Abstract
Objective
The aim of this study was to determine the role of α(1,2)-linked fucosylation of proteins by fucosyltransferase1 (fut1) in rheumatoid arthritis (RA) angiogenesis.
Methods
Analysis of α(1,2)-linked fucosylated proteins in synovial tissues (STs) was performed by immunohistological staining. α(1,2)-linked fucosylated angiogenic chemokine expression in synovial fluids (SFs) was determined by immunoprecipitation and lectin blotting. To determine the angiogenic role of α(1,2)-linked fucosylated proteins in RA, we performed human dermal microvascular endothelial cell (HMVEC) chemotaxis and Matrigel assays using nondepleted and α(1,2)-linked fucosylated protein depleted RA SFs. To examine the production of proangiogenic chemokines by fucosyltransferase 1 (fut1) in HMVECs, cells were transfected with fut1 sense or antisense oligonucleotides, and enzyme-linked immunosorbent assay was performed. We then studied mouse lung endothelial cell (MLEC) chemotaxis using wild type and fut1 gene deficient MLECs.
Results
α(1,2)-linked fucosylated proteins on RA ST endothelial cells (ECs) were highly expressed compared to normal ST. α(1,2)-linked fucosylated monocyte chemoattract protein-1 (MCP-1)/CCL2 was present in RA SFs, and was significantly elevated compared to osteoarthritis SFs. Depletion of α(1,2)-linked fucosylated proteins in RA SFs induced less HMVEC migration and tube formation compared to nondepleted RA SFs. We found that blocking fut1 expression in ECs resulted in decreased MCP-1/CCL2 and regulated upon activation and normal T cell expressed and secreted (RANTES)/CCL5 production. Finally, we showed that fut1 regulates EC migration in response to vascular endothelial cell growth factor.
Conclusions
α(1,2)-linked fucosylation by fut1 may be an important new target for angiogenic diseases like RA.
Keywords: α(1,2)-linked fucosylation; Fucosyltransferase 1; Rheumatoid Arthritis; Endothelial Cells; Angiogenesis
Introduction
Posttranslational protein modification can lead to alteration of biological properties of proteins (1, 2). Glycosylation is one of these, and many proteins are glycosylated in eukaryotes (3). For example, oligosaccharide antigens are used as tumor markers and oligosaccharides in tumors modulate functions such as tumor cell adhesion and invasion (4). It has also been shown that sialyl Lewisx is elevated in gastric cancers, and several antibodies raised against sialyl Lewisx are used for diagnosis of cancers (4). Fucosylation of glycoproteins involves the biological functions of adhesion molecules and growth factor receptors (5). Fucosylated glycans are synthesized by thirteen fucosyltransferases (futs). Terminal fucose can be linked to α(1,2)-, α(1,3)-, α(1,4)-, or α(1,6)-orientations by these futs. In addition, expression of futs has been reported in some cancers such as prostatic and pancreatic cancer (6, 7).
Fucosyltransferase 1 (fut1) and fut2 are α(1,2)-fucosyltransferases responsible for synthesis of the H blood group antigen (8, 9). The expression of cancer-associated carbohydrate antigens is modified by abnormal control by glycosyltransferases. We have previously shown that the soluble form of E-selectin mediates angiogenesis via its endothelial ligand sialyl Lewisx (10), and have shown that a related antigen, Lewisy-6/H/5-2 (Ley/H), synthesized by fut1, and its glucose analog, 2-fucosyllactose, mediate angiogenesis and inflammatory cell adhesion (11, 12).
Progression of rheumatoid arthritis (RA) is characterized by the appearance of inflammatory cells in both the pannus and joint fluid and by eventual tissue destruction. The RA synovium contains elevated levels of proangiogenic cytokines and inflammatory cells, such as lymphocytes and monocytes (13, 14). One of the most effective current therapies is designed to block tumor necrosis factor-α (TNF-α) (15). Some TNF-α blocking biologics are composed of IgG, and glycosylation in the Fc region plays an important role in the stability and effectiveness of such therapeutics (16).
Angiogenesis is critical in vasculoproliferative processes, including tumor growth, and inflammatory states such as psoriasis and RA (17). The process of angiogenesis contributes to RA progression, and may be an appropriate therapeutic target (18). Here we demonstrate the expression of α(1,2)-linked fucosylated proteins in RA and their role in angiogenesis and cell recruitment. We also show that blocking of fut1 in endothelial cells (ECs) reduced production of proangiogenic chemokines and EC migration.
Materials and Methods
Patients
RA and osteoarthritis (OA) synovial tissues (STs) were obtained from patients undergoing arthroplasty or synovectomy. Normal (NL) ST samples were obtained from a National Disease Research Interchange and Cooperative Human Tissue Network. RA and OA synovial fluids (SFs) were obtained from patients. NL skin tissues were obtained from the University of Michigan Tissue Procurement Service. All specimens were collected following approval from the Institutional Review Board.
Generation of fut1 gene deficient mice
Fut1 gene deficient mice have been generated (19–21). Mice were viable without gross defects, and were bred onto a C57BL/6 background (19–21). Fut1 gene deficient mice were later obtained from the Consortium for Functional Glycomics. C57BL/6 wild type mice were bred in house or purchased from the National Cancer Institute. All animal experiments were approved by the University Committee on Use and Care of Animals.
Cell culture
Human dermal microvascular endothelial cells (HMVECs) were purified from digested skin tissues using mouse anti-human CD31 MicroBeads (Miltenyi Biotec, Cambridge, MA), according to the manufacturer’s protocol. Cells were cultured in EC basal medium (Lonza, Walkersville, MD) with all growth factors. In order to confirm EC purification, we examined EC markers on these cells using antibodies to von Willebrand factor (vWF) and CD31.
Isolation and confirmation of EC purity in mouse lung preparations
Mouse lung ECs (MLECs) were harvested from wild type and fut1 gene deficient mouse lungs using rat anti-mouse CD146 MicroBeads (Miltenyi Biotec), according to the manufacturer’s protocol, and cultured in EC basal medium with all growth factors (Lonza) (22).
Flow cytometry
In order to confirm MLEC purity, we performed flow cytometry using CD146-FITC antibody (Miltenyi Biotec, Auburn, CA). Mouse lung ECs (3×106 cells/ml) were suspended in blocking buffer and incubated at 4°C for 15 minutes. The cells were incubated with rat IgG-FITC or CD146-FITC at 4°C for 30 minutes. Flow cytometry was performed as previously described (23).
Cell treatment
HMVECs and MLECs were seeded in 6-well plates (BD Biosciences, Bedford, MA) at a density of 2 × 105 cells per well. Cells were then maintained in EC basal medium with 5% fetal bovine serum (FBS). After overnight serum starvation, cells were treated with TNF-α for various time points (R&D Systems, Minneapolis, MN, 25 ng/ml) or interleukin-1β (IL-1β, R&D Systems, 25 ng/ml) as we have done previously (24, 25). Cell conditioned medium was collected and used in assays.
Immunofluorescence
Immunofluorescence staining was performed as previously described (26). To determine if α(1,2)-linked fucosylated proteins were expressed on RA, OA, and NL ST ECs, rabbit anti-human vWF (Dako, Carpinteria, CA) and goat anti-Ulex Europeaus Agglutinin 1 lectin (UEA-1, Vector Laboratories, Burlingame, CA) were used. RA, OA, and NL ST slides were fixed with cold acetone for 20 minutes. Then slides were blocked with 20% FBS and 5% donkey serum for 1 hour at 37°C, and incubated with UEA-1 lectin (2 µg/ml, Vector Laboratories) for 1 hour at 37°C. UEA-1 lectin binds specifically to α(1,2)-fucose, the terminal sugar of blood group antigens H and Lewisy. Goat anti-UEA-1 and rabbit anti-human vWF were used as primary antibodies. Fluorescently conjugated donkey anti-goat (for UEA-1 lectin) and anti-rabbit (for vWF) secondary antibodies were purchased from Life Technologies (Carlsbad, CA). For nuclear staining, 4’,6-diamidino-2-phenylindole (DAPI) was used. Images were taken at 200× magnification. Anti-vWF positive vessels were shown by fluorescent green staining, and fucosylation was shown by fluorescent red. Yellow vessels were a result of merger of the green and red fields. We determined the percentage of α(1,2)-fucosylated vessels in RA, OA and NL ST by counting the total number of yellow vessels, and divided this value by the total number of vessels (green) in each tissue section. Each section was evaluated by an observer blinded to the experimental conditions.
Depletion of rheumatoid factor (RF) from SFs
To avoid any possible confounding effects of RF on assays, RF was immunodepleted from SF samples using anti-IgM antibodies coupled to agarose beads (Sigma-Aldrich) as previously described (23). Removal of RF was determined by randomly choosing 6 RA SF samples and measuring RF levels before and after immunodepletion using an RF enzyme-linked immunosorbent assay (ELISA) kit (Alpha Diagnostic Intl. Inc, San Antonio, TX). Before immunodepletion, RF levels were measured and ranged from 21 IU/ml to 132 IU/ml. After immunodepletion, all samples had RF levels below 10 IU/ml (data not shown).
Immunoprecipitation and lectin blotting
RF depleted RA and OA SFs were incubated with mouse anti-human monocyte chemoattract protein-1 (MCP-1)/CCL2 antibody (R&D System) for 2 hours. The antibody-protein complexes were incubated with protein A agarose beads (Millipore, Billerica, MA) overnight at 4°C. The next day, antibody-protein-agarose bead complexes were washed five times with Tris-buffered saline (TBS, 50 mM Tris-Cl (pH 7.6) and 150 mM NaCl), and resuspended in Laemmli sample buffer.
For lectin blotting, samples were loaded onto two 10% polyacrylamide gels under reducing conditions. One gel was stained with Coomassie Brilliant Blue R-250 (Bio-Rad). The other gel was electrotransferred onto a polyvinylidene difluoride membrane. After transfer, the membrane was washed with TBS containing 0.05% Tween-20 (TBS-T), blocked with 2% bovine serum albumin in TBS-T overnight at 4°C, and incubated with 5 µg/ml of biotinylated-UEA-1 lectin (Vector Laboratories) for 1 hour at room temperature. After washing with TBS-T, the blot was incubated with 1/50,000 diluted streptavidin-HRP (BD Biosciences) for 1 hour at room temperature. Finally, the blot was washed with TBS-T and the color was developed using BCIP/NBT Liquid Substrate System (Sigma-Aldrich). As a loading control, the blots were stripped and probed with an anti-MCP-1/CCL2 antibody (R&D System). Densitometric analysis of the bands was performed using Un-Scan-It software, version 5.1 (Silk Scientific, Orem, UT).
Neutralization of α(1,2)-linked fucosylated proteins in RA SFs
To determine the role of α(1,2)-linked fucosylated proteins in RA SFs, RA SFs were depleted using UEA-1 lectin conjugated agarose beads (Vector Laboratories). RA SFs (1:50 diluted with PBS) were mixed overnight with UEA-1 lectin conjugated or nonspecific agarose beads (Sigma-Aldrich). The following day, SFs were centrifuged to pellet the lectin glycoprotein complex and depleted SF supernatants were collected. Depletion of α(1,2)-linked fucosylated proteins was confirmed by lectin blotting.
ELISAs for MCP-1/CCL2 and regulated and normal T cell expressed and secreted (RANTES)/CCL5
ELISAs were performed in a manner as described previously (23). To determine α(1,2)-linked fucosylated MCP-1/CCL2 and total MCP-1/CCL2 in RA SFs, we measured MCP-1/CCL2 from sham depleted and α(1,2)-linked fucosylated protein depleted SFs by using UEA-1 conjugated agarose beads. α(1,2)-linked fucosylated MCP-1/CCL2 in SFs was quantitated by measuring total MCP-1/CCL2 in SFs that were either sham depleted, or depleted of α(1,2)-linked fucosylated proteins with UEA-1 conjugated agarose beads, as described above. Differences in the values of the sham and α(1,2)-linked fucosylated protein depleted SFs gave the value of α(1,2)-linked fucosylated MCP-1/CCL2 in SFs. Levels of MCP-1/CCL2 and RANTES/CCL5 in TNF-α or IL-1β stimulated HMVEC conditioned medium were measured following the manufacturer’s protocol (R&D Systems). Levels of MCP-1/CCL2 and RANTES/CCL5 in TNF-α or IL-1β stimulated MLEC conditioned medium were measured by the University of Michigan Cancer Center Immunology Core or ELISA kits from commercial sources (R&D Systems, Minneapolis, MN).
In vitro HMVEC chemotaxis assay
Chemotaxis assays were performed using a 48-well modified Boyden chamber system as previously described (23, 27). To examine the bioactivity of α(1,2)-linked fucosylated proteins in RA SFs, we performed HMVEC chemotaxis assays using α(1,2)-linked fucosylated protein depleted or sham depleted RA SFs. Each test group was assayed in quadruplicate. Three high-power (400×) fields were counted in each replicate well, and results were expressed as cells per high power fields (hpfs).
In vitro Matrigel tube formation assay
Matrigel tube formation assays using growth factor-reduced Matrigel (BD Biosciences) were performed (28, 29). To examine the bioactivity of α(1,2)-linked fucosylated proteins in RA SFs, we performed HMVEC tube formation assays using α(1,2)-linked fucosylated protein depleted or sham depleted RA SFs. The controls used were vascular endothelial cell growth factor (VEGF, R&D Systems, 10 nM) as a positive and PBS as a negative control. HMVECs (1.8 × 104 cells/ 400 µl) were plated on Matrigel in the presence of 150 µg/ml sham or α(1,2)-linked fucosylated protein depleted RA SF for 6 hours at 37°C. Photographs (100×) were taken and tubes were counted by a blinded observer. Tubes were defined as elongated connecting branches between two identifiable HMVECs.
After finding that fut1 plays an important role in TNF-α-induced MCP-1/CCL2 and RANTES/CCL5 secretion, we examined if MCP-1/CCL2 and RANTES/CCL5 were involved in TNF-α-mediated tube formation. We performed HMVEC tube formation using TNF-α as a stimulus in the presence or absence of neutralizing antibodies against MCP-1/CCL2 and RANTES/CCL5 (R&D Systems).
Oligonucleotide (ODN) transfection
HMVECs were seeded in 6-well plates at 1 × 105 cells per well. ECs were maintained in EC basal medium with 5% FBS. Upon becoming 70% confluent, 2 µg/ml fut1 sense or antisense ODNs and TransIT-Oligo transfection reagent (Mirus, Madison, WI) was mixed according to manufacturer’s instructions and overlaid on the cells. Cells were incubated with the ODNs/TransIT-Oligo transfection reagent for 24 hours at 37°C. Fut1 sense and antisense ODNs were purchased from Integrated DNA Technologies (Coralville, IA). The following fut1 sequences were used; fut1 sense ODN sequence: TTTCTTCCACCATCTCCGGGAA, and fut1 antisense ODN sequence: CCTTCTCTCCAACTCTCCCA. The percent knockdown of fut1 expression was performed by using quantitative polymerase chain reaction (qPCR).
RNA extraction and qPCR
RNA extraction and qPCR were performed as previously described (30). Total RNA was isolated from HMVECs transfected with sense or antisense ODNs directed against fut1 using RNAeasy mini RNA isolation kits in conjunction with QIAshredders (Qiagen, Valencia, CA) following the manufacturer’s protocol. In our hands, we routinely yield approximately 40ng/µl of RNA from 1 × 105 cells. Following isolation, RNA (2µl) is quantified and checked for purity using a spectrophotometer (Nanodrop Technologies, Wilmington, DE). Fut1 and β-actin primer pairs were purchased from Integrated DNA Technologies. The following primers were used; fut1 forward 5’-GTGCCCGTATCCAGAGTGAT-3’; reverse 5’-AGGACCCAGGGGAGAGTAAA-3’; β-actin forward 5’-GCTAGGCAGCTCGTAGCTCT-3’; reverse 5’-GCCATGTACGTTGCTATCCA-3’. All samples were run in duplicate and analyzed using Applied Biosystems software (Life Technologies, Carlsbad, CA).
EC chemotaxis with fut1 gene deficient and wild type MLECs
MLECs (passage 2–4) were maintained in EC basal medium (Lonza) and 5% FBS. For the assay, the cells were fed one night before with EC basal medium with 5% FBS, harvested, and resuspended at 5 × 105 cells/ml in EC basal medium with 0.1% FBS. EC chemotaxis was performed using gelatin-coated polycarbonate membranes (8 µm pore size) (28, 29). The chambers were inverted and incubated for 2 hours to allow mouse EC attachment. The chambers were reinverted, test substances added, and incubation continued for 1 hour more. VEGF (Life Technologies) was used as a stimulus and PBS was used as a negative control.
HMVEC chemotaxis assay with fut1 sense or antisense ODN transfected EC conditioned medium
To further examine the role of fut1 in ECs, we performed HMVEC chemotaxis assays using fut1 sense or antisense ODN transfected HMVEC conditioned medium to determine whether HMVEC fut1 inhibition alters the secretion of chemotactic substances by cultured ECs. We used PBS as a negative control and VEGF (10 nM, R&D Systems) as a positive control for these studies.
Cell lysis and immunoblotting with MLECs
Cell lysis and immunoblotting were performed as described previously (29). Wild type and fut1 MLECs were stimulated with TNF-α (25 ng/ml) for various time periods. At the end of each time period, cell lysates were prepared. The protein concentration of all the samples was measured with a bicinchoninic acid protein assay kit (Pierce, Rockford, IL). Protein lysates (10 µg) were run on 10% SDS-PAGE and transblotted to nitrocellulose membranes (Bio-Rad, Hercules, CA). Blots were probed for rabbit anti-mouse for various VEGF receptors (VEGFRs). Blots were stripped and reprobed with β-actin to verify equal loading of proteins.
Statistical analysis
Data was analyzed using a Student’s t-test assuming equal variances. Data are reported as the mean ± SEM. P values less than 0.05 were considered statistically significant.
Results
Expression of α(1,2)-linked fucosylated proteins on RA ST ECs
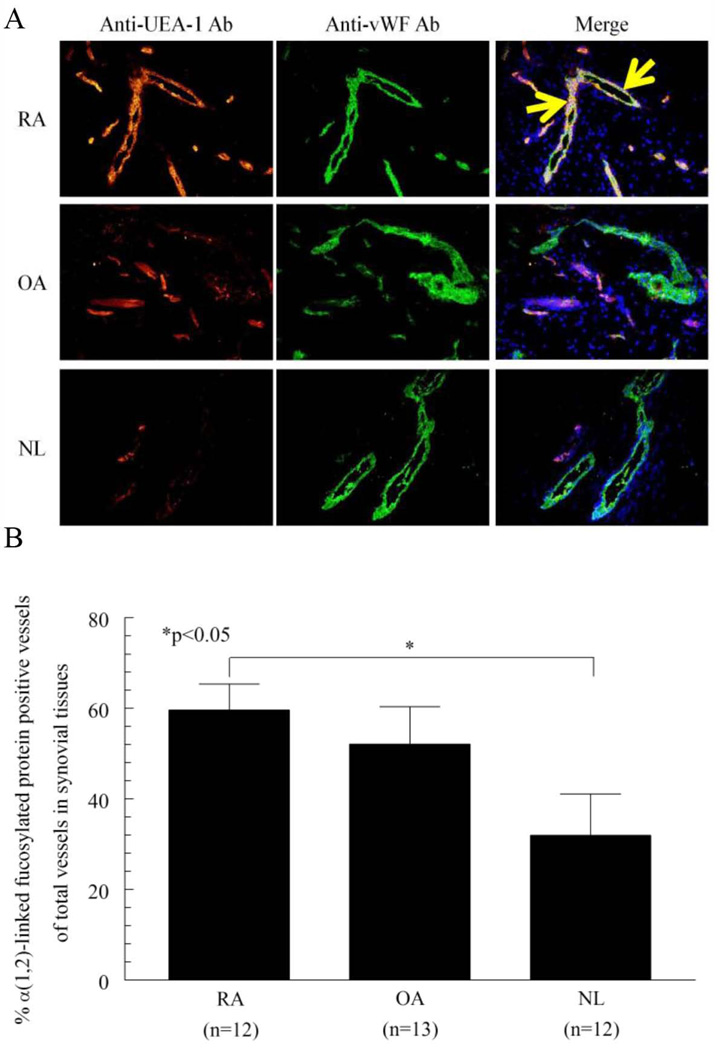
We examined the expression of α(1,2)-linked fucosylated proteins on RA, OA, and NL STs. We used UEA-1 lectin and antibody to UEA-1 lectin for immunohistology, because UEA-1 lectin binds specifically to α(1,2)-fucose (31). We found that α(1,2)-linked fucosylated proteins were expressed on ST ECs (Figure 1A). In addition, α(1,2)-linked fucosylated proteins in RA STs were highly expressed compared to NL STs (percent α(1,2)-linked fucosylated protein positive tubes of total tubes ± SEM; 60 ± 6% and 32 ± 9%, p<0.05, Figure 1B). These results suggest that α(1,2)-linked fucosylation may be important for EC angiogenic activity in RA ST.
Figure 1.
Expression of α(1,2)-linked fucosylated proteins in ST ECs. A) The left panel shows ST staining with UEA-1 lectin and goat anti-UEA-1. The middle panel shows staining with rabbit anti-vWF. The right panel shows merging of the left panel and middle panel. The yellow color indicates α(1,2)-linked fucosylated proteins associated with ST ECs (original magnification 200×). Arrows indicate α(1,2)-linked fucosylated protein positive ECs. B) The percentages of α(1,2)-linked fucosylated protein positive vessels in each of the tissues were calculated and graphed. We determined the percentage of positive vessels in RA, OA and NL ST by counting the total number of yellow vessels, and divided this value by the total number of vWF positive vessels (green) in each tissue section. α(1,2)-linked fucosylated protein containing vessels were significantly higher in RA compared to NL ST (n=number of patients). IgG control staining for all three groups did not show staining (data not shown).
Expression of α(1,2)-linked fucosylated chemokines in RA SFs
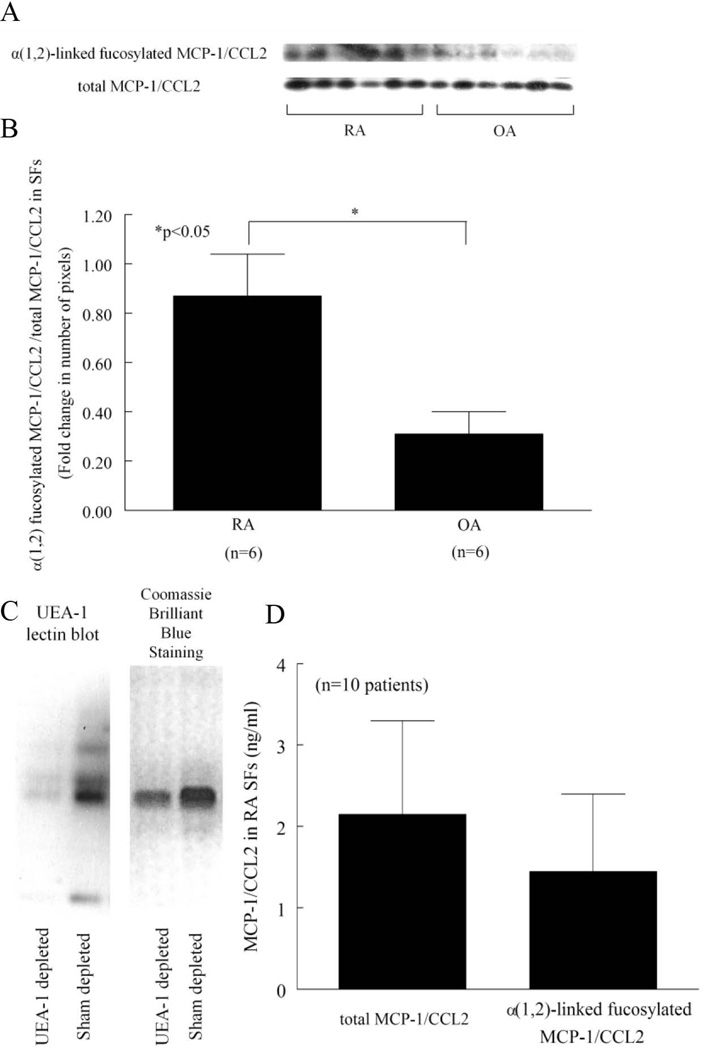
We and others have shown chemokines to be important in RA (32–34). To determine if α(1,2)-linked fucosylated chemokines were present in RA, we performed α(1,2)-linked fucosylated MCP-1/CCL2 immunoprecipitation. Figure 2A shows α(1,2)-linked fucosylated MCP-1/CCL2 in RA and OA SFs (Figure 2A). We found that α(1,2)-linked fucosylated MCP-1/CCL2 in RA SFs was significantly elevated compared to OA SFs (Figure 2B). In order to measure the α(1,2)-linked fucosylated MCP-1/CCL2 in RA SFs, SFs were depleted of α(1,2)-linked fucosylated proteins using UEA-1 conjugated agarose beads. We then determined whether α(1,2)-linked fucosylated proteins were depleted in SFs by lectin blotting. We confirmed that α(1,2)-linked fucosylated proteins were decreased after depletion (Figure 2C). We then measured MCP-1/CCL2 by ELISA. α(1,2)-linked fucosylated MCP-1/CCL2 and total MCP-1/CCL2 in RA SFs were 1.4 ± 1.0 ng/ml and 2.1 ± 1.2 ng/ml, respectively (n=10 patients) (Figure 2D). The percent of α(1,2)-linked fucosylated MCP-1/CCL2 of total MCP-1/CCL2 was 76 ± 9% (n=10 patients). These results indicate that α(1,2)-linked fucosylated MCP-1/CCL2 is found in SFs and is upregulated in RA compared to OA SF.
Figure 2.
Expression of α(1,2)-linked fucosylated MCP-1/CCL2 in SFs. A) α(1,2)-linked fucosylated MCP-1/CCL2 was detected by immunoprecipitation and lectin blotting. Six RA and 6 OA patients SFs were analyzed. B) α(1,2)-linked fucosylated MCP-1/CCL2 in RA SFs was significantly elevated compared to OA SFs, normalized to total MCP-1/CCL2. The data is graphed as the ratio of the densitometry of α(1,2)-linked fucosylated MCP-1/CCL2 to total MCP-1/CCL2 shown in 2A for RA and OA SFs. C) The left panel shows that α(1,2)-linked fucosylated proteins in RA SF are depleted using UEA-1 conjugated agarose beads by lectin blotting. The right panel shows Coomassie Brilliant Blue staining. α(1,2)-linked fucosylated proteins are decreased after depletion. D) Sham and α(1,2)-linked fucosylated protein depleted RA SFs were measured in an MCP-1/CCL2 ELISA. Percent α(1,2)-linked fucosylated MCP-1/CCL2 of total MCP-1/CCL2 was 76 ± 9 % (mean ± SEM, n=10; n=number of patients).
Function of α(1,2)-linked fucosylated proteins in RA SFs
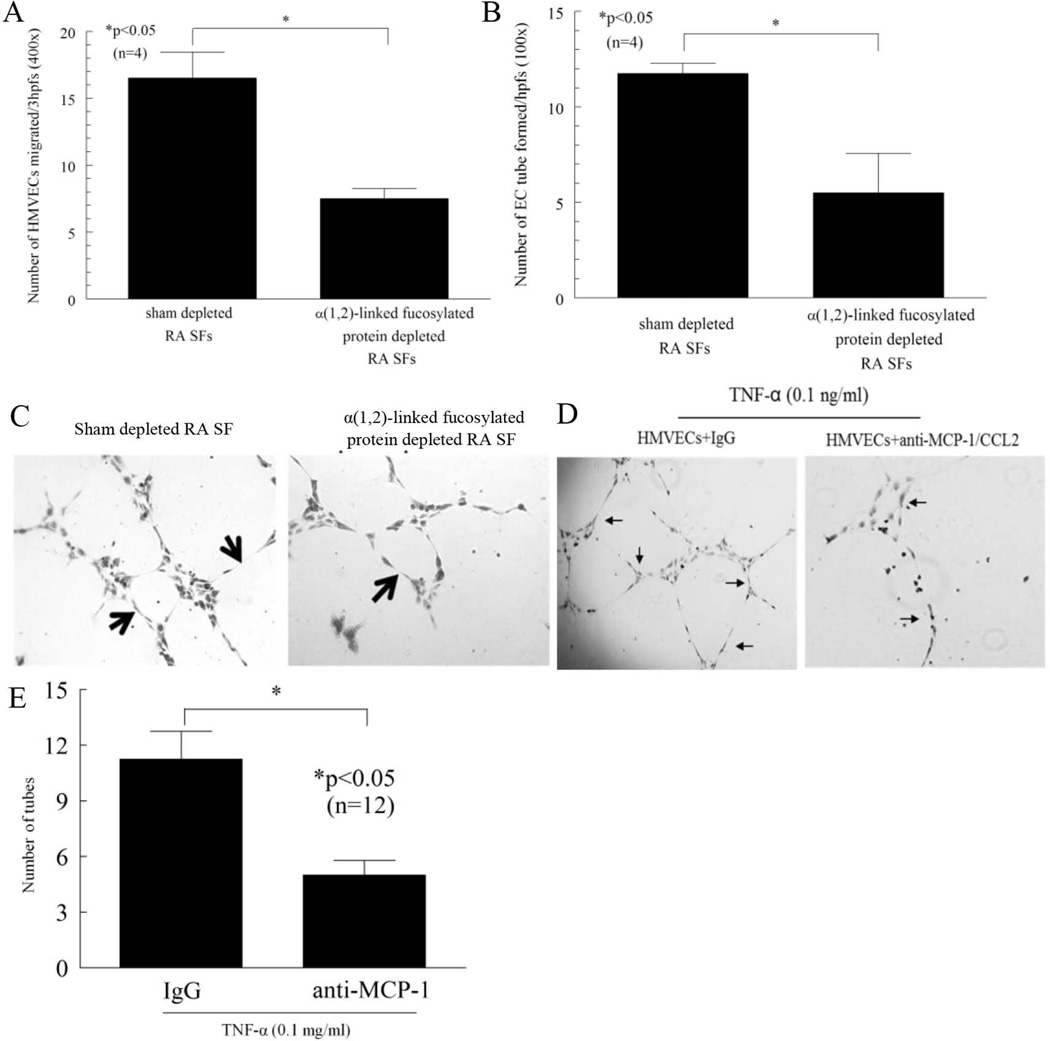
In order to demonstrate the function of α(1,2)-linked fucosylated proteins in RA SFs, SFs neutralized of α(1,2)-linked fucosylated proteins were assayed for the ability induce HMVEC chemotaxis and tube formation. Because EC chemotaxis is an initial step in the angiogenic process, we performed in vitro HMVEC chemotaxis assays using sham or α(1,2)-linked fucosylated protein depleted RA SFs. We found that α(1,2)-linked fucosylated protein depleted RA SFs showed a 54 ± 2% (n=4 patients) decrease in EC migratory activity compared to sham depleted controls (number of HMVECs migrated ± SEM; 8 ± 1 and 17 ± 2 cells migrated, p<0.05, Figure 3A). In addition, α(1,2)-linked fucosylated protein depleted RA SFs showed a 50 ± 21% (n=4 patients) decrease in EC tube forming activity compared to sham depleted controls (number of HMVEC tubes formed ± SEM; 6 ± 2 and 12 ± 1, p<0.05, Figure 3B).
Figure 3.
Chemotactic and tube formation activity of α(1,2)-linked fucosylated proteins in RA SFs. A) HMVEC migration was measured in a chemotaxis assay using sham or α(1,2)-linked fucosylated protein depleted RA SFs. α(1,2)-linked fucosylated protein depletion resulted in a 54 ± 2% (n=4) reduction in RA SF-induced HMVEC migration. B) α(1,2)-linked fucosylated protein depleted RA SFs show less HMVEC tube forming activity compared to sham depleted RA SFs (n=number of patients). C) The left panel shows HMVEC tubes formed by sham depleted RA SFs. The right panel shows HMVEC tubes formed by α(1,2)-linked fucosylated protein depleted RA SFs. Arrows indicate tubes (original magnification 100x). D and E) Blocking antibody against MCP-1/CCL2 inhibited TNF-α-mediated HMVEC tube formation on Matrigel. Arrows indicate the number of tubes formed. Results are expressed as mean ± SEM and *p<0.05 was considered significant (n=number of replicates).
Total length of the formed tubes and the number of formed nodes showed a similar trend. α(1,2)-linked fucosylated protein depleted RA SFs showed a 72 ± 7% decrease in total length of the formed tubes compared to sham depleted controls (length of the formed HMVEC tubes ± SEM; 21 ± 6 and 71 ± 4, p<0.05, Figure 3C). α(1,2)-linked fucosylated protein depleted RA SFs also showed a 75 ± 13% decrease in number of nodes compared to sham depleted controls (number of nodes ± SEM; 2 ± 1 and 6 ± 0, p<0.05, Figure 3C). These findings suggest that α(1,2)-linked fucosylated proteins in RA SFs have more potent EC angiogenic activity compared to non-α(1,2)-linked fucosylated proteins. After finding that MCP-1/CCL2 was decreased in fut1 gene deficient MLECs compared to wild type MLECs, we examined if MCP-1/CCL2 was involved in TNF-α-mediated tube formation in HMVECs. We found that TNF-α induced HMVEC tube formation was significantly decreased in the presence of neutralizing antibody to MCP-1/CCL2, suggesting that MCP-1/CCL2 is involved in TNF-α-stimulated angiogenesis in part (Figure 3D and E). We did not see a similar effect with RANTES, suggesting that this chemokine is regulated differently than MCP-1/CCL2 in this system (data not shown).
Validation of the purity of the MLECs by flow cytometry
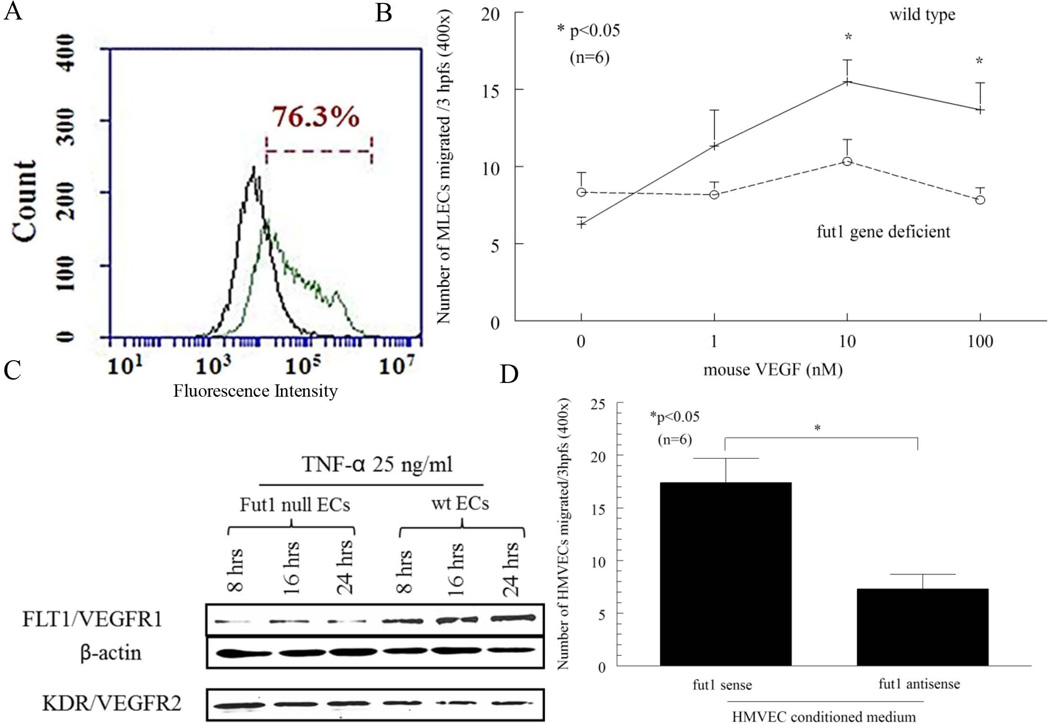
To validate the purity of the EC population, flow cytometry was performed using FITC conjugated CD146, a mouse EC marker. ECs were 76% CD146 positive (Figure 4A).
Figure 4.
A) Purity of wild type MLECs via flow cytometry. Purity of C57BL/6 wild type MLECs was 76.3%, as determined by CD146 staining via flow cytometric analysis. The green graph represents CD146 positive cells while the black graph is control IgG. This graph is representative of two independent assays. Blocking fut1 in ECs reduces EC chemotaxis. B) A complete lack of fut1 expression in ECs reduces cell migration. Fut1 gene deficient MLECs had significantly less migration compared to wild type MLECs in response to mouse VEGF (10 and 100 nM). C) TNF-α (25 ng/ml) induced Flt1/VEGFR1 was markedly increased in wild type MLECs compared to fut1 gene deficient MLECs at 8, 16, and 24 hours (representative of two independent assays). D) Fut1 antisense ODN transfected HMVEC conditioned medium reduces HMVEC migration compared to fut1 sense ODN transfected HMVEC conditioned medium. The negative and positive controls were PBS and VEGF respectively (number of HMVECs migrating towards PBS was 5 ± 1, and VEGF was 40 ± 6; n=number of experiments).
Fut1 deficient MLECs show decreased EC chemotaxis versus wild type ECs
To determine the role of fut1 in angiogenesis, we performed in vitro chemotaxis assays. Fut1 gene deficient and wild type MLECs were assayed for their chemotactic response to VEGF in a modified Boyden chamber. We found that fut1 gene deficient MLECs showed decreased migration compared to wild type MLECs towards VEGF [number of MLECs migrated ± SEM; 10 nM (n=6), 10 ± 1 and 16 ± 1; 100 nM (n=6), 8 ± 1 and 14 ± 2, respectively, p<0.05 (Figure 4B)]. After finding that VEGF induces migration of MLECs, we examined the expression of angiogenic VEGFRs in fut1 gene deficient and wild type MLECs. We found a marked increase in FMS-like tyrosine kinase 1 (Flt1)/VEGFR1 in a time dependent manner in wild type MLECs compared to fut1 gene deficient MLECs when stimulated with TNF-α. This suggests a mechanism by which fut1 gene deficient MLECs migrate less in response to VEGF (Figure 4C). However, we did not find a decrease in Kinase insert domain receptor (KDR)/VEGFR-2, another receptor for VEGF, in response to TNF-α in fut1 gene deficient MLECs compared to wild type MLECs (Figure 4C).
Blocking fut1 expression in ECs reduces EC migration to conditioned medium
To further examine the function of fut1 in ECs, we used HMVECs transfected with sense or antisense ODNs directed against fut1. Percent knockdown of fut1 in antisense ODN transfected HMVECs was 41 ± 7% (n=3). We performed HMVEC chemotaxis towards fut1 sense or antisense ODN transfected HMVEC conditioned medium, and found that fut1 antisense ODN transfected HMVEC conditioned medium resulted in a significant decrease in the chemotactic activity for HMVECs compared to fut1 sense ODN transfected HMVEC conditioned medium (number of HMVECs migrated ± SE; 7 ± 1 and 17 ± 2, p<0.05, Figure 4D).
Blocking fut1 expression in ECs reduces expression of proangiogenic chemokines
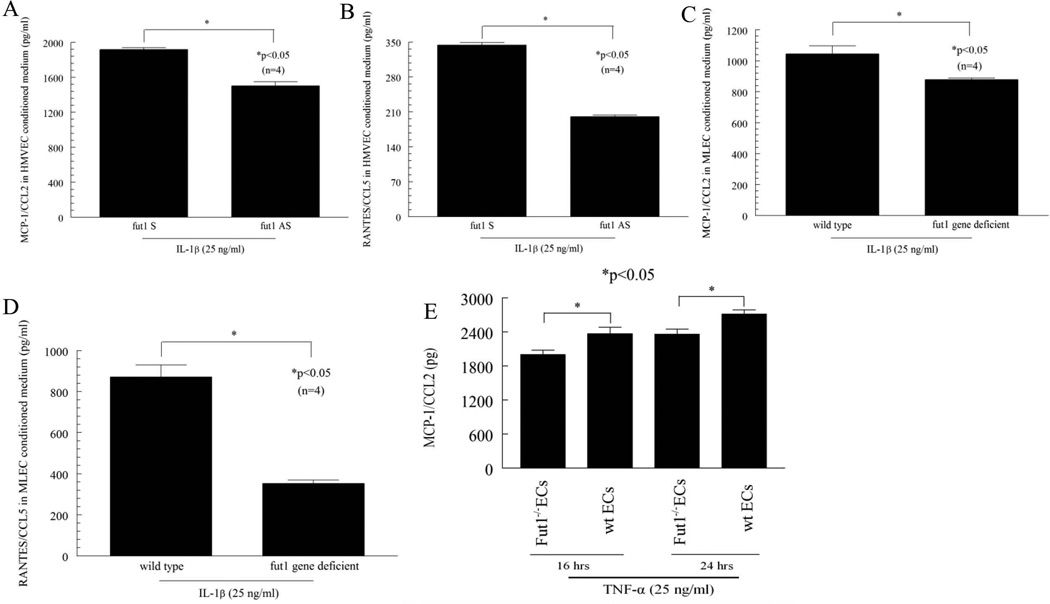
To determine whether angiogenic chemokines were present in EC conditioned medium and whether their expression was regulated by proinflammatory cytokines, ELISAs were performed on conditioned medium. HMVECs were transfected with fut1 antisense ODNs and then stimulated with IL-1β to induce chemokine production. This conditioned medium contained much less MCP-1/CCL2 than medium from sense transfected HMVECs when stimulated with IL-1β (Figure 5A) [non-stimulated (NS) fut1 sense 1578 ± 35 (average ± SEM) pg/ml, n=4; NS fut1 anti-sense 343 ± 30, n=4]. RANTES/CCL5 in fut1 antisense ODN transfected HMVEC conditioned medium was also decreased compared to fut1 sense ODN transfected HMVEC conditioned medium in response to IL-1β (Figure 5B; NS fut1 sense 51 ± 2 pg/ml, n=4; NS fut1 anti-sense 44 ± 4, n=4). We also found that MCP-1/CCL2 in IL-1β stimulated fut1 gene deficient MLEC conditioned medium was significantly decreased compared to wild type MLEC conditioned medium (Figure 5C; NS wild type 316 ± 28 pg/ml, n=4; NS fut gene deficient 302 ± 79, n=4). RANTES/CCL5 in fut1 gene deficient MLEC conditioned medium was also significantly decreased compared to wild type MLEC conditioned medium in response to IL-1β (Figure 5D; NS wild type 85 ± 22 pg/ml, n=4; NS fut gene deficient 9 ± 5, n=4). However, MCP-1/CCL2 and RANTES/CCL5 were not significantly decreased in conditioned medium using TNF-α as a stimulus with similar culture conditions (data not shown). We also measured VEGF in fut1 deficient and wild type MLEC conditioned medium, and did not find statistical differences between fut1 deficient and wild type MLEC conditioned medium (data not shown). These findings indicate that fut1 inhibition can regulate C-C chemokine production in ECs.
Figure 5.
Blocking fut1 expression in ECs reduces expression of proangiogenic chemokines. Cells were treated with IL-1β for 72 hours, and conditioned medium assayed. A) MCP-1/CCL2 in IL-1β stimulated fut1 antisense ODN transfected HMVEC conditioned medium is 21 ± 3 % (n=4) decreased compared to IL-1β stimulated fut1 sense ODN transfected HMVEC conditioned medium. B) RANTES/CCL5 in IL-1β stimulated fut1 antisense ODN transfected HMVEC conditioned medium is 41 ± 0 % (n=4) decreased compared to IL-1β stimulated fut1 sense ODN transfected HMVEC conditioned medium. C) MCP-1/CCL2 in IL-1β stimulated fut1 gene deficient MLEC conditioned medium is 15 ± 5 % (n=4) decreased compared to IL-1β stimulated wild type MLEC conditioned medium. D) RANTES/CCL5 in IL-1β stimulated fut1 gene deficient MLEC conditioned medium is 59 ± 1 % (n=4) decreased compared to IL-1β stimulated wild type MLEC conditioned medium (n=number of replicates). E) TNF-α (25 ng/ml) induced MCP-1/CCL2 expression was significantly less in fut1 gene deficient MLEC conditioned medium compared to wild type MLEC conditioned medium at 16 and 24 hours (n=number of samples).
To examine the possibility that peak chemokine induction stimulated with TNF-α may occur at an earlier time point than 24 hours, we measured the expression of MCP-1/CCL2 in conditioned media collected from fut1 gene deficient MLEC and wild type MLECs stimulated with TNF-α at 16 and 24 hours. TNF-α-induced significantly higher MCP-1/CCL2 secretion in wild type MLECs compared to fut1 gene deficient MLEC at both 16 and 24 hours (Figure 5E). However, we did not see this difference at 8 hours (data not shown).
Discussion
Glycosylation is involved in various events such as cell growth, cell migration, and tumor invasion. Approximately 50% of all proteins are glycosylated (35), suggesting that glycosylation is critical for proper protein function. Interestingly, it has also been shown that disregulated protein glycosylation can lead to enhanced pathologic responses. For example, oligosaccharides on glycoproteins are altered in tumorigenesis, and play a role in regulating the metastatic potential of tumor cells (36).
Alpha-fetoprotein and prostate-specific antigen are commonly used tumor markers. However, elevated levels of these markers occur not only in cancer but in benign diseases as well, whereas some groups reported that fucosylated tumor markers were more specific markers for active disease (6, 37). The present study demonstrates that α(1,2)-linked fucosylated proteins are expressed in RA, and are highly elevated compared to NL STs. We also show that α(1,2)-linked fucosylated MCP-1/CCL2 is present in RA SFs, and is significantly elevated compared to OA SFs. Przybysz et al. reported that the fucosylation of fibronectin in SFs and blood plasma were related to RA progression (38). Ferens-Sieczkowska et al. noted that fucosylation of synovial glycoconjugates could be a reliable clinical marker for RA and juvenile idiopathic arthritis (JIA) (39). All fucosylated proteins in RA, JIA, and traumatized knee SFs were detected with Aleuria aurantia lectin, one of the fucose specific lectins. Fucosylated proteins in RA and JIA were also elevated compared to traumatized knee SFs. However, no one has shown the role of fucosylation in RA. Our findings support the notion that α(1,2)-linked fucosylation is important in RA pathogenesis.
We examined the α(1,2)-linked fucosylation of MCP-1/CCL2 because this chemokine is an important monocyte chemotactic factor in RA and an angiogenic factor (32, 33, 40). We measured α(1,2)-linked fucosylated MCP-1/CCL2 using UEA-1 conjugated agarose beads, and found that the percent of α(1,2)-linked fucosylated MCP-1/CCL2 of total MCP-1/CCL2 in RA SFs was 76 ± 9%. Our finding of elevated α(1,2)-linked fucosylated MCP-1/CCL2 in RA SF suggests that post-translational modification of this chemokine is increased in inflammatory environments such as the RA joint.
We previously found that fut1 is expressed in the synovial lining of the RA ST (41), and that α(1,2)-linked fucosylated proteins were overexpressed in RA. Because of this, we sought to determine if these proteins in RA SFs had chemotactic and angiogenic activity. We found that RA SFs depleted of α(1,2)-linked fucosylated proteins induced less HMVEC migration compared to sham depleted RA SFs, suggesting that α(1,2)-linked fucosylation may have an important function in RA angiogenesis. Interestingly, Proost et al. reported that glycosylated MCP-1/CCL2 was two- to three fold less chemotactic for monocytes and THP-1 cells than nonglycosylated MCP-1/CCL2 (42). This discrepancy might be explained by the fact that they examined different cells and studied total glycosylation of MCP-1/CCL2, whereas we examined only α(1,2)-linked fucosylation. Carlyon et al. reported that fut4 and fut7 gene deficient mouse neutrophils exhibited decreased secretion of the chemokines macrophage inflammatory protein-2 (MIP-2)/CXCL2 and keratinocyte derived chemokine compared to wild type mouse neutrophils (43). However, production of angiogenic chemokines in fut1 deficient ECs has not been demonstrated. Mathieu et al. reported that tumor vascularization was decreased after fut1 inhibition (44), and supported the finding of Palumberi et al. who showed that adhesion of human epidermoid carcinoma cells to fut1 and fut2 siRNA transfected ECs was decreased compared with control siRNA transfected ECs. This group also reported that fut1 and fut2 siRNA treated human epidermoid carcinoma cells have reduced cell proliferation when transfected with fut1 or fut2 siRNA (45). We have previously shown that fut2 gene deficient MLECs have less chemotactic activity compared to wild type MLECs towards basic fibroblast growth factor, and that fut2 gene deficient MLECs have less fibroblast growth factor receptor (FGFR) 2 expression (46). Here, we found that MLECs isolated from fut1 gene deficient mice had decreased migration compared to wild type MLECs towards VEGF, indicating that fut1 plays an important role in EC chemotaxis. We also found that fut1 antisense ODN transfected HMVEC conditioned medium had less HMVEC chemotactic activity compared to fut1 sense ODN transfected HMVEC conditioned medium. This result indicates that fut1 affects EC migration not only directly but indirectly, as it appears that angiogenic factors may be secreted from HMVECs, mediated by fut1 expression.
MCP-1/CCL2 and RANTES/CCL5 are well known chemokines that induce angiogenesis and leukocyte recruitment (34, 40). We found that MCP-1/CCL2 and RANTES/CCL5 secretion were upregulated in HMVECs by IL-1β. We also found that MCP-1/CCL2 and RANTES/CCL5 in IL-1β stimulated fut1 gene deficient MLEC conditioned medium were decreased compared to similarly treated control conditioned medium. These findings point to fut1 as a critical intermediary in expression of some angiogenic chemokines known to be important in RA pathogenesis.
In summary, our study demonstrates that α(1,2)-linked fucosylated proteins are expressed on RA ST ECs, and α(1,2)-linked fucosylated proteins in RA SFs have angiogenic ability. We also found that α(1,2)-linked fucosylated MCP-1/CCL2 was present in RA SFs, and that α(1,2)-linked fucosylated MCP-1/CCL2 in RA SF was significantly elevated compared to OA SFs. Finally, we show that blocking fut1 in ECs reduced C-C chemokine production and EC migration. Taken together, these results demonstrate the importance of α(1,2)-linked fucosylation by fut1 in RA angiogenesis.
Acknowledgments
We thank Consortium for Functional Glycomics for providing fut1 gene deficient mice. We also thank the Cancer Center Immunology Core of University of Michigan for some chemokine ELISA assays.
Grant support: This work was supported by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs, the Frederick G. L. Huetwell and William D. Robinson, MD Professorship in Rheumatology.
Footnotes
Author Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version. Dr. Isozaki had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design: Isozaki, Amin, Ruth, Campbell, Koch.
Acquisition of data: Isozaki, Tsou, Ha, Domino, Stinson, Amin.
Analysis and interpretation of data: Isozaki, Amin, Ruth, Campbell, Koch.
References
- 1.Moremen KW, Tiemeyer M, Nairn AV. Vertebrate protein glycosylation: diversity, synthesis and function. Nat Rev Mol Cell Biol. 2012;13(7):448–462. doi: 10.1038/nrm3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moelants EA, Mortier A, Grauwen K, Ronsse I, Van Damme J, Proost P. Citrullination of TNF-alpha by peptidylarginine deiminases reduces its capacity to stimulate the production of inflammatory chemokines. Cytokine. 2012 doi: 10.1016/j.cyto.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Hart GW. Glycosylation. Curr Opin Cell Biol. 1992;4(6):1017–1023. doi: 10.1016/0955-0674(92)90134-x. [DOI] [PubMed] [Google Scholar]
- 4.Kannagi R, Izawa M, Koike T, Miyazaki K, Kimura N. Carbohydrate-mediated cell adhesion in cancer metastasis and angiogenesis. Cancer Sci. 2004;95(5):377–384. doi: 10.1111/j.1349-7006.2004.tb03219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyoshi E, Moriwaki K, Nakagawa T. Biological function of fucosylation in cancer biology. J Biochem. 2008;143(6):725–729. doi: 10.1093/jb/mvn011. [DOI] [PubMed] [Google Scholar]
- 6.Fukushima K, Satoh T, Baba S, Yamashita K. alpha1,2-Fucosylated and beta-N-acetylgalactosaminylated prostate-specific antigen as an efficient marker of prostatic cancer. Glycobiology. 2010;20(4):452–460. doi: 10.1093/glycob/cwp197. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida M, Takimoto R, Murase K, Sato Y, Hirakawa M, Tamura F, et al. Targeting anticancer drug delivery to pancreatic cancer cells using a fucose-bound nanoparticle approach. PLoS One. 2012;7(7):e39545. doi: 10.1371/journal.pone.0039545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly RJ, Rouquier S, Giorgi D, Lennon GG, Lowe JB. Sequence expression of a candidate for the human Secretor blood group alpha(1,2)fucosyltransferase gene (FUT2). Homozygosity for an enzyme-inactivating nonsense mutation commonly correlates with the non-secretor phenotype. J Biol Chem. 1995;270(9):4640–4649. doi: 10.1074/jbc.270.9.4640. [DOI] [PubMed] [Google Scholar]
- 9.Larsen RD, Ernst LK, Nair RP, Lowe JB. Molecular cloning, sequence, and expression of a human GDP-L-fucose:beta-D-galactoside 2-alpha-L-fucosyltransferase cDNA that can form the H blood group antigen. Proc Natl Acad Sci U S A. 1990;87(17):6674–6678. doi: 10.1073/pnas.87.17.6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koch AE, Halloran MM, Haskell CJ, Shah MR, Polverini PJ. Angiogenesis mediated by soluble forms of E-selectin and vascular cell adhesion molecule-1. Nature. 1995;376(6540):517–519. doi: 10.1038/376517a0. [DOI] [PubMed] [Google Scholar]
- 11.Halloran MM, Carley WW, Polverini PJ, Haskell CJ, Phan S, Anderson BJ, et al. Ley/H: an endothelial-selective, cytokine-inducible, angiogenic mediator. J Immunol. 2000;164(9):4868–4877. doi: 10.4049/jimmunol.164.9.4868. [DOI] [PubMed] [Google Scholar]
- 12.Zhu K, Amin MA, Kim MJ, Katschke KJ, Jr, Park CC, Koch AE. A novel function for a glucose analog of blood group H antigen as a mediator of leukocyte-endothelial adhesion via intracellular adhesion molecule 1. J Biol Chem. 2003;278(24):21869–21877. doi: 10.1074/jbc.M213052200. [DOI] [PubMed] [Google Scholar]
- 13.Koch AE, Kunkel SL, Burrows JC, Evanoff HL, Haines GK, Pope RM, et al. Synovial tissue macrophage as a source of the chemotactic cytokine IL-8. J Immunol. 1991;147(7):2187–2195. [PubMed] [Google Scholar]
- 14.Ritchlin C. Fibroblast biology. Effector signals released by the synovial fibroblast in arthritis. Arthritis Res. 2000;2(5):356–360. doi: 10.1186/ar112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48(1):35–45. doi: 10.1002/art.10697. [DOI] [PubMed] [Google Scholar]
- 16.Mori K, Iida S, Yamane-Ohnuki N, Kanda Y, Kuni-Kamochi R, Nakano R, et al. Non-fucosylated therapeutic antibodies: the next generation of therapeutic antibodies. Cytotechnology. 2007;55(2–3):109–114. doi: 10.1007/s10616-007-9103-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992;267(16):10931–10934. [PubMed] [Google Scholar]
- 18.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438(7070):932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 19.Domino SE, Zhang L, Gillespie PJ, Saunders TL, Lowe JB. Deficiency of reproductive tract alpha(1,2)fucosylated glycans and normal fertility in mice with targeted deletions of the FUT1 or FUT2 alpha(1,2)fucosyltransferase locus. Mol Cell Biol. 2001;21(24):8336–8345. doi: 10.1128/MCB.21.24.8336-8345.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Domino SE, Zhang L, Lowe JB. Molecular cloning, genomic mapping, and expression of two secretor blood group alpha (1,2)fucosyltransferase genes differentially regulated in mouse uterine epithelium and gastrointestinal tract. J Biol Chem. 2001;276(26):23748–23756. doi: 10.1074/jbc.M100735200. [DOI] [PubMed] [Google Scholar]
- 21.Iwamori M, Domino SE. Tissue-specific loss of fucosylated glycolipids in mice with targeted deletion of alpha(1,2)fucosyltransferase genes. Biochem J. 2004;380(Pt 1):75–81. doi: 10.1042/BJ20031668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marelli-Berg FM, Peek E, Lidington EA, Stauss HJ, Lechler RI. Isolation of endothelial cells from murine tissue. J Immunol Methods. 2000;244(1–2):205–215. doi: 10.1016/s0022-1759(00)00258-1. [DOI] [PubMed] [Google Scholar]
- 23.Ruth JH, Volin MV, Haines GK, 3rd, Woodruff DC, Katschke KJ, Jr, Woods JM, et al. Fractalkine, a novel chemokine in rheumatoid arthritis and in rat adjuvant-induced arthritis. Arthritis Rheum. 2001;44(7):1568–1581. doi: 10.1002/1529-0131(200107)44:7<1568::AID-ART280>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 24.Isozaki T, Rabquer BJ, Ruth JH, Haines GK, 3rd, Koch AE. ADAM-10 is overexpressed in rheumatoid arthritis synovial tissue and mediates angiogenesis. Arthritis Rheum. 2013;65(1):98–108. doi: 10.1002/art.37755. [DOI] [PubMed] [Google Scholar]
- 25.Marotte H, Tsou PS, Rabquer BJ, Pinney AJ, Fedorova T, Lalwani N, et al. Blocking of interferon regulatory factor 1 reduces tumor necrosis factor alpha-induced interleukin-18 bioactivity in rheumatoid arthritis synovial fibroblasts by induction of interleukin-18 binding protein a: role of the nuclear interferon regulatory factor 1-NF-kappaB-c-jun complex. Arthritis Rheum. 2011;63(11):3253–3262. doi: 10.1002/art.30583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rabquer BJ, Pakozdi A, Michel JE, Gujar BS, Haines GK, 3rd, Imhof BA, et al. Junctional adhesion molecule C mediates leukocyte adhesion to rheumatoid arthritis synovium. Arthritis Rheum. 2008;58(10):3020–3029. doi: 10.1002/art.23867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volin MV, Woods JM, Amin MA, Connors MA, Harlow LA, Koch AE. Fractalkine: a novel angiogenic chemokine in rheumatoid arthritis. Am J Pathol. 2001;159(4):1521–1530. doi: 10.1016/S0002-9440(10)62537-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park CC, Morel JC, Amin MA, Connors MA, Harlow LA, Koch AE. Evidence of IL-18 as a novel angiogenic mediator. J Immunol. 2001;167(3):1644–1653. doi: 10.4049/jimmunol.167.3.1644. [DOI] [PubMed] [Google Scholar]
- 29.Amin MA, Volpert OV, Woods JM, Kumar P, Harlow LA, Koch AE. Migration inhibitory factor mediates angiogenesis via mitogen-activated protein kinase and phosphatidylinositol kinase. Circ Res. 2003;93(4):321–329. doi: 10.1161/01.RES.0000087641.56024.DA. [DOI] [PubMed] [Google Scholar]
- 30.Marotte H, Ruth JH, Campbell PL, Koch AE, Ahmed S. Green tea extract inhibits chemokine production, but up-regulates chemokine receptor expression, in rheumatoid arthritis synovial fibroblasts and rat adjuvant-induced arthritis. Rheumatology (Oxford) 2010;49(3):467–479. doi: 10.1093/rheumatology/kep397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macartney JC. Fucose-containing antigens in normal and neoplastic human gastric mucosa: a comparative study using lectin histochemistry and blood group immunohistochemistry. J Pathol. 1987;152(1):23–30. doi: 10.1002/path.1711520104. [DOI] [PubMed] [Google Scholar]
- 32.Koch AE, Kunkel SL, Harlow LA, Johnson B, Evanoff HL, Haines GK, et al. Enhanced production of monocyte chemoattractant protein-1 in rheumatoid arthritis. J Clin Invest. 1992;90(3):772–779. doi: 10.1172/JCI115950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hachicha M, Rathanaswami P, Schall TJ, McColl SR. Production of monocyte chemotactic protein-1 in human type B synoviocytes. Synergistic effect of tumor necrosis factor alpha and interferon-gamma. Arthritis Rheum. 1993;36(1):26–34. doi: 10.1002/art.1780360106. [DOI] [PubMed] [Google Scholar]
- 34.Volin MV, Shah MR, Tokuhira M, Haines GK, Woods JM, Koch AE. RANTES expression and contribution to monocyte chemotaxis in arthritis. Clin Immunol Immunopathol. 1998;89(1):44–53. doi: 10.1006/clin.1998.4590. [DOI] [PubMed] [Google Scholar]
- 35.Apweiler R, Hermjakob H, Sharon N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim Biophys Acta. 1999;1473(1):4–8. doi: 10.1016/s0304-4165(99)00165-8. [DOI] [PubMed] [Google Scholar]
- 36.Hakomori S. Aberrant glycosylation in tumors and tumor-associated carbohydrate antigens. Adv Cancer Res. 1989;52:257–331. doi: 10.1016/s0065-230x(08)60215-8. [DOI] [PubMed] [Google Scholar]
- 37.Nakagawa T, Uozumi N, Nakano M, Mizuno-Horikawa Y, Okuyama N, Taguchi T, et al. Fucosylation of N-glycans regulates the secretion of hepatic glycoproteins into bile ducts. J Biol Chem. 2006;281(40):29797–29806. doi: 10.1074/jbc.M605697200. [DOI] [PubMed] [Google Scholar]
- 38.Przybysz M, Maszczak D, Borysewicz K, Szechinski J, Katnik-Prastowska I. Relative sialylation and fucosylation of synovial and plasma fibronectins in relation to the progression and activity of rheumatoid arthritis. Glycoconj J. 2007;24(9):543–550. doi: 10.1007/s10719-007-9049-9. [DOI] [PubMed] [Google Scholar]
- 39.Ferens-Sieczkowska M, Kossowska B, Gancarz R, Dudzik D, Knas M, Popko J, et al. Fucosylation in synovial fluid as a novel clinical marker for differentiating joint diseases--a preliminary study. Clin Exp Rheumatol. 2007;25(1):92–95. [PubMed] [Google Scholar]
- 40.Salcedo R, Ponce ML, Young HA, Wasserman K, Ward JM, Kleinman HK, et al. Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood. 2000;96(1):34–40. [PubMed] [Google Scholar]
- 41.Isozaki T, Ruth JH, Amin MA, Campbell PL, Tsou PS, Ha CM, et al. Fucosyltransferase 1 mediates angiogenesis, cell adhesion and rheumatoid arthritis synovial tissue fibroblast proliferation. Arthritis Res Ther. 2014;16(1):R28. doi: 10.1186/ar4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Proost P, Struyf S, Couvreur M, Lenaerts JP, Conings R, Menten P, et al. Posttranslational modifications affect the activity of the human monocyte chemotactic proteins MCP-1 and MCP-2: identification of MCP-2(6-76) as a natural chemokine inhibitor. J Immunol. 1998;160(8):4034–4041. [PubMed] [Google Scholar]
- 43.Carlyon JA, Akkoyunlu M, Xia L, Yago T, Wang T, Cummings RD, et al. Murine neutrophils require alpha1,3-fucosylation but not PSGL-1 for productive infection with Anaplasma phagocytophilum. Blood. 2003;102(9):3387–3395. doi: 10.1182/blood-2003-02-0621. [DOI] [PubMed] [Google Scholar]
- 44.Mathieu S, Gerolami R, Luis J, Carmona S, Kol O, Crescence L, et al. Introducing alpha(1,2)-linked fucose into hepatocarcinoma cells inhibits vasculogenesis and tumor growth. Int J Cancer. 2007;121(8):1680–1689. doi: 10.1002/ijc.22797. [DOI] [PubMed] [Google Scholar]
- 45.Palumberi D, Aldi S, Ermini L, Ziche M, Finetti F, Donnini S, et al. RNA-mediated gene silencing of FUT1 and FUT2 influences expression and activities of bovine and human fucosylated nucleolin and inhibits cell adhesion and proliferation. J Cell Biochem. 2010;111(1):229–238. doi: 10.1002/jcb.22692. [DOI] [PubMed] [Google Scholar]
- 46.Tsou PS, Ruth JH, Campbell PL, Isozaki T, Lee S, Marotte H, et al. A novel role for inducible Fut2 in angiogenesis. Angiogenesis. 2013;16(1):195–205. doi: 10.1007/s10456-012-9312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]