Abstract
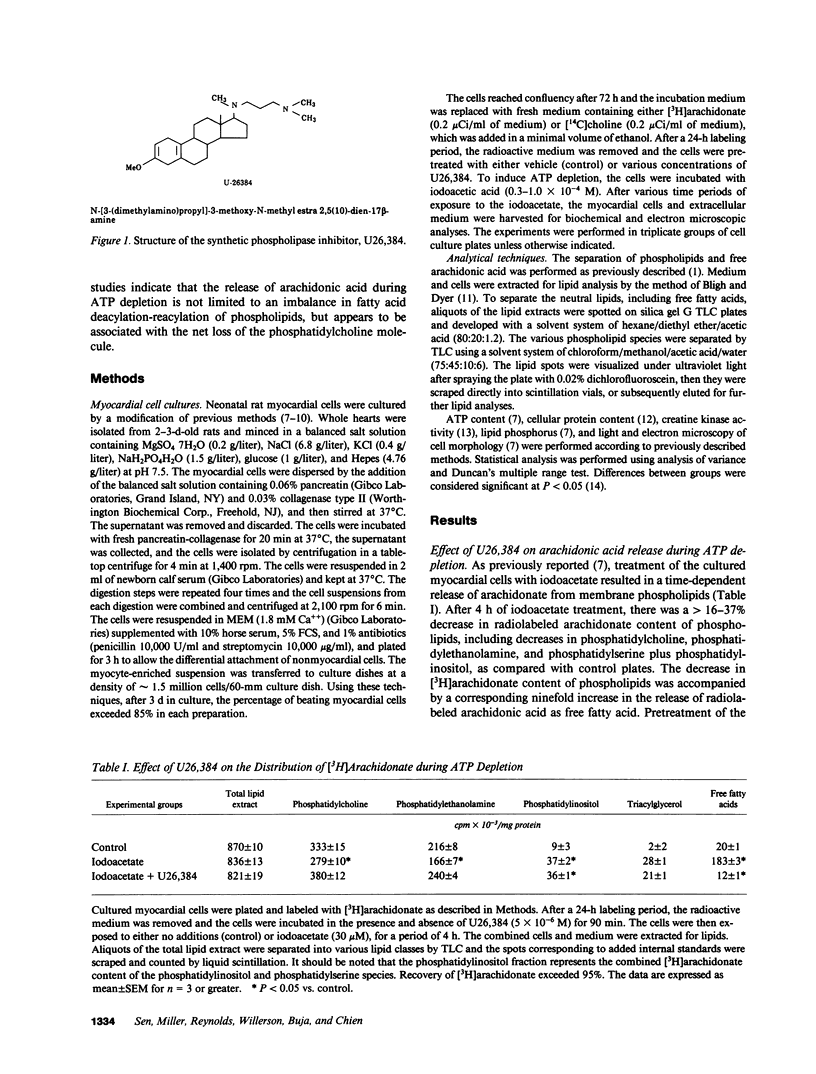
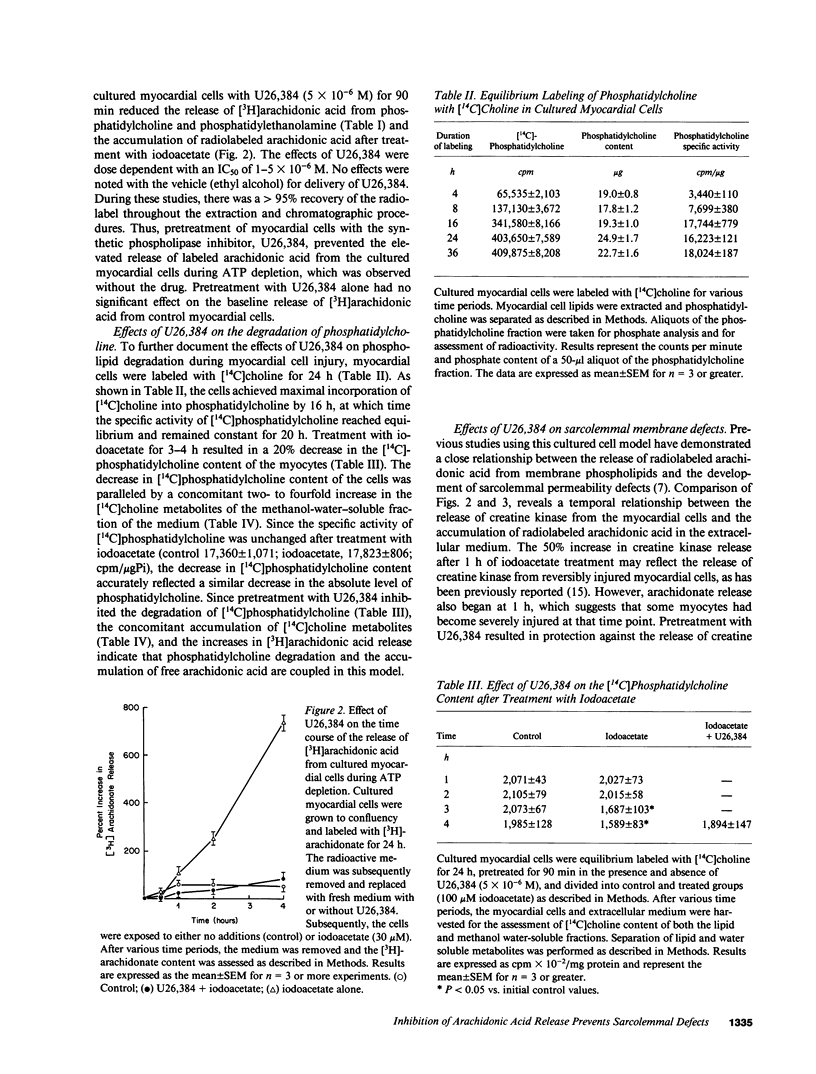
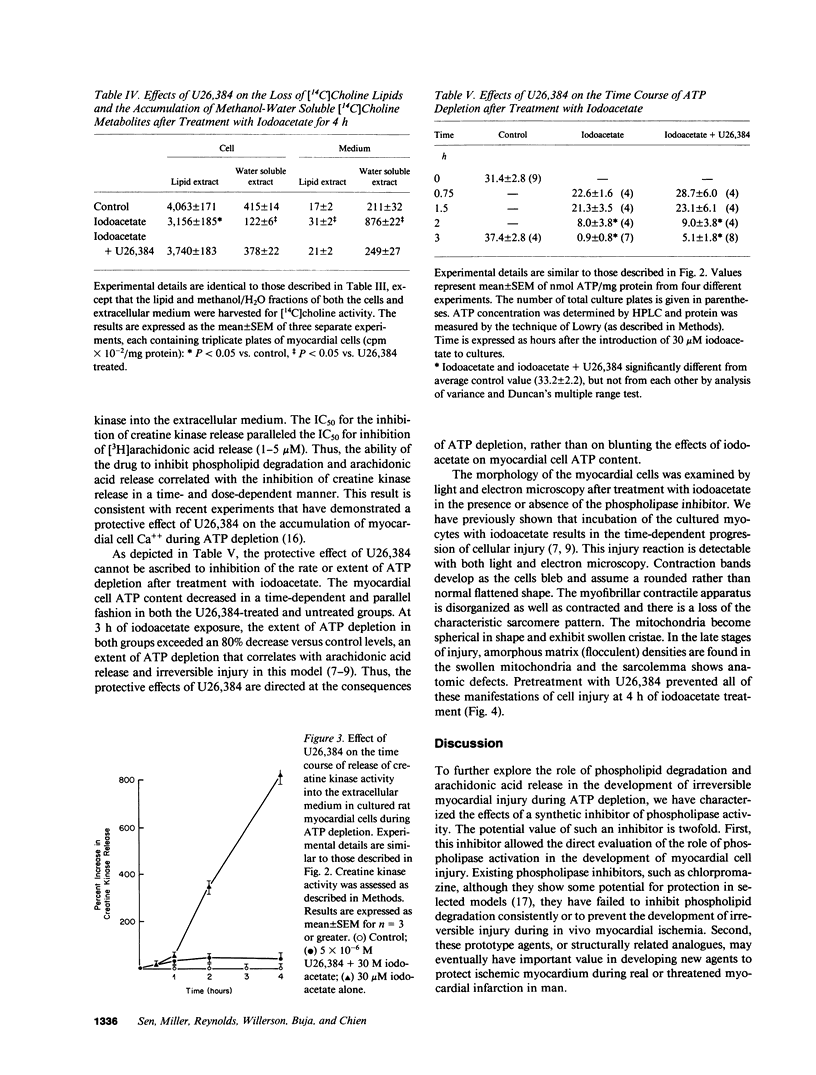
Previous studies have suggested that phospholipid degradation is closely associated with the development of sarcolemmal membrane injury. This study was initiated to characterize the effects of synthetic inhibitors of phospholipase activities using a cultured myocardial cell model in which arachidonic acid is liberated after treatment with the metabolic inhibitor, iodoacetate. Pretreatment with a steroidal diamine (U26,384) blocked the degradation of labeled phosphatidylcholine and the release of arachidonic acid in cultured myocardial cells during ATP depletion. Inhibition of phospholipid degradation by U26,384 prevented the development of sarcolemmal membrane defects and the release of creatine kinase from the cultured myocardial cells during ATP depletion. Pretreatment with U26,384 had no significant effect on the extent of ATP depletion after iodoacetate treatment, which indicates that the activity of this compound could not be simply ascribed to a sparing effect on ATP concentration. These results support the hypothesis that the development of sarcolemmal membrane injury and the associated loss of cell viability are causally related to progressive phospholipid degradation. In addition, these studies indicate that the release of arachidonic acid during ATP depletion is associated with the net loss of the phosphatidylcholine molecule.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Beaudry G. A., King L., Daniel L. W., Waite M. Stimulation of deacylation in Madin-Darby canine kidney cells. Specificity of deacylation and prostaglandin production in 12-O-tetradecanoylphorbol-13-acetate-treated cells. J Biol Chem. 1982 Sep 25;257(18):10973–10977. [PubMed] [Google Scholar]
- Buja L. M., Hagler H. K., Parsons D., Chien K., Reynolds R. C., Willerson J. T. Alterations of ultrastructure and elemental composition in cultured neonatal rat cardiac myocytes after metabolic inhibition with iodoacetic acid. Lab Invest. 1985 Oct;53(4):397–412. [PubMed] [Google Scholar]
- Burton K. P., Hagler H. K., Templeton G. H., Willerson J. T., Buja L. M. Lanthanum probe studies of cellular pathophysiology induced by hypoxia in isolated cardiac muscle. J Clin Invest. 1977 Dec;60(6):1289–1302. doi: 10.1172/JCI108888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien K. R., Abrams J., Pfau R. G., Farber J. L. Prevention by chlorpromazine of ischemic liver cell death. Am J Pathol. 1977 Sep;88(3):539–557. [PMC free article] [PubMed] [Google Scholar]
- Chien K. R., Abrams J., Serroni A., Martin J. T., Farber J. L. Accelerated phospholipid degradation and associated membrane dysfunction in irreversible, ischemic liver cell injury. J Biol Chem. 1978 Jul 10;253(13):4809–4817. [PubMed] [Google Scholar]
- Chien K. R., Crie J. S., Decker R. S., Wildenthal K. Influence of chlorpromazine on lysosomal alterations during myocardial ischaemia. Cardiovasc Res. 1983 Jul;17(7):407–414. doi: 10.1093/cvr/17.7.407. [DOI] [PubMed] [Google Scholar]
- Chien K. R., Han A., Sen A., Buja L. M., Willerson J. T. Accumulation of unesterified arachidonic acid in ischemic canine myocardium. Relationship to a phosphatidylcholine deacylation-reacylation cycle and the depletion of membrane phospholipids. Circ Res. 1984 Mar;54(3):313–322. doi: 10.1161/01.res.54.3.313. [DOI] [PubMed] [Google Scholar]
- Chien K. R., Reeves J. P., Buja L. M., Bonte F., Parkey R. W., Willerson J. T. Phospholipid alterations in canine ischemic myocardium. Temporal and topographical correlations with Tc-99m-PPi accumulation and an in vitro sarcolemmal Ca2+ permeability defect. Circ Res. 1981 May;48(5):711–719. doi: 10.1161/01.res.48.5.711. [DOI] [PubMed] [Google Scholar]
- Chien K. R., Sen A., Reynolds R., Chang A., Kim Y., Gunn M. D., Buja L. M., Willerson J. T. Release of arachidonate from membrane phospholipids in cultured neonatal rat myocardial cells during adenosine triphosphate depletion. Correlation with the progression of cell injury. J Clin Invest. 1985 Jun;75(6):1770–1780. doi: 10.1172/JCI111889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corr P. B., Gross R. W., Sobel B. E. Amphipathic metabolites and membrane dysfunction in ischemic myocardium. Circ Res. 1984 Aug;55(2):135–154. doi: 10.1161/01.res.55.2.135. [DOI] [PubMed] [Google Scholar]
- Gross R. W., Sobel B. E. Lysophosphatidylcholine metabolism in the rabbit heart. Characterization of metabolic pathways and partial purification of myocardial lysophospholipase-transacylase. J Biol Chem. 1982 Jun 25;257(12):6702–6708. [PubMed] [Google Scholar]
- HERDSON P. B., SOMMERS H. M., JENNINGS R. B. A COMPARATIVE STUDY OF THE FINE STRUCTURE OF NORMAL AND ISCHEMIC DOG MYOCARDIUM WITH SPECIAL REFERENCE TO EARLY CHANGES FOLLOWING TEMPORARY OCCLUSION OF A CORONARY ARTERY. Am J Pathol. 1965 Mar;46:367–386. [PMC free article] [PubMed] [Google Scholar]
- Heyndrickx G. R., Amano J., Kenna T., Fallon J. T., Patrick T. A., Manders W. T., Rogers G. G., Rosendorff C., Vatner S. F. Creatine kinase release not associated with myocardial necrosis after short periods of coronary artery occlusion in conscious baboons. J Am Coll Cardiol. 1985 Dec;6(6):1299–1303. doi: 10.1016/s0735-1097(85)80216-3. [DOI] [PubMed] [Google Scholar]
- Higgins T. J., Allsopp D., Bailey P. J. The effect of extracellular calcium concentration and Ca-antagonist drugs on enzyme release and lactate production by anoxic heart cell cultures. J Mol Cell Cardiol. 1980 Sep;12(9):909–927. doi: 10.1016/0022-2828(80)90059-0. [DOI] [PubMed] [Google Scholar]
- Hostetler K. Y., Hall L. B. Phospholipase C activity of rat tissues. Biochem Biophys Res Commun. 1980 Sep 16;96(1):388–393. doi: 10.1016/0006-291x(80)91227-9. [DOI] [PubMed] [Google Scholar]
- Hsueh W., Isakson P. C., Needleman P. Hormone selective lipase activation in the isolated rabbit heart. Prostaglandins. 1977 Jun;13(6):1073–1091. doi: 10.1016/0090-6980(77)90135-6. [DOI] [PubMed] [Google Scholar]
- Irvine R. F. How is the level of free arachidonic acid controlled in mammalian cells? Biochem J. 1982 Apr 15;204(1):3–16. doi: 10.1042/bj2040003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee H. R., Henderson S. A., Reynolds R., Dunnmon P., Yuan D., Chien K. R. Alpha 1-adrenergic stimulation of cardiac gene transcription in neonatal rat myocardial cells. Effects on myosin light chain-2 gene expression. J Biol Chem. 1988 May 25;263(15):7352–7358. [PubMed] [Google Scholar]
- Nalbone G., Hostetler K. Y. Subcellular localization of the phospholipases A of rat heart: evidence for a cytosolic phospholipase A1. J Lipid Res. 1985 Jan;26(1):104–114. [PubMed] [Google Scholar]
- van der Vusse G. J., Roemen T. H., Prinzen F. W., Coumans W. A., Reneman R. S. Uptake and tissue content of fatty acids in dog myocardium under normoxic and ischemic conditions. Circ Res. 1982 Apr;50(4):538–546. doi: 10.1161/01.res.50.4.538. [DOI] [PubMed] [Google Scholar]




