Targeted genomic integration typically requires designer nucleases that augment gene targeting efficiencies by creating a double-strand DNA (dsDNA) break at the target locus. This break provides the “molecular trigger” necessary to boost the efficiency of homologous recombination by several orders of magnitude. Eliminating the nuclease once it has done its job is highly desirable but remains a challenge. In a recent issue of Nature, Barzel et al. described a promising nuclease-free method for gene targeting by homologous recombination with unexpected high efficiency.1 Promoterless adeno-associated virus (AAV) vectors were designed to specifically target the albumin locus, which is highly expressed in the liver. Consequently, targeted genomic integration of a coagulation factor IX (FIX) complementary DNA into the albumin locus directed sustained FIX expression at therapeutic levels and corrected the bleeding diathesis in hemophilic mice. This approach may ultimately pave the way toward a safer and efficacious liver-directed gene therapy that minimizes the risk of insertional oncogenesis and off-target effects. This is particularly relevant for diseases that manifest themselves in infants and require early treatment by gene therapy.
The ability to integrate therapeutic genes at a specific location in the human genome represents an attractive paradigm for gene therapy of monogenic diseases. Efficient targeted genomic integration into a so-called “safe harbor” locus2,3 would obviate safety concerns associated with random genomic integration by reducing the risk of insertional oncogenesis.4,5 To achieve targeted genomic integration into a predefined locus, one can exploit homologous recombination between the donor DNA and the target site in the genome. This technology has been widely used to genetically modify embryonic stem cells and generate gene knockout mice—the basis for the Nobel Prize in Physiology or Medicine 2007 awarded to Mario R. Capecchi, Sir Martin J. Evans, and Oliver Smithies (http://www.nobelprize.org/nobel_prizes/medicine/laureates/2007). However, enrichment of cells harboring the desired genetic modification requires elaborate selection schemes that are not readily amenable to gene therapy applications. Initial attempts to achieve in vivo gene targeting (e.g., in liver) using AAV vectors resulted in modest targeting efficiencies, typically in the range of 1 per 105 cells.6 The efficiency of gene targeting could be dramatically increased—by up to 10,000-fold—by inducing a dsDNA break at the target locus. To achieve such a targeted dsDNA break, rare-cutting designer nucleases have been developed that recognize the desired nucleotide sequence.7 Typically, this has been accomplished using designer zinc-finger nucleases (ZFNs) composed of a nonspecific nuclease (e.g., FokI) genetically engineered with an array of zinc-finger (ZF) protein domains. These domains recognize and bind to the desired DNA sequence and consequently allow FokI to cleave the DNA sequence at this location.
Alternatively, transcription activator–like effector nucleases (TALENs) have been used, whereby the FokI nuclease is coupled to transcription activator–like effector domains that confer the nucleotide sequence specificity. More recently, gene targeting has also been accomplished using an RNA-based targeting paradigm based on the bacterial CRISPR/Cas9 system that cleaves DNA at a locus defined by a guide RNA that coaxes the Cas9 protein to the DNA target site to catalyze DNA cleavage.8,9 Designer nucleases, which have been explored for both ex vivo and in vivo gene therapy applications, were shown to result in increased gene targeting into predefined chromosomal loci with minimal off-target effects.7 Specifically, to achieve efficient in vivo gene targeting in the liver, AAV vectors have been employed to codeliver the gene-targeting construct with ZFNs specifically designed to achieve a dsDNA break at the target locus.10,11 This resulted in gene replacement through both homology-directed and homology-independent targeted gene insertion at the ZFN-specified locus. The level of gene targeting achieved (1–3%) was sufficient to partially correct the bleeding disorder in a mouse model of hemophilia B consistent with about 5% of normal FIX levels. In this specific case, targeted gene correction was critically dependent upon codelivery of the specific AAV-ZFN. ZFN-mediated targeting could also be achieved in the absence of homology arms, indicating that targeted integration occurred predominantly via nonhomologous end joining.
Although nuclease-mediated gene targeting remains promising, it also raises some important safety and/or efficacy issues. Specifically, expression of these designer nucleases after in vivo gene therapy might provoke unwanted immune reactions that could result in the immune clearance of the gene-modified cells. Although this has not yet been observed in conventional small-animal disease models, it cannot be excluded in human subjects. The expression of designer nucleases could theoretically result in an uncontrolled DNA damage response, off-target dsDNA breaks and mutagenesis, or even chromosomal translocations and other cytogenetic abnormalities. This may depend, at least in part, on the level and/or duration of expression, the type of nuclease used, the target cell type, and other unknown extrinsic and intrinsic factors. Transient expression of the designer nucleases would therefore be mandatory to minimize the risk of long-term side effects. However, the current vector platforms most commonly used to express the designer nucleases, particularly AAV and integration-defective lentiviral vectors, typically result in relatively prolonged expression after in vivo gene therapy.10,12 Moreover, it is unclear how to prevent inadvertent genomic integration of the designer nuclease genes.
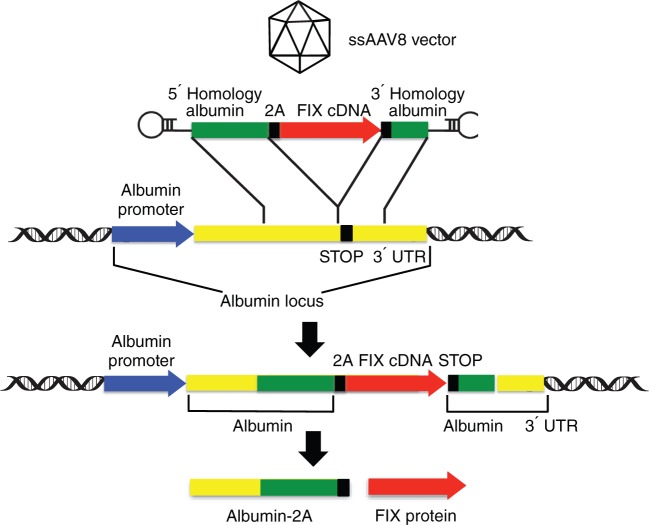
In the new study, Barzel and colleagues developed an alternative in vivo gene targeting strategy that does not require any designer nucleases.1 To achieve this, they designed an AAV vector that contains a promoterless FIX construct flanked by sequences homologous to the albumin locus, which is highly expressed in the liver. The construct was specifically designed to achieve targeted integration by homologous recombination of the FIX sequences into the albumin locus, just upstream of the albumin stop codon (Figure 1), resulting in a genetic coupling between the endogenous albumin gene and the exogenous FIX sequences and consequently linking FIX expression to the intrinsic high albumin expression in hepatocytes. As an extra precaution, to minimize disruption and dysregulation of the albumin gene, a 2A-peptide was included in the targeting construct. This caused ribosomal skipping, so that the transcribed bicistronic albumin-FIX messenger RNA was translated into individual albumin and FIX proteins. This targeting strategy was relatively efficient, yielding up to 0.5% hepatocytes harboring the desired genetic modification. Most importantly, by co-opting the potent endogenous albumin promoter, robust FIX expression levels could be achieved in the range of 7–20% of normal FIX levels sufficient to partially correct the bleeding phenotype in FIX-deficient hemophilia B mice.
Figure 1.
Nuclease-free adeno-associated virus (AAV)-mediated site-specific integration into the albumin locus. See text for details. cDNA, complementary DNA; FIX, coagulation factor IX; ssAAV8, single-stranded AAV serotype 8; UTR, untranslated region.
This study has important implications for gene therapy. A nuclease-free approach may greatly increase the overall safety of gene targeting by avoiding unwanted immune reactions to the designer nuclease and/or the gene-modified cells and minimizing the risk of off-target and other undesirable effects. More remarkably, the relatively robust targeting efficiency was several orders of magnitude higher than what had been reported in the pioneering studies of Miller and colleagues.6 The exact reason for this difference is not fully understood, but it may be due at least in part to the high expression potential of the albumin locus and its associated chromatin configuration, the design of the targeting construct itself, and/or the use of more efficient hepatotropic AAV serotypes (AAV8 vs. AAV6). It would be reassuring to test the general applicability of this approach using other genes and constructs so as to confirm the high targeting efficiencies using PCR-independent quantification methods. Whether the AAV episomes that did not engage in targeted integration at the albumin locus could still integrate randomly into preexisting dsDNA breaks in the target genome remains to be addressed.13 Interestingly, previous studies showed that targeted integration into an artificial chromosomal locus required de novo expression of designer nucleases,10,11 in contrast to the findings of Barzel et al. One likely explanation for this apparent discrepancy is that targeted integration in the absence of any homology arms occurred via nonhomologous end joining instead of by homology-directed repair.
Despite their promise, the overall efficiency of these novel integrating AAV vectors still lags behind that of conventional AAV vectors. For instance, the latest-generation AAV vector designs containing optimized liver-specific promoters showed that FIX activity levels approaching 50% of normal levels could be attained with a relatively low vector dose corresponding to only 109 genome copies per mouse (i.e., 5 × 1010 vector genomes/kg).14 By contrast, in the study by Barzel et al., 1012 genome copies per mouse (i.e., 5 × 1013 vector genomes/kg) yielded only 20% FIX, and doses below 1011 genome copies per mouse were subtherapeutic. This amounts to a >2,500-fold difference in overall efficacy, compared with state-of-the-art conventional AAVs. A hyperactive FIX transgene14,15 or alternative hepatotropic AAV capsids16 might further improve efficacy.
Nevertheless, even with these improvements, higher AAV vector doses will still be required to achieve efficient site-specific targeting. Such doses increase the likelihood of inducing AAV vector–specific cytotoxic T-cell-mediated immune responses that could clear the gene-modified cells and cause acute liver toxicity.17,18,19 However, this obstacle could potentially be overcome by using transient immune suppression.19,20 Despite these potential immune complications, the use of a site-specific integrating AAV vector may overcome potential concerns of insertional oncogenesis given that that AAV is known to integrate at a low but measurable rate (0.1–1% transduction events). Nevertheless, with the exception of the emergence of hepatocellular carcinoma in neonatal mucopolysaccharidosis VII mice undergoing AAV gene therapy,5 the overall oncogenic risk of using conventional AAV for gene therapy appears to be very low,21 although this remains a largely unresolved issue.22
Although hepatic expression from conventional AAV vectors seems relatively stable based on the currently available clinical data19,20 as well as on the many preclinical studies in a variety of small- and large-animal models, it is not known whether therapeutic expression will last for the entire life span of a treated patient. Expression from conventional AAV vectors typically declines as a result of hepatocyte turnover, albeit slowly. Consequently, this new AAV-based targeting approach may be most relevant for genetic diseases that manifest themselves in infants and therefore require stable genomic integration to offset the loss of episomes in dividing hepatocytes. Another advantage of the current promoterless AAV approach is that it not only improves the overall safety of the construct but also co-opts a robust endogenous hepatocyte-specific promoter to drive expression of the therapeutic transgene. Consequently, there is more space available in the AAV vector to accommodate larger transgenes (e.g., factor VIII) that cannot be easily packaged into AAV unless smaller and typically weaker promoters are employed. In conclusion, these integrating AAV vectors further enhance the versatility of AAVs and may ultimately foster the development of new gene therapy applications that warrant further preclinical exploration in small- and large-animal models.
References
- Barzel A, Paulk NK, Shi Y, Huang Y, Chu K, Zhang F.et al. (2014Promoterless gene targeting without nucleases ameliorates haemophilia B in mice Naturee-pub ahead of print 29 October 2014. [DOI] [PMC free article] [PubMed]
- Lombardo A, Cesana D, Genovese P, Di Stefano B, Provasi E, Colombo DF.et al. (2011Site-specific integration and tailoring of cassette design for sustainable gene transfer Nat Methods 8861–869. [DOI] [PubMed] [Google Scholar]
- Sadelain M, Papapetrou EP., and, Bushman FD. Safe harbours for the integration of new DNA in the human genome. Nat Rev Cancer . 2011;12:51–58. doi: 10.1038/nrc3179. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P.et al. (2003LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1 Science 302415–419. [DOI] [PubMed] [Google Scholar]
- Donsante A, Miller DG, Li Y, Vogler C, Brunt EM, Russell DW.et al. (2007AAV vector integration sites in mouse hepatocellular carcinoma Science 317477. [DOI] [PubMed] [Google Scholar]
- Miller DG, Wang PR, Petek LM, Hirata RK, Sands MS., and, Russell DW. Gene targeting in vivo by adeno-associated virus vectors. Nat Biotechnol. 2006;24:1022–1026. doi: 10.1038/nbt1231. [DOI] [PubMed] [Google Scholar]
- Kim H., and, Kim JS. A guide to genome engineering with programmable nucleases. Nat Rev Genet. 2014;15:321–334. doi: 10.1038/nrg3686. [DOI] [PubMed] [Google Scholar]
- Senís E, Fatouros C, Große S, Wiedtke E, Niopek D, Mueller AK.et al. (2014CRISPR/Cas9-mediated genome engineering: an adeno-associated viral (AAV) vector toolbox Biotechnol J 91402–1412. [DOI] [PubMed] [Google Scholar]
- Swiech L, Heidenreich M, Banerjee A, Habib N, Li Y, Trombetta J.et al. (2014In vivo interrogation of gene function in the mammalian brain using CRISPR-Cas9 Nat Biotechnole-pub ahead of print 19 October 2014. [DOI] [PMC free article] [PubMed]
- Li H, Haurigot V, Doyon Y, Li T, Wong SY, Bhagwat AS.et al. (2011In vivo genome editing restores haemostasis in a mouse model of haemophilia Nature 475217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anguela XM, Sharma R, Doyon Y, Miller JC, Li H, Haurigot V.et al. (2013Robust ZFN-mediated genome editing in adult hemophilic mice Blood 1223283–3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mátrai J, Cantore A, Bartholomae CC, Annoni A, Wang W, Acosta-Sanchez A.et al. (2011Hepatocyte-targeted expression by integrase-defective lentiviral vectors induces antigen-specific tolerance in mice with low genotoxic risk Hepatology 531696–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DG, Petek LM., and, Russell DW. Adeno-associated virus vectors integrate at chromosome breakage sites. Nat Genet. 2004;36:767–773. doi: 10.1038/ng1380. [DOI] [PubMed] [Google Scholar]
- Nair N, Rincon MY, Evens H, Sarcar S, Dastidar S, Samara-Kuko E.et al. (2014Computationally designed liver-specific transcriptional modules and hyperactive factor IX improve hepatic gene therapy Blood 1233195–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantore A, Nair N, Della Valle P, Di Matteo M, Màtrai J, Sanvito F.et al. (2012Hyperfunctional coagulation factor IX improves the efficacy of gene therapy in hemophilic mice Blood 1204517–4520. [DOI] [PubMed] [Google Scholar]
- Lisowski L, Dane AP, Chu K, Zhang Y, Cunningham SC, Wilson EM.et al. (2014Selection and evaluation of clinically relevant AAV variants in a xenograft liver model Nature 506382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ.et al. (2006Successful transduction of liver in hemophilia by AAV-factor IX and limitations imposed by the host immune response Nat Med 12342–347. [DOI] [PubMed] [Google Scholar]
- Mingozzi F, Maus MV, Hui DJ, Sabatino DE, Murphy SL, Rasko JE.et al. (2007CD8(+) T-cell responses to adeno-associated virus capsid in humans Nat Med 13419–422. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC.et al. (2011Adenovirus-associated virus vector–mediated gene transfer in hemophilia B N Engl J Med 3652357–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathwani AC, Reiss UM, Tuddenham EG, Rosales C, Chowdary P, McIntosh J.et al. (2014Long-term safety and efficacy of factor IX gene therapy in hemophilia B N Engl J Med 3711994–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Malani N, Hamilton SR, Schlachterman A, Bussadori G, Edmonson SE.et al. (2011Assessing the potential for AAV vector genotoxicity in a murine model Blood 1173311–3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdmanis PN, Lisowski L., and, Kay MA. rAAV-mediated tumorigenesis: still unresolved after an AAV assault. Mol Ther. 2012;20:2014–2017. doi: 10.1038/mt.2012.220. [DOI] [PMC free article] [PubMed] [Google Scholar]