Abstract
A 12-year-old boy with refractory acute lymphoblastic leukemia received a haploidentical transplant from his mother. As prophylaxis for Epstein-Barr virus (EBV), cytomegalovirus (CMV) and adenovirus, he received ex vivo expanded virus-specific donor T cells 3.5 months after transplant. Four weeks later leukemic blasts bearing the E2A deletion, identified by fluorescent in situ hybridization (FISH), appeared transiently in the blood followed by a FISH-negative hematological remission, which was sustained until a testicular relapse 3.5 months later. Clearance of the circulating leukemic cells coincided with a marked increase in circulating virus-specific T cells. The virus-specific cytotoxic T-cell (CTL) line showed strong polyfunctional reactivity with the patient's leukemic cells but not phytohemagglutinin (PHA) blasts, suggesting that virus-specific CTL lines may have clinically significant antileukemia activity.
Introduction
Refractory acute lymphoblastic leukemia (ALL) remains a disease with poor prognosis, and a small percentage of patients that undergo allogeneic stem cell transplantation will respond.1,2,3 Following allogeneic transplant, donor T cells may cause graft-versus-host (GVH) alloreactions but also confer immunity to viruses and exert a graft-versus-leukemia (GVL) effect contributing to maintenance of minimal residual disease and cure of leukemia.4,5,6 Control of viral disease can be enhanced by infusion of ex vivo expanded virus-specific T cells.7,8,9,10,11,12,13,14 Similar approaches to enhance the GVL effect are being developed to generate ex vivo donor T cells specific for the recipient's leukemia targeting leukemia antigens, minor histocompatibility antigens or leukemia cells, but clinical studies while promising are currently limited.15 Since some malignancies such as Epstein-Barr virus (EBV)-driven lymphoproliferative diseases can express viral antigens, they can be successfully eradicated by EBV-specific cytotoxic T-cell line (CTL).9,16 However cross-reactivity between virus-specific T cells and nonviral hematological malignancies has not been described. Here, we report for the first time an apparent GVL effect against acute lymphoblastic leukemia from ex vivo expanded multivirus-specific CTL lines in a patient receiving a haploidentical donor stem cell transplant.
Results
Patient history
A 12-year-old boy presented with high-risk precursor B ALL, (based on age and white blood cell count at the time of presentation) (Table 1). Cytogenetic evaluation showed loss of E2A (19p13). This abnormality without prognostic significance, may have been due to deletion of 5′ and 3′ E2A sequences, deletion of an intact E2A gene, or monosomy 19. Treatment under the COG AALL0232 protocol did not produce a complete remission and he subsequently received a reduced intensity allogeneic transplant from his haploidentical mother for his refractory ALL. Patient and donor high-resolution human leukocyte antigen (HLA) typing data are shown in Table 1. His conditioning regimen included total body irradiation 300cGy × 2 on day −6; fludarabine 30 mg/m2 from day −5 to day −2; and alemtuzumab 10 mg/dose from day −5 to day −2 per our institutional protocol (HIMSUM). Serology indicated prior exposure to EBV. The donor was seropositive for cytomegalovirus (CMV) and EBV. The patient received peripheral blood stem cells at a dose of 7.3 × 106 CD34+ cells/kg (T cell dose 4.8 × 104 CD3+ cells/kg) from his 43-year-old mother. Tacrolimus was started on day −2 for graft-versus-host disease (GVHD) prophylaxis and discontinued on day +38 since the patient did not have any evidence of GVHD. He engrafted on day +12. On day +27, a bone marrow showed complete hematological remission and 100% donor engraftment. Three and a half months post-transplant, he received 2 × 107 (1 × 107 cells/m2) T cells specific for CMV, EBV, and adenovirus.10 Six days prior to CTL infusion, a bone marrow biopsy was performed which confirmed complete remission since FISH analysis revealed that the hematopoietic cells were 100% donor (chromosomal analysis was 100% XX female) and no cells (0/200 cells) were identified that expressed the E2A deletion. However, 4 weeks later, circulating leukemic blasts were observed in the peripheral blood, which was confirmed by FISH analysis which revealed that 17/200 cells were now positive for the E2A deletion. At this time, circulating T cells specific for CMV and adenovirus mounted robust and persistent interferon-γ (IFN-γ) responses associated with a return to hematological remission and loss of the E2A deletion marker. Despite history of previous exposure to EBV, virus-specific immune reconstitution evaluation showed weak responses to EBV-transformed lymphoblastic cell lines (EBV-LCL) compared with T cell responses to CMV and adenovirus (Figure 1). He remained without detectable disease for a further 3.5 months until he developed a testicular relapse.
Table 1. Clinical information.
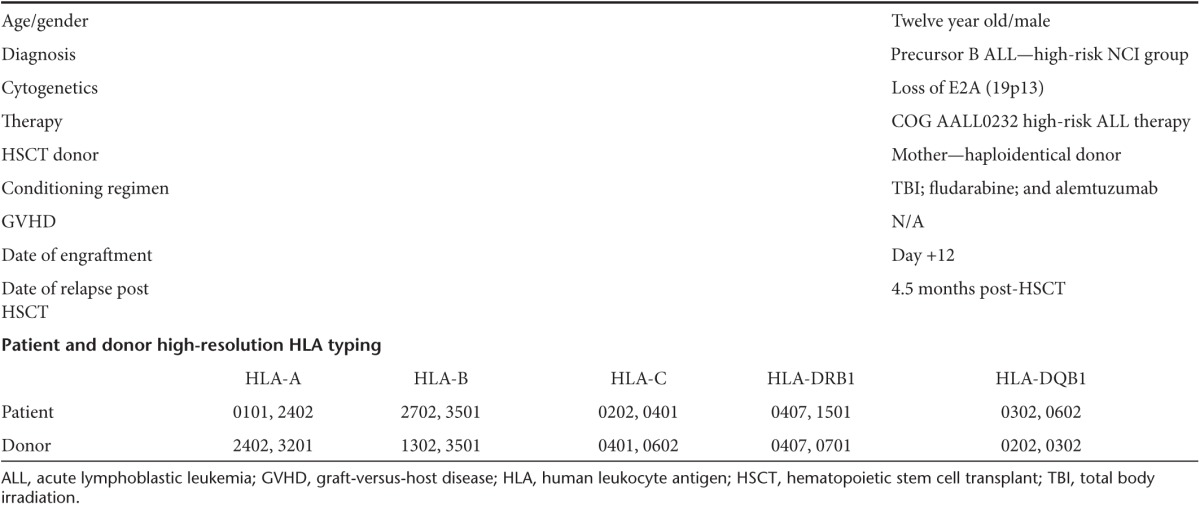
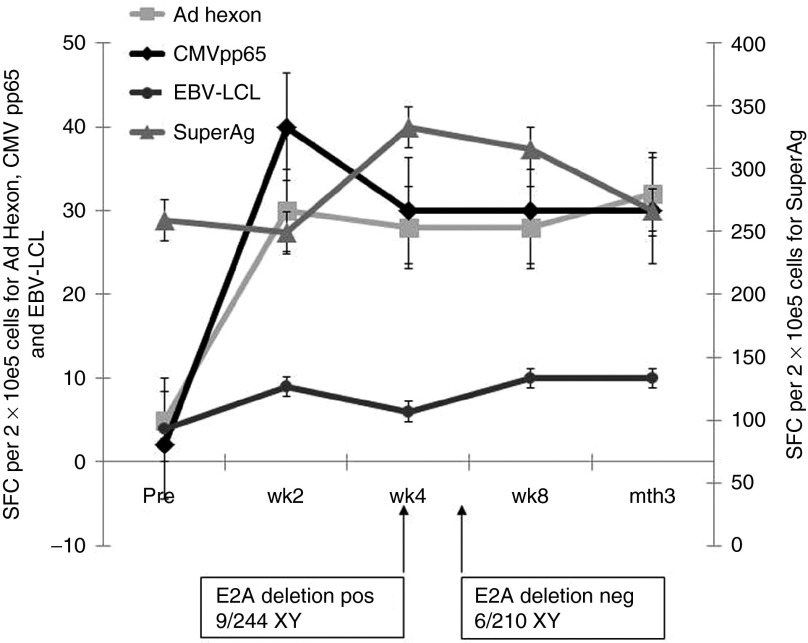
Figure 1.
Rapid reconstitution of antiviral immunity. The patient received a single dose of donor-derived trivirus-specific T cells 3.5 months following haploidentical transplant from the same donor. Peripheral blood samples were obtained pre- and post-cytotoxic T-cell line (CTL) infusion. Patient peripheral blood mononuclear cells obtained pre versus post infusion were incubated with Epstein-Barr virus-LCL, cytomegalovirus (CMV) pp65 pepmix and Adeno hexon pepmix, and interferon-γ responding T-cells quantified using ELISpot analysis (reported as spot-forming cells per 1 × 105 cells).
Virus-specific activity of the adoptively transferred cytotoxic T cells
The infused T cell line displayed predominant reactivity against CMV antigens (Figure 2a,b), and epitope mapping9,16 indicated broad reactivity with a number of pp65-derived epitopes (Figure 2c), including 1.88% of CD8+ T cells that recognized the HLA-A24-restricted pp65 epitope QYDPVAALF (QYD) (Supplementary Figure S1a,b). Within 2 weeks, post-CTL infusion markedly increased frequencies of CMV, and adenovirus-specific T cells were detectable in the patient (Figure 1), suggesting that the virus specific T-cells expanded in vivo.
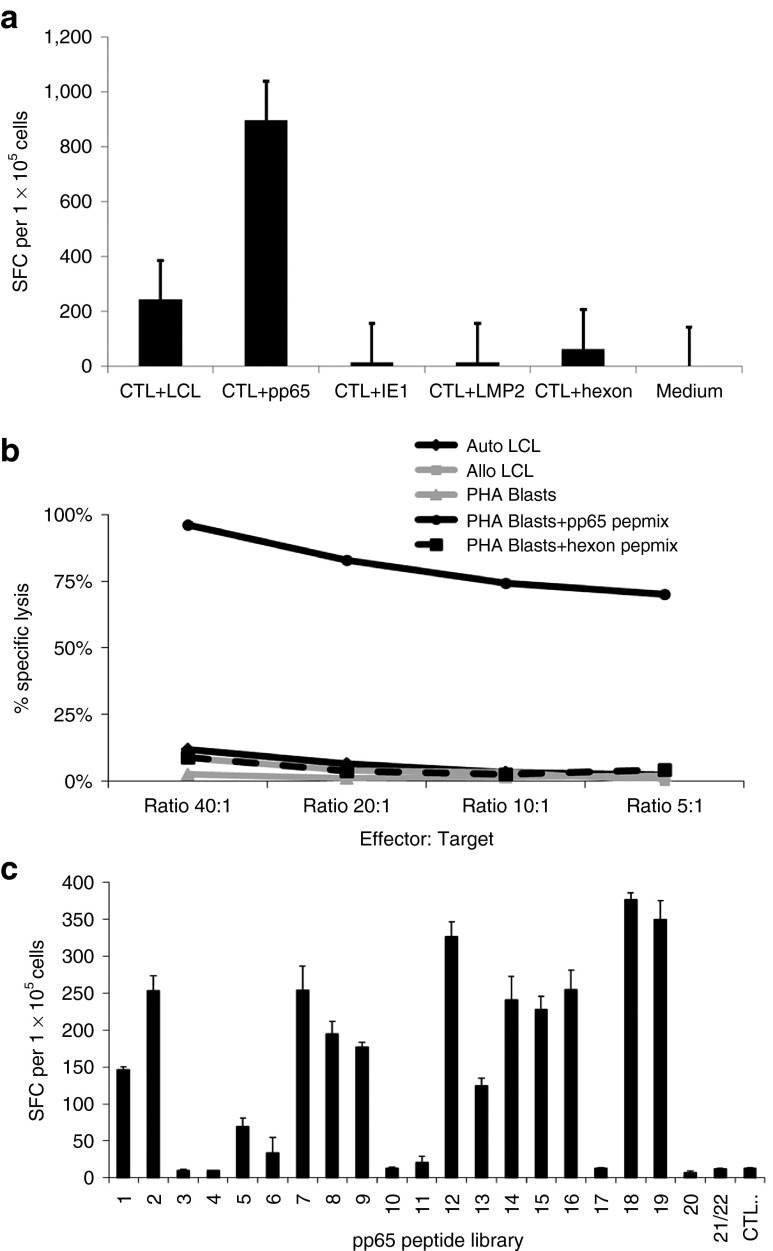
Figure 2.
Virus- and viral epitope specificity of the cytotoxic T-cell line (CTL). (a) Virus specificity was assessed in an ELISpot assay in which the virus-specific CTLs were challenged with the lymphoblastic cell lines (LCLs) from donor, or peptide libraries covering the open reading frames of cytomegalovirus (CMV) pp65, CMV IE1, Epstein-Barr virus (EBV) LMP2, or adenovirus hexon antigen. (b) Cytotoxic activity of the CTL line was evaluated in a 51Cr-release assay against viral peptide-pulsed phytohemagglutinin (PHA) blasts. The trivirus-specific CTL were incubated with 51Cr-loaded, peptide library (CMV pp65 or adenovirus hexon) pulsed or unpulsed PHA blasts, adenovirus hexon peptide library-pulsed PHA blasts or CMV pp65 peptide library-loaded PHA blasts, EBV-LCL from the patient or donor for 4 hours at 37 °C, after which supernatants were harvested to determine lysis. To determine whether the CTL line exhibited reactivity against healthy cells (i.e., alloreactivity), unpulsed PHA blasts derived from the patient were also used as targets. (c) Epitope mapping of the CTL indicated reactivity against known CD8 restricted epitopes for EBV and adenovirus as well as broad reactivity with known CD8+ epitopes as well as multiple unidentified epitopes in the pp65 antigen.
Adoptively transferred virus-specific T cells exhibit leukemia-specific activity
The occurrence of leukemic relapse followed by remission at the peak of virus-specific immune reconstitution suggested that virus-specific T cells were reactive against host leukemia. The observation conformed more with a GVL rather than a GVH effect since the patient did not develop GVHD following the T cell infusion and no antirecipient CTL activity was demonstrated (Figure 2b). To explore leukemia-specific targeting by the virus-specific CTL, the T cell line was incubated with patient PHA blasts, patient ALL blasts (obtained at diagnosis), and with donor EBV-LCL loaded with the CMV pp65 peptide library.17,18,19 As shown in Figure 3a,b, strong reactivity against pp65-pulsed autologous EBV-LCL by CD4+ and CD8+ T cells was seen. There was no recognition of patient PHA blasts. However, CD4+ T cells, and, to a lesser extent CD8+ T cells, displayed a strong, polyfunctional reactivity against patient leukemia blasts, indicating that the virus-specific T cells were cross-reactive with the leukemia cells (Figure 3a,b). Additionally, as shown using IFN-γ ELISpot, the virus-specific T cells not only recognized viral-derived overlapping peptides, but also peptides derived from the tumor-associated antigens, Wilm's tumor-1 (WT-1), preferentially expressed antigen in melanoma, and melanoma-associated antigen-3 (MAGEA3) known to be expressed at high frequencies on ALL blasts (Figure 3c).20 In order to eliminate the possibility that the effect was mediated by antileukemic natural killer cells, determination of CD4+ T cell reactivity was performed after gating CD4+ and CD8+ T cell subpopulations from the CTL line, separately. Despite a temporary remission, our patient eventually relapsed in the testis, recognized as an immune privileged leukemia sanctuary site21 and at a time when CMV-, EBV-, or AdV-specific T cells were no longer detectable in the peripheral blood (data not shown).
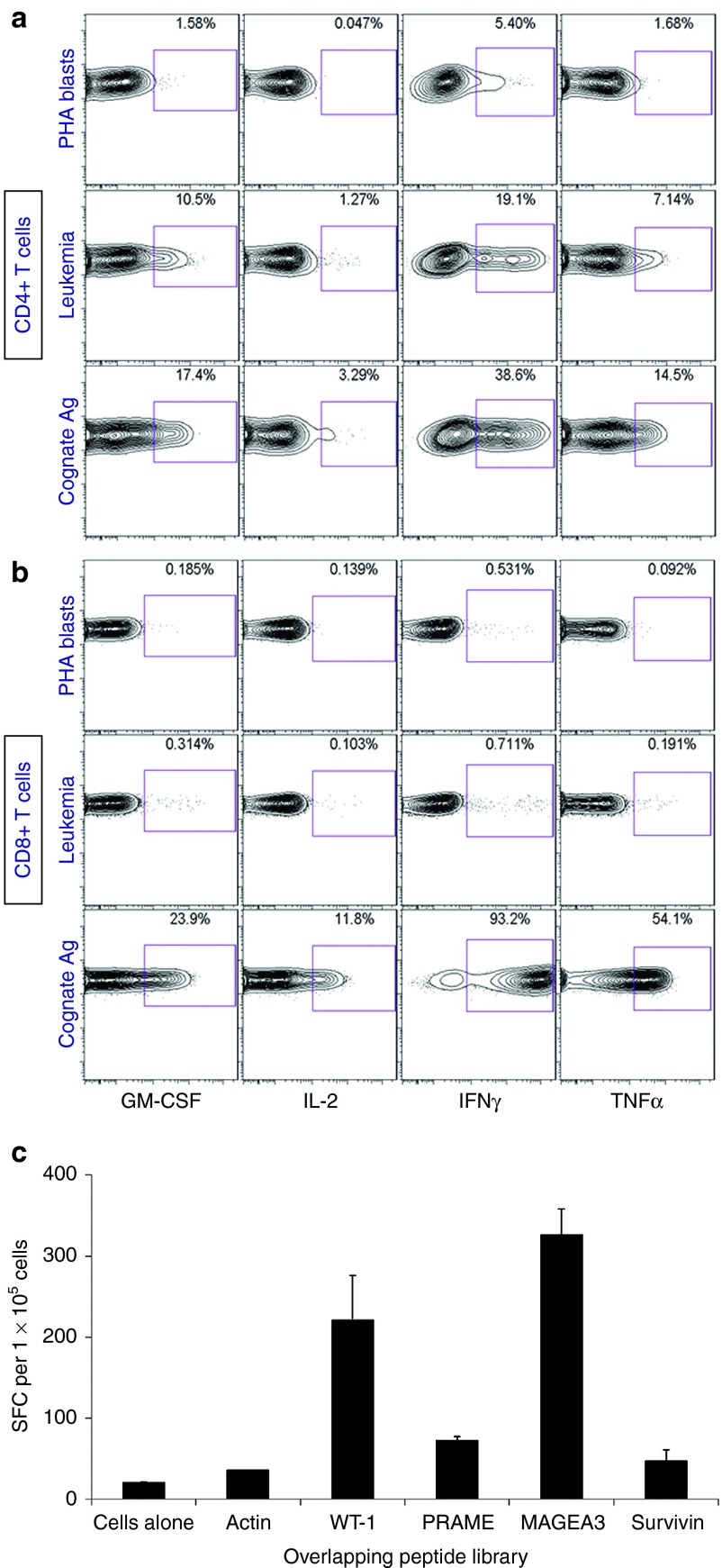
Figure 3.
Recognition of patient leukemic cells by the virus-specific donor T cell line. The trivirus-specific T cell line was labeled with carboxyfluorescein diacetate, succinimidyl ester and incubated with patient phytohemagglutinin (PHA) blasts, patient bone marrow, containing 85% leukemia blasts, or with cytomegalovirus (CMV)-pulsed Epstein-Barr virus (EBV)-LCL (cognate Ag) for 6 hours in the presence of cytokine secretion inhibitors at 37 °C, 5% CO2, and subsequently examined for the production of granulocyte-macrophage colony stimulating factor, interleukin (IL)-2, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α within CD4+ (a) and CD8+ (b) T cells by intracellular cytokine detection as outlined in the Materials and Methods section. Indicated in each plot is the percentage of the respective subset producing cytokine in response to each separate stimulation. The data show a strong reactivity of donor CD4+ T cells with patient leukemic marrow but not with patient PHA blasts, while CD8+ T cells show only marginal antileukemia cross-reactivity. (c) Reactivity against known leukemia-associated tumor-associated antigens was tested in an IFN-γ ELISPOT assay using overlapping peptides for tumor-associated antigens. Cells alone indicate cells without any antigen, and actin represents an irrelevant mixture of overlapping peptides. Error bars indicate standard deviation from the mean.
Discussion
Alloreactions in haploidentical donor:recipient pairs involve both major and minor major histocompatibility complex (MHC) antigens as well as other poorly defined antigens. We recently showed that T cell alloreactivity against non-self peptide-HLA complexes in CD4+ and CD8+ T cells are present within both the naive and memory T cell compartment.22 Furthermore, we showed that virus-specific T cell lines cross-react with allogeneic peptide-MHC complexes,23 in line with a previous report.24 However, a retrospective analysis of virus-specific T cell infusions in HLA-mismatch settings showed that despite clearly detectable in vitro alloreactivity against the recipient cells, no patients developed de novo GVHD. In this study, while the absence of alloreactivity against the patient's PHA blasts predictably correlated with the absence of GVHD, our in vitro and in vivo findings suggest that the virus-specific CTL line had clinically significant leukemia-specific reactivity.
Over the past few years, evidence for a role of virus reactivation in reducing leukemia relapses post-allogeneic transplant has accumulated.25,26 This is, to our knowledge, the first report of a virus-specific T cell line with heterologous reactivity against ALL cells. We found that the virus- specific T cells cross-reacted in vitro with the patient's leukemia, recognized tumor-associated antigens known to be expressed by leukemia cells20 and may have contributed to the remission of disease. The absence of GVHD at the time of maximum CTL expansion coinciding with a remission of the leukemia suggests a GVL rather than a nonspecific GVHD mechanism. Such T cell reactivity by the CTL line could be directed against either leukemia-specific antigens or minor histocompatibility antigens restricted to the B cell leukemia, or viral antigens expressed by the leukemia especially since normal B cell numbers continued to rise as would be expected.27,28 Our flow-based approach also eliminated the possibility that the antileukemia effect was due to natural killer cells. Thus, it seems unlikely that the effect could represent a CMV-driven natural killer cytotoxicity as has been previously described. Furthermore, antileukemic natural killer cell reactivity has only been reported against myeloid malignancies.29,30,31
While the observed antileukemic effect may have been due to residual alloreactive or tumor-associated antigen-reactive clones in the CTL line, the absence of GVHD and the lack of detectable tumor-specific T cells in the line suggest that this report underlines the need for further studies to identify how frequently virus-specific CTL lines can recognize and kill leukemia. Such studies are important because our findings suggest an unexpected further antileukemic benefit from the use of virus-specific CTL to treat or prevent reactivation from common viruses.
Materials and Methods
Patient. This patient was treated on a protocol under an established Investigational New Drug approved by the US Food and Drug Administration, the Institutional Review Boards, and the National Marrow Donor Program's Institutional Review Boards. The protocol included recipients of allogeneic donor stem cell transplants at risk for CMV reactivation with a CMV seropositive stem cell donor, who had no less than 50% donor chimerism in either peripheral blood or bone marrow or relapse of their original disease, no evidence of GVHD >Grade II at the time of enrollment, a life expectancy >30 days, no severe intercurrent infection or organ disease, no antiviral therapy and ≥ 30 days post-transplant with no evidence of significant CMV reactivation or evidence of CMV disease.10
Cells. Bone marrow and peripheral blood were obtained from the patient at diagnosis. The marrow contained >85% leukemic blasts. Both patient and donor cells were obtained under Institutional Review Boards-approved protocols and in accordance with the Declaration of Helsinki. Mononuclear cells were isolated using Ficoll-Hypaque density-gradient centrifugation and cryopreserved in the vapor phase of liquid nitrogen using standard procedures. EBV-LCL and PHA-stimulated T cell blasts (PHA blasts) were generated from patient pretransplant and from the donor peripheral blood mononuclear cells following standard procedures,10 and a trivirus- (EBV, CMV- and adenovirus) specific T cell line was generated from donor peripheral blood mononuclear cells as previously described.10 The EBV-LCL was cultured in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, glutamine, penicillin, and streptomycin (complete medium), and the PHA blasts in complete medium plus 100 IU interleukin (IL)-2/ml.
Flow cytometry reagents. The following fluorochrome-conjugated monoclonal antibodies (mAbs) were purchased from commercial vendors: (i) anti-CD8 H7-allophycocyanin (H7APC), anti-CD4 V500, anti-granulocyte-macrophage colony stimulating factor phycoerythrin, anti-tumor necrosis factor-α Cy7PE (BD Biosciences, San Diego, CA); (ii) anti-CD3 Quantum Dot-605, anti-CD14 Pacific blue, anti-IFN-γ APC, anti-IL-2 eFluor 710-peridinin chlorophyll protein (Invitrogen, Burlingame, CA). The fixable violet amine reactive dye (ViViD; Invitrogen/Molecular Probes, Eugene, OR) was used to eliminate dead cells from the analysis.32 For intracellular cytokine detection-mixed lymphocyte reaction experiments, the CTL was labeled with the green fluorescent dye carboxyfluorescein diacetate, succinimidyl ester (Invitrogen) to allow discrimination from stimulator cell populations as described.23 The Pentamer QYD was purchased from Proimmune (Oxford, UK).
Enzyme-linked immunospot (ELISpot) assay. ELISpot assay was used to determine the frequency of IFN-γ secreting T cells after exposure to EBV, adenoviral, and CMV antigens as previously described33 as well as the tumor-associated antigens WT-1, MAGEA3, preferentially expressed antigen in melanoma, and Survivin (JPT, Berlin, Germany). Spot-forming cells and input cell numbers were plotted, and the frequency of T cells specific to each antigen was expressed as specific spot-forming cells per 105 input cells.
Intracellular cytokine detection assay. Intracellular cytokine detection was performed as previously described.23 In brief, the carboxyfluorescein diacetate, succinimidyl ester-labeled CTL line was stimulated for 6 hours with irradiated patient bone marrow mononuclear cells, patient PHA blasts, and CMV pp65-pulsed donor EBV-LCL in the presence of monensin and brefeldin A to prevent cytokine secretion. Next, cells were stained with ViViD, surface antigens, fixed and permeabilized, and stained intracellularly with mAb against cytokines. The cells were washed, fixed one last time, and acquired on a Becton Dickinson LSRII Fortessa. The fixation and permeabilization buffers contained 1 mmol/l EDTA to prevent quenching of the quantum dot and eFluor fluorochromes due to the presence of heavy metals in the fixatives.34 Data were analyzed using FlowJo version 7.6.3 (Treestar, San Carlos, CA) as previously described.23
Cytotoxicity assay. Donor and patient PHA blasts and donor derived EBV-LCL, either unpulsed or pulsed with CMV pp65 or adenovirus hexon pepmixes, were used as targets of the T cell line in a standard 51Cr release assay as described.35 The targets were incubated with the T cell line at 40:1, 20:1, 10:1, and 5:1 ratios for 4 hours, after which supernatants were harvested to determine the amount of 51Cr with a scintillation counter. Spontaneous and maximum 51Cr release were determined by culturing the 51Cr loaded targets alone and in the presence of Triton X-100, respectively. Percentage specific lysis was calculated as follows: 100 × (experimental release cpm − spontaneous release cpm)/(maximum release cpm − spontaneous release cpm).
SUPPLEMENTARY MATERIAL Figure S1. T cell recognition of Viral Antigens.
Acknowledgments
The clinical trial was supported in part by the Production Assistance for Cellular Therapies program (NHLBI contract #HHSN268201000007C). We also appreciate the support of shared resources by Dan L Duncan Cancer Center support grant P30CA125123.
Supplementary Material
References
- Hilden JM, Dinndorf PA, Meerbaum SO, Sather H, Villaluna D, Heerema NA, et al. Children's Oncology Group Analysis of prognostic factors of acute lymphoblastic leukemia in infants: report on CCG 1953 from the Children's Oncology Group. Blood. 2006;108:441–451. doi: 10.1182/blood-2005-07-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribera JM, Ortega JJ, Oriol A, Bastida P, Calvo C, Pérez-Hurtado JM, et al. Comparison of intensive chemotherapy, allogeneic, or autologous stem-cell transplantation as postremission treatment for children with very high risk acute lymphoblastic leukemia: PETHEMA ALL-93 Trial. J Clin Oncol. 2007;25:16–24. doi: 10.1200/JCO.2006.06.8312. [DOI] [PubMed] [Google Scholar]
- Saarinen-Pihkala UM, Heilmann C, Winiarski J, Glomstein A, Abrahamsson J, Arvidson J, et al. Pathways through relapses and deaths of children with acute lymphoblastic leukemia: role of allogeneic stem-cell transplantation in Nordic data. J Clin Oncol. 2006;24:5750–5762. doi: 10.1200/JCO.2006.07.1225. [DOI] [PubMed] [Google Scholar]
- Chakraverty R, Eom HS, Sachs J, Buchli J, Cotter P, Hsu R, et al. Host MHC class II+ antigen-presenting cells and CD4 cells are required for CD8-mediated graft-versus-leukemia responses following delayed donor leukocyte infusions. Blood. 2006;108:2106–2113. doi: 10.1182/blood-2006-03-007427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiden PL, Flournoy N, Thomas ED, Prentice R, Fefer A, Buckner CD, et al. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N Engl J Med. 1979;300:1068–1073. doi: 10.1056/NEJM197905103001902. [DOI] [PubMed] [Google Scholar]
- Weiden PL, Sullivan KM, Flournoy N, Storb R, Thomas ED. Antileukemic effect of chronic graft-versus-host disease: contribution to improved survival after allogeneic marrow transplantation. N Engl J Med. 1981;304:1529–1533. doi: 10.1056/NEJM198106183042507. [DOI] [PubMed] [Google Scholar]
- Gerdemann U, Katari UL, Papadopoulou A, Keirnan JM, Craddock JA, Liu H, et al. Safety and clinical efficacy of rapidly-generated trivirus-directed T cells as treatment for adenovirus, EBV, and CMV infections after allogeneic hematopoietic stem cell transplant. Mol Ther. 2013;21:2113–2121. doi: 10.1038/mt.2013.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson A, Levitsky V, Zou JZ, Frisan T, Dalianis T, Ljungman P, et al. Epstein-Barr virus (EBV) load in bone marrow transplant recipients at risk to develop posttransplant lymphoproliferative disease: prophylactic infusion of EBV-specific cytotoxic T cells. Blood. 2000;95:807–814. [PubMed] [Google Scholar]
- Heslop HE, Slobod KS, Pule MA, Hale GA, Rousseau A, Smith CA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115:925–935. doi: 10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leen AM, Myers GD, Sili U, Huls MH, Weiss H, Leung KS, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006;12:1160–1166. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- Micklethwaite KP, Clancy L, Sandher U, Hansen AM, Blyth E, Antonenas V, et al. Prophylactic infusion of cytomegalovirus-specific cytotoxic T lymphocytes stimulated with Ad5f35pp65 gene-modified dendritic cells after allogeneic hemopoietic stem cell transplantation. Blood. 2008;112:3974–3981. doi: 10.1182/blood-2008-06-161695. [DOI] [PubMed] [Google Scholar]
- Riddell SR, Watanabe KS, Goodrich JM, Li CR, Agha ME, Greenberg PD. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257:238–241. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- Walter EA, Greenberg PD, Gilbert MJ, Finch RJ, Watanabe KS, Thomas ED, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333:1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- Heslop HE, Brenner MK, Rooney CM. Donor T cells to treat EBV-associated lymphoma. N Engl J Med. 1994;331:679–680. doi: 10.1056/NEJM199409083311017. [DOI] [PubMed] [Google Scholar]
- Saglio F, Hanley PJ, Bollard CM. The time is now: moving toward virus-specific T cells after allogeneic hematopoietic stem cell transplantation as the standard of care. Cytotherapy. 2014;16:149–159. doi: 10.1016/j.jcyt.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doubrovina E, Oflaz-Sozmen B, Prockop SE, Kernan NA, Abramson S, Teruya-Feldstein J, et al. Adoptive immunotherapy with unselected or EBV-specific T cells for biopsy-proven EBV+ lymphomas after allogeneic hematopoietic cell transplantation. Blood. 2012;119:2644–2656. doi: 10.1182/blood-2011-08-371971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley PJ, Cruz CR, Savoldo B, Leen AM, Stanojevic M, Khalil M, et al. Functionally active virus-specific T cells that target CMV, adenovirus, and EBV can be expanded from naive T-cell populations in cord blood and will target a range of viral epitopes. Blood. 2009;114:1958–1967. doi: 10.1182/blood-2009-03-213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley PJ, Shaffer DR, Cruz CR, Ku S, Tzou B, Liu H, et al. Expansion of T cells targeting multiple antigens of cytomegalovirus, Epstein-Barr virus and adenovirus to provide broad antiviral specificity after stem cell transplantation. Cytotherapy. 2011;13:976–986. doi: 10.3109/14653249.2011.575356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern F, Faulhaber N, Frömmel C, Khatamzas E, Prösch S, Schönemann C, et al. Analysis of CD8 T cell reactivity to cytomegalovirus using protein-spanning pools of overlapping pentadecapeptides. Eur J Immunol. 2000;30:1676–1682. doi: 10.1002/1521-4141(200006)30:6<1676::AID-IMMU1676>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Weber G, Caruana I, Rouce RH, Barrett AJ, Gerdemann U, Leen AM, et al. Generation of tumor antigen-specific T cell lines from pediatric patients with acute lymphoblastic leukemia–implications for immunotherapy. Clin Cancer Res. 2013;19:5079–5091. doi: 10.1158/1078-0432.CCR-13-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chessells JM, Veys P, Kempski H, Henley P, Leiper A, Webb D, et al. Long-term follow-up of relapsed childhood acute lymphoblastic leukaemia. Br J Haematol. 2003;123:396–405. doi: 10.1046/j.1365-2141.2003.04584.x. [DOI] [PubMed] [Google Scholar]
- Melenhorst JJ, Leen AM, Bollard CM, Quigley MF, Price DA, Rooney CM, et al. Allogeneic virus-specific T cells with HLA alloreactivity do not produce GVHD in human subjects. Blood. 2010;116:4700–4702. doi: 10.1182/blood-2010-06-289991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melenhorst JJ, Scheinberg P, Williams A, Ambrozak DR, Keyvanfar K, Smith M, et al. Alloreactivity across HLA barriers is mediated by both naïve and antigen-experienced T cells. Biol Blood Marrow Transplant. 2011;17:800–809. doi: 10.1016/j.bbmt.2010.12.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir AL, D'Orsogna LJ, Roelen DL, van Loenen MM, Hagedoorn RS, de Boer R, et al. Allo-HLA reactivity of virus-specific memory T cells is common. Blood. 2010;115:3146–3157. doi: 10.1182/blood-2009-07-234906. [DOI] [PubMed] [Google Scholar]
- Behrendt CE, Rosenthal J, Bolotin E, Nakamura R, Zaia J, Forman SJ. Donor and recipient CMV serostatus and outcome of pediatric allogeneic HSCT for acute leukemia in the era of CMV-preemptive therapy. Biol Blood Marrow Transplant. 2009;15:54–60. doi: 10.1016/j.bbmt.2008.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmaagacli AH, Steckel NK, Koldehoff M, Hegerfeldt Y, Trenschel R, Ditschkowski M, et al. Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood. 2011;118:1402–1412. doi: 10.1182/blood-2010-08-304121. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Prentice HG, Grundy JE. Natural killer cell lysis of cytomegalovirus (CMV)-infected cells correlates with virally induced changes in cell surface lymphocyte function-associated antigen-3 (LFA-3) expression and not with the CMV-induced down-regulation of cell surface class I HLA. J Immunol. 1998;161:2365–2374. [PubMed] [Google Scholar]
- Hermouet S, Sutton CA, Rose TM, Greenblatt RJ, Corre I, Garand R, et al. Qualitative and quantitative analysis of human herpesviruses in chronic and acute B cell lymphocytic leukemia and in multiple myeloma. Leukemia. 2003;17:185–195. doi: 10.1038/sj.leu.2402748. [DOI] [PubMed] [Google Scholar]
- Della Chiesa M, Falco M, Podestà M, Locatelli F, Moretta L, Frassoni F, et al. Phenotypic and functional heterogeneity of human NK cells developing after umbilical cord blood transplantation: a role for human cytomegalovirus. Blood. 2012;119:399–410. doi: 10.1182/blood-2011-08-372003. [DOI] [PubMed] [Google Scholar]
- Foley B, Cooley S, Verneris MR, Pitt M, Curtsinger J, Luo X, et al. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood. 2012;119:2665–2674. doi: 10.1182/blood-2011-10-386995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savani BN, Rezvani K, Mielke S, Montero A, Kurlander R, Carter CS, et al. Factors associated with early molecular remission after T cell-depleted allogeneic stem cell transplantation for chronic myelogenous leukemia. Blood. 2006;107:1688–1695. doi: 10.1182/blood-2005-05-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melenhorst JJ, Scheinberg P, Chattopadhyay PK, Gostick E, Ladell K, Roederer M, et al. High avidity myeloid leukemia-associated antigen-specific CD8+ T cells preferentially reside in the bone marrow. Blood. 2009;113:2238–2244. doi: 10.1182/blood-2008-04-151969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdemann U, Keirnan JM, Katari UL, Yanagisawa R, Christin AS, Huye LE, et al. Rapidly generated multivirus-specific cytotoxic T lymphocytes for the prophylaxis and treatment of viral infections. Mol Ther. 2012;20:1622–1632. doi: 10.1038/mt.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarkowsky D, Lamoreaux L, Chattopadhyay P, Koup RA, Perfetto SP, Roederer M. Heavy metal contaminants can eliminate quantum dot fluorescence. Cytometry A. 2011;79:84–89. doi: 10.1002/cyto.a.20986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melenhorst JJ, van Luxemburg-Heijs SA, Landegent JE, Willemze R, Fibbe WE, Falkenburg JH. Aplastic anaemia in donor cells 14 years after bone-marrow transplant. Lancet. 1999;353:2037–2038. doi: 10.1016/s0140-6736(99)01926-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.