological deficits. These results not only imply brain tissue protection afforded by G-CSF in stroke rats in the context of delayed tPA-induced HT, but also suggest that treatment with G-CSF may augment the efficacy and extend the therapeutic time window of tPA therapy for stroke. Of note, a recent study reported potentiation of hemorrhage when G-CSF was combined with tPA in experimental stroke.30 Nevertheless, comparing the results of the current study to those of the previous report is somewhat difficult given the differences in experimental procedures (e.g., time point of administering tPA), the manner by which tPA was administered, species of animals, and dosages of G-CSF used. Moreover, while our primary goal was to investigate whether G-CSF can attenuate delayed tPA-induced HT, the previous study30 evaluated whether G-CSF can be combined with tPA to enhance thrombolysis.
Disruption of the BBB accompanies stroke31, 32 and is further exacerbated following delayed administration of tPA.5 Given that BBB disruption is one of the mechanisms responsible for the development of HT after delayed tPA therapy, counteracting BBB breakdown may be an important step to prevent unwanted side effects (notably HT) associated with delayed treatment of tPA. Considering that the BBB is composed mainly of endothelial cells,5, 31 enhancing endothelial cell survival or proliferation coupled with facilitating their migration toward the site of BBB damage and their differentiation into mature endothelial cells has been considered as a rational strategy to prevent BBB breakdown post stroke and/or following delayed tPA therapy.5, 31 It has been suggested that exploiting this approach may not only result in better neuroprotection but also reduction of HT during tPA-induced reperfusion post ischemia.5, 31 In vitro studies have documented the ability of G-CSF to enhance endothelial cell proliferation, migration, and survival.13 In this study, we found attenuation of delayed tPA-induced HT in rats administered with G-CSF, indicating that the drug (G-CSF) might have exerted its effect via enhancement of endothelial cell survival or through activation of endothelial cells. Indeed, these findings lend support to the assumed beneficial effects of endothelial cell preservation to protect the BBB, and consequently to prevent HT after delayed tPA therapy. Nevertheless, further studies are required to determine whether G-CSF enhances endothelial cell survival or proliferation in the setting of tPA-induced HT, and also to discover the underlying mechanism(s) of action. Additional studies are warranted to reveal the functionality (i.e., attenuation of BBB breakdown) of G-CSF-activated endothelial cells to establish that G-CSF preserves the integrity of the BBB when given in conjunction with tPA after the therapeutic time window, thereby reducing HT.
Angiogenesis, defined as the outgrowth of new vessels from pre-existing vasculature, is a complex process involving endothelial cell activation, disruption of vascular basement membranes, migration and proliferation of endothelial cells, and the subsequent formation and maturation of blood vessels.26, 27, 28, 29 Meanwhile, vasculogenesis involves the formation of a primitive vessel network by stem and progenitor cells, such as EPCs.27, 28 Previous studies showed ability of G-CSF to induce endothelial cells to express activation/differentiation programs including proliferation and migration, related to angiogenesis.13 In line with this, we observed increased expression of angiogenesis marker Ang-2, but not Ang-1 expression in the ischemic hemisphere of G-CSF+tPA-treated rats, coinciding with the reported differential influence of G-CSF on the expression patterns of these angiopoietins in the brain of stroke rats.17 Although both Ang-1 and Ang-2 have been shown to participate in regulating ischemia-induced angiogenesis in rat brains, Ang-2 reportedly facilitated rapid increase in capillary diameter, the remodeling of the basal lamina, and growth of new blood vessels.17, 33 Along with increased angiogenesis, we also observed enhanced vasculogenesis, as evidenced by increased expression of vasculogenesis marker vWF in the ischemic hemisphere in G-CSF+tPA-treated rats, in accordance with the reported neovascularization (i.e., enhanced vWF expression) within infarcted regions of brain in G-CSF-treated stroke animals.11 Altogether, the above findings substantiate enhancement of endothelial cell activation, proliferation, or survival exerted by G-CSF, thereby preserving the BBB and abrogating HT after delayed tPA treatment. Moreover, increased angiogenesis and vasculogenesis in the ischemic hemisphere may underlie improvement of neurological functions in G-CSF+tPA-treated rats compared with those given vehicle or tPA alone, in view of functional recovery post stroke mediated by enhancement of neovascularization or blood vessel formation especially in brain regions associated with motor and neurological functions.11, 17, 18 Of note, improving neurological deficits post stroke is an important consideration for potential stroke treatments, and therefore, should also be exerted by drugs that could attenuate delayed tPA-induced HT.34 The VEGF signaling pathway has a key role in physiological angiogenesis.35 Blockade of the VEGF pathway reportedly abrogated G-CSF-induced angiogenesis,36 and our in vitro data using the antibody neutralizing paradigm coupled with quantitative reverse transcription-PCR analyses (see Supplementary File) showed that sunitinib, inhibitor of VEGF and angiogenesis, reduced G-CSF-induced upregulation of angiogenesis marker genes (VEGFR-2, VE-cadherin, and vWF), providing evidence for direct modulation of angiogenesis by G-CSF with a mechanism of action potentially involving the VEGF pathway.
EPCs have been showed to migrate and be a home to affected tissue following injury (e.g., ischemic stroke) via cytokine gradients, where they act in a paracrine manner, leading to endothelial cell proliferation and stabilization or through transformation into endothelial cells.11, 25, 26 In rat models of cerebral ischemia, transplantation of EPCs improved functional outcomes37 and promoted tissue regeneration owing to increased angiogenesis25 or vasculogenesis.18 Moreover, in clinical studies, circulating levels of EPCs has been assumed to reflect the temporal profile of recovery from 7 to 14 days after stroke onset.38, 39 Previous studies have shown the capacity of G-CSF to stimulate the release of EPCs from the bone marrow to local sites of ischemia and vascular damage.40 Furthermore, in experimental stroke studies, G-CSF-recruited EPCs have been shown to localize to the injured brain area and subsequently differentiate into endothelial cells to increase vessel formation, indicating that bone marrow-derived EPCs can contribute to brain tissue repair via homing to the ischemic brain. Identifying expression of surface markers CD34+ and VEGFR-2 has been routinely used to characterize and distinguish various types of EPCs.16, 25, 26 Moreover, mobilization of EPCs from the bone marrow has been described to be dependent upon the production of NO, which is synthesized by eNOS.26, 28 Our data showed increased phosphorylation of eNOS in the ischemic cortex of rats administered with G-CSF+tPA compared with those given vehicle and tPA only, indicating promotion and proliferation of endothelial cells and probably, recruitment of EPCs. Immunohistochemistry also showed enhanced expression of CD34+ and VEGFR-2 in the ischemic hemisphere of G-CSF+tPA-treated rats compared with those rats given tPA only, supporting further G-CSF induced recruitment of EPCs and subsequently localization of these cells in the ischemic brain. Correlating increases in EPC markers with enhanced angiogenesis or vasculogenesis in ischemic sites, the functional recovery post stroke, and importantly, reduction of HT in G-CSF-treated animals, may be attributed to the proliferative or reparative effects of G-CSF mobilized EPCs, in addition to direct effects of G-CSF on endogenous endothelial cells. Specifically, attenuation of delayed tPA-induced HT can be attributed to the postulated effects of recruited EPCs to reconstitute the BBB after ischemic injury.25 Nevertheless, other mechanisms may be involved in light of the multi-faceted actions (e.g., anti-inflammatory, neuroprotective, neurotrophic, and regenerative) of G-CSF in stroke as well as in other types of injury.6, 7, 8, 9, 10, 11, 12
In summary, we showed that G-CSF reduced delayed tPA-induced HT post stroke likely via enhancement of endothelial cell survival or owing to the effects of G-CSF-recruited EPCs and their speculated actions to abrogate BBB breakdown, and increase neovascularization or blood vessel formation in ischemic tissues. Enhanced angiogenesis and vasculogenesis may also explain improvement of neurological deficits in G-CSF+tPA-treated rats compared with subjects treated with tPA alone at 24 hours post MCAo. Nevertheless, it remains to be understood whether neurological recovery will also be observed in rats subjected to this combination therapy using other behavioral assays, which are more sensitive to ischemic injury (e.g., rotarod, sticky tests and grip strength, etc.). Additional studies are also warranted to explore whether the acute cerebrovascular preservation exerted by G-CSF is sustained for longer periods of recovery to verify whether effects of G-CSF are transient or permanent. Identifying the cellular and molecular mechanisms by which G-CSF interacts with the effects of delayed tPA treatment after stroke and how it enhances the therapeutic time window of tPA will also be interesting subjects for future studies. In addition, finding the optimum dosage of G-CSF, as well as testing the effects of the combination therapy in other experimental stroke models will facilitate future clinical application of this approach to reduce HT and other complications associated with delayed tPA treatment. Nevertheless, based on the findings of this study, we believe that G-CSF is an attractive therapeutic intervention to reduce detrimental effects associated with delayed tPA administration, most especially HT, and/or extend the thrombolytic efficacy of tPA in stroke patients.
Acknowledgments
This research was funded by the National Institutes of Health, National Institute of Neurological Disorders and Stroke 1R01NS071956-01A1, 1R21NS089851-01, and the James and Esther King Biomedical Research Foundation 1KG01-33966. We thank Genentech for providing the tPA.
CV Borlongan has patents and patent applications on stem cell therapy. All other authors have no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Supplementary Material
References
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics-2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nature Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A. Thrombolysis with alteplase 3 to 4.5 h after acute ischemic stroke. N Eng J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- The NINDS rt-PA Stroke Study Group Intracerebral hemorrhage after intravenous tPA therapy for ischemic stroke. Stroke. 1997;2007;28:2109–2118. doi: 10.1161/01.str.28.11.2109. [DOI] [PubMed] [Google Scholar]
- Wang X, Tsuji K, Lee SR, Ning M, Furie KL, Buchan AM, et al. Mechanisms of hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke. Stroke. 2004;35:2726–2730. doi: 10.1161/01.STR.0000143219.16695.af. [DOI] [PubMed] [Google Scholar]
- Hartung T. Anti-inflammatory effects of granulocyte colony-stimulating factor. Curr Opin Hematol. 1998;5:221–225. doi: 10.1097/00062752-199805000-00013. [DOI] [PubMed] [Google Scholar]
- Schabitz WR, Kollmar R, Schwaninger M, Juettler E, Bardutzky J, Scholzke MN, et al. Neuroprotective effect of granulocyte colony-stimulating factor after focal cerebral ischemia. Stroke. 2003;34:745–751. doi: 10.1161/01.STR.0000057814.70180.17. [DOI] [PubMed] [Google Scholar]
- Schneider A, Kuhn HG, Schabitz WR. A role for G-CSF (granulocyte-colony stimulating factor) in the central nervous system. Cell Cycle. 2005;4:1753–1757. doi: 10.4161/cc.4.12.2213. [DOI] [PubMed] [Google Scholar]
- Han JL, Blank T, Schwab S, Kollmar R. Inhibited glutamate release by granulocyte-colony stimulating factor after experimental stroke. Neurosci Lett. 2008;432:167–169. doi: 10.1016/j.neulet.2007.07.056. [DOI] [PubMed] [Google Scholar]
- Solaroglu I, Tsubokawa T, Cahill J, Zhang JH. Anti-apoptotic effect of granulocyte-colony stimulating factor after focal cerebral ischemia in the rat. Neuroscience. 2006;143:965–974. doi: 10.1016/j.neuroscience.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu WC, Lin SZ, Yang HI, Tzeng YS, Pang CY, Yen PS, et al. Functional recovery of stroke rats induced by granulocyte colony-stimulating factor-stimulated stem cells. Circulation. 2004;110:1847–1854. doi: 10.1161/01.CIR.0000142616.07367.66. [DOI] [PubMed] [Google Scholar]
- Schneider A, Kruger C, Steigleder T, Weber D, Pitzer C, Laage R, et al. The hematopoietic factor GCSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J Clin Invest. 2005;115:2083–2098. doi: 10.1172/JCI23559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussolino F, Ziche M, Wang JM, Alessi D, Morbidelli L, Cremona O, et al. In vitro and in vivo activation of endothelial cells by colony-stimulating factors. J Clin Invest. 1991;87:986–995. doi: 10.1172/JCI115107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu WC, Lin SZ, Lee CC, Liu DD, Li H. Granulocyte colony-stimulating factor for acute ischemic stroke: a randomized controlled trial. CMAJ. 2006;174:927–933. doi: 10.1503/cmaj.051322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawada H, Takizawa S, Takanashi T, Morita Y, Fujita J, Fukuda K, et al. Administration of hematopoietic cytokines in the subacute phase after cerebral infarction is effective for functional recovery facilitating proliferation of intrinsic neural stem/progenitor cells and transition of bone marrow-derived neuronal cells. Circulation. 2006;113:701–710. doi: 10.1161/CIRCULATIONAHA.105.563668. [DOI] [PubMed] [Google Scholar]
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;175:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Lee ST, Chu K, Jung KH, Ko SY, Kim EH, Sinn DI, et al. Granulocyte colony-stimulating factor enhances angiogenesis after focal cerebral ischemia. Brain Res. 2005;1058:120–128. doi: 10.1016/j.brainres.2005.07.076. [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Tajiri N, Shinozuka K, Vasconcellos J, Kaneko Y, Lee HJ, et al. Vasculogenesis in experimental stroke after human cerebral endothelial cell transplantation. Stroke. 2013;4:3473–3481. doi: 10.1161/STROKEAHA.113.001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won S, Lee KH, Wali B, Stein D, Sayeed I. Progesterone attenuates hemorrhagic transformation after delayed tPA treatment in an experimental model of stroke in rats: involvement of the VEGF-MMP pathway. J Cereb Blood Flow Metab. 2014;34:72–80. doi: 10.1038/jcbfm.2013.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro M, Kawasaki K, Suzuki Y, Ishizuka F, Mishiro K, Egashira Y, et al. A Rho kinase (ROCK) inhibitor, fasudil, prevents matrix metalloproteinase-9-related hemorrhagic transformation in mice treated with tissue plasminogen activator. Neuroscience. 2012;220:302–312. doi: 10.1016/j.neuroscience.2012.06.015. [DOI] [PubMed] [Google Scholar]
- Ishiguro M, Mishiro K, Fujiwara Y, Chen H, Izuta H, Tsuruma K, et al. Phosphodiesterase-III inhibitor prevents hemorrhagic transformation induced by focal cerebral ischemia in mice treated with tPA. PLoS ONE. 2010;5:e15178. doi: 10.1371/journal.pone.0015178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta SA, Tajiri N, Shinozuka K, Ishikawa H, Sanberg PR, Sanchez-Ramos J, et al. Combination therapy of human umbilical cord blood cells and granulocyte colony stimulating factor reduces histopathological and motor impairments in an experimental model of chronic traumatic brain injury. PLoS One. 2014;129:e90953. doi: 10.1371/journal.pone.0090953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Z, Karabiyikoglu M, Hua Y, Silbergleit R, He Y, Keep RF, et al. Hyperbaric oxygen-induced attenuation of hemorrhagic transformation after experimental focal transient cerebral ischemia. Stroke. 2007;38:1362–1367. doi: 10.1161/01.STR.0000259660.62865.eb. [DOI] [PubMed] [Google Scholar]
- Matsukawa N, Yasuhara T, Hara K, Xu L, Maki M, Yu G, et al. Therapeutic targets and limits of minocycline neuroprotection in experimental ischemic stroke. BMC Neurosci. 2009;6:126. doi: 10.1186/1471-2202-10-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlongan CV. Bone marrow stem cell mobilization in stroke: a 'bonehead' may be good after all! Leukemia. 2011;25:1674–1686. doi: 10.1038/leu.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- Liman TG, Endres M. New vessels after stroke: postischemic neovascularization and regeneration. Cerebrovasc Dis. 2012;33:492–999. doi: 10.1159/000337155. [DOI] [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiongenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Endres M, Laufs U, Liao JK, Moskowitz MA. Targeting eNOS for stroke protection. Trends Neurosci. 2004;27:283–289. doi: 10.1016/j.tins.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Gautier S, Ouk T, Tagzirt M, Lefebvre C, Laprais M, Pétrault O, et al. Impact of the neutrophil response to granulocyte colony-stimulating factor on the risk of hemorrhage when used in combination with tissue plasminogen activator during the acute phase of experimental stroke. J Neuroinflammation. 2014;11:96. doi: 10.1186/1742-2094-11-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlongan CV. Blood brain barrier in stroke. Curr Pharm Des. 2012;18:3613–3614. doi: 10.2174/138161212802002751. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Ramsauer M, D'Amore PA. Getting Tie(2)d up in angiogenesis. J Clin Invest. 2002;110:1615–1617. doi: 10.1172/JCI17326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishrat T, Pillai B, Ergul A, Hafez S, Fagan SC. Candesartan reduces the hemorrhage associated with delayed tissue plasminogen activator treatment in rat embolic stroke. Neurochem Res. 2013;38:2668–2677. doi: 10.1007/s11064-013-1185-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56:549–580. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- Ohki Y, Heissig B, Sato Y, Akiyama H, Zhu Z, Hicklin DJ, et al. Granulocyte colony-stimulating factor promotes neovascularization by releasing vascular endothelial growth factor from neutrophils. FASEB J. 2005;19:2005–2007. doi: 10.1096/fj.04-3496fje. [DOI] [PubMed] [Google Scholar]
- Fan Y, Shen F, Frenzel T, Zhu W, Ye J, Liu J, et al. Endothelial progenitor cell transplantation improves long-term stroke outcome in mice. Ann Neurol. 2010;67:488–497. doi: 10.1002/ana.21919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Sobrino M, Rosell A, Hernandez-Guillamon M, Penalba A, Ribo M, Alvarez-Sabin J, et al. Mobilization, endothelial differentiation and functional capacity of endothelial progenitor cells after ischemic stroke. Microvasc Res. 2010;80:317–323. doi: 10.1016/j.mvr.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Navarro-Sobrino M, Rosell A, Hernandez-Guillamon M, Penalba A, Boada C, Domingues-Montanari S, et al. A large screening of angiogenesis biomarkers and their association with neurological outcome after ischemic stroke. Atherosclerosis. 2011;216:205–211. doi: 10.1016/j.atherosclerosis.2011.01.030. [DOI] [PubMed] [Google Scholar]
- Toth ZE, Leker RR, Shahar T, Pastorino S, Szalayova I, Asemenew B, et al. The combination of granulocyte colony-stimulating factor and stem cell factor significantly increases the number of bone marrow-derived endothelial cells in brains of mice following cerebral ischemia. Blood. 2008;111:5544–5552. doi: 10.1182/blood-2007-10-119073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.