Abstract
Selective serotonin reuptake inhibitors (SSRIs) are associated with significant sexual side effects. By definition, all SSRIs increase overall serotonin (5HT) by binding to serotonin autoreceptors (5HTI A); however, each SSRI has a unique portfolio of secondary binding properties to other neurotransmitters such as norepinephrine (NE). As 5HTI A receptors mediate NE neurotransmission, SSRIs that are highly selective for 5HTI A are more likely to reduce NE efficiency; however, in SSRIs that are less selective for 5HTI A, this could be counteracted by secondary binding to NE. Norepinephrine is the major neurotransmitter of the sympathetic nervous system (SNS), which has been shown to mediate genital arousal in women; thus, it is possible that increasing SNS activity in women taking SSRIs that are highly selective for 5HTI A may counteract sexual side effects in those women. To test this hypothesis, we conducted a reanalysis of Meston (2004)’s 8-week, double-blind, cross-over, placebocontrolled study of the effects of ephedrine (50 mg taken 1 h prior to sexual activity) on self-reported sexual functioning of women taking paroxetine (N = 5), sertraline (N = 7), or fluoxetine (N = 7). As predicted, women taking SSRIs, which are highly selective for 5HTI A (sertraline, paroxetine), showed improvement in sexual arousal and orgasm. By contrast, women taking SSRIs, which are less selective for 5HTI A relative to NE (fluoxetine), showed no change or decrease in sexual functioning. These findings have implications for treating certain SSRI-induced sexual side effects.
It is well known that despite their beneficial effects in treating depression and other psychological disorders, selective serotonin reuptake inhibitors (SSRIs) often carry a burden of sexual side effects. It is estimated that about 90% of men and 96% of women taking SSRIs experience sexual side effects in at least one area of sexual functioning (Clayton, Keller, & McGarvey, 2006). Approximately 20–50% of people using SSRIs experience a sexual side effect that is deemed significant enough to warrant separate clinical attention (Rosen, Lane, & Menza, 1999). Given that sexual side effects are cited by patients as one of the top reasons for discontinuing SSRI treatment (Bull et al., 2002), an investigation into ways to diminish or manage adverse sexual side effects is clearly warranted.
Previous work on SSRI-induced sexual side effects has stipulated that they stem from SSRI’s effects on serotonin neurotransmission in the central nervous system (CNS; Gitlin, 2003). As such, previously investigated treatment strategies have often concentrated on reducing excess serotonin in the CNS through reducing the action of SSRIs (e.g., in a “drug holiday” (Rothschild, 1995) or using anti-serotonergic agents (e.g., addition of busprione (Balon, 2006). These treatments, however, have been met with limited success and moreover can be detrimental to the main therapeutic action of SSRIs (Gregorian, 2002). It would seem that a more circuitous route to affecting sexual functioning—one that bypasses the therapeutic central action of the SSRI—may be ultimately more beneficial.
To this end, based on laboratory findings showing 50 mg of ephedrine increased sexual arousal compared to placebo (Meston & Heiman, 1998), Meston (2004) examined whether ephedrine might be beneficial in counteracting SSRI-induced sexual side effects—in particular, impaired arousal. Ephedrine acts peripherally as an alpha and beta adrenergic agonist, and increases sympathetic nervous system (SNS) activity which enhances genital arousal in women (Meston & Gorzalka, 1996a, 1996b). Given ephedrine acts primarily via peripheral mechanisms, it would be unlikely to diminish the therapeutic effects of SSRIs that are more centrally mediated. In this study, 19 women receiving psychopharmacological treatment for depression with sertraline, paroxetine, or fluoxetine participated in an 8-week, randomized, cross-over, placebo-controlled, at-home trial to determine if ephedrine alleviated SSRI-induced sexual side effects. Ephedrine was beneficial in improving sexual desire and orgasmic ability and intensity; however, these findings were not statistically significantly greater than the improvements seen with placebo.
One explanation for Meston’s (2004) null findings that was not mentioned by the author is that the analyses were conducted by collapsing all participants into a single group, regardless of the type of SSRI they were receiving. Recent research has shown that there are important differences between SSRIs that may warrant individual attention. Although the primary action of all SSRIs is inhibition of serotonin reuptake, each SSRI also has a unique portfolio of secondary binding properties which contribute to its distinctive side effect profile (Hyttel, 1993). That is, even though all SSRIs share the ability to change serotonin action, each may have additional, albeit lesser, abilities to change the action of other neurotransmitters. For example, fluoxetine acts not only as a serotonin reuptake inhibitor but also as a (relatively) weak norepinephrine (NE) reuptake inhibitor (Stahl, 1998a). In contrast, sertraline and paroxetine have much less of a direct effect on NE neurotransmission (Stahl, 1998b). Thus, grouping all SSRI users together may have obscured an interaction between ephedrine and these unique secondary properties.
Understanding the subtle differences between SSRIs in how they transmit neurochemical messages may elucidate ways in which adverse side effects may be counteracted at a peripheral level. According to the monoamine hypothesis, depression is due not only to a deficiency of serotonin (5HT) but also to a relative surplus of 5HT receptors (Stahl, 2000). In order to regulate the amount of 5HT produced in the neuron, the somatodendritic area of the cell body has a number of presynaptic autoreceptors, which when stimulated, send information to the cell nucleus to inhibit the amount of 5HT that is produced at the synapse. However, in a depressed individual, there are too many of these inhibitory receptors and thus the cell is constantly signaled to shut down production of 5HT. The most immediate response of SSRIs is to block the 5HT reuptake pump at the somatodendritic site. This keeps 5HT from returning to the neuron, thereby forcing it to stay in the synapse, where it can continue to activate receptors.
Information that there is a rapidly increased level of stimulation at the autoreceptors is communicated to the cell nucleus, which responds by downregulating (i.e., halting the production of new autoreceptors to slowly reduce the total number of receptors) and desensitizing autoreceptors. This allows the neuron to maintain balance between receptors and messages and avoids overstimulation of the cell. Because the autoreceptors have become less sensitive to 5HT, they do not transmit as many inhibitory signals to the rest of the neuron to stop 5HT production. Also, as there are fewer receptors available to receive “stop messages,” there is less inhibition overall. With less inhibition, there is more 5HT produced and released into the synapse that can communicate with the post-synaptic neuron. This helps to alleviate the relative deficiency overall. As this level of neuronal change mediated gradually through genetic expression, there is a delay of about 2 weeks between beginning SSRI treatment and the onset of therapeutic effect.
Serotonin autoreceptors are also associated with facilitatory effects on NE neurotransmission (Millan, Lejeune, & Gobert, 2000). Thus, as SSRIs desensitize and downregulate 5HT autoreceptors, they also reduce the amount of NE that is available in the synapse. In theory, as the release of NE decreases, the post-synaptic neuron will produce more receptors to try to catch the NE. This, in turn, will cause the NE that is released to be less “efficient” in sending messages. One way in which this less-efficient NE neurotransmission could be observed is through the SNS, which uses NE as its primary messenger for arousal cues. That is, SSRIs that are highly selective for 5HT may dull the sensitivity of the SNS. As previously mentioned, SNS activity has been shown to mediate sexual arousal in women (Meston & Gorzalka, 1996a, 1996b). It is possible, then, that a dulled SNS could contribute to impaired sexual arousal. Perhaps sexual stimuli, which previously caused enough NE to be released to send a message to the peripheral systems (e.g., to the vaginal walls to engorge with blood), no longer signals these events, causing impaired genital arousal. If this is the case, we should be able to observe this acutely by boosting the amount of NE in the system and seeing if it produces more “efficient” messages.
Furthermore, we would expect the dulling effect of an SSRI to differ depending on the secondary binding properties of the SSRI in question. SSRIs that are highly selective for 5HT (e.g., sertraline, paroxetine) would follow the mechanism outlined above. However, SSRIs that have a direct, albeit weak, effect of inhibiting NE reuptake (e.g., fluoxetine) might counteract dulling effects by increasing NE through similar mechanisms to those outlined above for serotonin reuptake inhibition. That is, although fluoxetine would decrease NE neurotransmission through downregulating 5HT autoreceptors, it would also (weakly) increase NE production through its secondary binding properties. Accordingly, fluoxetine would be less likely to dull NE efficiency than sertraline or paroxetine.
As outlined above, decreased NE is related to decreased SNS arousal and decreased SNS impairs genital arousal. Therefore, a temporary boost in SNS activation should increase sexual arousal more in women taking SSRIs that have a greater overall decrease in NE efficiency (sertraline or paroxetine) than for women taking SSRIs that have little to no overall effect on NE (fluoxetine). In fact, increasing SNS activity in women taking fluoxetine may elicit a slight decrease in sexual arousal due to overstimulation of the SNS leading to increased compensatory activation of the PNS.
In this manuscript, we present a reanalysis of the data reported in Meston (2004) to test these predictions. Specifically, it was hypothesized that for elements of sexual functioning that are thought to be at least partially mediated through peripheral neurotransmitters systems—sexual arousal, lubrication, orgasm frequency, and intensity—there would be differences between SSRIs in the effects of ephedrine. Specifically, women taking SSRIs that are highly selective for 5HT relative to NE (sertraline and paroxetine) would experience greater benefits than women taking SSRIs that are less selective for 5HT relative to NE (fluoxetine). For elements of sexual functioning that are presumably primarily centrally mediated—sexual desire and satisfaction—we predicted that there would not be differences between SSRIs in the effects of ephedrine on sexual responses.
METHOD
For a full report of participant demographics and experimental methods, see Meston (2004). Briefly, participants included women experiencing sexual dysfunction side effects secondary to treatment with fluoxetine (N = 7), sertraline (N = 7), or paroxetine (N = 5). All participants were screened to ensure that the sexual dysfunction symptoms were temporally linked to SSRI use (to ensure they were side effects of the medication) and long-standing (to reduce spontaneous remission of symptoms). Exclusion criteria included Axis I psychopathology, cardiovascular or endocrine dysfunction, current psychotherapy, or any other medical illness known to affect vascular or sexual functioning.
Participants reported their sexual functioning on a modified version of the Brief Index of Sexual Functioning for Women (BISF-W; Taylor, Rosen, & Leiblum, 1994). The modified BISF-W asked about sexual desire, sexual arousability, lubrication, orgasmic ability, orgasmic intensity/pleasure, and overall sexual satisfaction in the previous week. Participants then were randomized to receive either a 3-week supply of 50 mg ephedrine pills or a 3-week supply of placebo pills. They were instructed to take one pill orally at the same time every day, approximately 1 h previous to when sexual activity would usually occur. They also received three BISF-W forms to fill out and mail in once per week during the 3-week treatment period. Participants returned 3 weeks later to receive the crossover arm of the treatment (ephedrine or placebo) and additional BISF-W forms. Depression was assessed at baseline and at each treatment arm using the Beck Depression Inventory (BDI; Beck & Beamesderfer, 1974).
RESULTS
To ensure depression was not a significant determinant of change in sexual functioning between the SSRI groups, we conducted a repeated measures ANOVA with BDI scores at each assessment session (baseline, placebo, ephedrine) as the repeated measures variable and SSRI type (sertraline, paroxetine, fluoxetine) as a between-subjects variable. There was no significant change in depression scores overall, nor by SSRI, (F < 1.0).
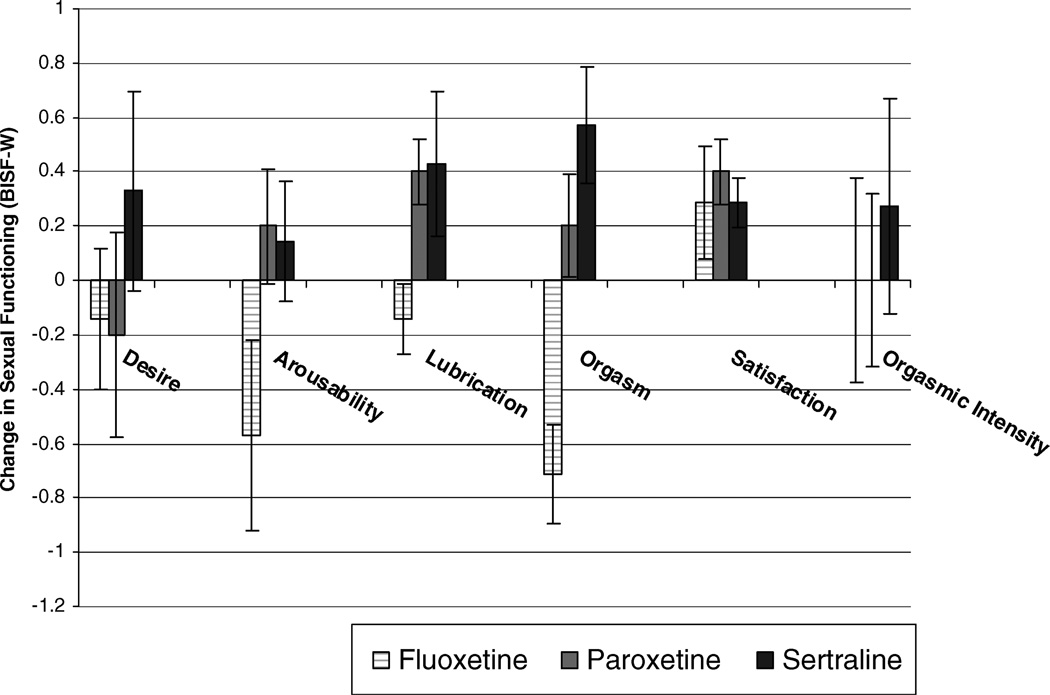
To investigate the differential effects of ephedrine between SSRIs, we conducted separate repeated measures ANOVA for each of the dependent variables (sexual desire, sexual arousability, lubrication, orgasmic ability, orgasm intensity/pleasure, and satisfaction) with assessment session as the repeated measures variable and SSRI as a between-subjects variable. (All repeated measures F values are approximations taken from Roy’s Largest Root of the between-subjects analysis). There was no significant difference between SSRI types in the effect of assessment session on sexual desire, satisfaction, and orgasmic intensity/pleasure (F < 1.0). However, there was a significant difference between SSRIs on the effects of ephedrine on lubrication, F (2, 17) = 3.28, p < 0.05, η2 = .29, and orgasmic ability, F (2, 17) = 6.92, p < 0.01, η2 = .37; there was marginal significance in sexual arousability, F (2, 17) = 2.78, p = 0.09, η2 = .14. Simple contrast follow-up analyses revealed that relative to placebo, women taking sertraline and paroxetine reported significantly greater sexual arousability, lubrication, and orgasmic ability during treatment with ephedrine than did women taking fluoxetine. As seen in Figure 1, women taking fluoxetine experienced a decrease in sexual arousability, lubrication, and orgasmic ability while taking ephedrine relative to placebo while women taking paroxetine and sertraline experienced an increase.
Figure 1.
Differential effects of ephedrine on sexual functioning in women taking sertraline, paroxetine, or fluoxetine, relative to placebo.
DISCUSSION
As hypothesized, even after accounting for the effects of placebo, women taking highly selective SSRIs (sertraline, paroxetine) reported significant increases in sexual arousal and orgasm while taking ephedrine. By contrast, women taking SSRIs with slight affinity to NE reuptake reported no change or deterioration in the same measures. Interestingly, the beneficial effects of ephedrine were strongest in the parts of the sexual response cycle in which the peripheral SNS is thought to play an important role (arousal, orgasm), but limited or deficient in areas of sexual functioning in which the CNS plays a more dominant role (e.g., desire, satisfaction). To our knowledge, this report is the first to document a differential account of the response to side effect management by SSRI type.
The original analyses by Meston (2004) conducted on all women combined found no significant difference between ephedrine and the effects of placebo on any measure of sexual functioning. In the case of sexual lubrication and orgasm, this may have been due to the fact that some women (those taking highly selective SSRIs) improved on ephedrine, while others worsened (those taking less selective SSRIs) thus creating a null effect. It may be the case that other prior studies reporting null results of interventions for sexual side effects in multiple SSRIs may have similarly masked important clinical effects.
Unexpectedly, while there was a difference in the effects of ephedrine on orgasmic frequency between SSRI groups, this was not also true of orgasmic intensity/pleasure. It is likely that ephedrine’s effects on orgasmic potential are mediated solely through the increase in genital arousal. That is, although ephedrine increases physical genital arousal, which allows a woman to reach the orgasmic threshold, it does not directly increase mental sexual arousal and thus does not, in the short run, increase the perception of sexual pleasure. Indeed, sexual satisfaction was not shown to be significantly different between ephedrine and placebo in any SSRI group.
There are limitations to the present study that deem the findings preliminary. Most notably, there were only 5–7 participants in each SSRI group, which may have limited the range in which significant effects could be observed. However, considering the medium-to-large effect sizes of those results that were significant, it is likely that replication in a larger sample would result in greater, not lesser, significance. Our null findings in the areas of sexual desire, satisfaction, and orgasmic intensity may have been due to insufficient power.
It should be noted that despite the positive findings presented here, we are not suggesting ephedrine be considered a standard route of intervention for sexual side effects in women taking sertraline and paroxetine. Ephedrine carries its own side effects, such as increased heart rate and insomnia (Physician’s Desk Reference, 2008) which may counter-indicate its long-term use, and could put persons with a history of cardiovascular diseases, diabetes, or glaucoma, among others, at risk.
In conclusion, the findings presented here provide a preliminary suggestion that decreased SNS activity may be a factor in SSRI-induced sexual side effects in women. They also demonstrate that the primary and secondary binding properties that differentiate the SSRIs from one another may have meaning at the level of clinical intervention. Specifically, they suggest that although interventions for sexual side effect management may be null (or beneficial) for one SSRI, this does not indicate that this will hold true for all SSRIs.
Footnotes
Copyright of Journal of Sex & Marital Therapy is the property of Routledge and its content may not be copied or emailed to multiple sites or posted to a listserv without the copyright holder's express written permission. However, users may print, download, or email articles for individual use.
REFERENCES
- Balon R. SSRI-associated sexual dysfunction. American Journal of Psychiatry. 2006;163:1504–1509. doi: 10.1176/ajp.2006.163.9.1504. [DOI] [PubMed] [Google Scholar]
- Beck AT, Beamesderfer A. Assessment of depression: The depression inventory. In: Pinchot P, editor. Modern problems in pharmacopsychiatry: Psychological measurements in psychopharmacology. Vol. 7. New York: Karger, Basel; 1974. pp. 151–169. [DOI] [PubMed] [Google Scholar]
- Bull SA, Hunkeler EM, Lee JY, Rowland CR, Williamson TE, Schwab JR, Hurt SW. Discontinuing or switching selective serotonin-reuptake inhibitors. The Annals of Pharmacotherapy. 2002;36:578–584. doi: 10.1345/aph.1A254. [DOI] [PubMed] [Google Scholar]
- Clayton A, Keller A, McGarvey EL. Burden of phase-specific sexual dysfunction with SSRIs. Journal of Affective Disorders. 2006;91:27–32. doi: 10.1016/j.jad.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Gitlin M. Sexual dysfunction with psychotropic drugs. Expert Opinion on Pharmacotherapy. 2003;4:2259–2269. doi: 10.1517/14656566.4.12.2259. [DOI] [PubMed] [Google Scholar]
- Gregorian RS. Antidepressant-induced sexual dysfunction. The Annals of Pharmacotherapy. 2002;36:1577–1589. doi: 10.1345/aph.1A195. [DOI] [PubMed] [Google Scholar]
- Hyttel J. Comparative pharmacology of selective serotonin re-uptake inhibitors (SSRIs) Nordic Journal of Psychiatry. 1993;47:5–12. [Google Scholar]
- Meston C. A randomized, placebo-controlled, crossover study of ephedrine for SSRI-induced female sexual dysfunction. Journal of Sex and Marital Therapy. 2004;30:57–68. doi: 10.1080/00926230490247093. [DOI] [PubMed] [Google Scholar]
- Meston CM, Gorzalka BB. The effects of immediate, delayed, and residual sympathetic activation on sexual arousal in women. Behaviour Research and Therapy. 1996a;34:143–148. doi: 10.1016/0005-7967(95)00050-x. [DOI] [PubMed] [Google Scholar]
- Meston CM, Gorzalka BB. Differential effects of sympathetic activation on sexual arousal in sexually dysfunctional and functional women. Journal of Abnormal Psychology. 1996b;105:582–591. doi: 10.1037//0021-843x.105.4.582. [DOI] [PubMed] [Google Scholar]
- Meston CM, Heiman JR. Ephedrine-activated physiological sexual arousal in women. Archives of General Psychiatry. 1998;55:652–656. doi: 10.1001/archpsyc.55.7.652. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Lejeune F, Gobert A. Reciprocal autoreceptor and heteroreceptor control of serotonergic, dopaminergic and noradrenergic transmission in the frontal cortex.: Relevance to the actions of antidepressant agents. Journal of Psychopharmacology. 2000;14:114–138. doi: 10.1177/026988110001400202. [DOI] [PubMed] [Google Scholar]
- Physicians’ Desk Reference. 62nd ed. Montvale, NJ: Thomson Healthcare; 2008. [Google Scholar]
- Rosen RC, Lane RM, Menza M. Effects of SSRIs on sexual function: A critical review. Journal of Clinical Psychopharmacology. 1999;19:67–85. doi: 10.1097/00004714-199902000-00013. [DOI] [PubMed] [Google Scholar]
- Rothschild AJ. Selective serotonin reuptake inhibitor-induced sexual dysfunction: Efficacy of a drug holiday. American Journal of Psychiatry. 1995;152:1514–1516. doi: 10.1176/ajp.152.10.1514. [DOI] [PubMed] [Google Scholar]
- Stahl SM. Essential psychopharmacology: Neuroscientific basis and practical applications. Cambridge, UK: Cambridge University Press; 2000. [Google Scholar]
- Stahl SM. Mechanism of action of serotonin selective reuptake inhibitors: Serotonin receptors and pathways mediate therapeutic effects and side effects. Journal of Affective Disorders. 1998a;51:215–235. doi: 10.1016/s0165-0327(98)00221-3. [DOI] [PubMed] [Google Scholar]
- Stahl SM. Using secondary binding properties to select a not so selective serotonin reuptake inhibitor. Journal of Clinical Psychiatry. 1998b;59:642–643. doi: 10.4088/jcp.v59n1201. [DOI] [PubMed] [Google Scholar]
- Taylor JF, Rosen RC, Leiblum SR. Self-report assessment of female sexual function: Psychometric evaluation of the brief index of sexual functioning for women. Archives of Sexual Behavior. 1994;23:627–643. doi: 10.1007/BF01541816. [DOI] [PubMed] [Google Scholar]