Abstract
Blunted cortisol responses to stress or trauma have been linked with genetic (familial) risk for both alcoholism and post-traumatic stress disorder (PTSD). Mouse lines selectively bred for high (HAP) or low (LAP) alcohol preference may be a relevant model of genetic risk for co-morbid alcoholism and PTSD in humans. HAP mice show greater fear-potentiated startle (FPS), a model used to study PTSD, than LAP mice. The relation between corticosterone (CORT) and FPS behavior was explored in four experiments. Naïve male and female HAP2 and LAP2 mice received fear-conditioning or control treatments, and CORT levels were measured before and immediately after fear-conditioning or FPS testing. In two other experiments, HAP2 mice received CORT (1.0, 5.0 or 10.0 mg/kg) or a glucocorticoid receptor antagonist (mifepristone; 25.0 and 50.0 mg/kg) 30 minutes before fear conditioning. HAP2 mice exposed to fear conditioning and to control foot shock exposures showed lower CORT after the fear-conditioning and FPS testing sessions than LAP2 mice. A trend toward higher FPS was seen in HAP2 mice pretreated with 10.0 mg/kg CORT, and CORT levels were the lowest in this group, suggesting negative feedback inhibition of CORT release. Mifepristone did not alter FPS. Overall, these results are consistent with data in humans and rodents indicating that lower cortisol/CORT levels after stress are associated with PTSD/PTSD-like behavior. These findings in HAP2 and LAP2 mice suggest that a blunted CORT response to stress may be a biological marker for greater susceptibility to develop PTSD in individuals with increased genetic risk for alcoholism.
Keywords: Alcohol, corticosterone, fear-potentiated startle, genetic correlation, mifepristone, PTSD
INTRODUCTION
Alcoholism has a high incidence of co-morbidity with post-traumatic stress disorder (PTSD) (Kessler et al. 1995). Accumulating evidence suggests that the risk for developing co-morbid alcoholism and PTSD stems from inherited genetic and biological factors that influence the risk for both conditions (Xian et al. 2000; Sartor et al. 2011). Risk for psychiatric disease is determined by complex gene × environment interactions. Exposure to environmental stressors is thought to increase the risk for developing both alcoholism and PTSD (McEwen 2000).
Stressors activate the hypothalamic–pituitary–adrenal (HPA) axis resulting in the release of glucocorticoids [cortisol in humans and corticosterone (CORT) in rodents] from the adrenal gland that bind to mineralocorticoid (MR) and glucocorticoid receptors (GR) in the brain. Through coordinated actions, MR and GR activation prepares the organism, both physiologically (de Kloet & Reul 1987) and psychologically (Putman & Roelofs 2011), to deal with the stressor and return the activated systems to a homeostatic level of functioning. Genes that regulate HPA axis function have been identified as important candidates for determining how environmental stressors may interact with genotype to influence vulnerability or resiliency to alcoholism (Clarke et al. 2008) and PTSD (Mehta & Binder 2012).
Both alcoholism (Adinoff et al. 2005; Sorocco et al. 2006) and PTSD (de Kloet et al. 2006) are associated with altered HPA axis responses to stress. Findings in the PTSD literature have led to the hypothesis that low cortisol response to trauma (Yehuda, McFarlane & Shalev 1998) and/or enhanced negative feedback inhibition of the HPA axis (Yehuda 2001) may be a risk factor for PTSD. Further, increased GR sensitivity has been proposed as a mechanism for the association between lower cortisol and PTSD (Yehuda 2001), and various alterations in GR signaling mechanisms have been linked with risk for PTSD (van Zuiden et al. 2012). However, it is not clear whether alterations in GR signaling are present before the onset of PTSD, occur as a consequence of PTSD (de Kloet et al. 2008) or both.
A functional relationship between cortisol and PTSD is supported by reports that administration of cortisol (hydrocortisone) to people during septic shock has been shown to reduce the subsequent development of PTSD (Schelling et al. 2001) and reduces PTSD symptoms in people who already have PTSD (Aerni et al. 2004). In rats, a blunted CORT response to stress is associated with PTSD-like behavior (King, Abend & Edwards 2001; Cohen et al. 2006; Zoladz, Fleshner & Diamond 2012), and CORT administration prior to stressor exposure reduced anxiety-related behaviors in an animal model of PTSD (Cohen et al. 2006, 2008). Evidence suggests that the therapeutic effects of glucocorticoid administration in both humans and rodents may be due to glucocorticoid-induced impairment of memory retrieval (de Quervain 2008) and/or facilitation of memory extinction via GR activation (Yang, Chao & Lu 2006). On the other hand, GR activation can also produce anxiogenesis (Jakovcevski, Schachner & Morellini 2011), and pharmacological antagonism of GR has been shown to reduce fear and anxiety-related behavior in rodents (Calvo & Volosin 2001; Zhou et al. 2010; Jakovcevski et al. 2011) and PTSD symptoms in humans (Golier et al. 2012).
Fear-conditioning models in rodents are commonly used to study fear-related behavior to understand processes that contribute to anxiety and fear-related disorders, such as PTSD (Kim & Jung 2006). In our laboratory, we utilize the fear-potentiated startle (FPS) procedure, which produces a classically conditioned fear behavior, evidenced by a potentiated acoustic startle response, in the presence of a light cue previously associated with foot shock (Davis et al. 1993). FPS has been suggested to be a particularly relevant model for associative learning processes that may contribute to PTSD symptomatology (Grillon, Southwick & Charney 1996).
Work in our laboratory has focused on characterizing biological and behavioral phenotypes in mouse lines selectively bred for high (HAP) or low (LAP) alcohol preference. Selectively bred rodent models are useful for identifying traits that are genetically related, or ‘correlated,’ and results from these models help to identify mechanisms that underlie the correlated traits. Our prior findings indicate a positive genetic correlation between FPS and alcohol preference in two separate sets of the HAP/LAP mouse lines (replicates 1 and 2) (Barrenha & Chester 2007; Barrenha, Coon & Chester 2011). These data suggest that common genes regulate the propensity toward alcohol preference and fear-related behavior, as measured by FPS. We posit that these mouse lines represent a unique and relevant model to explore genetic and biological mechanisms that may contribute to co-morbid alcoholism and PTSD in humans.
The purpose of the present study was to explore the genetic relationship between stress-induced CORT levels and FPS behavior in the HAP2 and LAP2 selected mouse lines. Based on findings in the human and rodent literature, it was hypothesized that HAP2 mice would show a reduced CORT response to fear conditioning and testing compared to LAP2 mice, indicating a negative genetic correlation between FPS/alcohol preference and CORT response to fear-conditioning and fear cues. We also examined the effect of administering exogenous CORT or a GR antagonist (mifepristone) on the acquisition of FPS in HAP2 mice based on the previously discussed literature indicating that both agonists and antagonists of GR reduce anxiety-related behavior in rodents and humans.
MATERIALS AND METHODS
Subjects
Subjects were alcohol-naïve adult male and female HAP2 and LAP2 mice. These mouse lines were selectively bred from a progenitor population of outbred HS/Ibg mice (Institute of Behavioral Genetics, Boulder, CO, USA) at the Indiana Alcohol Research Center in Indianapolis, IN (Oberlin et al. 2011). Subjects were derived from 69 different HAP2 families and 43 different LAP2 families from multiple generations of selection. In experiment 1, HAP2 mice were from generations 31, 34, 37 and 39; LAP2 mice were from generations 35, 37 and 39. In experiment 2, HAP2 mice were from generations 23, 27, 31, 37 and 39; LAP2 mice were from generations 23, 27, 31 and 39. In experiment 3, HAP2 mice were from generations 27, 29, 31 and 34. In experiment 4, HAP2 mice were from generation 42. Multiple replications of the experiments were conducted, and subject representation in each replication was balanced across line, sex, age and litter of origin to the best extent possible. On the first day of each experiment, mice were between 63 and 119 (HAP2: 63 and 119, LAP2: 63 and 118; experiment 1), 82 and 422 (HAP2: 82 and 422, LAP2: 84 and 347; experiment 2), 97 and 163 (experiment 3) and 55 and 129 (experiment 4) days old. Mice were housed in polycarbonate cages (29.2 × 19.0 × 12.7 cm) with aspen wood shavings in groups of two to four per cage. Ambient room temperature was maintained at 21 ± 2°C. Mice had free access to food (Rodent Lab Diet 5001, Purina Mills Inc., St Louis, MO, USA) and water in the home cage at all times, except when testing procedures took place. Experimental procedures were conducted during the light phase of a 12:12 light : dark cycle (lights off at 19:00).
Experimental procedures were approved by the Purdue Animal Care and Use Committee and conducted in accordance with the Guide for the Care and Use of Laboratory Animals.
Drugs
CORT (Sigma-Aldrich, St Louis, MO, USA) was dissolved in a 20% 2-Hydroxypropyl-β-cyclodextrin solution (Sigma-Aldrich) and administered IP at doses of 1.0, 5.0 and 10.0 mg/kg (10 ml/kg). Mifepristone (Sigma-Aldrich) was suspended in a 0.5% low-viscosity carboxymethylcellulose solution (Sigma-Aldrich) and administered IP at doses of 25.0 and 50.0 mg/kg (10 ml/kg).
Apparatus
FPS was assessed using two dark, sound-attenuated Coulbourn Instruments (Allentown, PA, USA) Animal Acoustic Startle System chambers, as previously described (Barrenha & Chester 2007). Startle stimuli consisted of 100 dB, 40 milliseconds white noise bursts (frequency range: 20 Hz–20 kHz). Both the acoustic startle response and tactile startle response were measured as the amount of force in grams exerted against a weight-sensitive platform during the 200 milliseconds after the onset of each acoustic stimulus or foot shock stimulus, respectively. The force measurement does not include the subject’s bodyweight. A ventilating fan provided continuous 70–71 dB background noise.
FPS procedures
FPS procedures consisted of one conditioning and one test session separated by 24 hours. During each conditioning session, fear-conditioned (FC) groups received 40 trials of a 30-second, 7 W light stimulus paired with a 0.5-second, 0.8 mA foot shock (2-minute intertrial interval). The foot shock occurred during the last 0.5 second of the light stimulus presentation. Control (CON) groups (experiments 1 and 2) received the same number and sequence of light and shock presentations as the FC group, but these stimuli were explicitly unpaired during each of the 40 2-minute intervals (interstimulus range 1–118 seconds). Tactile startle responses were measured during conditioning sessions. The FPS test session consisted of a 5-minute habituation period followed by 36 total trials (2-minute intertrial interval) presented on a random schedule (range: 12–108 seconds) to reduce habituation to any single trial type. Twelve of the trials were blank (no stimuli; 40 milliseconds), 12 were noise-alone (100 dB, 40 milliseconds), and 12 were light (7 W, 30 seconds) + noise (100 dB, 40 milliseconds). On light + noise trials, the noise stimulus was presented immediately after the light stimulus ended. FPS parameters were chosen based on our previous work in HAP2/LAP2 mice (Barrenha et al. 2011).
Blood collection
Blood samples were obtained from the submandibular vein (Golde, Gollobin & Rodriguez 2005). A 5.0-mm sterile GoldenRod Animal Lancet (MEDIpoint, Inc., Mineola, NY, USA) was used to puncture the skin at the hinge of the jawbones and approximately 0.05–0.1 ml of whole blood was collected within < 1 minute into heparinized capillary tubes (VWR International, West Chester, PA, USA). Samples were kept on ice until centrifugation and plasma extraction, and then plasma was frozen at −80°C until CORT analyses.
CORT analyses
CORT levels in blood were determined using a competitive enzyme (sheep polyclonal antibody to CORT) immunoassay kit from Enzo Life Sciences (Farmingdale, NY, USA). Optical densities were read on a microplate reader at 405 nm wavelength. CORT values were interpolated from standard curves using a multiple parameter curve fitting program (Assay Blaster!, Enzo Life Sciences). Samples were analyzed in duplicate and averaged. Duplicate samples with a coefficient of variation greater than 30%, or samples with a duplicate value of less than 0, were subjected to the Dixon Extreme Score Test (Dixon 1950). Each of the two flagged values in the pair was compared to the average CORT values for that particular subgroup. If neither flagged value passed the outlier test, the average of the two values was used. If one of the two values passed the outlier test, the other (non-outlier) value for that subject was used as the valid value. If both of the two values passed as outliers, the subject was removed from all analyses. Following this procedure, CORT values from each sample timepoint were ranked within line/sex and treatment group subgroups, where applicable, and screened for outliers using the Dixon Extreme Score Test. Subjects were removed from all analyses if a CORT value passed the outlier test. Outlier analyses resulted in the removal of five subjects from experiment 1, 10 from experiment 2, four from experiment 3 and one from experiment 4.
Study procedures
General timeframes for blood sampling and FPS procedures
Baseline blood samples were taken 22–24 hours before (experiments 1, 3, 4) or immediately before (experiment 2) the fear-conditioning session, between 08:00 and 12:00, during the light portion of the light : dark cycle when circulating CORT levels are low (de Kloet & Reul 1987). Three additional blood samples were taken at 0 (immediately after), 1 and 2 hours after the end of the fear-conditioning session (experiments 1, 3 and 4) or after the FPS test session (experiment 2). However, to simplify data presentation, only CORT levels measured immediately after the conditioning or test session are reported later, given that the additional CORT measurements at 1 and 2 hours showed a similar pattern to that seen at the 0 hour timepoint. Drug or vehicle treatments were administered 30 minutes before the start of the fear-conditioning sessions in experiments 3 and 4.
Experiment 1: CORT in relation to fear conditioning
Ninety-five HAP2 (52 males, 43 females) and 89 LAP2 (40 males, 49 females) mice were randomly assigned to either FC (paired light + shock), CON (unpaired light and shock) or no-shock group (NS; light only).
Experiment 2: CORT in relation to fear testing
One hundred and nine HAP2 (52 males, 57 females) and 105 LAP2 (50 males, 55 females) mice were randomly assigned to either an FC or CON group.
Experiment 3: effects of CORT treatment before fear conditioning on FPS
One hundred and two HAP2 (50 males, 52 females) mice were randomly assigned to one of four CORT dose groups: vehicle, 1.0 mg/kg, 5.0 mg/kg or 10.0 mg/kg and all groups were FC.
Experiment 4: effects of GR antagonist (mifepristone) treatment before fear conditioning on FPS
Forty-one HAP2 (21 males, 20 females) mice were randomly assigned to one of three mifepristone dose groups: vehicle, 25.0 mg/kg or 50.0 mg/kg, and all groups were FC.
Statistical analyses
All 12 startle responses on each trial type (noise-alone, light + noise) were averaged for each mouse. Mice were removed from all analyses if their average startle response value on either the noise-alone or light + noise trials did not exceed the average value obtained on blank (no stimulus) trials. Applying this criterion resulted in the removal of four subjects from experiment 1 and eight from experiment 2. The % FPS measure was obtained using proportional change scores calculated using the following formula: {[(startle amplitude on light + noise trials − startle amplitude on noise-alone trials)/startle amplitude on noise-alone trials] × 100}. Thus, the % FPS measure adjusts for individual and group differences in startle response magnitude that may be observed on noise-alone trials and is an accurate method for detecting selective effects of pharmacological compounds on FPS (Walker & Davis 2002).
Data were analyzed using analysis of variance (ANOVA) with line (HAP2, LAP2), sex (male, female), conditioning group (FC, CON, NS) and treatment group (vehicle, 1.0, 5.0, 10.0 mg/kg CORT or vehicle, 25.0, and 50.0 mg/kg mifepristone) as between-group factors. In some cases, only higher-order interactions are reported from the overall ANOVAs to simplify data presentation. Lower-order ANOVAs and Tukey’s t-test were used to explore interactions and main effects. Pearson product moment correlation coefficients were generated to assess relationships between variables. Analysis of covariance (ANCOVA) was used in some cases when significant correlations with age and body weight were found. Probability values ≤ 0.05 were considered to be significant. In some cases, trends with a P value less than 0.1 were investigated with lower-order ANOVAs to increase power to detect significant differences between relevant groups.
RESULTS
Experiment 1: CORT in relation to fear conditioning
% FPS
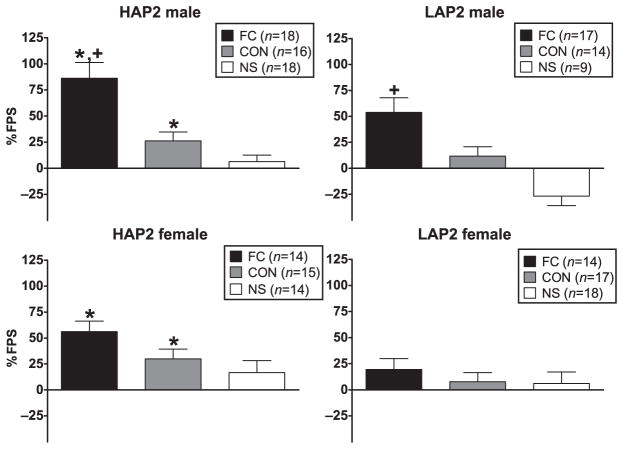
ANOVA (line × sex × conditioning group) indicated significant main effects of line [F(1,183) = 15.2, P < 0.01; HAP2 > LAP2] and conditioning group [F(2,183) = 23.9, P < 0.01; FC > CON and FC > NS] and a sex × conditioning group interaction [F(2,183) = 5.9, P < 0.01]. Post hoc one-way ANOVAs of the conditioning group within each sex indicated that this interaction was due to a significantly greater % FPS in males than females in the FC group only [F(1,62) = 5.6, P < 0.05]. Line effects were also examined within each conditioning group, which showed a main effect of line (HAP2 > LAP2) in both the FC [F(1,62) = 6.5, P = 0.01] and the CON [F(1,61) = 4.4, P < 0.05] group (Fig. 1).
Figure 1.
Mean (±SEM) % FPS in male and female HAP2 (left panels) and LAP2 (right panels) mice in the fear-conditioned (FC; paired light + shock), control (CON; unpaired light and shock) and no-shock (NS; light only) groups for experiment 1. *P < 0.05, HAP2 > LAP2; +P < 0.05, males > females
Startle on preconditioning and noise-alone trials
For startle on preconditioning trials, ANOVA (line × sex) showed a main effect of line [F(1,183) = 26.3, P < 0.01; HAP2 > LAP2]. For startle on noise-alone trials, ANOVA (line × sex × conditioning group) indicated a line × conditioning group interaction [F(2,183) = 5.5, P < 0.01]. Post hoc analyses showed line effects (HAP2 > LAP2) within each conditioning group [FC: F(1,62) = 11.9, P < 0.01; CON: F(1,61) = 26.2, P < 0.01; NS:F(1,58) = 6.3, P < 0.05] and a conditioning group effect in HAP2 [F(2,94) = 9.6, P < 0.01] due to greater startle on noise-alone trials in both the FC and CON groups relative to the NS (Ps < 0.05). Startle on preconditioning and noise-alone trials was not correlated with % FPS.
Tactile startle response to foot shock
Tactile startle was positively correlated with body weight (r = 0.5, P < 0.01). Mean body weights for subgroups were as follows: 25.8 ± 0.4 (HAP2 male), 23.0 ± 0.3 (HAP2 female), 25.5 ± 0.3 (LAP2 male) and 22.0 ± 0.3 (LAP2 female), with ANOVA showing main effects of line [F(1183 = 4.0 P < 0.05; HAP2 > LAP2] and sex [F(1,183) = 92.6, P < 0.01; male > female]. ANCOVA (line × sex × conditioning group with body weight as a co-factor) on tactile startle response showed a line × sex interaction [F(1,124) = 4.8, P < 0.05] due to greater tactile startle response in HAP2 than LAP2 males [F(1,64) = 4.6, P < 0.05] and in HAP2 males than HAP2 females [F(1,62) = 5.3, P < 0.05]. Tactile startle was not correlated with % FPS.
Baseline CORT
Table 1 shows baseline CORT as a function of line and sex. Baseline CORT was negatively correlated with body weight (r = −0.2, P < 0.05). ANCOVA (line × sex with body weight as a co-factor) indicated a line × sex [F(1,183) = 15.2, P < 0.01] interaction. Post hoc one-way ANOVAs indicated a line effect in females only [F(1,91) = 9.1, P < 0.01; HAP2 > LAP2] and a sex effect in HAP2 only [F(1,94) = 19.9, P < 0.01; female > male]. Baseline CORT was not correlated with % FPS.
Table 1.
Baseline CORT for each experiment.
| Experiment 1 | Experiment 2 | Experiment 3 | Experiment 4 | |
|---|---|---|---|---|
| HAP2 male | 8.6 ± 1.4 | 8.8 ± 1.4 | 6.1 ± 1.2 | 8.0 ± 2.5 |
| HAP2 female | 30.3 ± 4.1*,** | 13.6 ± 2.1* | 13.2 ± 1.5* | 13.8 ± 4.7 |
| LAP2 male | 15.3 ± 3.7 | 9.5 ± 1.9 | NA | NA |
| LAP2 female | 15.3 ± 1.7 | 16.1 ± 2.3* | NA | NA |
Mean (± standard error of the mean) baseline CORT values (ng/ml) for experiments 1, 2, 3 and 4. CORT = corticosterone; HAP = high alcohol preference; LAP = low alcohol preference; NA = not applicable.
P < 0.05, female > male, collapsed across line;
P < 0.05, HAP2 female > LAP2 female.
CORT (ng/ml) immediately after the conditioning session
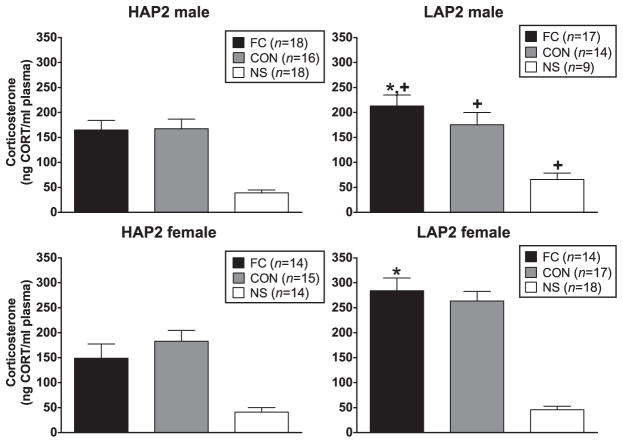
CORT after the conditioning session was negatively correlated with body weight (r = −0.2, P < 0.01). Also, because there were line and sex differences in baseline CORT [even though there was no significant correlation between baseline CORT and CORT levels after the conditioning session (r = −0.1, P = 0.06), baseline CORT and body weight was included as a co-factor in the following analyses]. ANCOVA (line × sex × conditioning group) indicated line × sex [F(1,183) = 3.8, P = 0.05] and line × conditioning group [F(2,183) = 4.6, P = 0.01] interactions. The interactions were due to line effects (LAP2 > HAP2) in males [F(1,91) = 4.7, P < 0.05] and in the FC group [F(1,62) = 13.1, P < 0.01] (Fig. 2). CORT after the conditioning session was not correlated with % FPS.
Figure 2.
Mean (±SEM) CORT (ng/ml) immediately following the conditioning session in male and female HAP2 (left panels) and LAP2 (right panels) mice in the fear-conditioned (FC; paired light + shock), control (CON; unpaired light and shock) and no-shock (NS; light only) groups for experiment 1. *P < 0.05, LAP2 > HAP2, in FC collapsed across sex; +P < 0.05, LAP2 > HAP2, in males, collapsed across group
Experiment 2: CORT in relation to fear testing
% FPS
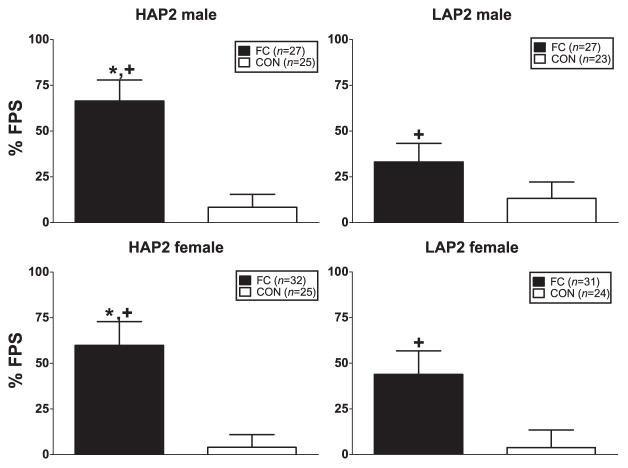
ANOVA (line × sex × conditioning group) indicated a significant main effect of conditioning group [F(1,213) = 32.3, P < 0.01; FC > CON] and a line × conditioning group interaction close to significance [F(1,213) = 3.1, P = 0.08]. Line comparisons within each conditioning group showed a line effect in the FC groups [F(1, 116) = 3.9, P = 0.05; HAP2 > LAP2] but not CON groups. Conditioning group comparisons within each line showed conditioning group effects in each line (Fs > 7.8, Ps < 0.01; FC > CON) (Fig. 3).
Figure 3.
Mean (±SEM) % FPS in male and female HAP2 (left panels) and LAP2 (right panels) mice in the fear-conditioned (FC; paired light + shock) and control (CON; unpaired light and shock) groups for experiment 2. *P = 0.05, HAP2 > LAP2 in FC collapsed across sex; +P < 0.01, FC > CON within each line, collapsed across sex
Startle on preconditioning and noise-alone trials
Due to an experimental error, 39/214 mice were not weighed in this experiment. Based on an n of 175, preconditioning startle was negatively correlated with body weight (r = −0.2, P = 0.01). Analyses were conducted with and without body weight as a co-factor yielding line effects in both analyses (Fs > 19.0, Ps < 0.01; HAP2 > LAP2) and a sex effect with body weight as a co-factor [F(1,174) = 13.6, P < 0.01; male > female]. Noise-alone startle was positively correlated with age (r = 0.2, P = 0.01). ANCOVA (line × sex × conditioning group with age as a co-factor) on noise-alone startle showed a main effect of line [F(1,213) = 31.6, P < 0.01; HAP2 > LAP2]. Startle on preconditioning and noise-alone trials was not correlated with % FPS.
Tactile startle response to foot shock
Tactile startle was positively correlated with age (r = 0.3, P < 0.01) and body weight (r = 0.4, P < 0.01). Mean body weights for subgroups were as follows: 28.0 ± 0.5 (HAP2 male), 25.1 ± 0.4 (HAP2 female), 27.9 ± 0.7 (LAP2 male) and 25.6 ± 0.7 (LAP2 female), with ANOVA showing a main effect of sex [F(1,174) = 18.4, P < 0.01; male > female]. ANCOVA (line × sex × conditioning group with age as a co-factor) on tactile startle response indicated a significant main effect of sex [F(1,213) = 38.7, P < 0.01; male > female]; this effect was still found when body weight was also included as a co-factor. Tactile startle was not correlated with % FPS.
Baseline CORT
ANOVA (line × sex) indicated a significant main effect of sex [F(1,213) = 8.2, P < 0.01; female > male] (Table 1). Baseline CORT was not correlated with % FPS.
CORT (ng/ml) immediately after FPS test session
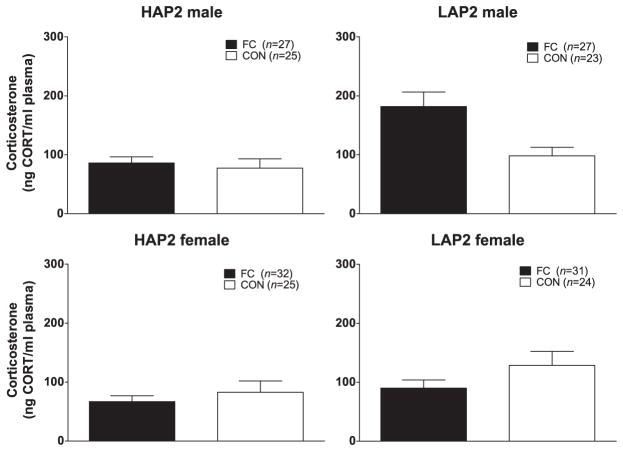
CORT immediately after the FPS test session was positively correlated with age (r = 0.2, P < 0.05) and body weight (r = 0.3, P < 0.01). Also, because there were sex differences in baseline CORT (even though there was no correlation between baseline CORT and CORT after the test session), baseline CORT, age and body weight were included as co-factors in the following analyses. ANCOVA (line × sex × conditioning group) indicated a main effect of line [F(1,174) = 5.9, P < 0.05; LAP2 > HAP2] (Fig. 4). CORT after the FPS test session was not correlated with % FPS.
Figure 4.
Mean (±SEM) CORT (ng/ml) immediately following the FPS test session in male and female HAP2 (left panels) and LAP2 (right panels) mice in the fear-conditioned (FC; paired light + shock) and control (CON; unpaired light and shock) groups for experiment 2
Experiment 3: effects of CORT treatment before fear conditioning on FPS
% FPS
% FPS was negatively correlated with age (r = −0.2, P < 0.05). ANCOVA (sex × dose group with age as a co-factor) of % FPS indicated no significant effects (Fig. 5).
Figure 5.
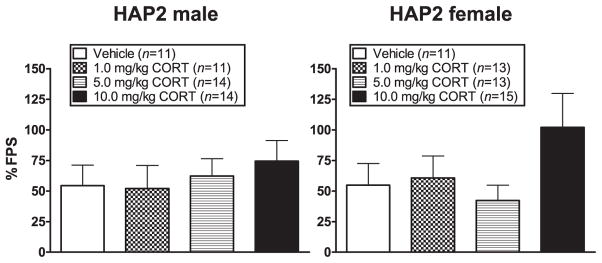
Mean (±SEM) % FPS in male (left panel) and female (right panel) HAP2 mice in the vehicle, 1.0 mg/kg, 5.0 mg/kg and 10.0 mg/kg CORT groups for experiment 3. Mice received vehicle or CORT 30 minutes prior to the fear-conditioning session
Startle on preconditioning and noise-alone trials
Preconditioning and noise-alone startle were positively correlated with age (rs = 0.4, Ps < 0.01). ANCOVAs (sex × dose group with age as a co-factor) showed no effects on preconditioning startle and a main effect of sex [F(1,101) = 4.3, P < 0.01; male > female] on noise-alone startle. Startle on preconditioning (r = −0.2, P < 0.05) and noise-alone (r = −0.3, P < 0.01) trials was negatively correlated with % FPS.
Tactile startle response to foot shock
Tactile startle was positively correlated with body weight (r = 0.2, P < 0.05). Mean body weights for subgroups were as follows: 27.9 ± 0.4 (HAP2 male) and 25.0 ± 0.3 (HAP2 female), with ANOVA showing a main effect of sex [F(1,101) = 34.7, P < 0.01; male > female]. ANCOVA (sex × dose group with body weight as a co-factor) on tactile startle response indicated a significant main effect of sex [F(1,101) = 6.4, P = 0.01; male > female]. Tactile startle was not correlated with % FPS.
Baseline CORT
Baseline CORT was negatively correlated with age (r = −0.3, P = 0.01) and body weight (r = −0.2, P = 0.05). ANCOVA (sex with age and body weight as a co-factors) indicated a significant main effect of sex [F(1,101) = 10.4, P < 0.01; female > male] (Table 1). Baseline CORT was not correlated with % FPS.
CORT (ng/ml) immediately after conditioning session
CORT after the conditioning session was negatively correlated with body weight (r = −0.3, P < 0.01). Because there were sex differences in baseline CORT (even though there was no correlation between baseline CORT and CORT after the conditioning session), baseline CORT and body weight were included as a covariate in the following analyses. ANCOVA (sex × dose group) indicated a main effect of treatment [F(3,101) = 4.0, P = 0.01; Tukey’s indicated 5.0 and 10.0 mg/kg groups < vehicle group (P < 0.05)] (Fig. 6). CORT after the conditioning session was not correlated with % FPS.
Figure 6.
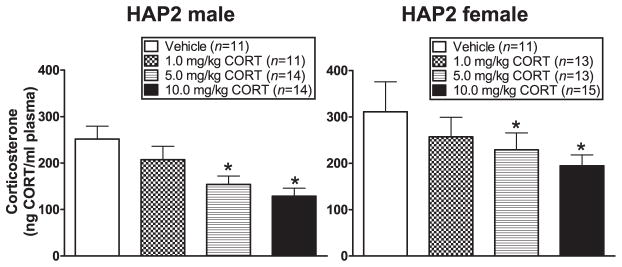
Mean (±SEM) CORT (ng/ml) immediately following the conditioning session in male (left panel) and female (right panel) HAP2 mice in the vehicle, 1.0 mg/kg, 5.0 mg/kg and the 10.0 mg/kg CORT groups for experiment 3. Mice received vehicle or CORT 30 minutes prior to the fear-conditioning session. *P < 0.05, 5.0 mg/kg and 10.0 mg/kg CORT groups < vehicle group, collapsed across sex
Experiment 4: effects of GR antagonist (mifepristone) treatment before fear conditioning on FPS
% FPS
% FPS was negatively correlated with baseline CORT (r = −0.3, P = 0.05). ANCOVA (sex × dose group with baseline CORT as a co-factor) of % FPS indicated no significant effects (Fig. 7).
Figure 7.
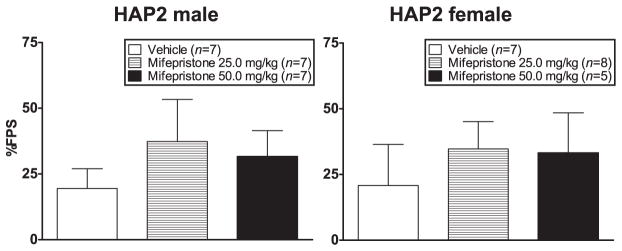
Mean (±SEM) % FPS in male (left panel) and female (right panel) HAP2 mice in the vehicle, 25.0 mg/kg and 50.0 mg/kg mifepristone groups for experiment 4. Mice received vehicle or mifepristone 30 minutes prior to the fear-conditioning session
Startle on preconditioning and noise-alone trials
ANOVAs (sex × dose group) showed no significant effects.
Tactile startle response to foot shock
Mean body weights for subgroups were as follows: 26.9 ± 0.5 (HAP2 male) and 24.0 ± 0.6 (HAP2 female), with ANOVA showing a main effect of sex [F(1,40) = 11.9, P < 0.01; male > female]. ANOVA (sex × dose group) on tactile startle response indicated a main effect of sex [F(1,40) = 10.7, P < 0.01; male > female]. Tactile startle was not correlated with % FPS.
Baseline CORT
Baseline CORT was negatively correlated with body weight (r = −0.3, P < 0.05). ANCOVA (sex with body weight as a co-factor) showed no significant effects. Baseline CORT was negatively correlated with % FPS (r = −0.3, P < 0.05).
CORT (ng/ml) immediately after conditioning session
ANOVA (sex × dose group) showed a sex × dose group interaction [F(2,40) = 3.9, P < 0.05] due to a treatment effect in females close to significance [F(2,19) = 3.3, P = 0.06; Tukey’s indicated vehicle group < 25.0 mg/kg group (P = 0.056)]. CORT after the conditioning session was not correlated with % FPS (Fig. 8).
Figure 8.
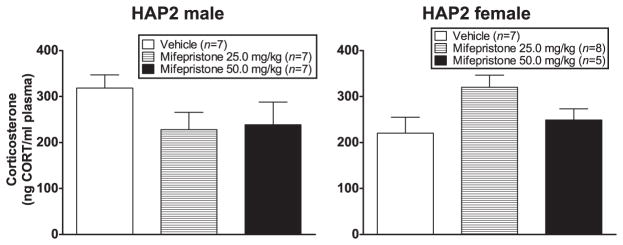
Mean (±SEM) CORT (ng/ml) immediately following the conditioning session in male (left panel) and female (right panel) HAP2 mice in the vehicle, 25.0 mg/kg and the 50.0 mg/kg mifepristone groups for experiment 4. Mice received vehicle or mifepristone 30 minutes prior to the fear-conditioning session
DISCUSSION
Animal models are helpful in identifying and disentangling the mechanisms of gene × environment interactions and how they influence the expression of co-morbid disorders in humans. Rodents selectively bred for HAP or LAP, such as the HAP2 and LAP2 lines, have long been used to identify genetically correlated traits and associated mechanisms related to alcoholism in humans (Crabbe, Phillips & Belknap 2010). Our prior work in HAP/LAP lines indicates that common genes regulate alcohol preference and propensity toward fear-related behavior. HAP lines show greater FPS than LAP lines (Barrenha & Chester 2007; Barrenha et al. 2011; Barrenha & Chester 2012), and this correlated trait was again demonstrated in the current study.
We found support for our hypothesis that HAP2 mice would show a reduced CORT response to fear conditioning and testing compared to LAP2 mice. These data are consistent with reports in rats in which blunted CORT response to stress is associated with PTSD-like behavior (King et al. 2001; Cohen et al. 2006; Zoladz et al. 2012). Lower stress-induced CORT in HAP2 mice is also consistent with a report by Prasad & Prasad (1995) who showed that rats selectively bred for alcohol preference [alcohol-preferring (P)] had lower levels of stress-induced CORT compared to rats selectively bred for alcohol non-preference [alcohol-non-preferring (NP)].
Our findings are also consistent with human studies in which people with increased genetic risk for alcoholism (Adinoff et al. 2005; Sorocco et al. 2006) and with PTSD (Yehuda et al. 1998; de Kloet et al. 2006), show blunted cortisol responses to stress, trauma or situational reminders. In humans, however, it is still an open question as to whether the blunted cortisol response is present before the onset of PTSD, occurs as a consequence of PTSD (e.g. de Kloet et al. 2008) or both. Many studies indicate that abnormalities are present before the trauma and subsequent development of PTSD. For example, low cortisol levels have been found in healthy individuals who are at greater risk for developing PTSD (family history positive; Yehuda 2001), and several reports indicate that acute cortisol responses in people who developed PTSD were blunted in the immediate hours after the trauma (Yehuda et al. 1998). It should be noted that we did not find consistent line differences in baseline CORT (HAP2 females > LAP2 females in experiment 1 only), and baseline CORT was not correlated with % FPS in any study. This is an important point because low baseline cortisol has been reported in people at risk for developing PTSD (Yehuda 2001). Taken together with the human and rat data, these results in HAP2/LAP2 mice are consistent with the idea that lower CORT response to stressful and anxiogenic stimuli may be a pre-morbid biological marker for risk to develop co-morbid alcoholism and PTSD.
Neither CORT (experiment 3) nor mifepristone (experiment 4) altered FPS when administered to HAP2 mice prior to fear conditioning. Data from experiment 3 do not support findings in rats where exogenous CORT treatment prior to stressor exposure reduced PTSD-like behaviors (Cohen et al. 2006, 2008) or in humans where administration of cortisol (during or after trauma) reduced the development of, and symptoms associated with, PTSD (Schelling et al. 2001; Aerni et al. 2004). However, a trend toward higher FPS was seen in the group pretreated with 10.0 mg/kg CORT. CORT levels were significantly lower in the 5.0 and 10.0 mg/kg CORT dose groups compared to the vehicle group, reflecting negative feedback inhibition of CORT release (CORT was measured 2.5 hours after injection). This trend toward higher FPS and lower CORT levels in this group, although not statistically significant, is consistent with our other results where lower CORT levels are associated with greater FPS. In a recent human study, cortisol enhanced memory retrieval in subjects with PTSD (Wingenfeld et al. 2012). In rats, the acquisition of contextual fear learning was facilitated with repeated prior administration of CORT (Thompson et al. 2004). Interestingly, Brinks, de Kloet & Oitzl (2009) showed that CORT (0.25 mg/kg) administration 5 minutes before fear conditioning facilitated extinction of conditioned fear in BALB/c mice but enhanced fear behavior in C57BL/6J mice, indicating that the effect of CORT on the acquisition of fear-related behavior may depend on genetic background.
The lack of effect of mifepristone in experiment 4 is consistent with some reports but not others. As mentioned in the Introduction section, this drug has been shown to reduce stress-induced anxiety-related behavior in rats (Calvo & Volosin 2001) and mice (Jakovcevski et al. 2011), including conditioned freezing to a context (Zhou et al. 2010), and PTSD symptoms in humans (Golier et al. 2012). On the other hand, other reports show no effect of mifepristone on stress-induced anxiety-related behavior (Korte et al. 1996; Zhou et al. 2011). It is quite likely that methodological differences, including route of administration, type of stress/anxiety model and timing of drug administration, account for these discrepant results. For example, mifepristone administered prior to training in a fear-conditioning procedure reduced freezing to context when tested 24 hours after training but enhanced freezing to a tone cue when tested 4 hours after training (Zhou et al. 2010). In contrast, mifepristone had no effect on freezing behavior when administered prior to re-exposure to the fear context or tone cue (Zhou et al. 2011). Future studies are necessary to explore the relative roles of MRs and GRs in modulating FC behavior in the HAP/LAP lines using experimental designs that assess consolidation, retrieval and extinction mechanisms of fear-related memory.
The current experiments included both male and female mice to explore whether there were sex differences in the variables of interest. In experiment 1, but not in experiments 2, 3 or 4, FC males showed significantly greater % FPS than females. This finding is consistent with a recent review of data from animal models of PTSD, which indicated that, of the few studies that have compared males and females, male animals were more susceptible toward stress-induced anxiety (Cohen & Yehuda 2011). Males also showed greater anxiety-related behavior than females as measured by other indices, including greater startle on preconditioning (experiment 2) and noise-alone trials (experiment 3) and greater tactile startle responses (all 4 experiments). Greater startle reactivity in males is consistent with our prior data in the HAP2/LAP2 lines (Barrenha & Chester 2007; Chester & Barrenha 2007) and with other reports in both rats (Lehmann, Pryce & Feldon 1999) and mice (Plappert, Rodenbucher & Pilz 2005).
Baseline CORT was higher in females than males in experiments 1 (HAP2 only), 2, 3, but not 4. Sex differences in CORT response to fear-conditioning/FPS testing were inconsistent across studies. A line difference (LAP2 > HAP2) was seen in males in experiment 1 (Fig. 2). LAP2 males also showed greater CORT after the FPS test session than females (Fig. 4), but the effect was not statistically significant with ANCOVA. However, females showed greater CORT after the conditioning session in experiment 3, and no sex difference was seen in experiment 4. We will continue to explore sex differences in HAP/LAP mice given that there are sex differences in response to stress-induced anxiety (Wang et al. 2007).
There is significant evidence that the lower cortisol measurements associated with PTSD are due to enhanced negative feedback inhibition on both the pituitary and central brain sites (Yehuda 2001; McFarlane et al. 2011), perhaps driven by increased sensitivity of GRs. It has been suggested that enhanced negative feedback inhibition can occur relatively quickly following the trauma (McFarlane et al. 2011). Fast negative feedback can occur within 15 minutes of glucocorticoid exposure and occurs via non-genomic mechanisms (Hinz & Hirschelmann 2000). Thus, the inverse relationship between CORT levels following stress and subsequent anxiety-related behavior seen in this and other studies may in fact reflect a fast negative feedback inhibition of CORT, rather than reduced initial output of CORT. This hypothesis could help explain discrepancies in the literature where PTSD has been linked with enhanced cortisol release (de Kloet et al. 2006). The current data set cannot address whether the reduced CORT levels in HAP2 versus LAP2 mice are due to reduced initial output of CORT or enhanced negative feedback inhibition (blood sampling occurred after a 1-hour FPS test or after a 2-hour fear-conditioning session). Future studies need to address this issue.
HAP2 mice showed greater anxiety-related behavior than LAP2 mice as evidenced by greater startle responses on preconditioning and noise-alone trials in experiments 1 and 2, and greater tactile startle responses in experiment 1. In addition, in experiment 1, HAP2 mice in the unpaired foot shock (CON) groups showed greater ‘FPS’ behavior compared to HAP2 mice that did not receive foot shock (NS groups), due to enhanced startle reactivity on light + noise trials (Fig. 1). This behavior likely reflects some form of pseudo-conditioning, perhaps due to accidental pairings of light and foot shock during randomized stimuli presentations or to contextual conditioning that enhanced startle reactivity to light. The fact that HAP2 mice in the FC and CON groups showed enhanced startle responses on noise-alone trials in this experiment provides additional evidence for contextual conditioning (Guscott, Cook & Bristow 2000) and suggests that HAP2 mice may be more prone to develop this form of anxiety/fear-related behavior. Interestingly, although FPS was greater in FC than CON groups, these groups showed similar levels of CORT after conditioning (Fig. 2). But in experiment 2, there was an interesting trend seen in male FC mice, with LAP2 mice showing greater CORT after the FPS test session than HAP2 mice (Fig. 4). This result may suggest that fear-related cues during the FPS test were stressful/anxiogenic as reflected by CORT levels in LAP2 FC males and that this response was blunted, or negative feedback was enhanced, in the HAP2 FC males.
In summary, these data in selectively bred mouse lines suggest that lower CORT response to stressful/anxiogenic stimuli is correlated with genetic propensity toward HAP and high fear-related behavior, suggesting that common genes may regulate these phenotypes. The current data in this mouse model are consistent with a large body of literature indicating that blunted cortisol responses to stress or trauma are linked with genetic (familial) risk for both alcoholism and PTSD. The present and prior findings in these selected lines suggest they are a unique and relevant animal model for increased genetic risk for developing co-morbid alcoholism and PTSD. Additional research in these lines is warranted and should include additional measures of anxiety-related behavior as well as responses to alcohol. Furthermore, future work should include assessments using both HAP2/LAP2 and HAP3/LAP3 lines of mice (an independently replicated line from the same progenitor population) to provide further confirmation of the observed genetic correlation between lower CORT response to stressful/anxiogenic stimuli and genetic propensity toward HAP. This research may help identify biological targets for the effective pharmacological treatment of co-morbid alcoholism and PTSD in humans.
Acknowledgments
Supported by AA016843 (J.A.C.). We are grateful to Dr. Nicholas J. Grahame for providing the breeders for the HAP1/LAP1 and HAP2/LAP2 lines, made possible by grant AA015512 awarded to Dr. Lawrence Lumeng.
Footnotes
Authors Contributions
JAC was responsible for the concept and design of these studies, for supervising the data collection and for drafting the manuscript. AMK and GDB were responsible for data collection, technical and administrative support. AMK and GDB also made intellectual contributions to the project and revisions to the manuscript. All authors have critically reviewed the manuscript and approve the final submitted version.
References
- Adinoff B, Junghanns K, Kiefer F, Krishnan-Sarin S. Suppression of the HPA axis stress-response: implications for relapse. Alcohol Clin Exp Res. 2005;29:1351–1355. doi: 10.1097/01.ALC.0000176356.97620.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aerni A, Traber R, Hock C, Roozendaal B, Schelling G, Papassotiropoulos A, Nitsch RM, Schnyder U, de Quervain DJ. Low-dose cortisol for symptoms of posttraumatic stress disorder. Am J Psychiatry. 2004;161:1488–1490. doi: 10.1176/appi.ajp.161.8.1488. [DOI] [PubMed] [Google Scholar]
- Barrenha GD, Chester JA. Genetic correlation between innate alcohol preference and fear-potentiated startle in selected mouse lines. Alcohol Clin Exp Res. 2007;31:1081–1088. doi: 10.1111/j.1530-0277.2007.00396.x. [DOI] [PubMed] [Google Scholar]
- Barrenha GD, Chester JA. Effects of cross-fostering on alcohol preference and correlated responses to selection in high- and low-alcohol-preferring mice. Alcohol Clin Exp Res. 2012;36:2065–2073. doi: 10.1111/j.1530-0277.2012.01839.x. [DOI] [PubMed] [Google Scholar]
- Barrenha GD, Coon LE, Chester JA. Effects of alcohol on the acquisition and expression of fear-potentiated startle in mouse lines selectively bred for high and low alcohol preference. Psychopharmacology (Berl) 2011;218:191–201. doi: 10.1007/s00213-011-2285-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinks V, de Kloet ER, Oitzl MS. Corticosterone facilitates extinction of fear memory in BALB/c mice but strengthens cue related fear in C57BL/6 mice. Exp Neurol. 2009;216:375–382. doi: 10.1016/j.expneurol.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Calvo N, Volosin M. Glucocorticoid and mineralocorticoid receptors are involved in the facilitation of anxiety-like response induced by restraint. Neuroendocrinology. 2001;73:261–271. doi: 10.1159/000054643. [DOI] [PubMed] [Google Scholar]
- Chester JA, Barrenha GD. Acoustic startle at baseline and during acute alcohol withdrawal in replicate mouse lines selectively bred for high or low alcohol preference. Alcohol Clin Exp Res. 2007;31:1633–1644. doi: 10.1111/j.1530-0277.2007.00462.x. [DOI] [PubMed] [Google Scholar]
- Clarke TK, Treutlein J, Zimmermann US, Kiefer F, Skowronek MH, Rietschel M, Mann K, Schumann G. HPA-axis activity in alcoholism: examples for a gene-environment interaction. Addict Biol. 2008;13:1–14. doi: 10.1111/j.1369-1600.2007.00084.x. [DOI] [PubMed] [Google Scholar]
- Cohen H, Matar MA, Buskila D, Kaplan Z, Zohar J. Early post-stressor intervention with high-dose corticosterone attenuates posttraumatic stress response in an animal model of posttraumatic stress disorder. Biol Psychiatry. 2008;64:708–717. doi: 10.1016/j.biopsych.2008.05.025. [DOI] [PubMed] [Google Scholar]
- Cohen H, Yehuda R. Gender differences in animal models of posttraumatic stress disorder. Dis Markers. 2011;30:141–150. doi: 10.3233/DMA-2011-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H, Zohar J, Gidron Y, Matar MA, Belkind D, Loewenthal U, Kozlovsky N, Kaplan Z. Blunted HPA axis response to stress influences susceptibility to posttraumatic stress response in rats. Biol Psychiatry. 2006;59:1208–1218. doi: 10.1016/j.biopsych.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Belknap JK. The complexity of alcohol drinking: studies in rodent genetic models. Behav Genet. 2010;40:737–750. doi: 10.1007/s10519-010-9371-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Falls WA, Campeau S, Kim M. Fear-potentiated startle: a neural and pharmacological analysis. Behav Brain Res. 1993;58:175–198. doi: 10.1016/0166-4328(93)90102-v. [DOI] [PubMed] [Google Scholar]
- Dixon WJ. Analysis of extreme values. Ann Math Stat. 1950;21:488–506. [Google Scholar]
- Golde WT, Gollobin P, Rodriguez LL. A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab Anim (NY) 2005;34:39–43. doi: 10.1038/laban1005-39. [DOI] [PubMed] [Google Scholar]
- Golier JA, Caramanica K, Demaria R, Yehuda R. A pilot study of mifepristone in combat-related PTSD. Depress Res Treat. 2012 doi: 10.1155/2012/393251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Southwick SM, Charney DS. The psychobiological basis of posttraumatic stress disorder. Mol Psychiatry. 1996;1:278–297. [PubMed] [Google Scholar]
- Guscott MR, Cook GP, Bristow LJ. Contextual fear conditioning and baseline startle responses in the rat fear-potentiated startle test: a comparison of benzodiazepine/gamma-aminobutyric acid-A receptor agonists. Behav Pharmacol. 2000;11:495–504. doi: 10.1097/00008877-200009000-00006. [DOI] [PubMed] [Google Scholar]
- Hinz B, Hirschelmann R. Rapid non-genomic feedback effects of glucocorticoids on CRF-induced ACTH secretion in rats. Pharm Res. 2000;17:1273–1277. doi: 10.1023/a:1026499604848. [DOI] [PubMed] [Google Scholar]
- Jakovcevski M, Schachner M, Morellini F. Susceptibility to the long-term anxiogenic effects of an acute stressor is mediated by the activation of the glucocorticoid receptors. Neuropharmacology. 2011;61:1297–1305. doi: 10.1016/j.neuropharm.2011.07.034. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: a critical review. Neurosci Biobehav Rev. 2006;30:188–202. doi: 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JA, Abend S, Edwards E. Genetic predisposition and the development of posttraumatic stress disorder in an animal model. Biol Psychiatry. 2001;50:231–237. doi: 10.1016/s0006-3223(01)01071-x. [DOI] [PubMed] [Google Scholar]
- de Kloet CS, Vermetten E, Geuze E, Kavelaars A, Heijnen CJ, Westenberg HG. Assessment of HPA-axis function in posttraumatic stress disorder: pharmacological and non-pharmacological challenge tests, a review. J Psychiatr Res. 2006;40:550–567. doi: 10.1016/j.jpsychires.2005.08.002. [DOI] [PubMed] [Google Scholar]
- de Kloet CS, Vermetten E, Geuze E, Lentjes EG, Heijnen CJ, Stalla GK, Westenberg HG. Elevated plasma corticotrophin-releasing hormone levels in veterans with posttraumatic stress disorder. Prog Brain Res. 2008;167:287–291. doi: 10.1016/S0079-6123(07)67025-3. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Reul JM. Feedback action and tonic influence of corticosteroids on brain function: a concept arising from the heterogeneity of brain receptor systems. Psychoneuroendocrinology. 1987;12:83–105. doi: 10.1016/0306-4530(87)90040-0. [DOI] [PubMed] [Google Scholar]
- Korte SM, Korte-Bouws GA, Koob GF, de Kloet ER, Bohus B. Mineralocorticoid and glucocorticoid receptor antagonists in animal models of anxiety. Pharmacol Biochem Behav. 1996;54:261–267. doi: 10.1016/0091-3057(95)02172-8. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Pryce CR, Feldon J. Sex differences in the acoustic startle response and prepulse inhibition in Wistar rats. Behav Brain Res. 1999;104:113–117. doi: 10.1016/s0166-4328(99)00058-3. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22:108–124. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- McFarlane AC, Barton CA, Yehuda R, Wittert G. Cortisol response to acute trauma and risk of posttraumatic stress disorder. Psychoneuroendocrinology. 2011;36:720–727. doi: 10.1016/j.psyneuen.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Mehta D, Binder EB. Gene × environment vulnerability factors for PTSD: the HPA-axis. Neuropharmacology. 2012;62:654–662. doi: 10.1016/j.neuropharm.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Oberlin B, Best C, Matson L, Henderson A, Grahame N. Derivation and characterization of replicate high- and low-alcohol preferring lines of mice and a high-drinking crossed HAP line. Behav Genet. 2011;41:288–302. doi: 10.1007/s10519-010-9394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plappert CF, Rodenbucher AM, Pilz PK. Effects of sex and estrous cycle on modulation of the acoustic startle response in mice. Physiol Behav. 2005;84:585–594. doi: 10.1016/j.physbeh.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Prasad C, Prasad A. A relationship between increased voluntary alcohol preference and basal hypercorticosteronemia associated with an attenuated rise in corticosterone output during stress. Alcohol. 1995;12:59–63. doi: 10.1016/0741-8329(94)00070-t. [DOI] [PubMed] [Google Scholar]
- Putman P, Roelofs K. Effects of single cortisol administrations on human affect reviewed: coping with stress through adaptive regulation of automatic cognitive processing. Psychoneuroendocrinology. 2011;36:439–448. doi: 10.1016/j.psyneuen.2010.12.001. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ. Glucocorticoid-induced reduction of traumatic memories: implications for the treatment of PTSD. Prog Brain Res. 2008;167:239–247. doi: 10.1016/S0079-6123(07)67017-4. [DOI] [PubMed] [Google Scholar]
- Sartor CE, McCutcheon VV, Pommer NE, Nelson EC, Grant JD, Duncan AE, Waldron M, Bucholz KK, Madden PA, Heath AC. Common genetic and environmental contributions to post-traumatic stress disorder and alcohol dependence in young women. Psychol Med. 2011;41:1497–1505. doi: 10.1017/S0033291710002072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelling G, Briegel J, Roozendaal B, Stoll C, Rothenhausler HB, Kapfhammer HP. The effect of stress doses of hydrocortisone during septic shock on posttraumatic stress disorder in survivors. Biol Psychiatry. 2001;50:978–985. doi: 10.1016/s0006-3223(01)01270-7. [DOI] [PubMed] [Google Scholar]
- Sorocco KH, Lovallo WR, Vincent AS, Collins FL. Blunted hypothalamic-pituitary-adrenocortical axis responsivity to stress in persons with a family history of alcoholism. Int J Psychophysiol. 2006;59:210–217. doi: 10.1016/j.ijpsycho.2005.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BL, Erickson K, Schulkin J, Rosen JB. Corticosterone facilitates retention of contextually conditioned fear and increases CRH mRNA expression in the amygdala. Behav Brain Res. 2004;149:209–215. doi: 10.1016/s0166-4328(03)00216-x. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Quantifying fear potentiated startle using absolute versus proportional increase scoring methods: implications for the neurocircuitry of fear and anxiety. Psychopharmacology (Berl) 2002;164:318–328. doi: 10.1007/s00213-002-1213-0. [DOI] [PubMed] [Google Scholar]
- Wang J, Korczykowski M, Rao H, Fan Y, Pluta J, Gur RC, McEwen BS, Detre JA. Gender difference in neural response to psychological stress. Soc Cogn Affect Neurosci. 2007;2:227–239. doi: 10.1093/scan/nsm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingenfeld K, Driessen M, Terfehr K, Schlosser N, Fernando SC, Otte C, Beblo T, Spitzer C, Lowe B, Wolf OT. Cortisol has enhancing, rather than impairing effects on memory retrieval in PTSD. Psychoneuroendocrinology. 2012;37:1048–1056. doi: 10.1016/j.psyneuen.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Xian H, Chantarujikapong SI, Scherrer JF, Eisen SA, Lyons MJ, Goldberg J, Tsuang M, True WR. Genetic and environmental influences on posttraumatic stress disorder, alcohol and drug dependence in twin pairs. Drug Alcohol Depend. 2000;61:95–102. doi: 10.1016/s0376-8716(00)00127-7. [DOI] [PubMed] [Google Scholar]
- Yang YL, Chao PK, Lu KT. Systemic and intra-amygdala administration of glucocorticoid agonist and antagonist modulate extinction of conditioned fear. Neuropsychopharmacology. 2006;31:912–924. doi: 10.1038/sj.npp.1300899. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Biology of posttraumatic stress disorder. J Clin Psychiatry. 2001;62(Suppl 17):41–46. [PubMed] [Google Scholar]
- Yehuda R, McFarlane AC, Shalev AY. Predicting the development of posttraumatic stress disorder from the acute response to a traumatic event. Biol Psychiatry. 1998;44:1305–1313. doi: 10.1016/s0006-3223(98)00276-5. [DOI] [PubMed] [Google Scholar]
- Zhou M, Bakker EHM, Velzig EH, Berger S, Oitzl M, Joels M, Krugers HJ. Both mineralocorticoid and glucocorticoid receptors regulate emotional memory in mice. Neurobiol Learn Mem. 2010;94:530–537. doi: 10.1016/j.nlm.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Zhou M, Kindt M, Joels M, Krugers HJ. Blocking mineralocorticoid receptors prior to retrieval reduces contextual fear memory in mice. Plos ONE. 2011;6:e26220. doi: 10.1371/journal.pone.0026220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoladz PR, Fleshner M, Diamond DM. Psychosocial animal model of PTSD produces a long-lasting traumatic memory, an increase in general anxiety and PTSD-like glucocorticoid abnormalities. Psychoneuroendocrinology. 2012;37:1531–1545. doi: 10.1016/j.psyneuen.2012.02.007. [DOI] [PubMed] [Google Scholar]
- van Zuiden M, Kavelaars A, Geuze E, Olff M, Heijnen CJ. Predicting PTSD: pre-existing vulnerabilities in glucocorticoid-signaling and implications for preventative interventions. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.08.015. [DOI] [PubMed] [Google Scholar]