Abstract
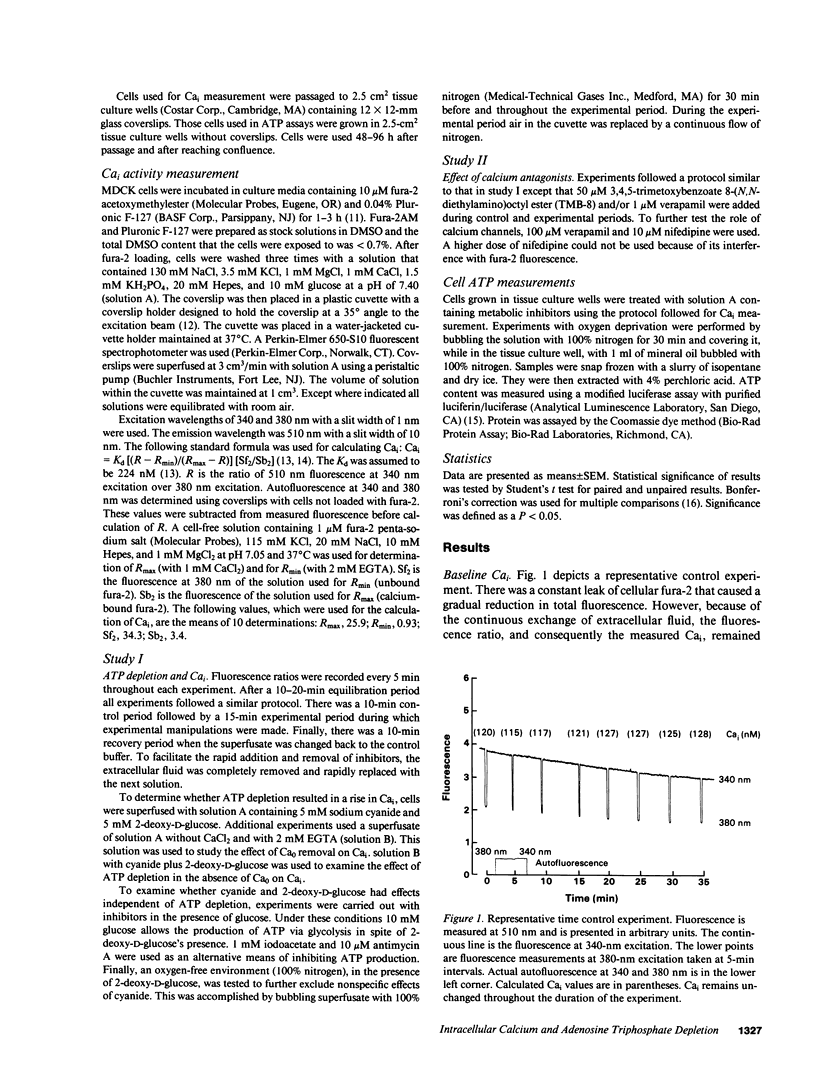
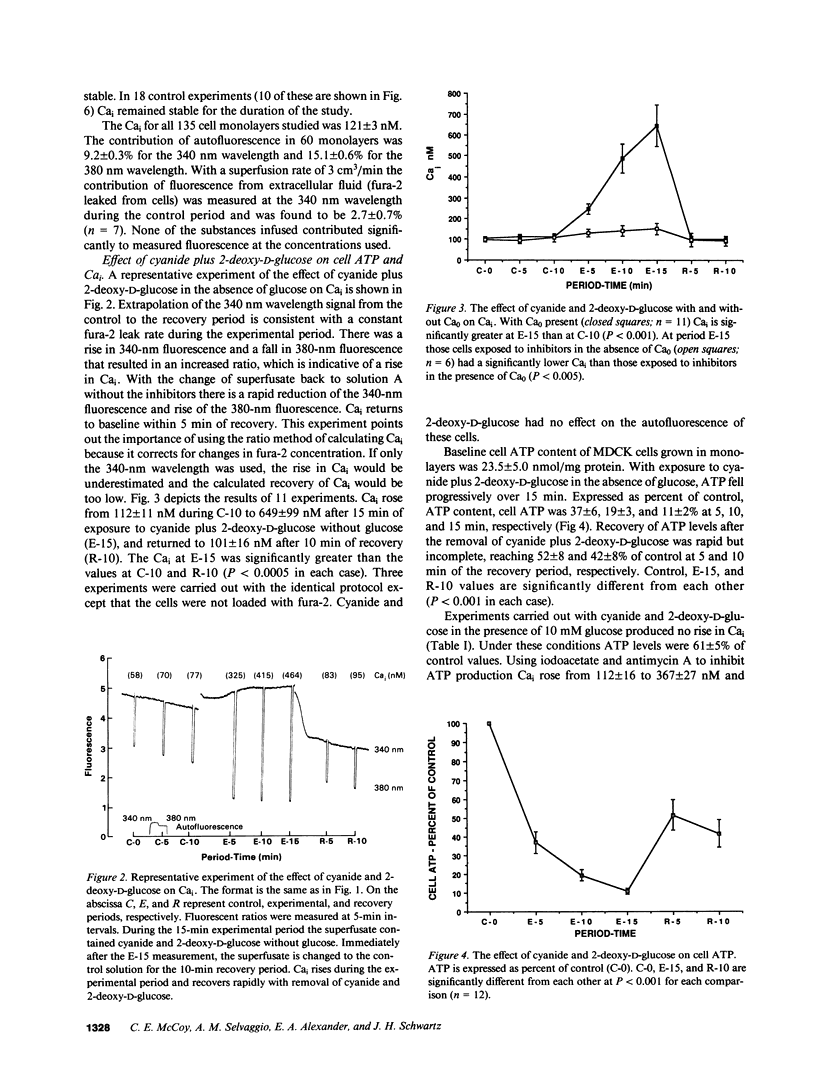
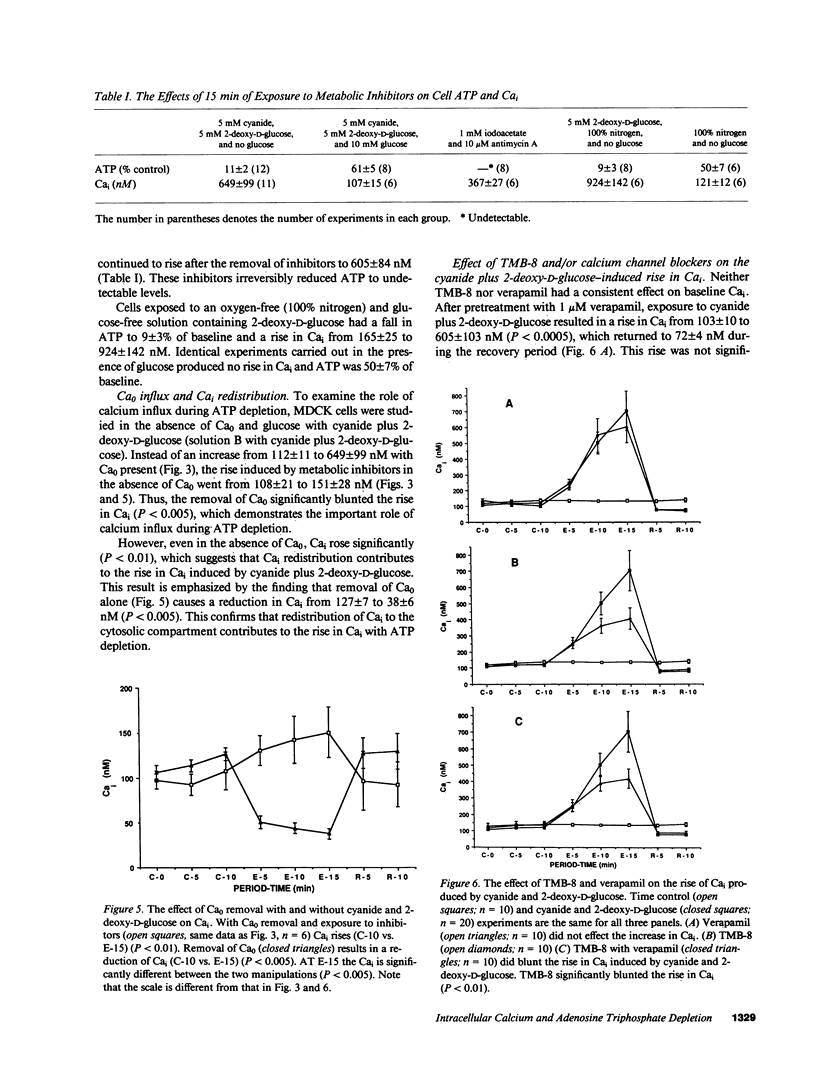
An elevation in cytosolic free calcium (Cai) produced by cellular ATP depletion may contribute to the initiation of cytotoxic events in renal ischemia. To evaluate whether ATP depletion results in a rise in Cai we examined the effect of cyanide and 2-deoxy-D-glucose on the Cai of Madin-Darby canine kidney cells. Exposure to the metabolic inhibitors resulted in a rise in Cai from 112 +/- 11 to 649 +/- 99 nM in 15 min. This combination of metabolic inhibitors also resulted in a decrement of cell ATP to 11 +/- 2% of control by 15 min. Experiments that were performed with other metabolic inhibitors confirm that the increment in Cai is due to inhibition of ATP synthesis. With the removal of cyanide and 2-deoxy-D-glucose, Cai recovered to 101 +/- 16 nM. In the absence of extracellular calcium activity (Ca0), Cai declined from 127 +/- 7 to 38 +/- 6 nM, whereas with cyanide plus 2-deoxy-D-glucose in the absence of Ca0 the Cai rose from 108 +/- 21 to 151 +/- 28 nM. Because the rise in Cai produced by ATP depletion in the absence of Ca0 is significantly less than that which occurs in the presence of Ca0, influx of Ca0 is necessary for the maximal rise of Cai. The rise in Cai that occurred in the absence of Ca0 suggests that the release of calcium from intracellular stores contributes to the increment in Cai seen with ATP depletion. TMB-8, an inhibitor of calcium release from intracellular stores, blunted the rise in Cai by nearly 50%. Neither verapamil nor nifedipine inhibited the rise in Cai. This study demonstrates that ATP depletion induced by the metabolic inhibitors cyanide and 2-deoxy-D-glucose is associated with a rapid and reversible increase in Cai. Both Ca0 influx and Cai redistribution contribute to this rise.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold P. E., Lumlertgul D., Burke T. J., Schrier R. W. In vitro versus in vivo mitochondrial calcium loading in ischemic acute renal failure. Am J Physiol. 1985 Jun;248(6 Pt 2):F845–F850. doi: 10.1152/ajprenal.1985.248.6.F845. [DOI] [PubMed] [Google Scholar]
- Bonventre J. V., Skorecki K. L., Kreisberg J. I., Cheung J. Y. Vasopressin increases cytosolic free calcium concentration in glomerular mesangial cells. Am J Physiol. 1986 Jul;251(1 Pt 2):F94–102. doi: 10.1152/ajprenal.1986.251.1.F94. [DOI] [PubMed] [Google Scholar]
- Burke T. J., Arnold P. E., Gordon J. A., Bulger R. E., Dobyan D. C., Schrier R. W. Protective effect of intrarenal calcium membrane blockers before or after renal ischemia. Functional, morphological, and mitochondrial studies. J Clin Invest. 1984 Nov;74(5):1830–1841. doi: 10.1172/JCI111602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli E., Zurini M., Niggli V., Krebs J. The calcium-transporting ATPase of erythrocytes. Ann N Y Acad Sci. 1982;402:304–328. doi: 10.1111/j.1749-6632.1982.tb25752.x. [DOI] [PubMed] [Google Scholar]
- Cheung J. Y., Bonventre J. V., Malis C. D., Leaf A. Calcium and ischemic injury. N Engl J Med. 1986 Jun 26;314(26):1670–1676. doi: 10.1056/NEJM198606263142604. [DOI] [PubMed] [Google Scholar]
- Cheung J. Y., Leaf A., Bonventre J. V. Mechanism of protection by verapamil and nifedipine from anoxic injury in isolated cardiac myocytes. Am J Physiol. 1984 Mar;246(3 Pt 1):C323–C329. doi: 10.1152/ajpcell.1984.246.3.C323. [DOI] [PubMed] [Google Scholar]
- Cheung J. Y., Leaf A., Bonventre J. V. Mitochondrial function and intracellular calcium in anoxic cardiac myocytes. Am J Physiol. 1986 Jan;250(1 Pt 1):C18–C25. doi: 10.1152/ajpcell.1986.250.1.C18. [DOI] [PubMed] [Google Scholar]
- Donowitz M., Cusolito S., Sharp G. W. Effects of calcium antagonist TMB-8 on active Na and Cl transport in rabbit ileum. Am J Physiol. 1986 May;250(5 Pt 1):G691–G697. doi: 10.1152/ajpgi.1986.250.5.G691. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Humes H. D. Role of calcium in pathogenesis of acute renal failure. Am J Physiol. 1986 Apr;250(4 Pt 2):F579–F589. doi: 10.1152/ajprenal.1986.250.4.F579. [DOI] [PubMed] [Google Scholar]
- Kimmich G. A., Randles J., Brand J. S. Assay of picomole amounts of ATP, ADP, and AMP using the luciferase enzyme system. Anal Biochem. 1975 Nov;69(1):187–206. doi: 10.1016/0003-2697(75)90580-1. [DOI] [PubMed] [Google Scholar]
- Kojima I., Shibata H., Ogata E. Action of TMB-8 (8-(N,N-diethylamino)octyl-3,4,5-trimethoxybenzoate) on cytoplasmic free calcium in adrenal glomerulosa cell. Biochim Biophys Acta. 1986 Aug 29;888(1):25–29. doi: 10.1016/0167-4889(86)90066-2. [DOI] [PubMed] [Google Scholar]
- Lemasters J. J., DiGuiseppi J., Nieminen A. L., Herman B. Blebbing, free Ca2+ and mitochondrial membrane potential preceding cell death in hepatocytes. Nature. 1987 Jan 1;325(6099):78–81. doi: 10.1038/325078a0. [DOI] [PubMed] [Google Scholar]
- Malis C. D., Cheung J. Y., Leaf A., Bonventre J. V. Effects of verapamil in models of ischemic acute renal failure in the rat. Am J Physiol. 1983 Dec;245(6):F735–F742. doi: 10.1152/ajprenal.1983.245.6.F735. [DOI] [PubMed] [Google Scholar]
- Molitoris B. A., Wilson P. D., Schrier R. W., Simon F. R. Ischemia induces partial loss of surface membrane polarity and accumulation of putative calcium ionophores. J Clin Invest. 1985 Dec;76(6):2097–2105. doi: 10.1172/JCI112214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poenie M., Alderton J., Steinhardt R., Tsien R. Calcium rises abruptly and briefly throughout the cell at the onset of anaphase. Science. 1986 Aug 22;233(4766):886–889. doi: 10.1126/science.3755550. [DOI] [PubMed] [Google Scholar]
- Rasgado-Flores H., Blaustein M. P. Na/Ca exchange in barnacle muscle cells has a stoichiometry of 3 Na+/1 Ca2+. Am J Physiol. 1987 May;252(5 Pt 1):C499–C504. doi: 10.1152/ajpcell.1987.252.5.C499. [DOI] [PubMed] [Google Scholar]
- Schwarz W., Passow H. Ca2+-activated K+ channels in erythrocytes and excitable cells. Annu Rev Physiol. 1983;45:359–374. doi: 10.1146/annurev.ph.45.030183.002043. [DOI] [PubMed] [Google Scholar]
- Schwertschlag U., Schrier R. W., Wilson P. Beneficial effects of calcium channel blockers and calmodulin binding drugs on in vitro renal cell anoxia. J Pharmacol Exp Ther. 1986 Jul;238(1):119–124. [PubMed] [Google Scholar]
- Selvaggio A. M., Schwartz J. H., Bengele H. H., Alexander E. A. Kinetics of the Na+-H+ antiporter as assessed by the change in intracellular pH in MDCK cells. Am J Physiol. 1986 Oct;251(4 Pt 1):C558–C562. doi: 10.1152/ajpcell.1986.251.4.C558. [DOI] [PubMed] [Google Scholar]
- Siegel N. J., Avison M. J., Reilly H. F., Alger J. R., Shulman R. G. Enhanced recovery of renal ATP with postischemic infusion of ATP-MgCl2 determined by 31P-NMR. Am J Physiol. 1983 Oct;245(4):F530–F534. doi: 10.1152/ajprenal.1983.245.4.F530. [DOI] [PubMed] [Google Scholar]
- Simons T. J. The role of calcium in the regulation of sugar transport in the pigeon red blood cell. J Physiol. 1983 May;338:501–525. doi: 10.1113/jphysiol.1983.sp014686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowdowne K. W., Ertel R. J., Borle A. B. Measurement of cytosolic calcium with aequorin in dispersed rat ventricular cells. J Mol Cell Cardiol. 1985 Mar;17(3):233–241. doi: 10.1016/s0022-2828(85)80006-7. [DOI] [PubMed] [Google Scholar]
- Snowdowne K. W., Freudenrich C. C., Borle A. B. The effects of anoxia on cytosolic free calcium, calcium fluxes, and cellular ATP levels in cultured kidney cells. J Biol Chem. 1985 Sep 25;260(21):11619–11626. [PubMed] [Google Scholar]
- Weinberg J. M., Humes H. D. Calcium transport and inner mitochondrial membrane damage in renal cortical mitochondria. Am J Physiol. 1985 Jun;248(6 Pt 2):F876–F889. doi: 10.1152/ajprenal.1985.248.6.F876. [DOI] [PubMed] [Google Scholar]
- Weinberg J. M. Oxygen deprivation-induced injury to isolated rabbit kidney tubules. J Clin Invest. 1985 Sep;76(3):1193–1208. doi: 10.1172/JCI112075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. R., Arnold P. E., Burke T. J., Schrier R. W. Mitochondrial calcium accumulation and respiration in ischemic acute renal failure in the rat. Kidney Int. 1984 Mar;25(3):519–526. doi: 10.1038/ki.1984.48. [DOI] [PubMed] [Google Scholar]



