Abstract
Objective
This phase II, multicenter, single-arm, two-stage study in platinum-resistant, advanced epithelial ovarian or primary peritoneal cancer evaluated the efficacy, safety, and tolerability of weekly single-agent volociximab. Pharmacokinetic/pharmacodynamic (PK/PD) studies were also performed.
Methods
Sixteen patients were enrolled in Stage 1. Volociximab was administered at 15 mg/kg IV qwk until progression of disease or drug intolerability. Tumor response was assessed every 8 weeks. Serum samples for PK or whole blood for the evaluation of circulating tumor cells, endothelial cells, and endothelial progenitor cells were obtained on Days 1, 8, 15, 29, and 50. Ascites from one patient was collected for volociximab concentration analysis. Archived tumor tissue was analyzed by immunohistochemistry (IHC) for α5 integrin expression.
Results
Safety data are available on all 16 patients; 14 were evaluable for efficacy. One patient had stable disease at 8 weeks. The remaining 13 progressed on treatment. Twelve patients (75%) experienced study-related adverse events (AEs); the most common (≥20%) were headache and fatigue. Three patients experienced possible study-related serious AEs (SAEs): reversible posterior leukoencephalopathy syndrome, pulmonary embolism, and hyponatremia. Peak serum concentrations of volociximab increased 2–3 fold from Day 1 to Day 50. Clinically relevant trough levels were achieved (>150 µg/mL). IHC analysis of archived tumor sections showed low-to-moderate expression of α5 integrin on all ovarian cancer tissue evaluated.
Conclusion
Despite insufficient clinical activity in this refractory patient population to continue the study, weekly volociximab was well tolerated, and the gained understanding of the mechanism of action of volociximab will inform future development efforts.
Keywords: Volociximab, Ovarian cancer, Platinum resistant, α5β1 integrin
Introduction
Volociximab is a high-affinity, chimeric antibody directed against human α5β1 integrin. Integrins are heterodimeric cell surface adhesion receptors consisting of α and β chains, and their ligands are often components of the extracellular matrix (ECM). Integrins are highly expressed on tumor vascular endothelial cells. While growth factors are required to elicit new blood vessel growth, interactions between cell surface integrin receptors and their ECM protein ligands provide migration, adhesion, and survival signals to activated endothelial cells [1,2]. Recent evidence suggests that the interaction between α5β1 integrin and its ligand fibronectin plays a pivotal role in the initial process of neovascularization. Antagonists of this interaction, such as volociximab, inhibit endothelial cell survival and proliferation in vitro and in vivo even when endothelial cells adhere to the ECM through other integrins [3,4].
Ovarian carcinoma is a highly vascular tumor type that expresses α5β1 integrin on its vasculature [5]. In addition, α5 integrin is also found on ovarian tumor cells [6]. Interfering with integrin–ligand binding may affect the ability of ovarian cancer tumor cells to spread and grow within the peritoneal cavity. Adhesion and migration of ovarian cancer cells are regulated by integrin-dependent mechanisms involving the interaction of integrins with the ECM and CD44 [7]. In the mouse SKOV-3ip1 ovarian cancer xenograft model, treatment with the murine parent antibody of volociximab IIA1 showed significant benefit compared with control IgG treatment [6]. The number of metastases, total tumor burden, and ascites production in mice treated with IIA1 was significantly decreased, while overall survival was increased compared with controls.
The phase I study of volociximab in solid tumors showed that volociximab could be safely administered at a dose of 15 mg/kg intravenously per week [8]. Of the 21 patients enrolled, one minor response was seen in a renal carcinoma patient, and stable disease was seen in one melanoma patient. There were no ovarian carcinoma patients enrolled in the phase I study. The present phase II study in platinum-resistant, advanced epithelial ovarian cancer or primary peritoneal cancer was warranted based on the strong preclinical data to evaluate the efficacy and safety of weekly infusions of single-agent volociximab at a dose of 15 mg/kg. Correlative studies were performed to evaluate the prevalence of α5-integrin expression in patients' tumors and to analyze the effects of volociximab on circulating tumor cells (CTCs), circulating endothelial cells (CECs), and circulating endothelial progenitor cells (CEPCs).
Patients and methods
Patient eligibility
Patients age 18 or older with advanced, histologically documented epithelial ovarian cancer or primary peritoneal cancer that had progressed during or within 6 months of discontinuing platinum-based chemotherapy and that had progressed during or following treatment with topotecan or liposomal doxorubicin were eligible to participate. Additional eligibility criteria included the presence of at least one measurable lesion in accordance with Response Evaluation Criteria in Solid Tumors (RECIST) [9], no more than 3 prior chemotherapy regimens, Eastern Cooperative Oncology Group (ECOG) performance status of ≤1 [10], life expectancy >12 weeks, and the availability of paraffin slides containing representative tumor. Patients were required to have adequate bone marrow, hepatic, and renal function. Patients were ineligible to participate if they had significant cardiovascular disease; active infection requiring systemic antibiotics, antivirals, or anti-fungals; known central nervous system disease; other malignancies within 3 years, excluding adequately treated carcinoma in situ of the cervix, ductal carcinoma in situ of the breast, or basal or squamous cell skin cancer; autoimmune disease; known human anti-murine antibodies (HAMA) or human anti-chimeric antibodies (HACA); or known hypersensitivity to murine or chimeric antibodies. Patients were required to provide written informed consent before enrollment. The study was conducted in compliance with good clinical practice guidelines and was approved by the Institutional Review Board from all centers participating in the study.
Study design and objectives
This was a phase II, single-arm, multi-institutional study using Simon's two-stage design [11]. Enrolled patients received weekly intravenous volociximab at a dose of 15mg/kg until progression or unacceptable toxicity. Tumor assessment via CT or MRI was performed after 8 weeks of volociximab treatment. The primary objective was to evaluate the efficacy of volociximab when administered at 15mg/kg qwk in patients with platinum-resistant, advanced epithelial ovarian cancer or primary peritoneal cancer. The secondary objectives were to evaluate the safety, tolerability, and pharmacokinetics (PK) of volociximab when administered at 15 mg/kg qwk.
Toxicity and response evaluation
Safety analyses were performed on the safety population, defined as all patients who received any part of an infusion of volociximab. Toxicity was classified and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE, Version 3.0). Efficacy was determined using RECIST [9]. Evaluable patients for response included those who had at least one on-study response assessment and received at least 4 infusions of volociximab or discontinued study treatment after fewer than 4 infusions for safety reasons, death, or lack of efficacy.
Pharmacokinetic assessment
Serum concentrations of volociximab were analyzed from patient samples drawn on Days 1, 15, 29, and 50 approximately 10 min before volociximab infusion and 1 h after volociximab infusion using colorimetric ELISA. The limit of quantitation of volociximab in human serum was 50 ng/mL.
Immunohistochemistry
Archived formalin-fixed, paraffin-embedded (FFPE) tumor tissue was obtained from 15 of the 16 patients enrolled. The α5 FFPE immunohistochemistry (IHC) was performed using a rabbit polyclonal anti-α5 reagent (clone H-104, Santa Cruz Biotechnology, Inc., Santa Cruz, CA; sc-10729). Deparaffinized FFPE tissue sections were subject to antigen-retrieval with EDTA and processed using an automated staining instrument (Dako, Carpinteria, CA). Binding of the primary anti-α5 antibody was detected with the HRP-labeled Envision-Dual Link system (Dako, Carpinteria, CA). Semi-quantitative assessment of expression of α5 integrin on malignant tumor cells was performed by a board-certified pathologist utilizing a composite H-score that encompasses both the intensity of staining and the frequency of cells that are positive for expression. The score was calculated by multiplying the intensity of the staining on a 0 to 3+ scale by the percentage of tumor cells exhibiting that level of staining, resulting in an H-score range from 0 to 300.
Whole blood circulating cellular biomarkers
Whole blood samples were collected at screening and on Day 1 prior to volociximab infusion (i.e., baseline samples) and on Days 8, 15, 29, and 50 (±2 days). Whole blood samples were analyzed for CTCs, CECs, and CEPCs. CTCs and CECs were enumerated using the Veridex CellSearch™ system in conjunction with the Circulating Tumor Cell IVD Kit or the Circulating Endothelial Cell Kit, respectively. CTCs were defined as DAPI+, CD45−, EpCAM+, and cytokeratins 8+ and/or 18+, and/or 19+. CTC results were reported as absolute CTC counts in 7.5 mL of whole blood. CECs were defined as being CD146+, DAPI+, CD105+, and CD45−. CEC results were reported as absolute CEC counts in 4.0 mL of whole blood. CEPCs were analyzed by flow cytometry using a Becton Dickinson FACSCaliber instrument. Cells were gated for CD34 moderately to highly positive. From this population, cells were plotted for CD133 versus VEGFR2. Cells that were CD34+, CD133+, and VEGFR2+, were reported as CEPCs. 7-AAD staining was also utilized to select only viable CEPCs for analysis. CEPC results were reported as percent of the total peripheral blood mononuclear cells in a sample.
Statistical considerations
The sample size was determined based on Simon's two-stage design [11]. A baseline response rate of 15% was chosen given prior studies in platinum-resistant ovarian cancer where response rates have ranged from 6.5 to 15.9% [12–14]. A 15% increase was sought over this baseline. In the language of Simon's optimal two-stage design, p0 = 15% and p1 = 30%, α = 0.10, and power = 80%. If at least ≥3/23 evaluable patients enrolled in Stage I achieved a partial response or complete response based on RECIST, then 33 additional evaluable patients would be enrolled in Stage 2. Efficacy and safety analyses, PK/PD, and exploratory studies were reported using descriptive statistics.
Results
Patient characteristics
Sixteen patients were enrolled from 9/2007 to 4/2008 (Table 1). All 16 patients received at least one volociximab infusion and were included in the safety analysis. Fifteen patients (94%) received 8 or fewer weekly infusions of volociximab. The median number of infusions was 7.5 (range, 1–16).
Table 1.
Patient characteristics (safety population N = 16).
| Characteristics | N |
|---|---|
| No. of patients | 16 |
| Median age, years (range) | 61 (54–80) |
| ECOG performance status | |
| 0 | 7 |
| 1 | 9 |
| Tumor type | |
| Epithelial ovarian | 12 |
| Primary peritoneal | 4 |
| Histological subtype | |
| Serous | 14 |
| Clear cell | 1 |
| Transitional cell | 1 |
| Stage of disease | |
| III | 9 |
| IV | 7 |
| No. of prior treatments, median (range) | 3 (2–4) |
ECOG, Eastern Cooperative Oncology Group.
Response evaluation
Fourteen patients were evaluable for response. Of the 2 remaining patients, 1 withdrew consent after 1 infusion and opted for supportive care; the other was removed from study by the treating physician after 2 weeks of treatment secondary to lack of efficacy. There were no complete or partial responses. Seven patients completed at least 1 cycle (8 weeks) of treatment. Six of these patients had progression of disease following the first cycle. One patient had stable disease and continued on therapy. She had progression of disease after 2 full cycles of therapy. Seven patients developed progression of disease prior to completing 8 weeks of therapy.
Of the 14 patients evaluable for response, 12 had baseline CA125 values >35 U/mL. Three of these patients, including the patient with stable disease at the end of cycle 1, had a decline in their CA125 during Week 4 of treatment. Of the remaining 2 patients, one patient's CA125 rapidly rose over the next 2 weeks to greater than the baseline value. The other patient did not have a repeat CA125 when coming off study for progression.
A review of the efficacy data for the first 13 evaluable patients revealed no responses. Under the alternative hypothesis (30% response rate), the probability of 0/13 responses in the first 13 evaluable patients was 0.01. Under the null hypothesis (15% response rate), the probability of observing 3 responses in the next 10 evaluable patients was 0.18. Biogen Idec and Facet Biotech decided to terminate the study as it was unlikely the efficacy criteria required to advance to Stage 2 of the study would be met.
Toxicity
All 16 patients were evaluable for safety. “Related AEs” were considered to be possibly related or related to study treatment. Twelve patients (75%) experienced at least one study-related AE (Table 2). The most common AEs were headache and fatigue, occurring in 4 patients each (25%). AEs were mild in most patients, with 9 (75%) of 12 having AEs ≤ grade 2.
Table 2.
Study-relateda adverse events (safety population N = 16).
| Event | Grade 1 |
Grade 2 |
Grade 3 |
Grade 4/5 |
n (%) |
|---|---|---|---|---|---|
| Abdominal discomfort | 1 | 1 (6) | |||
| Alopecia | 2 | 2 (13) | |||
| Anorexia | 1 | 1 | 2 (13) | ||
| Arthralgia, myalgia or musculoskeletal pain | 4 | 4 (25) | |||
| Candidiasis | 2 | 2 (13) | |||
| Chills | 1 | 1 (6) | |||
| Cough | 1 | 1 (6) | |||
| Dysgeusia | 1 | 1 (6) | |||
| Dyspnea | 1 | 1 (6) | |||
| Dysuria | 1 | 1 (6) | |||
| Fatigue | 2 | 2 | 4 (25) | ||
| Headache | 3 | 1 | 4 (25) | ||
| Hyponatremia | 1 | 1 (6) | |||
| Hypotension | 1 | 1 (6) | |||
| Insomnia | 1 | 1 (6) | |||
| Nausea | 1 | 2 | 3 (19) | ||
| Neuropathy | 1 | 1 (6) | |||
| Pruritus | 1 | 1 (6) | |||
| Pulmonary embolism | 1 | 1 (6) | |||
| Pyrexia | 2 | 2 (13) | |||
| Rash | 1 | 1 (6) | |||
| Respiratory failure | 1 (G 5) | 1 (6) | |||
| Reversible posterior leukoencephalopathy syndrome | 1 (G 4) | 1 (6) | |||
| Upper respiratory tract infection | 1 | 1 (6) | |||
| Vaginal bleeding | 1 | 1 (6) | |||
| Vomiting | 1 | 1 (6) |
G = grade.
An adverse event is considered study related if it is considered possibly related or related to volociximab.
Two patients experienced grade 3 related AEs: hyponatremia and pulmonary embolism (1 patient each). One patient experienced a grade 4 related AE of reversible posterior leukoencephalopathy syndrome. This patient had been treated with bevacizumab following the discontinuation of volociximab prior to the AE. One patient developed respiratory failure leading to death, which was initially reported as unlikely related to volociximab but was later listed as possibly related to volociximab.
Overall, 8 patients (50%) experienced AEs ≥ grade 3 (unrelated and related). Seven of these patients (44%) experienced a total of 19 grade 3 AEs, with 6 of these classified as serious AEs (SAEs): small intestinal obstruction (2 patients); and deep venous thrombosis, hyponatremia, pleural effusion, and pulmonary embolism (1 patient each). Two patients (12%) had grade 4 AEs: fatigue and reversible posterior leukoencephalopathy syndrome. Three patients died within 30 days of their last infusion of volociximab: 2 attributable to respiratory failure and 1 to disease progression.
Pharmacokinetics
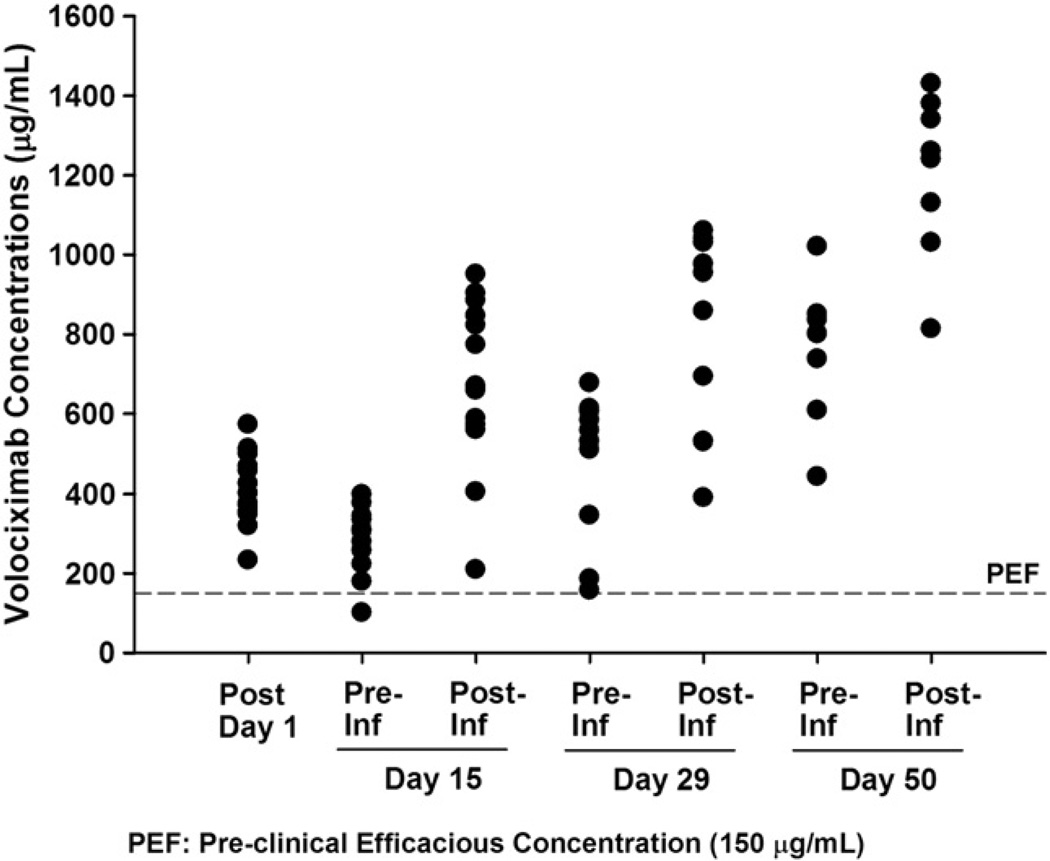
All patients had at least 1 measurable post-treatment serum concentration of volociximab. Serum concentrations accumulated after the first infusion, with trough concentrations continuing to increase through the fourth dose of treatment (Fig. 1). On Day 50, the mean observed trough concentration was 762 µg/mL and the post 1-h concentration was 1203 µg/mL (Table 3). Mean trough serum concentrations of volociximab were greater than 150 µg/mL throughout the treatment period. This concentration correlated with efficacy in preclinical xenograft studies. Only 1 patient had pre- and post-treatment ascites, sampled on cycle 1, Day 29. The concentration of volociximab in the post-treatment ascites sample was 83% of the corresponding serum sample (154.3 µg/mL in ascites, 186.0 µg/mL in serum).
Fig. 1.
Serum volociximab concentrations during treatment cycle 1 (PK population N = 16).
Table 3.
Serum volociximab concentrations (µg/mL) in treatment cycle 1 (PK population N = 16).
| Day | Infusion Day 1 | Infusion Day 15 | Infusion Day 29 | Infusion Day 50 | |||
|---|---|---|---|---|---|---|---|
| Post-dose | Pre | Post | Pre | Post | Pre | Post | |
| N | 16 | 13 | 13 | 10 | 10 | 8 | 8 |
| Mean ± SD (range) | 402 ± 84 (233–572) | 279 ± 88 (101–398) | 680 ± 215 (209–950) | 477 ± 183 (157–677) | 856 ± 238 (390–1060) | 762 ± 173 (443–1020) | 1203 ± 205 (813–1430) |
Immunohistochemistry
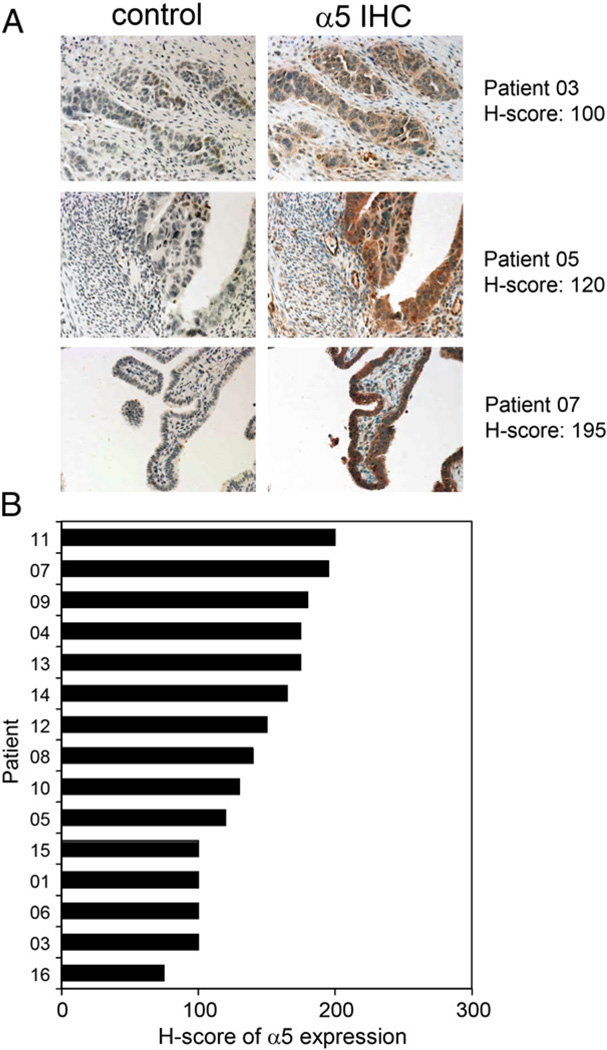
Archived tumor sections were obtained from 15/16 patients enrolled in the study. All 15 tumors exhibited low-to-moderate α5 integrin expression that was characterized by a predominantly diffuse or cytoplasmic pattern of staining (Fig. 2A), with occasional membrane-specific staining. Expression was evenly distributed among the 15 samples analyzed, with an H-score range from 75 to 200 and an observed mean H-score of 140 ± 40 (Fig. 2B). In the absence of objective clinical responses, assessment of potential correlations between α5 expression and clinical activity could not be made.
Fig. 2.
Analysis of α5 integrin expression on archived tumor tissue by immunohistochemical (IHC) staining. (A)Representative images from IHC staining of archived, formalin-fixed, paraffin-embedded ovarian carcinoma tissue sections demonstrate the range of staining intensity that was observed among all samples analyzed. The control represents IHC staining of serial sections using an isotype control reagent (rabbit IgG). (B) H-scores of α5 expression for all available tumor samples demonstrate an evenly distributed range of low-to-moderate expression (mean H-score 140 ± 40; range, 75–200).
Whole blood circulating cellular biomarkers
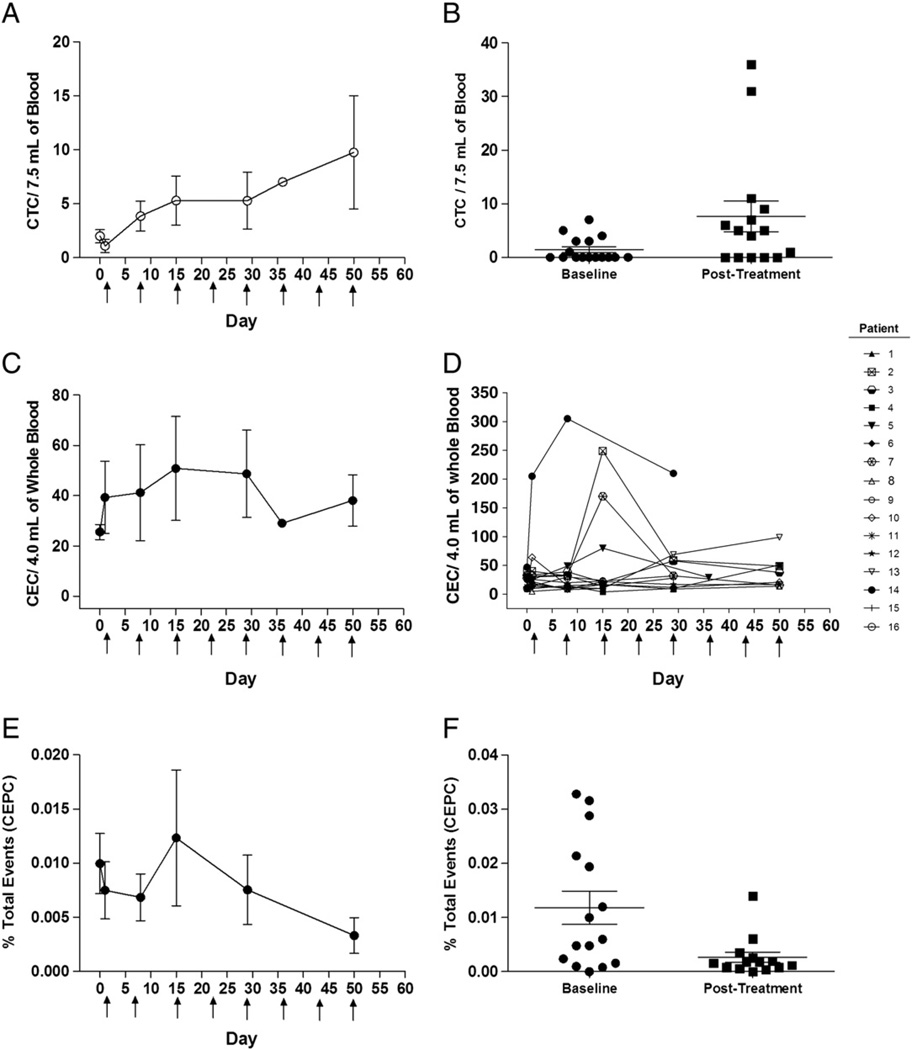
Nine of 16 patients had detectable CTC levels at baseline prior to receiving volociximab. The CTC values at baseline ranged from 0 to 7 CTC per 7.5 mL of whole blood (mean ± SD = 1.0 ± 2.2 CTC/7.5 mL). During the course of volociximab dosing, almost all patients (15/16) had detectable CTCs (Figs. 3A and B). Post-treatment CTC values ranged from 0–36 CTC per 7.5 mL of whole blood (mean ± SD = 9.8 ± 14.9 CTC/7.5 mL at Day 50). Post-treatment CTC values were higher than baseline values in most patients (p=0.0058).
Fig. 3.
Changes in circulating cellular biomarkers during treatment with volociximab. A significant increase in circulating tumor cells (CTCs) during the course of treatment with volociximab is seen with the mean values of CTCs for all patients (A) and the baseline compared to post-treatment sample (B). No significant change was seen in the mean values of circulating endothelial cells (CECs) during treatment (C). However, a transient increase in CECs was seen in some patients early in the treatment course (D). Circulating endothelial progenitor cells (CEPCs) did decline significantly over the course of treatment as shown in the mean values (E) in baseline and post-treatment samples (F).
All patients had detectable CECs at baseline, with a range of 9–205 CEC per 4.0 mL of whole blood (mean ± SD=39.3 ± 57.9 CEC/4.0 mL). Following volociximab, no obvious trend was observed collectively for all patients (Fig. 3C). However, 7 patients (44%) did show a transient increase in CEC levels after their first or second dose of volociximab (Fig. 3D).
Fifteen of 16 patients had detectable CEPCs at baseline prior to receiving volociximab. These samples had a range of 0.0004–0.0328% of total peripheral blood mononuclear cells (mean ± SD = 0.007 ± 0.009% total). Changes in CEPCs suggested a trend toward decreasing CEPCs following exposure to volociximab (Fig. 3E).When comparing baseline CEPC values with the values observed after longest duration of exposure to volociximab for each patient, a statistically significant (p = 0.02) decrease in CEPC frequency was observed (Fig. 3F).
Discussion
Advanced ovarian cancer carries a poor overall prognosis [15]. While platinum-based chemotherapy may be initially effective, responses to additional chemotherapy are limited when ovarian cancer recurs within 3–6 months after initial treatment [12,13,16–20]. Standard FDA-approved therapeutic options for patients who recur within 6 months of initial therapy include liposomal doxorubicin or topotecan. There are no approved therapies for use after the development of platinum resistance and progression following treatment with liposomal doxorubicin or topotecan.
Therapies targeting the tumor vasculature may be efficacious in this population. Initial phase II studies of the anti-angiogenic agent bevacizumab have shown promising response rates [14,21,22]. Volociximab is another anti-angiogenic agent blocking α5β1 integrin. Early human studies showed that volociximab monotherapy was well tolerated, as was the case in this study. In particular, the problematic side effects of hypertension, proteinuria, and bowel perforation previously reported with bevacizumab [14,21,22] were not seen in this study. One patient developed reversible posterior leukoencephalopathy syndrome, possibly related to bevacizumab. In the first 288 patients treated with volociximab across all studies, this is the only report of reversible posterior leukoencephalopathy syndrome. However, the elimination half-life of volociximab is estimated to be in excess of 21 days, so physician discretion is advised when considering giving bevacizumab following volociximab treatment.
Despite being well tolerated, volociximab was not efficacious in the present study. However, the patient population was challenging. Patients were required to be either platinum resistant or refractory and have had additional progression on either liposomal doxorubicin or topotecan. Alternative trial designs for patients with earlier stage disease or using volociximab in combination with cytotoxic chemotherapies would be beneficial to further explore the activity of volociximab in ovarian cancer. Alternative primary endpoints, such as progression-free survival, are also worthy of consideration when evaluating anti-angiogenic agents such as volociximab in the event that they stabilize disease but do not lead to tumor regression. Such designs have been used successfully with bevacizumab and anti-integrin therapies [22,23].
The correlative studies evaluating PK and circulating tumor and endothelial cells provide data to help guide future development of volociximab. The PK studies show that maintaining a concentration ≥150 µg/mL, which corresponds to pre-clinical activity, is achievable with this dosing regimen. Further evaluation of volociximab PK across early studies has shown that the half-life of the drug is on the order of 2–5 weeks, with models predicting an elimination half-life of 36 ± 19 days. At doses ≥ 10 mg/kg IV q2wk, the majority of α5β1 integrin is saturated (≤5% free) in monocytes [24]. Given these factors, maintaining sustained levels of volociximab ≥ 150 µg/mL is possible with q3wk dosing regimens using a 30 mg/kg or 20mg/kg dose with a Day 8 loading dose.
With regard to circulating cellular biomarkers, an increase in CTCs was observed in the majority of the patients. CTCs have been previously reported in ovarian cancer patients [25,26], and increased CTC numbers correlate with poorer prognoses as is consistent with the observations in this study. Whether the increase in CTCs had any relation to volociximab interference of tumor cell/peritoneal interactions is unknown. Tumor–peritoneal interactions were not assessed in this study, although analysis of ascites demonstrated accumulation of volociximab.
The present study provides the first report of CEC and CEPC levels in advanced ovarian cancer patients. CEPCs have been shown to play a critical role in tumor neovascularization and growth [27,28]. In preclinical studies, they contribute to the progression from micrometastases to lethal macrometastases and promote revascularization following acute cytotoxic therapy [29,30]. A subset of patients exhibited a transient increase in CECs on Day 8 or 15 after beginning treatment with volociximab. This may represent cells unable to integrate into the neovasculature secondary to volociximab blockade of the α5β1 integrin/fibronectin interaction. However, this change in CECs was not maintained throughout the study. The number of CEPCs declined over time on treatment with volociximab in this study, suggesting pharmacodynamic effects. This is similar to the decrease in CEPC levels and viability after treatment with the anti-angiogenic agent bevacizumab in other solid tumors [31].
The reduction in CEPCs during volociximab therapy was also seen in early studies in non-small cell lung cancer (NSCLC) and melanoma. In a phase Ib study of escalating doses of volociximab given with carboplatin and paclitaxel in Stage IIIB/IV NSCLC patients, 11/14 patients showed a reduction in CEPCs [32]. In the phase II study of volociximab monotherapy in metastatic melanoma [33], 8/13 patients had a reduction in CEPCs on treatment. This reduction of CEPCs may enhance the action of standard chemotherapeutics. In preclinical models, paclitaxel has been shown to rapidly induce CEPC mobilization. This effect appears to be chemotherapy specific, as several other chemotherapeutics including gemcitabine, cisplatin, and doxorubicin had no significant effect on CEPCs in animal models. Therapies preventing induction of CEPCs resulted in enhanced anti-tumor effects of paclitaxel [34]. Thus, volociximab may potentiate paclitaxel activity. Combination approaches using volociximab with paclitaxel-based regimens are worthy of consideration in ovarian cancer. Additional approaches using volociximab in the setting of low-volume residual disease following tumor debulking surgery or as a maintenance therapy to prevent recurrence of ovarian cancer would also be of interest. Blockade of α5β1 integrin function earlier in the course of disease may have a greater clinical impact on initial peritoneal seeding and tumor angiogenesis.
Acknowledgments
We would like to thank all participating 206OC201 study investigators, research staff, and patients. Biogen Idec would like to thank their development partner, Facet Biotech, for all their contributions to the program.
Steffan N. Ho: Employed by Biogen Idec.
Richard T. Penson: Research funds from PDL Biopharma Inc.
Ernst Lengyel: Received funding from PDL for experiments with the antibody. (Cancer Res. 2008 Apr 1;68(7):2329–39.)
Rameshraja Palaparthy: Employed by Biogen Idec. Owns stock of Biogen Idec.
Kye Gilder: Employed by Biogen Idec. Owns stock of Biogen Idec.
Artemios Vassos: Employed by Biogen Idec.
William McAuliffe: Employed by Biogen Idec.
Sara Weymer: Employed by Biogen Idec.
Jeremy Barton: Employed by Biogen Idec.
Footnotes
Conflict of interest statement
Katherine M. Bell-McGuinn: None.
Carolyn M. Matthews: None.
Minal Barve: None.
Lucy Gilbert: None.
Russell J. Schilder: None.
References
- 1.Francis SE, Goh KL, Hodivala-Dilke K, et al. Central roles of alpha5beta1 integrin and fibronectin in vascular development in mouse embryos and embryoid bodies. Arterioscler Thromb Vasc Biol. 2002;22:927–933. doi: 10.1161/01.atv.0000016045.93313.f2. [DOI] [PubMed] [Google Scholar]
- 2.Robinson EE, Foty RA, Corbett SA. Fibronectin matrix assembly regulates alpha5beta1-mediated cell cohesion. Mol Biol Cell. 2004;15:973–981. doi: 10.1091/mbc.E03-07-0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim S, Bakre M, Yin H, Varner JA. Inhibition of endothelial cell survival and angiogenesis by protein kinase A. J Clin Invest. 2002;110:933–941. doi: 10.1172/JCI14268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramakrishnan V, Bhaskar V, Law DA, et al. Preclinical evaluation of an anti-alpha5beta1 integrin antibody as a novel anti-angiogenic agent. J Exp Ther Oncol. 2006;5:273–286. [PubMed] [Google Scholar]
- 5.Markowska J, Szala S. Inhibitors of angiogenesis in therapy of ovarian cancers. Eur J Gynaecol Oncol. 2004;25:562–567. [PubMed] [Google Scholar]
- 6.Sawada K, Mitra AK, Radjabi AR, et al. Loss of E-cadherin promotes ovarian cancer metastasis via α5-integrin, which is a therapeutic target. Cancer Res. 2008;68:2329–2339. doi: 10.1158/0008-5472.CAN-07-5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gardner MJ, Jones LM, Catterall JB, Turner GA. Expression of cell adhesion molecules on ovarian tumour cell lines and mesothelial cells, in relation to ovarian cancer metastasis. Cancer Lett. 1995;91:229–234. doi: 10.1016/0304-3835(95)03743-g. [DOI] [PubMed] [Google Scholar]
- 8.Ricart AD, Tolcher AW, Liu G, et al. Volociximab, a chimeric monoclonal antibody that specifically binds alpha5beta1 integrin: a phase I, pharmacokinetic, and biological correlative study. Clin Can Res. 2008;14:7924–7929. doi: 10.1158/1078-0432.CCR-08-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Therasse P, Arbuck S, Eisenhauer E, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 10.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 11.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 12.ten Bokkel Huinink W, Gore M, Carmichael J, et al. Topotecan versus paclitaxel for the treatment of recurrent epithelial ovarian cancer. J Clin Oncol. 1997;15:2183–2193. doi: 10.1200/JCO.1997.15.6.2183. [DOI] [PubMed] [Google Scholar]
- 13.Gordon AN, Fleagle JT, Guthrie D, et al. Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol. 2001;19:3312–3322. doi: 10.1200/JCO.2001.19.14.3312. [DOI] [PubMed] [Google Scholar]
- 14.Cannistra SA, Matulonis UA, Penson RT, et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J Clin Oncol. 2007;25:5180–5186. doi: 10.1200/JCO.2007.12.0782. [DOI] [PubMed] [Google Scholar]
- 15.Bhoola S, Hoskins WJ. Diagnosis and management of epithelial ovarian cancer. Obstet Gynecol. 2006;107:1399–1410. doi: 10.1097/01.AOG.0000220516.34053.48. [DOI] [PubMed] [Google Scholar]
- 16.Blackledge G, Lawton F, Redman C, Kelly K. Response of patients in phase II studies of chemotherapy in ovarian cancer: implications for patient treatment and the design of phase II trials. Br J Cancer. 1989;59:650–653. doi: 10.1038/bjc.1989.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gershenson DM, Kavanagh JJ, Copeland LJ, Stringer CA, Morris M, Wharton JT. Retreatment of patients with recurrent epithelial ovarian cancer with cisplatin based therapy. Obstet Gynecol. 1989;73:798–802. [PubMed] [Google Scholar]
- 18.Gore ME, Fryatt I, Wiltshaw E, Dawson T. Treatment of relapsed carcinoma of the ovary with cisplatin or carboplatin following initial treatment with these compounds. Gynecol Oncol. 1990;36:207–211. doi: 10.1016/0090-8258(90)90174-j. [DOI] [PubMed] [Google Scholar]
- 19.Markman M, Rothman R, Hakes T, et al. Second-line platinum therapy in patients with ovarian cancer previously treated with cisplatin. J Clin Oncol. 1991;9:389–393. doi: 10.1200/JCO.1991.9.3.389. [DOI] [PubMed] [Google Scholar]
- 20.Petru E, Angleitner-Boubenizek L, Reinthaller A, et al. Combined PEG liposomal doxorubicin and gemcitabine are active and have acceptable toxicity in patients with platinum-refractory and -resistant ovarian cancer after previous platinum–taxane therapy: a phase II Austrian AGO study. Gynecol Oncol. 2006;102:226–229. doi: 10.1016/j.ygyno.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 21.Garcia AA, HIrte H, Fleming G, et al. Phase II clinical trial of bevacizumab and low-dose metronomic oral cyclophosphamide in recurrent ovarian cancer: a trial of the California, Chicago, and Princess Margaret Hospital phase II consortia. J Clin Oncol. 2008;26:76–82. doi: 10.1200/JCO.2007.12.1939. [DOI] [PubMed] [Google Scholar]
- 22.Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2007;25:5165–5171. doi: 10.1200/JCO.2007.11.5345. [DOI] [PubMed] [Google Scholar]
- 23.Stupp R, Goldbrunner R, Neyns B, et al. Phase I/IIa trial of cilengitide (EMD121974) and temozolomide with concomitant radiotherapy, followed by temozolomide and cilengitide maintenance therapy in patients with newly diagnosed glioblastoma. J Clin Oncol. 2007:25. doi: 10.1200/JCO.2009.26.6650. abstr 2000. [DOI] [PubMed] [Google Scholar]
- 24.Ng CM, Bai S, Takimoto CH, Tang MT, Tolcher AW. Mechanism-based receptor-binding model to describe the pharmacokinetic and pharmacodynamic of an anti-α5β1 integrin monoclonal antibody (volociximab) in cancer patients. Cancer Chemother Pharmacol. 2010;65:207–217. doi: 10.1007/s00280-009-1023-8. [DOI] [PubMed] [Google Scholar]
- 25.Fan T, Zhao Q, Chen JJ, Chen WT, Pearl ML. Clinical significance of circulating tumor cells detected by an invasion assay in peripheral blood of patients with ovarian cancer. Gynecol Oncol. 2009;112:185–191. doi: 10.1016/j.ygyno.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He W, Kularatne SA, Kalli KR, et al. Quantitation of circulating tumor cells in blood samples from ovarian and prostate cancer patients using tumor-specific fluorescent ligands. Int J Cancer. 2008;123:1968–1973. doi: 10.1002/ijc.23717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyden D, Hattori K, Dias S, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 28.Bertolini F, Mancuso P, Braidotti P, Shaked Y, Kerbel RS. The multiple personality disorder phenotype(s) of circulating endothelial cells in cancer. Biochem Bipphys Acta. 2009;1796:27–32. doi: 10.1016/j.bbcan.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319:1995–1998. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- 30.Shaked Y, Ciarrocchi A, Franco M, et al. Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science. 2006;22:1785–1787. doi: 10.1126/science.1127592. [DOI] [PubMed] [Google Scholar]
- 31.Willett CG, Boucher Y, di Tomaso E, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Besse B, Almokadem S, Planchard D, et al. Safety and early efficacy results from a phase I study of volociximab (V) in combination with carboplatin (C) and paclitaxel (P) in patients (pts) with advanced non small cell lung cancer (NSCLC) J Clin Oncol. 2009:27. abstr 13513. [Google Scholar]
- 33.Stephenson JJ, Cranmer L, Hodi S, et al. A multi-center phase II study of volociximab in patients with relapsed metastatic melanoma. J Clin Oncol. 2008:26. abstr 9051. [Google Scholar]
- 34.Shaked Y, Henke E, Roodhart JML, et al. Rapid chemotherapy-induced acute endothelial progenitor cell mobilization: implications for antiangiogenic drugs as chemosensitizing agents. Cancer Cell. 2008;14:263–273. doi: 10.1016/j.ccr.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]