Abstract
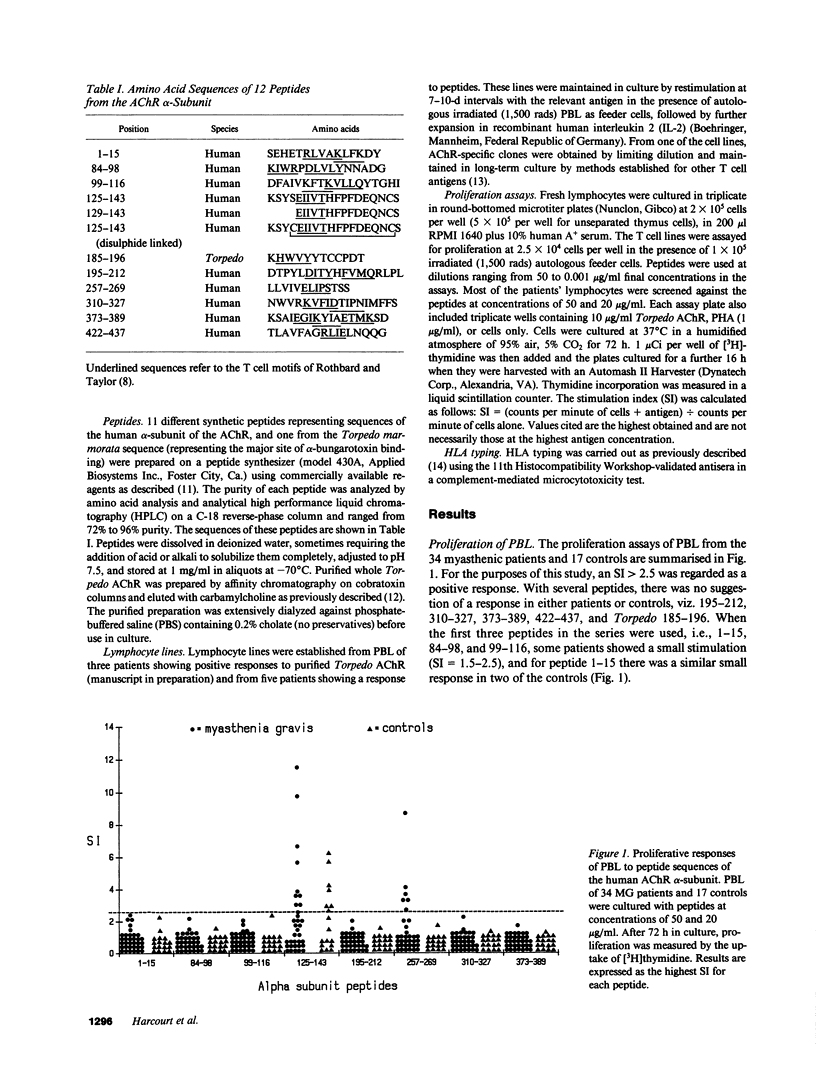
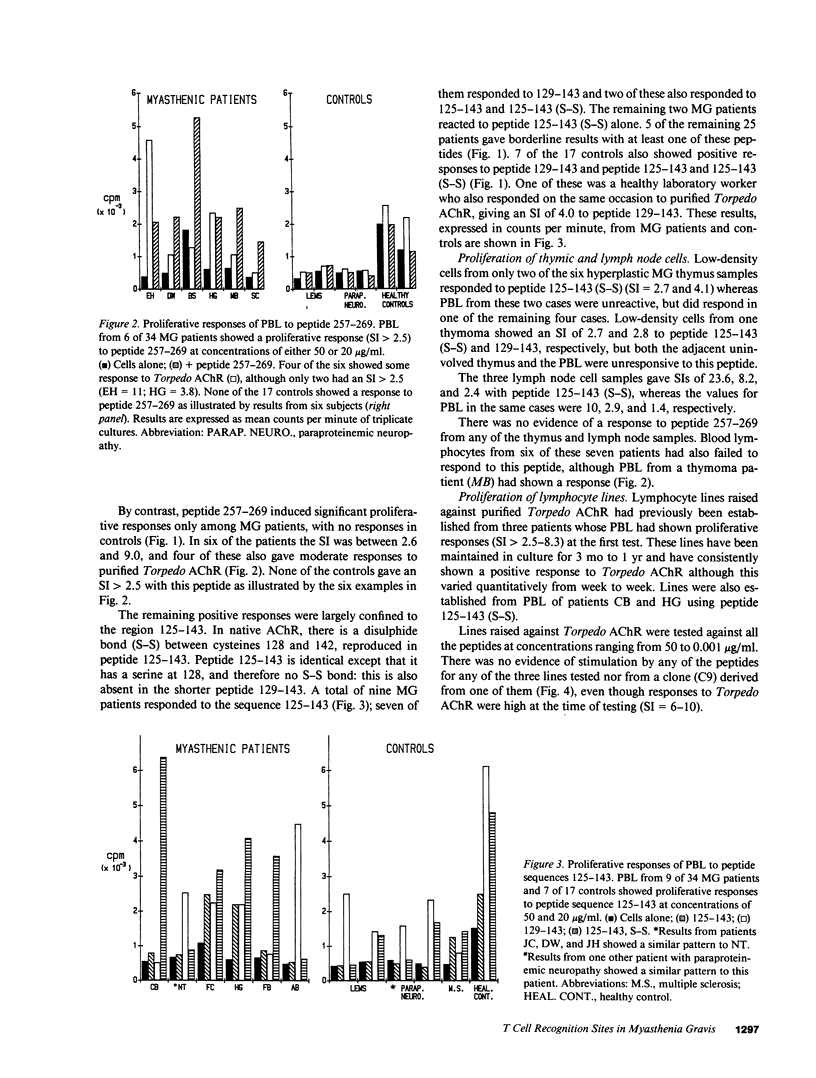
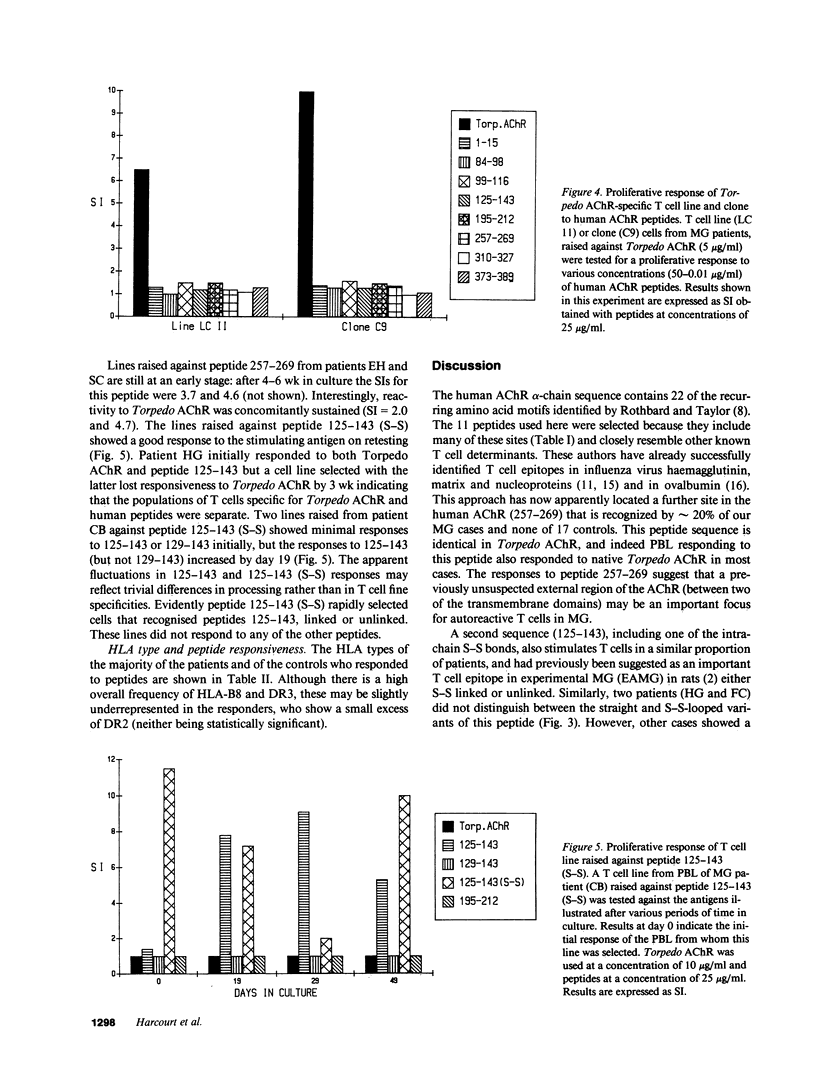
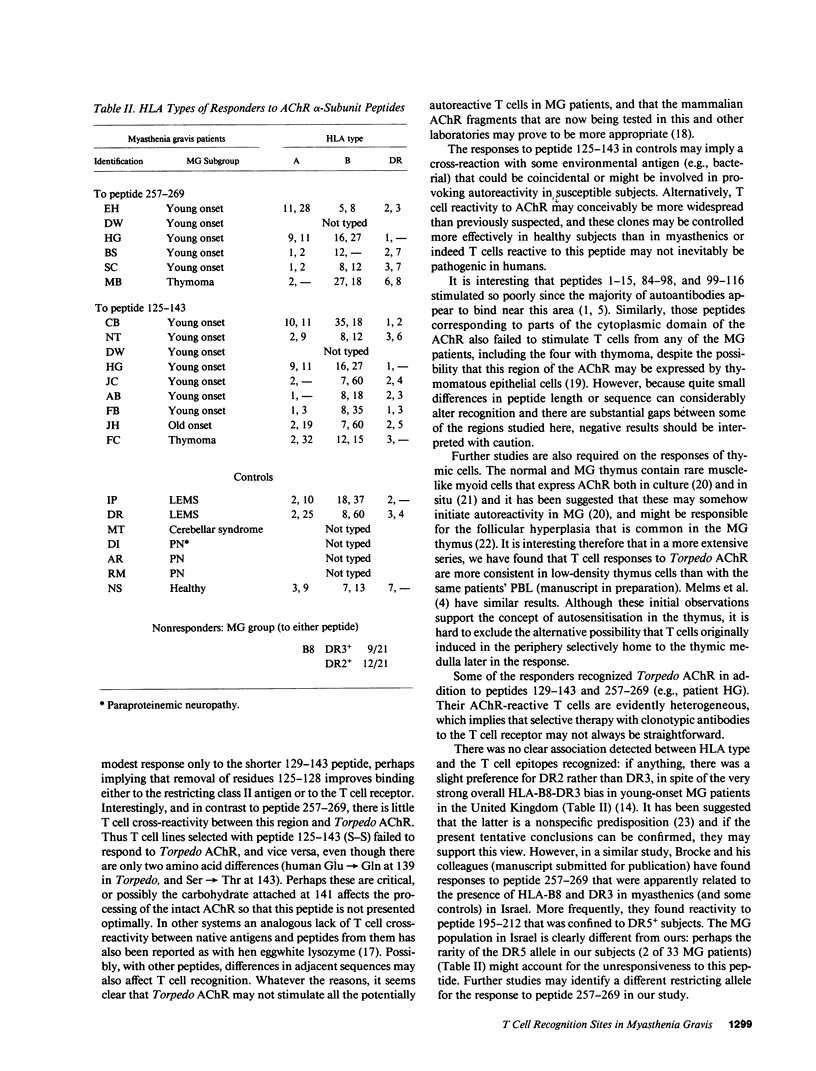
T cell proliferative responses to synthetic peptides taken from the human nicotinic acetylcholine receptor (AChR) alpha-chain sequence, or to whole AChR purified from electric fish (Torpedo marmorata), have been studied, using blood, thymus, and lymph node cells, from 34 patients with myasthenia gravis (MG) and 17 controls mostly with other neurological diseases. Peptides were selected because they contained amino acid motifs that recur in most defined T cell epitopes. Peptide 257-269 (from the extracellular loop of the AChR alpha-chain between the second and third trans-membrane domains) stimulated cells from six patients and no controls. Peptides from region 125-143 (from the main extracellular 1-210 stretch), which is thought to be an important T cell epitope in rats, provoked responses in 26% of patients and 41% of controls. Two patients responded both to these peptides and to peptide 257-269, thereby implying some heterogeneity of their reacting T cells. Whereas the initial blood T cell samples sometimes responded both to Torpedo AChR and to the 125-143 peptides, T cell lines selected with either antigen subsequently showed no response to the other. This observation suggests that it may be essential to use human AChR sequences for studying truly autoreactive T cells in MG. Finally, no strong association was found between any of the responses to peptides and the HLA types of the responding individuals.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton R., Goldschneider I., Bollum F. J. The distribution of terminal deoxynucleotidyl transferase (TdT) among subsets of thymocytes in the rat. J Immunol. 1976 Feb;116(2):462–468. [PubMed] [Google Scholar]
- Bofill M., Janossy G., Willcox N., Chilosi M., Trejdosiewicz L. K., Newsom-Davis J. Microenvironments in the normal thymus and the thymus in myasthenia gravis. Am J Pathol. 1985 Jun;119(3):462–473. [PMC free article] [PubMed] [Google Scholar]
- Compston D. A., Vincent A., Newsom-Davis J., Batchelor J. R. Clinical, pathological, HLA antigen and immunological evidence for disease heterogeneity in myasthenia gravis. Brain. 1980 Sep;103(3):579–601. doi: 10.1093/brain/103.3.579. [DOI] [PubMed] [Google Scholar]
- Gammon G., Shastri N., Cogswell J., Wilbur S., Sadegh-Nasseri S., Krzych U., Miller A., Sercarz E. The choice of T-cell epitopes utilized on a protein antigen depends on multiple factors distant from, as well as at the determinant site. Immunol Rev. 1987 Aug;98:53–73. doi: 10.1111/j.1600-065x.1987.tb00519.x. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R., Toyka K. V., Heininger K., Grosse-Wilde H., Kalies I. Autoimmune human T lymphocytes specific for acetylcholine receptor. Nature. 1984 Jul 19;310(5974):244–246. doi: 10.1038/310244a0. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R., Toyka K. V., Tzartos S. J., Carson W., Conti-Tronconi B. M. Human T-helper lymphocytes in myasthenia gravis recognize the nicotinic receptor alpha subunit. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5379–5383. doi: 10.1073/pnas.84.15.5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner T., Tzartos S., Hoppe F., Schalke B., Wekerle H., Müller-Hermelink H. K. Pathogenesis of myasthenia gravis. Acetylcholine receptor-related antigenic determinants in tumor-free thymuses and thymic epithelial tumors. Am J Pathol. 1988 Feb;130(2):268–280. [PMC free article] [PubMed] [Google Scholar]
- Lamb J. R., Eckels D. D., Lake P., Johnson A. H., Hartzman R. J., Woody J. N. Antigen-specific human T lymphocyte clones: induction, antigen specificity, and MHC restriction of influenza virus-immune clones. J Immunol. 1982 Jan;128(1):233–238. [PubMed] [Google Scholar]
- Lamb J. R., Green N. Analysis of the antigen specificity of influenza haemagglutinin-immune human T lymphocyte clones: identification of an immunodominant region for T cells. Immunology. 1983 Dec;50(4):659–666. [PMC free article] [PubMed] [Google Scholar]
- Lennon V. A., McCormick D. J., Lambert E. H., Griesmann G. E., Atassi M. Z. Region of peptide 125-147 of acetylcholine receptor alpha subunit is exposed at neuromuscular junction and induces experimental autoimmune myasthenia gravis, T-cell immunity, and modulating autoantibodies. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8805–8809. doi: 10.1073/pnas.82.24.8805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom J. Immunobiology of myasthenia gravis, experimental autoimmune myasthenia gravis, and Lambert-Eaton syndrome. Annu Rev Immunol. 1985;3:109–131. doi: 10.1146/annurev.iy.03.040185.000545. [DOI] [PubMed] [Google Scholar]
- Melms A., Schalke B. C., Kirchner T., Müller-Hermelink H. K., Albert E., Wekerle H. Thymus in myasthenia gravis. Isolation of T-lymphocyte lines specific for the nicotinic acetylcholine receptor from thymuses of myasthenic patients. J Clin Invest. 1988 Mar;81(3):902–908. doi: 10.1172/JCI113401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbard J. B., Taylor W. R. A sequence pattern common to T cell epitopes. EMBO J. 1988 Jan;7(1):93–100. doi: 10.1002/j.1460-2075.1988.tb02787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluep M., Willcox N., Vincent A., Dhoot G. K., Newsom-Davis J. Acetylcholine receptors in human thymic myoid cells in situ: an immunohistological study. Ann Neurol. 1987 Aug;22(2):212–222. doi: 10.1002/ana.410220205. [DOI] [PubMed] [Google Scholar]
- Sette A., Buus S., Colon S., Smith J. A., Miles C., Grey H. M. Structural characteristics of an antigen required for its interaction with Ia and recognition by T cells. 1987 Jul 30-Aug 5Nature. 328(6129):395–399. doi: 10.1038/328395a0. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Rothbard J., Gotch F. M., Bahadur G., Wraith D., McMichael A. J. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986 Mar 28;44(6):959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- Tzartos S. J., Seybold M. E., Lindstrom J. M. Specificities of antibodies to acetylcholine receptors in sera from myasthenia gravis patients measured by monoclonal antibodies. Proc Natl Acad Sci U S A. 1982 Jan;79(1):188–192. doi: 10.1073/pnas.79.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R. Antigen-presenting function of the macrophage. Annu Rev Immunol. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]
- Wekerle H., Ketelsen U. P. Intrathymic pathogenesis and dual genetic control of myasthenia gravis. Lancet. 1977 Mar 26;1(8013):678–680. doi: 10.1016/s0140-6736(77)92118-3. [DOI] [PubMed] [Google Scholar]
- Whiting P., Vincent A., Newsom-Davis J. Monoclonal antibodies to Torpedo acetylcholine receptor. Characterisation of antigenic determinants within the cholinergic binding site. Eur J Biochem. 1985 Aug 1;150(3):533–539. doi: 10.1111/j.1432-1033.1985.tb09054.x. [DOI] [PubMed] [Google Scholar]
- Willcox H. N., Newsom-Davis J., Calder L. R. Greatly increased autoantibody production in myasthenia gravis by thymocyte suspensions prepared with proteolytic enzymes. Clin Exp Immunol. 1983 Nov;54(2):378–386. [PMC free article] [PubMed] [Google Scholar]


