Abstract
Clostridium difficile toxin A and B (TcdA and TcdB) are the major virulence factors of the bacterium, both of which consist of two enzymatic domains: an effector glucosyltransferase domain (GTD) and a cysteine protease domain (CPD) responsible for autocleavage and release of GTD. Although the CPDs from both toxins share a similar structure and mechanism of hexakisphosphate (InsP6) -induced activation, TcdA is substantially less sensitive to the autocleavage as compared with TcdB. In this study, we provided evidence of inter-domain regulation of CPD activity of TcdA and its autoprocessing. The C-terminus combined repetitive oligo peptides (CROPs) of TcdA reduced the accessibility of TcdB CPD to its substrate in a chimeric toxin TxB-Ar, consequently blocking autoprocessing. Moreover, interference of antibodies with the CROPs of full-length TcdA efficiently enhanced its GTD release. In conclusion, by utilizing chimeric toxins and specific antibodies, we identified that the CROPs of TcdA plays a crucial role in controlling the InsP6-mediated activation of CPD and autocleavage of GTD. Our data provides insights on the molecular mode of action of the C. difficile toxins.
Keywords: Clostridium difficile, toxin A, autoprocessing, C-terminus combined repetitive oligo peptides (CROPs), cysteine protease domain (CPD)
1. Introduction
Clostridium difficile infection is the leading cause of antibiotic associated diarrhea for the past decade in North America and Europe. Two large exotoxins, TcdA (308 kDa) and TcdB (269 kDa) are the primary virulence factors of the disease. The two proteins are homologous to each other and have a similar domain structure containing at least four functional domains [1–3]: the N-terminus glucosyltransferase domain (GTD), a cysteine protease domain (CPD), a putative translocation domain, and a C-terminus receptor binding domain (RBD, also known as combined repetitive oligopeptides or CROPs). The CPDs of TcdA and TcdB autocleave and release their GTDs upon binding of allosteric cofactors inositol hexakis and heptakisphosphate (InsP6 and InsP7) [4–6], a process that is important but not required for the cytotoxicity of the toxins [7, 8].
Although TcdA and TcdB share a similar CPD structure and conserved InsP6-induced activation mechanism [9], the two toxins differ significantly in efficiency to undergo autoprocessing. InsP6-induced autoprocessing of TcdB holotoxin has been fully demonstrated in several studies [5, 6, 10, 11]. On the contrary, few reports showed only autoprocessing of full-length of TcdA in the presence of both InsP6 and DTT [8, 12, 13]. Since dithiothreitol (DTT) alone can trigger the autocleavage of TcdA [14], it is unknown whether TcdA holotoxin is sensitive to InsP6-induced autoprocessing. On the other hand, a fully autoprocessing was reported in C-terminus-truncated TcdA [8] or CPD fragment of TcdA containing the cleavage site [15]. The molecular mechanism underlying the insensitive of TcdA to InsP6-mediated autocleavage is unknown but a recent study indicates that CROPs may play some roles [12].
In this study, we found that a chimeric TcdB bearing the full-length of receptor binding domain (RBD) or CROPs from TcdA was no longer sensitive to InsP6-induced autoprocessing. Monoclonal antibodies that specifically bind to CROPs of TcdA significantly enhanced the InsP6-mediated autocleavage and the release of its GTD. Our study thus provided evidence that the C-terminus CROPs from TcdA affect the toxin’s autoprocessing and understanding on inter-domain interaction that may affect the molecular mechanism of toxin action.
2. Materials and Methods
2.1. C. difficile wild type toxins and chimera TxB-Ar
The generation and purification of recombinant wild type TcdA and TcdB were reported previously. The molecular cloning and purification of chimera TxB-Ar were as previously described [16]. The highly purified recombinant toxins that appeared as a single band on an SDS-PAGE gel were used in this study. Western blot was performed to detect various domains of toxins using mouse poly- and mono-clonal antibodies that were reported previously [17, 18]. These antibodies are: α-TcdA and α-TcdB-I are antibodies respectively against C terminus of TcdA and TcdB (BioDesign Inc.); α-TcdB-II is alpaca serum raised in the lab which is against full length of TcdB; monoclonal mouse antibody A1E6 raised in the lab recognizes the whole C terminus RBD of TcdA.
2.2. Cell lines and toxicity assay
The African green monkey kidney Vero cell line and mouse intestine carcinoma CT26 cell line were obtained from American Type Culture Collection (ATCC). Cells were maintained in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM L-glutamine, and 1 mM sodium pyruvate. The cytopathic/cytotoxic effects of toxins on cultured cells were assessed by cell rounding assays [16]. Vero or CT26 cells seeded in 96-well plates were treated with either wild type toxins or the chimera. Cell rounding was visualized by phase-contrast microscopy. Each toxin concentration was tested in triplicate for overall cell rounding, and the experiments were repeated three times. The glucosyltransferase activity was measured by detecting the glucosylation of Rac1 of Vero cells after toxin exposure as described previously [7, 19]. Vero cells seeded in 24-well plates were incubated with various doses of toxins for 4 hours. Then, cell pellets were collected and lyzed by SDS-loading buffer and heating at 95 °C for 5 minutes. Non-glucosylated Rac1 was detected by immunoblot. β-actin was using as an equal loading control.
2.3. InsP6 induced autoprocessing
TcdB and TxB-Ar were diluted in 20 μM Tris (pH 7.0) buffer to a concentration of 10 ng/μL in a final volume of 20 μL. Autoprocessing was initiated by the addition of 25 μM InsP6. Following incubation at 37 °C for 1 hour, the reactions were stopped by SDS sampling buffer and analyzed by Western blot using a VHH antibody E3 against GTD of TcdB [18]. Autoprocessing of TcdA induced by 100 μM InsP6 in 20 mM HEPES (pH8.0) in the presence or absence of 20 ng/μL of individual anti-CROPs of TcdA monoclonal antibodies or their mixture (20 ng/uL each) at 37 °C for 1 hour. These antibodies included mouse monoclonal antibody A1H3 and A1E6 [20], VHH antibodies AB8, A11G, AE1, AC1 and A3H [18]. An anti-GTD of TcdA VHH AH3 was used to detect the cleaved GTD fragment in a western blot [18]. All the antibodies used in this experiment were generated in this laboratory [18, 20].
2.4. AWP19 labeling
For CPD-activity based-labeling, 0.1 μg/μL of TcdB or TxB-Ar was incubated with different doses of AWP19 in 20 uM pH 7.0 Tris buffer in the presence of 25 uM InsP6 at 37 °C for 1 hour. Carboxytetramethylrhodamine (TAMRA) labeled and non-fluorescent AWP19 [21] were gifts from Dr. Aimee Shen (University of Vermont). The reaction was terminated with heating at 95 °C for 5 minutes in SDS-loading buffer. Samples were separated by 4–20% SDS-PAGE. AWP19-TAMRA labeled bands were detected using a G-Box Chemi (Syngene) with Green LED lights (520–550 nm) and UV06 filter (572–625nm ban pass). Coomassie blue staining was used to visualize total amount of loaded proteins and cleaved fragment.
3. Results
3.1. Structure and activity of chimera TxB-Ar
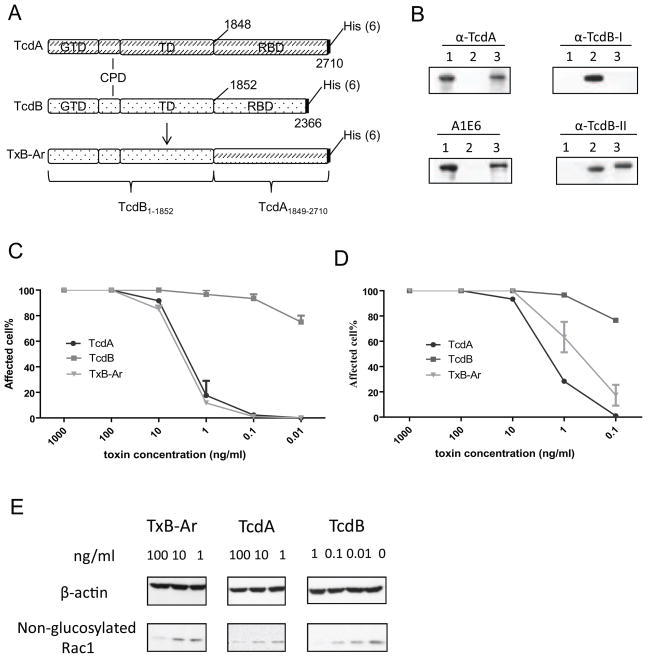
The chimera TxB-Ar is TcdB with its intact CROPs replaced by that from TcdA (Figure 1A) with a molecular weight of 300 kDa. TxB-Ar was recognized by antibodies against the CROPs of TcdA (α-TcdA and A1E6) but not antibody against the CROPs of TcdB (α-TcdB-I) (Figure 1B). TxB-Ar was also recognized by the poly-serum against full-length TcdB (αTcdB-II). TxB-Ar is less potent than TcdB in inducing cell rounding in either Vero (Figure 1C) or CT26 cells (Figure 1D), but comparable to TcdA. We further examined Rac1 glucosylation of Vero cells after exposure to the chimeric and wild type toxins. Consistent with the results of cytotoxicity assay, TcdA or TxB-Ar at 100 ng/mL was able to cause glucosyltation of most Rac1 whereas TcdB at 1 ng/mL induced a similar Rac1 glucosylation (Figure 1E). Furthermore, TxB-Ar is proinflammatory and capable of inducing TNF-a production by DCs [16]. These data indicate that TxB-Ar is a fully functional toxin.
Fig. 1.
Structure and activities of the chimera TxB-Ar. (A) Domain structure of TxB-Ar. TxB-Ar is TcdB with its the C-terminus CROPs replaced by the full-length CROPs from TcdA. (B) Western blot analysis of TxB-Ar. Western blot was performed to detect the domains of TcdA, TcdB, and TxB-Ar with various antibodies. Lane 1: TcdA, Lane 2: TcdB, and Lane 3: TxB-Ar. (C, D): Cytopathic effects of the chimera TxB-Ar, TcdA, or TcdB to Vero cells (C) or CT26 cells (D). The serially diluted toxins were applied to the sub-confluent cells for 16 hours. The percentage of rounding cells was examined under a phase contrast microscope. The experiments were performed three times and error bars indicate the standard error of mean (SEM). (E) Vero cells were incubated with the indicated doses of the toxins for 4 hours. Cells were collected and lysed by SDS sampling buffer for western blot to detect Rac1 glucosylation.
3.2. InsP6 induced autoprocessing of TxB-Ar is abolished
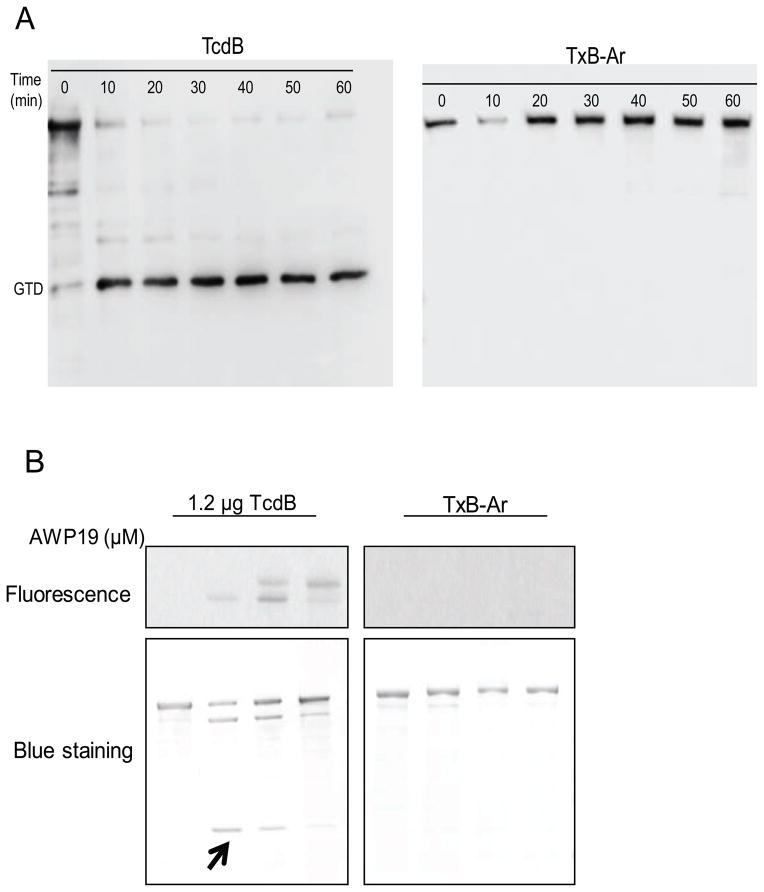
Since TxB-Ar is fully functional and has intact GTD and CPD as wild type TcdB, we expect that the chimera acts similarly as TcdB in response to InsP6 exposure. In the presence of InsP6, TcdB efficiently released its GTD after 10 min of incubation (Figure 2A). Surprisingly, the autoprocessing and releasing GTD from TxB-Ar were not detectable even after 60 min of InsP6 incubation (Figure 2A). Furthermore, a fluorescent probe AWP19 that mimics the substrate and specifically binds to the enzymatic pocket of TcdB cysteine protease [10, 21, 22] failed to bind to TxB-Ar whereas a dose-dependent binding showed in wild type TcdB, suggesting CPD activity sites of TxB-Ar were not accessible to its substrate (Figure 2B upper panel). As the result, the autocleavage and release of GTD from TcdB was inhibited by AWP19 in a dose-dependent manner whereas no autocleavage was seen in any conditions tested for TxB-Ar (Figure 2B lower panel).
Fig. 2.
Autoprocessing of chimera TxB-Ar. (A) InsP6 induced autoprocessing of TcdB and TxB-Ar. 10 ng/μL of TcdB or TxB-Ar was incubated in the reaction buffer containing 25 μM InsP6 at 37 °C for the indicated time. The reaction was terminated by SDS-sampling buffer and heating at 95 °C for 5 min. A VHH antibody (E3) against the GTD of TcdB was used for western blot analysis. (B) CPD conformational change probed by fluorescent AWP19. TcdB (0.1 μg/μL) was incubated with the indicated doses of AWP19 in 20 μM pH8.0 Tris buffer containing 25 μM InsP6 at 37 °C for 1 hour. The reactions were stopped by SDS sampling buffer and the samples were loaded on a SDS-PAGE. Fluorescence was measured using G-Box Chemi system and the total proteins were visualized on the gel after coomassie blue staining. The arrow indicates the cleaved GTD fragment.
3.3. Antibody binding to the CROPs affect autoprocessing of TcdA
The results that chimera TxB-Ar did not undergo detectable autoprocessing in response to InsP6 exposure prompt us to investigate the potential interaction of the CROPs from TcdA with the CPD thus affect the autoprocessing of the toxin. To test this hypothesis, we utilized a panel of antibodies specifically recognizing CROPs from TcdA [18, 20]. As shown in Figure 3, wild type TcdA underwent autoprocessing in response to InsP6 treatment and the GTD fragment was clearly detected when the toxin was incubated with A1E6, AB8, or the antibody mixture containing all the tested antibodies. A1H3, A11G, and A3H antibodies seemed to have partial effects whereas antibodies AE1 and AC1 had no detectable effects on triggering the autoprocessing of TcdA holotoxin. These data demonstrated that antibodies specific to CROPs of TcdA rendered the toxin sensitive to InsP6-induced autocleaveage, suggesting that the CROPs domain at the C-terminus of TcdA affects the function of CPD by blocking InsP6-mediated activation of the CPD and the subsequent autocleavage and release of N-terminal GTD fragment.
Fig. 3.
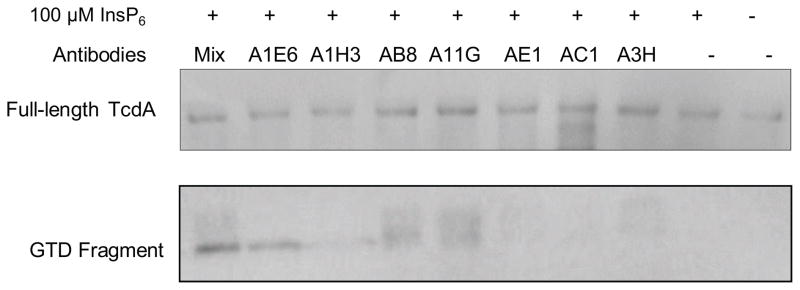
Antibody interference with the autoprocessing of TcdA. TcdA was incubated with different antibodies and their mixture at 37°C for 1 hour in autoprocessing reaction buffer containing 25 μM InsP6. The reaction was terminated by SDS-sampling buffer and heated at 95 °C for 5 min. Western blot was performed for detecting cleaved GTD fragment from TcdA using a GTD-specific VHH antibody AH3.
4. Discussions
The cysteine protease domains of TcdA and TcdB have been identified to mediate InsP6-induced autocleavage and release GTDs [6, 8, 14]. However, unlike TcdB, TcdA is highly resistant to InsP6-mediated autoprocessing while the two toxins share structurally similar CPDs and the same mechanism of InsP6-induced activation [8, 12, 14]. In this study, we found that the full-length RBD containing CROPs of TcdA regulates the InsP6-mediated activation of CPD and autocleavage of its GTD. Our data provides insights on the molecular mode of action of the C. difficile TcdA and TcdB.
To study whether other domains of the toxins affect InsP6-mediated autoprocessing, we compared the autocleavage and release of GTD from wild type TcdB and chimeric TcdB bearing the CROPs (TxB-Ar) from TcdA. We found that the chimera TxB-Ar was insensitive to InsP6 exposure, suggesting that the CROPs from TcdA may affect the InsP6-mediated CPD activation. To confirm whether this can be a potential mechanism underlying the insensitivity of wild type TcdA to InsP6-mediated activation, we exposed TcdA with a panel of monoclonal antibodies that binds to the CROPs of the toxins while treating with InsP6. Our results showed that the binding of specific antibodies to the CROPs of TcdA allowed the InsP6-mediated activation of CPD and subsequently autoprocessing and release GTD of the toxin.
Crystal structure studies revealed that InsP6 binding to both TcdA and TcdB could markedly shift the inactive CPD to an active and stable form exposing catalytic residues and intramolecular substrate docking [15, 22]. Thus, InsP6 treatment allows a fluorescent probe AWP19 mimicking CPD substrate to covalently modify the catalytic cysteine 698 residue of TcdB CPD [22]. The fact that TxB-Ar could not be labeled by AWP19 suggested that the protein was somehow folded to enclose the catalytic residues even in the presence of InsP6, indicating that the CROPs from TcdA might either affect InsP6 binding or the access of AWP19 to the CPD of TcdB.
In this study, the chimeric toxin TxB-Ar failed to efficiently undergo autocleaveage in the presence of InsP6, suggesting that CROPs from TcdA may affect CPD-mediated autoprocessing. Previously, Genisyuerek et al found a TcdB chimera with its C-terminus replaced by the receptor-binding domain of diphtheria toxin (DTRD) can efficiently undergo autoprocessing in the presence of InsP6 [23], thus the suppression of the autoprocessing in TxB-Ar may be specific to the CROPs from TcdA. Most recently, Olling et al. [12] reported a similar chimeric toxin as TcdB1-1852-TcdA1875-2710 underwent autoprocessing induced by InsP6 in the presence of DTT at a concentration alone can sufficiently induce autoprocessing of either TcdA or TcdB [14]. In addition, the CROPs from Olling et al study is shorter than TxB-Ar (TcdA1849-2710) in our study which may also account for its inability to block autoprocessing of the chimeric toxin.
To investigate whether the CROPs of TcdA indeed affect the holotoxin’s autoprocessing, we utilized a panel of monoclonal antibodies that recognize the CROPs of TcdA. The binding of several antibodies to CROPs, especially A1E6 and AB8, led to a significant increase of autocleavage and release of TcdA GT fragment in the presence of InsP6. Other antibodies such as AC1 that also bind to the CROPs but have no effects on CPD autoprocessing, suggesting that the interaction of CROPs with CPD or cleavage sites may be specific. The future study to identify the exact binding epitopes of these monoclonal antibodies may help us to elucidate the precise regions that affect CPD autoprocessing.
It is unclear how exactly the CROPs affect autoprocessing of TcdA. Negative stain EM showed a two-tailed structure of the two toxins [24]. One tail is corresponding to CROPs while the other is N-terminal GTD and CPD. The two tails are spatially adjacent to each other. Compared with TcdB, TcdA has longer CROPs that seems to interact with the glucosyltransferase (1–542) or intermediate (1102–1847) domain of TcdA [12]. It is likely that the long CROPs from TcdA may block the binding sites of InsP6, subsequently abolishing conformational reorganization and CPD activation induced by InsP6 [21]. The other potential mechanism is that the CROPs does not affect the InsP6 binding but the cleavage. CROPs from TcdA may interact CPD catalytic residue(s) or affect the access of substrate to the cleavage pocket. In the future studies, it is important to elucidate the exact mechanism that the CROPs of TcdA affects the toxin’s autoprocessing. Given our recent finding that CPD-mediated autoprocessing is not necessary for cytotoxicity of TcdB to cultured cells [7, 8], further study is needed to reveal the physiological importance of the differential regulation of CPD-mediated autoprocessing between these two toxins in relevant animal disease models.
In summary, we reported new evidence to support the potential functional inter-domain regulation in C. difficile toxins that the CROPs of TcdA may regulate CPD autoprocessing function. Our finding provides insights on the molecular mode of action of Clostridium difficile toxins.
Supplementary Material
Highlights.
CROPs of TcdA blocks CPD-mediated autoprocessing through inter-domain interaction.
The substrate is not accessible to the CPD of TxB-Ar carrying the CROPs from TcdA
TxB-Ar is insensitive to InsP6-induced activation.
Antibody binding to the CROPs of TcdA facilitates InsP6-induced activation.
Acknowledgments
This work was supported by awards R01AI088748, R01DK084509, R56AI99458, and U19 AI109776 funded from the National Institute of Allergy and Infectious Diseases and National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health (NIH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jank T, Aktories K. Structure and mode of action of clostridial glucosylating toxins: the ABCD model. Trends in microbiology. 2008;16:222–229. doi: 10.1016/j.tim.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 2.Albesa-Jove D, Bertrand T, Carpenter EP, Swain GV, Lim J, Zhang J, Haire LF, Vasisht N, Braun V, Lange A, von Eichel-Streiber C, Svergun DI, Fairweather NF, Brown KA. Four distinct structural domains in Clostridium difficile toxin B visualized using SAXS. Journal of Molecular Biology. 2010;396:1260–1270. doi: 10.1016/j.jmb.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Pruitt RN, Chambers MG, Ng KK, Ohi MD, Lacy DB. Structural organization of the functional domains of Clostridium difficile toxins A and B. Pro Natl Acad Sci US. 2010;107:13467–13472. doi: 10.1073/pnas.1002199107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savidge TC, Urvil P, Oezguen N, Ali K, Choudhury A, Acharya V, Pinchuk I, Torres AG, English RD, Wiktorowicz JE, Loeffelholz M, Kumar R, Shi L, Nie W, Braun W, Herman B, Hausladen A, Feng H, Stamler JS, Pothoulakis C. Host S-nitrosylation inhibits clostridial small molecule-activated glucosylating toxins. Nature medicine. 2011;17:1136–1141. doi: 10.1038/nm.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reineke J, Tenzer S, Rupnik M, Koschinski A, Hasselmayer O, Schrattenholz A, Schild H, von Eichel-Streiber C. Autocatalytic cleavage of Clostridium difficile toxin B. Nature. 2007;446:415–419. doi: 10.1038/nature05622. [DOI] [PubMed] [Google Scholar]
- 6.Egerer M, Giesemann T, Herrmann C, Aktories K. Autocatalytic processing of Clostridium difficile toxin B. Binding of inositol hexakisphosphate. The Journal of biological chemistry. 2009;284:3389–3395. doi: 10.1074/jbc.M806002200. [DOI] [PubMed] [Google Scholar]
- 7.Li S, Shi L, Yang Z, Feng H. Cytotoxicity of Clostridium difficile toxin B does not require cysteine protease-mediated autocleavage and release of the glucosyltransferase domain into the host cell cytosol. Pathogens and disease. 2013;67:11–18. doi: 10.1111/2049-632X.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kreimeyer I, Euler F, Marckscheffel A, Tatge H, Pich A, Olling A, Schwarz J, Just I, Gerhard R. Autoproteolytic cleavage mediates cytotoxicity of Clostridium difficile toxin A. Naunyn-Schmiedeberg’s archives of pharmacology. 2011;383:253–262. doi: 10.1007/s00210-010-0574-x. [DOI] [PubMed] [Google Scholar]
- 9.Pruitt RN, Lacy DB. Toward a structural understanding of Clostridium difficile toxins A and B. Frontiers in cellular and infection microbiology. 2012;2:28. doi: 10.3389/fcimb.2012.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lanis JM, Hightower LD, Shen A, Ballard JD. TcdB from hypervirulent Clostridium difficile exhibits increased efficiency of autoprocessing. Molecular microbiology. 2012;84:66–76. doi: 10.1111/j.1365-2958.2012.08009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rupnik M, Pabst S, Rupnik M, von Eichel-Streiber C, Urlaub H, Soling HD. Characterization of the cleavage site and function of resulting cleavage fragments after limited proteolysis of Clostridium difficile toxin B (TcdB) by host cells. Microbiology. 2005;151:199–208. doi: 10.1099/mic.0.27474-0. [DOI] [PubMed] [Google Scholar]
- 12.Olling A, Huls C, Goy S, Muller M, Krooss S, Rudolf I, Tatge H, Gerhard R. The combined repetitive oligopeptides of clostridium difficile toxin A counteract premature cleavage of the glucosyl-transferase domain by stabilizing protein conformation. Toxins. 2014;6:2162–2176. doi: 10.3390/toxins6072162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donald RG, Flint M, Kalyan N, Johnson E, Witko SE, Kotash C, Zhao P, Megati S, Yurgelonis I, Lee PK, Matsuka YV, Severina E, Deatly A, Sidhu M, Jansen KU, Minton NP, Anderson AS. A novel approach to generate a recombinant toxoid vaccine against Clostridium difficile. Microbiology. 2013;159:1254–1266. doi: 10.1099/mic.0.066712-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egerer M, Giesemann T, Jank T, Satchell KJ, Aktories K. Auto-catalytic cleavage of Clostridium difficile toxins A and B depends on cysteine protease activity. The Journal of biological chemistry. 2007;282:25314–25321. doi: 10.1074/jbc.M703062200. [DOI] [PubMed] [Google Scholar]
- 15.Pruitt RN, Chumbler NM, Rutherford SA, Farrow MA, Friedman DB, Spiller B, Lacy DB. Structural determinants of Clostridium difficile toxin A glucosyltransferase activity. The Journal of biological chemistry. 2012;287:8013–8020. doi: 10.1074/jbc.M111.298414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H, Sun X, Zhang Y, Li S, Chen K, Shi L, Nie W, Kumar R, Tzipori S, Wang J, Savidge T, Feng H. A chimeric toxin vaccine protects against primary and recurrent Clostridium difficile infection. Infection and immunity. 2012;80:2678–2688. doi: 10.1128/IAI.00215-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He X, Sun X, Wang J, Wang X, Zhang Q, Tzipori S, Feng H. Antibody-enhanced, Fc gamma receptor-mediated endocytosis of Clostridium difficile toxin A. Infection and immunity. 2009;77:2294–2303. doi: 10.1128/IAI.01577-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Z, Schmidt D, Liu W, Li S, Shi L, Sheng J, Chen K, Yu H, Tremblay JM, Chen X, Piepenbrink KH, Sundberg EJ, Kelly CP, Bai G, Shoemaker CB, Feng H. A novel multivalent, single-domain antibody targeting TcdA and TcdB prevents fulminant Clostridium difficile infection in mice. The Journal of infectious diseases. 2014;210:964–972. doi: 10.1093/infdis/jiu196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Shi L, Li S, Yang Z, Standley C, Yang Z, ZhuGe R, Savidge T, Wang X, Feng H. A segment of 97 amino acids within the translocation domain of Clostridium difficile toxin B is essential for toxicity. PloS one. 2013;8:e58634. doi: 10.1371/journal.pone.0058634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He X, Wang J, Steele J, Sun X, Nie W, Tzipori S, Feng H. An ultrasensitive rapid immunocytotoxicity assay for detecting Clostridium difficile toxins. Journal of microbiological methods. 2009;78:97–100. doi: 10.1016/j.mimet.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puri AW, Lupardus PJ, Deu E, Albrow VE, Garcia KC, Bogyo M, Shen A. Rational design of inhibitors and activity-based probes targeting Clostridium difficile virulence factor TcdB. Chemistry & biology. 2010;17:1201–1211. doi: 10.1016/j.chembiol.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen A, Lupardus PJ, Gersch MM, Puri AW, Albrow VE, Garcia KC, Bogyo M. Defining an allosteric circuit in the cysteine protease domain of Clostridium difficile toxins. Nature structural & molecular biology. 2011;18:364–371. doi: 10.1038/nsmb.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Genisyuerek S, Papatheodorou P, Guttenberg G, Schubert R, Benz R, Aktories K. Structural determinants for membrane insertion, pore formation and translocation of Clostridium difficile toxin B. Molecular microbiology. 2011;79:1643–1654. doi: 10.1111/j.1365-2958.2011.07549.x. [DOI] [PubMed] [Google Scholar]
- 24.Pruitt RN, Chambers MG, Ng KK, Ohi MD, Lacy DB. Structural organization of the functional domains of Clostridium difficile toxins A and B. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:13467–13472. doi: 10.1073/pnas.1002199107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.