Abstract
MicroRNA 122 (miR-122) is a tumor suppressor for hepatocellular carcinoma (HCC) but is lowly expressed in HCC cells. MiR-151 is aberrantly overexpressed in HCC cells and promotes HCC metastasis yet its roles on HCC tumorigenicity are unknown. To combat HCC tumorigenicity/metastasis, we developed Sleeping Beauty (SB)-based hybrid baculovirus (BV) vectors that expressed (i) miR-122 precursors (pre-miR-122), (ii) miR-151 sponges, or (iii) pre-miR-122 and miR-151 sponges. Transduction of aggressive HCC cells (Mahlavu) with the pre-miR-122-expressing BV tremendously enhanced miR-122 levels for >6 weeks, suppressed the levels of downstream effectors (e.g., ADAM10 and Bcl-w), proliferation, anchorage-independent growth, motility and migration/invasion in vitro. Intratumoral injection of the pre-miR-122-expressing BV attenuated the HCC growth/metastasis. The miR-151 sponges-expressing BV diminished the miR-151 levels for 6 weeks, enhanced RhoGDIA expression, suppressed RhoGTPases, as well as motility and migration/invasion of Mahlavu cells. Intratumoral injection of the miR-151 sponge-expressing BV impeded not only HCC metastasis but also cell proliferation, MMP expression and tumor growth in vivo. The BV co-expressing pre-miR-122 and miR-151 sponges also simultaneously enhanced miR-122 expression and inhibited miR-151, and conferred antitumor/anti-metastasis effects albeit lack of synergism. These data implicate the potentials of the SB-based hybrid BV for persistently modulating miRNA and suppressing HCC tumorigenicity/metastasis.
Introduction
Hepatocellular carcinoma (HCC) is one of the most malignant tumors, causing more than 600,000 deaths per year. The high mortality rate of HCC is chiefly related to metastases through multistep processes including cell proliferation, migration, invasion and extracellular matrix degradation by matrix metalloproteinase (MMP).1 MicroRNAs (miRNAs) are small non-coding RNAs that regulate gene expression and may act as oncogenes or tumor suppressors.2 miRNAs may be upregulated or downregulated in distinct types of cytogenetic abnormalities and such aberrant miRNA expression can lead to transformation and malignancy.2
Among the wide array of tumor suppressing miRNAs, miR-122 is abundantly expressed in hepatocytes but is downregulated in more than 70% of HCC cells. MiR-122 can inhibit tumorigenic properties of HCC cells by directly targeting ADAM10 (a distintegrin and metalloprotease family 10), serum response factor (SRF), insulin-like growth factor 1 receptor (IGF-1R)3 and Bcl-w.4 Furthermore, miR-122 suppresses tumors and regulates intrahepatic metastasis via targeting ADAM17.5 By modulating cyclin G1 (CCNG1), miR-122 also influences p53 protein stability and reduces the invasiveness of HCC-derived cell lines.6
Conversely, miR-151 was discovered to be highly expressed together with its host gene focal adhesion kinase (FAK) in HCC cells (Huh-7 and MHCC-LM3) and increases cell migration/invasion in vitro and in vivo.7 MiR-151 exerts this function by directly targeting RhoGDIA, a RhoGTPase inhibitor that can attenuate HCC cell migration/invasion. MiR-151 upregulation in HCC cells directly suppresses RhoGDIA expression and in turn activates RhoGTPases such as Rac1, Cdc42, and Rho A to promote cell migration/invasion and HCC metastasis.7
Baculovirus (BV) is a promising gene delivery vector and has been exploited for various applications, including tissue engineering,8,9,10 mediation of RNA interference11,12,13 and cancer therapy.14,15 However, BV typically mediates short-term expression because BV does not replicate within transduced cells and BV DNA integration into host chromosomes is extremely rare.16 The transient expression nature would preclude the use of BV vectors for cancer therapy for which stable expression is desired. To enable long-term expression, we developed a Sleeping Beauty (SB) transposon-based hybrid BV system, which comprises 2 BV vectors: one expressing SB100X (a hyperactive SB transposase) and the other harboring the genetic cargo flanked by the inverted repeats (IR)/directed repeats (DR) elements.15 Within the cells co-transduced with the two BV vectors, the SB transposase binds to the IR/DR sequences and mediates the transgene excision from the donor BV and subsequent integration into the chromosome.15 Similar SB-based BV vector was also constructed for gene delivery into mouse eyes.17
Due to the roles of miRNAs in cancer, restoring normal miRNA programs in cancer cells may rewire the cell connectivity map and reverse cancer phenotypes. Given the potential of BV as a gene delivery vector, this study aimed to develop SB-based hybrid BV vectors for miRNA modulation and HCC therapy. Since miR-122 downregulation is associated with HCC tumorigenesis/metastasis, we constructed hybrid BV vectors that enabled persistent miR-122 overexpression. Because miR-151 overexpression contributes to HCC metastasis, to saturate miR-151 we constructed hybrid BV vectors that exploited miRNA sponges, which are transcripts with miRNA binding sites capable of sequestering miRNAs from endogenous targets.18 The effects of sustained miR-122 overexpression and miR-151 inhibition on Mahlavu cell tumorigenicity/invasiveness and HCC tumor growth/metastasis were compared in vitro and in vivo. Whether simultaneously overexpressing miR-122 and repressing miR-151 synergistically suppressed HCC cell tumorigenicity and tumor growth/metastasis was also evaluated.
Results
Confirmation of BV-mediated modulation of miR-122/miR-151
We first examined whether miR-122 downregulation and miR-151 overexpression indeed occurred in different cells by qRT-PCR. Compared with the levels in primary human hepatocytes (HH), the miR-122 levels in HCC (SNU-449, Mahlavu, HepG2) and non-HCC (HEK293, A549) cells were significantly lower (Figure 1a), whereas miR-151 levels were significantly higher in HCC cells (Figure 1b). In particular, Mahlavu had the highest miR-151 level, suggesting its high metastasis potential.7
Figure 1.
Expression profiles of miR-122 and miR-151. (a) Endogenous miR-122 levels in primary human hepatocytes (HH), HCC and non-HCC cell lines. (b) Endogenous miR-151 levels. (c) Schematic illustrations of BV vectors, the expressed miR-151 sponge and target miRNA. (d) miR-122 levels after BV transduction. (e) miR-151 levels after BV transduction. The miR-122/miR-151 levels were determined by TaqMan qRT-PCR using U6 small nuclear RNA as an internal control. For endogenous expression, the data were normalized to those in HH. For miRNA levels after BV transduction, the data were normalized to those of mock-transduced cells. All data represent the means ± SD of three independent culture experiments. CMV, CMV promoter; W, WPRE (woodchuck hepatitis virus post-transcriptional regulatory element) sequence; pA, polyadenylation signal; d2EGFP, destabilized green fluorescent protein.
To explore the feasibility of augmenting miR-122 and inhibiting miR-151 by BV, we constructed 2 BV vectors (Figure 1c). Bac-m122 harbored eight tandem copies of miR-122 precursors (pre-miR-122) flanked by the splicing donor and branch/splicing acceptor. Bac-s151 carried six tandem copies of sponges comprised of binding sites for miR-151 and a 4 bp bulge (ATCT). The qRT-PCR analysis depicted that in all three HCC cells Bac-m122 transduction at MOI 100 or 500 remarkably elevated mature miR-122 levels (Figure 1d) while Bac-s151 transduction significantly decreased the mature miR-151 levels (Figure 1e), when compared to the Mock and Bac-CdE (a d2EGFP-expressing BV as control)19 transduction. Compared with MOI 100, MOI 500 resulted in higher miR-122 levels in Mahlavu and lower miR-151 levels in SNU-449 and HepG2, thus MOI 500 was used in all following experiments.
Development of SB-based hybrid BV vectors for sustained miR-122/miR-151 modulation
To enable long-term miRNA modulation, we next constructed Bac-SB that expressed SB100X and three hybrid BV vectors with transgene cassettes flanked by IR/DR elements (Figure 2a): Bac-T2-CdE (expressing d2EGFP as control), Bac-T2-m122 (expressing the clustered pre-miR-122), and Bac-T2-s151 (expressing the tandem miR-151 sponges). We also constructed Bac-T2-Dual for persistently expressing clustered pre-miR-122 and miR-151 sponges, in attempts to simultaneously modulate miR-122/miR-151. Among the three HCC lines, Mahlavu had low miR-122 levels, high miR-151 levels (Figure 1a,b) and high metastatic potentials.20 Thus we chose the aggressive Mahlavu cells for testing and co-transduced Mahlavu cells with Bac-SB (MOI 500) and the hybrid BV (MOI 500). This co-transduction condition enabled the transgene transposition into cells and stable transgene expression for >35 days (Supplementary Figure S1). qRT-PCR analyses delineated that mock transduction (Mock group) and Bac-SB/Bac-T2-CdE co-transduction (SB/CdE group) did not significantly alter the mature miR-122 (Figure 2b) and miR-151 (Figure 2c) levels throughout the experiments. Yet Bac-SB/Bac-T2-m122 co-transduction (SB/m122 group) remarkably enhanced the miR-122 levels while Bac-SB/Bac-T2-s151 co-transduction (SB/s151 group) significantly attenuated the miR-151 levels for 6 weeks. Notably, Bac-SB/Bac-T2-Dual co-transduction (SB/Dual group) simultaneously upregulated miR-122 expression and repressed miR-151 levels for 6 weeks.
Figure 2.

Sustained miR-122/miR-151 modulation in Mahlavu cells by the hybrid BV vectors. (a) Illustrations of BV vectors. (b) miR-122 expression. (c) miR-151 suppression. The miR-122/miR-151 levels at different times were measured by qRT-PCR as in Figure 1 and normalized to the levels at 1 dpt. All data represent the means ± SD of three independent culture experiments.
Effects of miR-122/miR-151 modulation on downstream protein levels
MiR-122 regulates metastasis of HCC cells via targeting ADAM10, ADAM17, Bcl-w, CCNG1, IGF-1R, and SRF.21 Compared with the Mock group, both SB/m122 and SB/Dual groups significantly (P < 0.05) reduced the protein levels of ADAM10, ADAM17, Bcl-w, CCNG1, IGF-1R, and SRF, as verified by western blot and scanning densitometry (Figure 3a). Conversely, miR-151 overexpression in HCCs negatively regulates RhoGDIA, and hence upregulates RhoGTPases such as Cdc42, Rac1, and RhoA.7 In Mahlavu cells, miR-151 inhibition in the SB/s151 and SB/Dual groups significantly restored the RhoGDIA protein level and concomitantly diminished the protein levels of FAK and active forms of Cdc42 (Cdc42-GTP), Rac1 (Rac1-GTP), and RhoA (RhoA-GTP) (Figure 3b).
Figure 3.
Effects of miR-122/miR-151 modulation on downstream protein levels in Mahlavu cells. (a) Protein levels of ADAM10, ADAM17, Bcl-w, cyclin G1 (CCNG1), IGF-1R, and SRF. (b) Protein levels of RhoGDIA, FAK/PTK2, Cdc42-GTP, Rac1-GTP, and RhoA-GTP. Mahlavu cells were transduced with the BV vectors and analyzed by western blot at 1 dpt. The band intensity in each group was normalized to that in the mock-transduced cells to yield the relative quantity. All data represent the means ± SD of three independent culture experiments.
Effects of miR-122/miR-151 modulation on HCC cell behavior
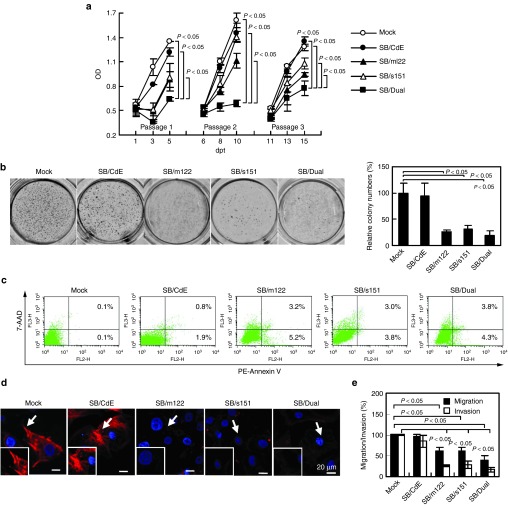
To explore the effects of miR-122/miR-151 modulation on cell proliferation, the transduced cells were cultured and passaged at 6 and 11 days post-transduction (dpt). The BrdU proliferation assay (Figure 4a) attested that the SB/Dual group more effectively decelerated the proliferation than the Mock and SB/CdE groups throughout the experiments. The SB/m122 and SB/s151 groups also suppressed the proliferation albeit to a lesser extent after passaging.
Figure 4.
Effects of miR-122/miR-151 modulation on Mahlavu cell behavior. (a) Proliferation rates. (b) Anchorage-independent growth. (c) Apoptosis. (d) Motility. (e) Migration and invasion. For proliferation rate analysis, the cells were cultured and passaged at 6 and 11 dpt. Cell densities were measured by the BrdU proliferation assay and are expressed as OD450. For the anchorage-independent growth determined by colony formation assay, the colony number of each group was counted and normalized to that of the Mock group. Cell apoptosis was analyzed by Annexin V-PE/7-AAD staining coupled with flow cytometry. Percentages of apoptotic (lower right) and late apoptotic/dead (upper right) cells are shown. The cell motility, as judged by F-actin polymerization and hence stress fiber assembly, was determined by rhodamine-phalloidin staining. Images representative of three independent culture experiments are shown. Migration/invasion was evaluated using transwell assays at 1 dpt. All quantitative data are means ± SD of three independent culture experiments.
The anchorage-independent growth was examined by the colony formation assay, which demonstrated significantly fewer colonies in the SB/m122, SB/s151, and SB/Dual groups than in the control groups (Figure 4b). The apoptosis assays (Figure 4c) revealed negligible apoptosis in the Mock (0.2 ± 0.1%) and SB/CdE groups (2.7 ± 1.1%), but elevated cell apoptosis in the SB/m122 (8.7 ± 0.7%), SB/s151 (6.8 ± 1.9%), and SB/Dual (8.1 ± 2.9%) groups. Furthermore, Mahlavu cell motility was assessed by rhodamine phalloidin staining of F-actin (Figure 4d). Compared to the Mock and SB/CdE groups in which stress fiber essential for motility was evident, the SB/m122, SB/s151, and SB/Dual groups resulted in severe reduction of stress fiber formation. In accord, the SB/m122, SB/s151, and SB/Dual groups led to less migration and invasion than the Mock and SB/CdE groups as determined by transwell assays (Figure 4e). Figure 4b–e collectively confirmed that forced miR-122 expression and/or miR-151 inhibition in Mahlavu cells slightly enhanced apoptosis and suppressed anchorage-independent growth, motility and migration/invasion. However, no significant differences existed between the SB/m122, SB/s151, and SB/Dual groups. In addition to Mahlavu, in another HCC cell line (SNU-449) the SB/m122, SB/s151, and SB/Dual groups also regulated the downstream genes, which diminished cell proliferation, enhanced apoptosis and attenuated migration/invasion (Supplementary Figure S2), indicating that these vectors were applicable to different HCC cells.
HCC tumor suppression by in vivo modulation of miR-122/miR-151
To examine the efficacy of miR-122/miR-151 modulation for tumor suppression, we injected Mahlavu cells subcutaneously into nude mice, and co-injected Bac-SB (1 × 109 pfu) and one of the hybrid BV vectors (1 × 109 pfu) intratumorally when the tumors became palpable. Compared with PBS injection (n = 5), the SB/CdE group (n = 6) decelerated the tumor growth (Figure 5a), which was attributed to the BV-elicited innate immune responses22 that attenuated tumor growth.23 The SB/m122 (n = 8), SB/s151 (n = 8), and SB/Dual (n = 8) groups further suppressed the tumor growth (left panel, Figure 5a). At 30 days after injection, the tumor volume (right panel, Figure 5a) in the SB/m122 (308.6 ± 176.4 mm3), SB/s151 (461.3 ± 140.3 mm3), and SB/Dual (141.3 ± 104.2 mm3) groups was significantly (P < 0.05) lower than that in the PBS (1566.9 ± 370.0 mm3) and SB/CdE (832.4 ± 210.8 mm3) groups. The tumor volume in the SB/Dual group was lower, despite statistically insignificantly, than that in the SB/s151 (P = 0.06) and SB/m122 (P = 0.23) groups. The volume in the SB/m122 group was also lower than that in the SB/s151 groups despite the lack of statistical difference (P = 0.34). Meanwhile, we observed no significant variations in the body weight in all groups (Supplementary Figure S3), indicating negligible side effects of these BV vectors.
Figure 5.
Effects of miR-122/miR-151 modulation on HCC tumorigenesis. (a) Tumor growth (left panel) and tumor volume at 30 days after BV injection (right panel). (b) In vivo cell proliferation analysis by Ki-67 immunostaining. (c) In vivo apoptosis analysis by caspase-3 immunostaining. (d) MMP-2 immunostaining. (e) MMP-9 immunostaining. Mahlavu cells were injected subcutaneously into nude mice and tumor volume reached ≈10 mm3 in 7 days. The mice received intratumoral injection of 100 μl PBS or BV vectors (n = 5–8) at this time (defined as day 0). The tumor volume was recorded for another 30 days and the data represented means ± SD. All mice were sacrificed at day 30 and the tumor sections were subjected to immunohistochemical staining and DAPI counterstaining. The images representative of sections from five animals are shown. The numbers of Ki-67+, caspase-3+, MMP-2+, or MMP-9+ cells were quantified from 15 fields (from five tumors each group) and divided by the total number of cells (DAPI+ cells) to yield the percentage of specific protein-positive cells.
At day 30, tumors were sectioned and analyzed by immunohistochemical staining. Figure 5b illustrates high percentages of Ki-67+ cells in the PBS (54.2 ± 17.5%) and SB/CdE (45.8 ± 13.3%) groups, indicating active cell proliferation within the tumors. The percentage of Ki-67+ cells in the SB/s151 group (23.1 ± 10.3%) was statistically (P < 0.05) lower than those in the PBS and SB/CdE groups, but was higher (P < 0.05) than those in the SB/m122 (9.0 ± 2.5%) and SB/Dual (8.5 ± 3.4%) groups. Caspase-3 staining, an indicator of apoptosis, was faint and rare in the PBS, SB/CdE and SB/s151 groups (Figure 5c). However, the percentage of caspase-3+ cells was significantly elevated in the SB/m122 (22.4 ± 9.1%) and SB/Dual (35.5 ± 12.3%) groups, indicating the induction of apoptosis within the tumors. Moreover, MMP-2 (Figure 5d) and MMP-9 (Figure 5e) expressions, both being required for metastasis, were evident in the PBS and SB/CdE groups, but were markedly repressed in the SB/m122, SB/s151, and SB/Dual groups.
Effects of miR-122/miR-151 modulation on pulmonary metastasis
To further confirm whether regulation of miR-122/miR-151 was able to suppress pulmonary metastasis, we injected 1 × 106 mock-transduced or transduced Mahlavu cells through tail vein into nude mice. Eight weeks after injection, lungs were removed from mice and examined for metastatic nodules by visual observation (Figure 6a) and H&E staining (Figure 6b). As shown, metastatic nodules were evidently observed in two out of two mice injected with CT26 (colon cancer cells as the control), five out of six mice in the Mock group and four out of six mice in the SB/CdE group. The SB/s151 group partially suppressed lung metastasis as only four out of eight mice developed metastatic nodules. Metastasis was only observed in one out of eight mice in the SB/m122 and SB/Dual groups, demonstrating that SB/m122 and SB/Dual groups suppressed the pulmonary metastasis induced by the aggressive Mahlavu cells.
Figure 6.
Gross and microscopic views of metastatic nodules in nude mice. (a) Pictures of metastatic nodules (indicated by arrows) in the lung of nude mice. (b) H&E staining of lung sections. CT26 or Mahlavu cells were injected into nude mice via tail vein and the lungs were removed at week 8 for visual observation and sectioning/H&E staining.
Discussion
This study aimed to develop a BV-based gene therapy approach for forced miRNA expression and/or miRNA inhibition in order to suppress HCC tumorigenesis/metastasis. Given the miR-122 downregulation and miR-151 overexpression in HCC cells (Figure 1a,b), we constructed Bac-m122 and Bac-s151 (Figure 1c). We inserted eight copies of pre-miR-122 in Bac-m122 because multimerization of miRNA for up to eight tandem copies potentiates the inhibition of its cognate mRNA.24 We also added the splicing donor and branch/splicing acceptor to flank the clustered pre-miR-122 to form a chimeric intron because intronic miRNAs are more efficiently processed.25 This design allowed for robust production of mature miR-122 in 3 HCC cells (Figure 1d), indicating effective pre-miR-122 expression and processing. To decoy miR-151, we inserted six copies of miR-151 sponges in Bac-s151 because the optimal number of tandem miRNA sponges is 4–10 yet increasing the number may have diminishing marginal utility.26 Since the spacing between the miRNA sponges influences the binding affinity between the sponges and their cognate miRNA, and a central bulge opposite to position 10–11 of the miRNA prevents endonucleolytic target cleavage,27 the miRNA sponges were designed to be separated by a 3-nt linker and contain a 4-nt bulge. Such design effectively diminished the miR-151 levels in the three HCC cells (Figure 1e). Besides sponge, TuD (tough decoy) RNA is another miRNA inhibitor and was reported to outduel sponge with regard to miRNA inhibition.28 Such a TuD design may be incorporated into the BV vectors to substantiate the miRNA inhibition.
These designs were further combined with the SB transposon for constructing SB-based hybrid BV vectors (Figure 2a), which remarkably enhanced the miR-122 expression (SB/m122 group) while repressing miR-151 levels (SB/s151 group) for at least 6 weeks (Figure 2b,c). Consistent with previous reports,3,5,21 the hybrid BV-mediated ectopic miR-122 expression successfully knocked down the proteins levels of ADAM10, ADAM17, Bcl-w, CCNG1, IGF-1R, and SRF (Figure 3a). ADAM family proteins are involved in cell adhesion, migration and invasion.29 Bcl-w plays an important role in the resistance of cancer cells to intrinsic apoptosis.21 CCNG1 regulates cell cycle and is closely linked to cancer development. IGF-1R is a receptor tyrosine kinase and stimulates cell growth and proliferation30 while SRF is a transcription factor that regulates cell proliferation, differentiation and cytoskeleton reorganization.31 Concomitant with the knockdown of these effector proteins, the BV-mediated miR-122 expression by the SB/m122 group enhanced Mahlavu apoptosis and repressed Mahlavu proliferation, anchorage-independent growth, motility and migration/invasion (Figure 4). Consequently, injection of SB/m122 group into the aggressive HCC (Mahlavu) tumors attenuated the growth and metastasis thanks to the enhanced cell apoptosis, repressed cell proliferation and decreased MMP activities (Figures 5 and 6). The SB/m122 group also upregulated E-cadherin and downregulated vimentin in vitro (data not shown), suggesting that ectopic miR-122 expression suppressed epithelial-mesenchymal transition, which at least partly accounted for the inhibited metastasis. These results confirmed that restoration of miR-122 expression by the hybird BV vector mitigated the tumorigenic/metastatic potentials of Mahlavu cells and suppressed HCC growth/metastasis, which agreed with results from ectopic miR-122 expression reported previously.3,5
Conversely, the BV-mediated miR-151 inhibition (SB/s151 group) restored RhoGDIA expression and suppressed the RhoGTPases (Cdc42, Rac1, and RhoA) (Figure 3b) that play crucial roles in actin reorganization, cytoskeleton rearrangement and cancer metastasis.1 As a result, the SB/s151 group compromised the Mahlavu's anchorage-independent growth, motility, migration and invasion (Figure 4). It should be stressed that very little is known about the target genes and physiological roles of miR-151, despite the recent findings that miR-151 upregulation stimulates HCC metastasis by targeting RhoGDIA7 and miR-151 downregulation contributes to increased susceptibility to arrhythmogenesis.32 Furthermore, how miR-151 affects cancer development remains poorly understood. Here we unveiled that BV-mediated miR-151 inhibition also suppressed FAK expression (Figure 3b) and slightly enhanced apoptosis while marginally impeded Mahlavu proliferation (Figure 4). Importantly, we uncovered that in vivo miR-151 inhibition also impeded HCC cell proliferation, MMP expression and tumor development (Figures 5 and 6). Since each miRNA potentially targets a large number of genes,33 it was likely that miR-151 also targeted other genes involved in cell tumorigenicity and cancer development. For instance, it was recently found that miR-151 is associated with cyclin E1 expression and nasopharyngeal carcinoma.34 However, further investigations are needed to unveil other target gene(s) of miR-151 and the molecular targets contributing to the tumor-suppressing effects.
In comparison with ectopic miR-122 expression, miR-151 inhibition was similarly effective in inducing apoptosis and suppressing anchorage-independent growth, motility and migration/invasion in vitro (Figure 4). However, in vivo miR-151 inhibition was less effective in triggering apoptosis and suppressing cell proliferation, tumor growth and pulmonary metastasis (Figures 4–6). The relative superiority of forced miR-122 expression over miR-151 inhibition was likely because miR-151 inhibition exerted its effects primarily via suppression of RhoGTPases-associated pathway essential for metastasis, whereas ectopic miR-122 expression concurrently knocked down multiple genes involved in various pathways (e.g., proliferation, migration, invasion, apoptosis, etc.), leading to more potent antitumor/anti-metastasis effects.
Intriguingly, although the hybrid BV vector harboring both miR-122 cassette and miR-151 sponges concomitantly enhanced miR-122 expression and inhibited miR-151 (Figure 2), the SB/Dual group did not impart significant advantages over the SB/m122 group with regard to anchorage-independent growth and migration/invasion. Neither did the SB/Dual group confer more pronounced antitumor and anti-metastasis efficacy than the SB/m122 group (Figures 5–6). Therefore, co-modulation of two miRNAs does not necessarily confer synergistic effects. Intuitively, this might stem from the saturation of miRNA processing pathway due to the co-expression of multiple copies of pre-miR-122 and miR-151 sponges. However, the SB/Dual group regulated the molecular pathways governed by miR-122 and miR-151 as effectively as the SB/m122 and SB/s151 groups (Figure 3), thus ruling out this possibility. As such, the insignificant synergism might be ascribed to two reasons: (i) miR-122 expression alone already imparted very potent antitumor and anti-metastasis effects, thus masking the advantage of the SB/Dual group. (ii) Individual miRNAs may regulate hundreds of target genes to coordinate complex gene expression programs. Simultaneous regulation of miR-122 and miR-151 might have evoked unexpected disturbance of global gene regulation network, thus offsetting the synergistic therapeutic effects. Future bioinformatic analyses and comprehensive miRNA profiling are needed to confirm this hypothesis.
miRNA-based therapy holds promise for the treatment of a wide spectrum of cancers2 and virus infections,35 but how to effectively manage miRNA remains to be challenging. miRNA-based gene silencing can be achieved by delivery of synthetic miRNA mimics36 or pri-miRNA mimics while miRNA itself may be decoyed by inhibitors such as antagomirs, sponges33 or mirvirsen.37 However, transfection of these synthetic oligonucleotides or miRNA-encoding plasmid suffers from low delivery efficiency (especially into HCC cells) and requires repeated administration to achieve persistent miRNA modulation.28 In contrast, viral vector-based approach is advantageous in terms of delivery efficiency, and adenoviral,21 adeno-associated viral (AAV)38 and lentiviral39 vectors have been widely used. AAV and lentiviral vectors confer stable expression, thus being able to modulate the miRNA repertoire for a long-term. However, their cloning capacity is low and their production is cumbersome and costly due to the need to transfect multiple plasmids into packaging cells.40 Furthermore, AAV and lentiviral vectors induce transgene integration into chromosomes41,42 and may cause insertional mutagenesis/malignancy in animals.42,43 Conversely, adenovirus is a powerful vector, but adenovirus mounts potent immune responses and its clinical efficacy, similar to AAV, may be hindered by pre-existing immunity.
In contrast, BV is non-pathogenic and humans do not possess pre-existing immunity against BV. BV has a large cloning capacity (at least 38 kb) and can be readily produced to high titers. Importantly, BV efficiently transduces mammalian cells including liver and HCC cells (for review see ref. 44,45). These attributes render BV a promising vector for miRNA delivery into HCC tumors. However, BV typically mediates transient expression. This problem has been tackled by the development of hybrid BV based on minicircle formation9,16 or replicon.46 The transgenes delivered by these vectors, however, are episomal and the expression is still transient. The hybrid BV we developed here overcame the problem and enabled persistent miR-122/miR-151 modulation by exploiting the SB-mediated transposition. Unlike gammaretrovirus and lentivirus that have the propensity to integrate transgenes into transcription start sites or transcription units,42 SB results in fairly unbiased genomic integration and reduces the risk of insertional mutagenesis.47 Moreover, SB seems to trigger significantly milder epigenetic changes at the genomic insertion site, hence minimizing the potential genotocxicity.47
In summary, we developed SB-based hybrid BV vectors that enabled persistent miRNA modulation and inhibition of HCC tumorigenesis/metastasis, by ectopic miR-122 expression and/or by miR-151 inhibition, which suggested the potential of the hybrid BV for persistently augmenting tumor suppressing miRNA (e.g., miR-26a) and/or antagonizing overexpressed oncomirs (e.g., miR-21). These results altogether implicate the potential of such SB-based hybrid BV vectors for the therapy of HCC and other cancers.
Materials and Methods
Cells, recombinant BV preparation and transduction. Human hepatocytes (HH) were purchased from Lonza (Walkersville, MD). Human cell lines were obtained from Bioresource Collection and Research Center, Taiwan. A549, HEK293, HepG2 were cultured in DMEM (Sigma) containing 10% fetal bovine serum (FBS, Biological Industries) and 1% penicillin/streptomycin (P/S). Mahlavu cells were cultured in DMEM containing 10% FBS, 1% L-glutamine (Life Technologies), 1% MEM non-essential amino acids (Life Technologies) and 1% P/S. SNU-449 and CT-26 (murine colon cancer) cells were maintained in RPMI-1640 medium (Life Technologies) containing 10% FBS and 1% P/S.
Construction of recombinant BV vectors Bac-m122, Bac-s151, Bac-SB, Bac-T2-CdE, Bac-T2-m122, Bac-T2-s151 and Bac-T2-Dual is described in Supplementary Methods. Primers used for BV vectors construction, sequence of the chimeric intron, and synthetic tandem copies of miR-151-binding sites are listed in Supplementary Tables S1–S3. BV vectors were amplified by infecting Sf-9 cells and BV titers (plaque forming units (pfu) per milliliter) were determined by end-point dilution method.48 HCC cells were transduced in six-well plates with BV as described16 using different multiplicity of infection (MOI, pfu/cell). For co-transduction in each well, the two viruses were mixed at certain volume depending on the MOI and diluted to 100 μl with TNM-FH medium. The virus solution was mixed with 400 μl NaHCO3-deficient DMEM (pH 7.4) and added to cells to initiate the transduction. Mock transduction was performed by treating cells with a mixture of 100 μl TNM-FH medium and 400 μl NaHCO3-deficient DMEM medium. After 6 hours transduction on the rocking plate at room temperature, we replaced the virus mixture with fresh medium and cultured the cells in medium containing 3 mmol/l sodium butyrate for 24 hours. Alternatively, at 1 dpt the medium was replaced with complete medium without sodium butyrate and continued to be cultured.
Quantitative real-time reverse transcription PCR (qRT-PCR) and western blot. Total RNA was isolated using Trizol (Life Technologies) and stored at −80 °C until analysis. The levels of mature miRNA were quantified based on quantitative stem loop PCR using the TaqMan MicroRNA Assays kit (Applied Biosystems) with U6 RNA as an internal control. Threshold cycle (Ct) values of each miRNA were corrected by the Ct values of the internal control to yield −ΔCt values. All data were normalized to those of HH or mock-transduced cells at 1 dpt. Proteins were analyzed by western blot as described in Supplementary Methods.
Cell proliferation and apoptosis assays. For proliferation assay, transduced cells were seeded to 96-well plates (2 × 103 cells/well), cultured and passaged to new 96-well plates (5 × 103 cells/well) at 6 and 11 dpt. The cell densities were analyzed at different times using the BrdU cell proliferation assay kit (Roche) and are expressed as optical densities at 450 nm (OD450). Apoptosis was assessed using the PE Annexin V Apoptosis Detection Kit I (BD Pharmingen) coupled with flow cytometry analysis.
Colony formation assay. Agarose solution (1.2% (w/v)) was mixed with 2× DMEM containing 20% FBS in a 1:1 ratio and added to six-well plates (1.5 ml/well) to form the bottom layer. In parallel, trypsinized cells were resuspended in 2× DMEM containing 20% FBS and mixed with equal volume of 0.8% (w/v) agarose solution. The cell-containing mixture (1.5 ml containing 1 × 104 cells per well) was overlaid to the bottom agarose layer as the top layer. The plates were incubated at 37 °C for 14 days and colonies were counted using the GeneGnome HR scanner (Syngene).
Rhodamine-phalloidin staining. Cells were seeded onto cover slips in the six-well plates and transduced. At 1 dpt, cells were fixed and stained for F-actin with rhodamine-phalloidin (1 U/ml, Invitrogen) at room temperature for 15 minutes,49 followed by counterstaining with 4′,6-diamidino-2-phenylindole (DAPI, Vector Laboratories). The fluorescent images were captured using a confocal microscope (Nikon TE2000 equipped with a confocal upgrade laser kit).
Cell migration and invasion assays. Cell migration and invasion were evaluated in 24-well plates using transwell assays (BD Biosciences). For migration assays, 2.5 × 104 cells were added to the top chamber of each insert. For invasion assays, 2.5 × 104 cells were added to the upper chamber of each Matrigel-coated insert. After 24 hours of incubation at 37 °C, the cells migrating/invading through the membrane were fixed, stained by DAPI and visualized with a confocal microscope. Dividing the number of migrated/invaded cells in each group by the number of cells in the mock-transduction group yielded the percentages of migration/invasion. The mean ± SD from 10 fields per chamber was calculated from three chambers per group, and the experiment was repeated three times.
Tumor growth and metastasis in mice. All animal experiments were performed in compliance with the Guide for the Care and Use of Laboratory Animals (Ministry of Science and Technology, Taiwan). Mahlavu cells (5 × 106) were suspended in 100 μl PBS and injected subcutaneously into the flanks of 6–8 weeks old BALB/c nude mice. The length (L) and width (W) of the tumor were measured with a caliper and the tumor volume was calculated with the formula: V (mm3) = (L × W2) × (π/6). When the tumor volume reached ≈10 mm3, the mice were randomly grouped and 100 μl BV (2 × 109 pfu/mouse) which was concentrated by ultracentrifugation as described48 or PBS was injected into the tumor. All mice were sacrificed at 30 days after injection and the tumor specimens were removed, cryosectioned, fixed and subjected to immunohistochemical staining as described in details in Supplementary Methods. The sections were counterstained with DAPI. The confocal images were captured and the numbers of Ki-67+, capase-3+, MMP-2+, and MMP-9+ cells were counted from 15 fields (three random fields/tumor, five different tumors/group) using Image-Pro Plus 6.0 (Media Cybernetics). The percentage of specific protein-positive cells (e.g., Ki-67+) was defined by dividing the number of positive cells by the total number of DAPI+ cells.
For the in vivo metastasis assay, 1 × 106 cells were injected into the tail vein of 6–8 weeks old nude mice. The mice were sacrificed after 8 weeks and the lung sections were stained with hematoxylin and eosin (H&E).
Statistical analysis. All in vitro data are expressed as means ± SD of at least three independent experiments. Statistical significance was evaluated by Student's t-tests and P values <0.05 were considered significant.
SUPPLEMENTARY MATERIAL Figure S1. SB-based hybrid BV vector enabled integration and stable expression. Figure S2. BV-mediated miR-122/miR-151 modulation and their effects on molecular pathways and cell behavior in SNU-449 cells. Figure S3. Body weight of mice. Table S1. Primer sequences used for the construction of recombinant BVs. Table S2. Sequences of the chemically synthetic chimeric intron. Table S3. Sequences of the synthetic miR-151 binding sites. Supplementary Methods.
Acknowledgments
The authors thank Perry B Hackett of University of Minnesota and Zsuzsanna Izsvak of Max Delbruck Center for kindly providing the plasmids coding for the IR/DRs sequences and SB100X transposase. The authors also acknowledge the support from the National Tsing Hua University (Toward World-Class University Project 100N2050E1, 102N2051E1, 103N2051N1, and NTHU-CGMH Joint Research Program 102N2766E1, 103N2758E1) and Ministry of Science and Technology (NSC 101-2923-E-007-002-MY3, 102-2622-E-007-022-CC1), Taiwan.
Supplementary Material
References
- Wong CC, Wong CM, Au SL, Ng IO. RhoGTPases and Rho-effectors in hepatocellular carcinoma metastasis: ROCK N'Rho move it. Liver Int. 2010;30:642–656. doi: 10.1111/j.1478-3231.2010.02232.x. [DOI] [PubMed] [Google Scholar]
- Garzon R, Marcucci G, Croce CM. Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discov. 2010;9:775–789. doi: 10.1038/nrd3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai S, Nasser MW, Wang B, Hsu SH, Datta J, Kutay H, et al. MicroRNA-122 inhibits tumorigenic properties of hepatocellular carcinoma cells and sensitizes these cells to sorafenib. J Biol Chem. 2009;284:32015–32027. doi: 10.1074/jbc.M109.016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CJ, Gong HY, Tseng HC, Wang WL, Wu JL. miR-122 targets an anti-apoptotic gene, Bcl-w, in human hepatocellular carcinoma cell lines. Biochem Biophys Res Commun. 2008;375:315–320. doi: 10.1016/j.bbrc.2008.07.154. [DOI] [PubMed] [Google Scholar]
- Tsai WC, Hsu PW, Lai TC, Chau GY, Lin CW, Chen CM, et al. MicroRNA-122, a tumor suppressor microRNA that regulates intrahepatic metastasis of hepatocellular carcinoma. Hepatology. 2009;49:1571–1582. doi: 10.1002/hep.22806. [DOI] [PubMed] [Google Scholar]
- Fornari F, Gramantieri L, Giovannini C, Veronese A, Ferracin M, Sabbioni S, et al. MiR-122/cyclin G1 interaction modulates p53 activity and affects doxorubicin sensitivity of human hepatocarcinoma cells. Cancer Res. 2009;69:5761–5767. doi: 10.1158/0008-5472.CAN-08-4797. [DOI] [PubMed] [Google Scholar]
- Ding J, Huang S, Wu S, Zhao Y, Liang L, Yan M, et al. Gain of miR-151 on chromosome 8q24.3 facilitates tumour cell migration and spreading through downregulating RhoGDIA. Nat Cell Biol. 2010;12:390–399. doi: 10.1038/ncb2039. [DOI] [PubMed] [Google Scholar]
- Lu CH, Chang YH, Lin SY, Li KC, Hu YC. Recent progresses in gene delivery-based bone tissue engineering. Biotechnol Adv. 2013;31:1695–1706. doi: 10.1016/j.biotechadv.2013.08.015. [DOI] [PubMed] [Google Scholar]
- Liao YH, Chang YH, Sung LY, Li KC, Yeh CL, Yen TC, et al. Osteogenic differentiation of adipose-derived stem cells and calvarial defect repair using baculovirus-mediated co-expression of BMP-2 and miR-148b. Biomaterials. 2014;35:4901–4910. doi: 10.1016/j.biomaterials.2014.02.055. [DOI] [PubMed] [Google Scholar]
- Yeh TS, Fang YH, Lu CH, Chiu SC, Yeh CL, Yen TC, et al. Baculovirus-transduced, VEGF-expressing adipose-derived stem cell sheet for the treatment of myocardium infarction. Biomaterials. 2014;35:174–184. doi: 10.1016/j.biomaterials.2013.09.080. [DOI] [PubMed] [Google Scholar]
- Lin J, Teo S, Lam DH, Jeyaseelan K, Wang S. MicroRNA-10b pleiotropically regulates invasion, angiogenicity and apoptosis of tumor cells resembling mesenchymal subtype of glioblastoma multiforme. Cell Death Dis. 2012;3:e398. doi: 10.1038/cddis.2012.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CL, Luo WY, Lo WH, Lin KJ, Sung LY, Shih YS, et al. Development of hybrid baculovirus vectors for artificial MicroRNA delivery and prolonged gene suppression. Biotechnol Bioeng. 2011;108:2958–2967. doi: 10.1002/bit.23250. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Tamai N, Habu Y, Chang MO, Takaku H. Suppression of hepatitis C virus replication by baculovirus vector-mediated short-hairpin RNA expression. FEBS Lett. 2008;582:3085–3089. doi: 10.1016/j.febslet.2008.07.056. [DOI] [PubMed] [Google Scholar]
- Wang S, Balasundaram G. Potential cancer gene therapy by baculoviral transduction. Curr Gene Ther. 2010;10:214–225. doi: 10.2174/156652310791321251. [DOI] [PubMed] [Google Scholar]
- Luo WY, Shih YS, Hung CL, Lo KW, Chiang CS, Lo WH, et al. Development of the hybrid Sleeping Beauty: baculovirus vector for sustained gene expression and cancer therapy. Gene Ther. 2012;19:844–851. doi: 10.1038/gt.2011.129. [DOI] [PubMed] [Google Scholar]
- Sung LY, Chen CL, Lin SY, Hwang SM, Lu CH, Li KC, et al. Enhanced and prolonged baculovirus-mediated expression by incorporating recombinase system and in cis elements: a comparative study. Nucleic Acids Res. 2013;41:e139. doi: 10.1093/nar/gkt442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turunen TA, Laakkonen JP, Alasaarela L, Airenne KJ, Ylä-Herttuala S. Sleeping Beauty-baculovirus hybrid vectors for long-term gene expression in the eye. J Gene Med. 2014;16:40–53. doi: 10.1002/jgm.2756. [DOI] [PubMed] [Google Scholar]
- Ebert MS, Sharp PA. Emerging roles for natural microRNA sponges. Curr Biol. 2010;20:R858–R861. doi: 10.1016/j.cub.2010.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CY, Chang YH, Lin KJ, Yen TC, Tai CL, Chen CY, et al. The healing of critical-sized femoral segmental bone defects in rabbits using baculovirus-engineered mesenchymal stem cells. Biomaterials. 2010;31:3222–3230. doi: 10.1016/j.biomaterials.2010.01.030. [DOI] [PubMed] [Google Scholar]
- Yang MH, Chen CL, Chau GY, Chiou SH, Su CW, Chou TY, et al. Comprehensive analysis of the independent effect of twist and snail in promoting metastasis of hepatocellular carcinoma. Hepatology. 2009;50:1464–1474. doi: 10.1002/hep.23221. [DOI] [PubMed] [Google Scholar]
- Ma L, Liu J, Shen J, Liu L, Wu J, Li W, et al. Expression of miR-122 mediated by adenoviral vector induces apoptosis and cell cycle arrest of cancer cells. Cancer Biol Ther. 2010;9:554–561. doi: 10.4161/cbt.9.7.11267. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Oo Chang M, Kitajima M, Takaku H. Induction of antitumor immunity against mouse carcinoma by baculovirus-infected dendritic cells. Cell Mol Immunol. 2010;7:440–446. doi: 10.1038/cmi.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo WY, Shih YS, Lo WH, Chen HR, Wang SC, Wang CH, et al. Baculovirus vectors for antiangiogenesis-based cancer gene therapy. Cancer Gene Ther. 2011;18:637–645. doi: 10.1038/cgt.2011.35. [DOI] [PubMed] [Google Scholar]
- Chung KH, Hart CC, Al-Bassam S, Avery A, Taylor J, Patel PD, et al. Polycistronic RNA polymerase II expression vectors for RNA interference based on BIC/miR-155. Nucleic Acids Res. 2006;34:e53. doi: 10.1093/nar/gkl143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BD, Naldini L. Exploiting and antagonizing microRNA regulation for therapeutic and experimental applications. Nat Rev Genet. 2009;10:578–585. doi: 10.1038/nrg2628. [DOI] [PubMed] [Google Scholar]
- Ebert MS, Sharp PA. MicroRNA sponges: progress and possibilities. RNA. 2010;16:2043–2050. doi: 10.1261/rna.2414110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert MS, Neilson JR, Sharp PA. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat Methods. 2007;4:721–726. doi: 10.1038/nmeth1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak RO, Hollensen AK, Mikkelsen JG. Managing microRNAs with vector-encoded decoy-type inhibitors. Mol Ther. 2013;21:1478–1485. doi: 10.1038/mt.2013.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel CP. ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol. 2005;6:32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- Tognon CE, Sorensen PH. Targeting the insulin-like growth factor 1 receptor (IGF1R) signaling pathway for cancer therapy. Expert Opin Ther Targets. 2012;16:33–48. doi: 10.1517/14728222.2011.638626. [DOI] [PubMed] [Google Scholar]
- Posern G, Treisman R. Actin' together: serum response factor, its cofactors and the link to signal transduction. Trends Cell Biol. 2006;16:588–596. doi: 10.1016/j.tcb.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang R, Du W, Wang S, Yang L, Pan Z, et al. Downregulation of miR-151-5p contributes to increased susceptibility to arrhythmogenesis during myocardial infarction with estrogen deprivation. PLoS One. 2013;8:e72985. doi: 10.1371/journal.pone.0072985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley CR, Seow Y, Wood MJ. Novel RNA-based strategies for therapeutic gene silencing. Mol Ther. 2010;18:466–476. doi: 10.1038/mt.2009.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Cai H, Liu J, Fan H, Wang Z, Wang Q, et al. A miR-151 binding site polymorphism in the 3'-untranslated region of the cyclin E1 gene associated with nasopharyngeal carcinoma. Biochem Biophys Res Commun. 2013;432:660–665. doi: 10.1016/j.bbrc.2013.02.024. [DOI] [PubMed] [Google Scholar]
- Yang X, Marcucci K, Anguela X, Couto LB. Preclinical evaluation of an anti-HCV miRNA cluster for treatment of HCV infection. Mol Ther. 2013;21:588–601. doi: 10.1038/mt.2012.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi S, Iwasaki J, Kumazaki M, Mori T, Maruo K, Sakai H, et al. Chemically modified synthetic microRNA-205 inhibits the growth of melanoma cells in vitro and in vivo. Mol Ther. 2013;21:1204–1211. doi: 10.1038/mt.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebert LF, Rebhan MA, Crivelli SE, Denzler R, Stoffel M, Hall J. Miravirsen (SPC3649) can inhibit the biogenesis of miR-122. Nucleic Acids Res. 2014;42:609–621. doi: 10.1093/nar/gkt852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borel F, Kay MA, Mueller C. Recombinant AAV as a platform for translating the therapeutic potential of RNA interference. Mol Ther. 2014;22:692–701. doi: 10.1038/mt.2013.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amendola M, Passerini L, Pucci F, Gentner B, Bacchetta R, Naldini L. Regulated and multiple miRNA and siRNA delivery into primary cells by a lentiviral platform. Mol Ther. 2009;17:1039–1052. doi: 10.1038/mt.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan B, Jayandharan GR. Basic biology of adeno-associated virus (AAV) vectors used in gene therapy. Curr Gene Ther (epub ahead of print) (doi:10.2174/1566523214666140302193709) 2014. [DOI] [PubMed]
- Nowrouzi A, Penaud-Budloo M, Kaeppel C, Appelt U, Le Guiner C, Moullier P, et al. Integration frequency and intermolecular recombination of rAAV vectors in non-human primate skeletal muscle and liver. Mol Ther. 2012;20:1177–1186. doi: 10.1038/mt.2012.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe M, Modlich U, Schambach A. Biosafety challenges for use of lentiviral vectors in gene therapy. Curr Gene Ther. 2013;13:453–468. doi: 10.2174/15665232113136660006. [DOI] [PubMed] [Google Scholar]
- Donsante A, Miller DG, Li Y, Vogler C, Brunt EM, Russell DW, et al. AAV vector integration sites in mouse hepatocellular carcinoma. Science. 2007;317:477. doi: 10.1126/science.1142658. [DOI] [PubMed] [Google Scholar]
- Airenne KJ, Hu YC, Kost TA, Smith RH, Kotin RM, Ono C, et al. Baculovirus: an insect-derived vector for diverse gene transfer applications. Mol Ther. 2013;21:739–749. doi: 10.1038/mt.2012.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Lin CY, Chen GY, Hu YC. Baculovirus as a gene delivery vector: recent understandings of molecular alterations in transduced cells and latest applications. Biotechnol Adv. 2011;29:618–631. doi: 10.1016/j.biotechadv.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Xu F, Fang L, Xu J, Li B, Jiang Y, et al. Enhanced immunogenicity induced by an alphavirus replicon-based pseudotyped baculovirus vaccine against porcine reproductive and respiratory syndrome virus. J Virol Methods. 2013;187:251–258. doi: 10.1016/j.jviromet.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Hackett PB, Largaespada DA, Cooper LJ. A transposon and transposase system for human application. Mol Ther. 2010;18:674–683. doi: 10.1038/mt.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly D, Miller L, Luckow V. Baculovirus Expression Vectors: A Laboratory Manual. W.H. Freeman and Co; New York; 1992. [Google Scholar]
- Zhou Q, Anderson C, Zhang H, Li X, Inglis F, Jayagopal A, et al. Repression of choroidal neovascularization through actin cytoskeleton pathways by microRNA-24. Mol Ther. 2014;22:378–389. doi: 10.1038/mt.2013.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.