Abstract
Introduction
Regenerative endodontic protocols recommend White Mineral Trioxide Aggregate (WMTA) as a capping material due to its osteoinductive properties. Stem Cells from the Apical Papilla (SCAP) are presumed to be involved in this regenerative process, but the effects of WMTA on SCAP are largely unknown. Our hypothesis is that WMTA induces proliferation and migration of SCAP.
Methods
Here, we used an unsorted population of SCAP (passages 3 to 5) characterized by high CD24, CD146 and Stro-1 expression. The effect of WMTA on SCAP migration was assessed using transwells and its effect on proliferation was determined by the WST-1 assay. Fetal bovine serum (FBS) and calcium-chloride enriched media were used as positive controls.
Results
The SCAP analyzed here showed a low percentage of STRO-1+ and CD24+ cells. Both set and unset WMTA significantly increased the short-term migration of SCAP after 6 hours (P<0.05), whereas calcium-chloride enriched medium did after 24 hours of exposure. Set WMTA significantly increased proliferation on days 1 to 5 while calcium-enriched media showed a significant increase on day 7 with a significant reduction on proliferation afterwards. SCAP migration and proliferation were significantly and steadily induced by the presence of 2% and 10% FBS
Conclusions
Collectively, these data demonstrate that WMTA induced an early short-term migration and proliferation of a mixed population of stem cells from apical papilla as compared to a later and longer-term induction by calcium-chloride or FBS.
Keywords: MTA, SCAP, stem cells, chemotaxis, dental, calcium
Introduction
Interest in regenerative endodontics has increased substantially in recent years encouraged by successful clinical case reports of treated immature necrotic teeth (1–3). A number of these cases have shown that following disinfection with triple antibiotic paste or calcium hydroxide, the use of mineral trioxide aggregate (MTA) as capping material over an induced blood clot will continue root development (2–4). Lovelace and colleagues have shown that the induction of bleeding at the apex results on a higher concentration of mesenchymal stem cells (MSCs) than is found in the systemic circulation (1). While the exact source of these stem cells is as yet unknown, the apical papilla is the likely origin (1). The understanding of the effects of MTA on the stem cells from the apical papilla is critically important to optimizing clinical regenerative endodontics protocols.
Stem Cells from the Apical Papilla (SCAP) have been isolated from immature permanent human teeth during root formation (5, 6). The apical papilla appears during the early stages of tooth development and plays an important role moving apically to allow continued formation of the radicular dentin and dental pulp (7). SCAP are an excellent source of cells for regeneration of the pulpo-dentin complex (8). Studies by Sonoyama et al (5) have shown that SCAP proliferate 2–3 times faster than other pulp cells and they are as potent as MSCs for both osteogenic and odontogenic differentiation (5, 6). Recent studies have shown that MSCs and human dental pulp cells react favorably to the presence of some dental materials such MTA (9–13).
The main components of unset white MTA (WMTA) are tricalcium silicate, dicalcium silicate, tricalcium aluminate, bismuth oxide, and calcium sulphate dehydrate. The composition of gray MTA is similar, except that it contains tetracalcium aluminoferrite (14,15). The set composition of MTA is mostly calcium silicate hydrate (C-S-H) and calcium hydroxide (Ca(OH)2). A substantial release of calcium ions results from the dissociation of Ca(OH)2 and the decomposition of the C-S-H (14, 16, 17). The start of Ca(OH)2 formation is greatest at 4 to 24 hours with the highest total concentration occurring after 7 days. The formation of Ca(OH)2 begins to decline at day 30 but continues over 5 weeks (14, 17, 18). MTA has excellent biocompatibility and has been used in root end fillings, perforation repair, pulp capping, and apexification procedures (9, 14, 19). Calcium ions have been shown to be potent signaling molecules and to play a role in the regulation of most cellular activities (20–26). The migration of mesenchymal stem cells, bone marrow-derived progenitor cells, and tumor cells are affected by calcium ion concentrations (21–23). Human dental pulp cells increase proliferation when exposed to media containing MTA and media containing exogenous calcium ions (20). The mineralization potential of human periodontal ligament cells and adipose-derived stem cells is promoted with increased calcium ion concentrations (24, 25). The differentiation of pulpal cells into odontoblasts may also be influenced by the levels of calcium ions present in the surrounding environment (26). The ability of calcium ions to influence cell migration and proliferation makes it logical to explore its role element in regenerative procedures.
In a mixed population of pulp cells MTA stimulates hard tissue formation and induces migration, proliferation, and differentiation into odontoblast-like cells (9, 10, 12). WMTA increases gene expression in human pulp cells exposed for 72 hours with the up-regulation of 109 genes and down-regulation of 69 genes (11). Seo, et at (27) have recently shown up-regulation of genes related to migration in dental pulp stem cells from permanent teeth. Although, how MTA activates pulp cells remains unclear, the high calcium ion release from MTA suggests that calcium initiates the biological response (20). The purpose of this study was to determine if WMTA plays a role in the migration and proliferation of a mixed population of SCAP and to determine whether calcium chloride or fetal bovine serum have the same effect.
Materials and Methods
Cell Culture
The SCAP used for all experiments were provided by Dr. Songtao Shi (University of Southern California, USC, Los Angeles, USA). These human cells were obtained and experiments carried out with the approval of the USC Institutional Review Board. Cells were cultured in minimal essential medium alpha Eagle (α-MEM; Invitrogen, Carlsbad, CA, USA). This medium was used for all the experimental conditions as in its plain form or supplemented with 2% or 10% FBS, 250 g/ml L-glutamine, and 1% penicillin/streptomycin (Gibco, Grand Island, NY)and cells incubated at 37°C in 5% CO2 and 100% humidity. Cells used were between passages 3 and 5.
Flow Cytometric Analysis
SCAP from passages 2, 4 and 10 were evaluated by Flow Cytometry to characterize the cell population at different passages. 5×105 cells were collected and antibody staining performed in the dark with human CD146-APC antibodies (Milenyi Biotec, Auburn, CA, USA), PE mouse antihuman CD24 antibodies (BD Pharmingen, San Jose, CA, USA), and mouse anti-STRO-1 primary antibodies (Invitrogen Corp., Carlsbad, CA) with FITC rabbit anti-mouse IgG conjugate antibodies (Invitrogen). Controls of untreated cells and cells stained with either the primary STRO-1 or conjugate FITC antibody were used. Cells were incubated in triplicates in the dark on ice for 30 minutes. 4 independent experiments were run. The samples were read on a BD FACSAria3 Flow Cytometry machine using the BD FACSDiva software (BD Falcon, San Jose, CA, USA) at 10,000 events.
MTA Preparation
White MTA (ProRoot, Dentsply Endodontics, Tulsa, OK, USA) was mixed following the manufacturer’s instruction. WMTA pellets of 10 mg were form using a ratio of 100 mg of WMTA powder and 35µl of sterile water under a laminar flow hood in a sterile dappen dish (Keystone Industries, Cherry Hill, NJ, USA) for approximately 1 minute. Pellets were formed using a Lee MTA pellet forming block (G. Hartzell & Son, Concord, CA, USA). The pellets were allowed to set for 1 hour or 24 hours at 37°C in 5% CO2 and 100% humidity under sterile conditions.
Calcium Enriched Media
The calcium-enriched media was prepared with anhydrous CaCl2 (1M) dissolved in minimal essential plain medium alpha Eagle (α-MEM; Invitrogen). Serial dilutions of 3.0mmol, 0.3mmol, and 0.03mmol CaCl2 were made.
Trans-well Migration Assay
SCAP migration was assessed using a trans-well migration assay after the cells were prestained with Cell Tracker Green (Invitrogen). 1×105 cells in 250µl of plain media were seeded into the upper chambers of trans-well 8-µm pore FluoroBlok of 24 multi-well system membrane filters (BD Falcon) and allowed to attach for 4 hours before exposure to test conditions. The lower chambers of the 24 multi-well system contained the following test conditions: plain α- MEM, plain α-MEM with 1 hour set WMTA or 24 hour set WMTA, plain α-MEM with 3.0mmol CaCl2 or 0.3mmol CaCl2 or 0.03mmol CaCl2. 2% FBS α-MEM with 1 hour set WMTA or 24 hour set WMTA, , 2% FBS α-MEM or 10% FBS α-MEM. One pellet of WMTA was placed into each well of the test groups. Cells were cultured at 37°C in 5% CO2 and 100% humidity. Cells that migrated to the bottom of the FluoroBlok inserts were read by fluorescence at 485/585 nm using a microplate reader (Genius; Tecan, Grödig, Austria) at 0.5, 1, 3, 6, 12, 24, 48, and 72 hours.
WST-1 Proliferation Assay
Proliferation was evaluated by WST-1 proliferation assay. 4×103 SCAP were seeded into 12 well companion plates (BD Falcon) with 1.5ml of α-MEM in the same test conditions as used for the migration assay. A 3-µm-pore size trans-well cell culture insert (BD Falcon) with two pellets of either 1 or 24 hours set WMTA were used. The plates were incubated at 37°C in 5% CO2 and 100% humidity with the media changed every 2–3 days. The plates were evaluated at 1, 3, 5, 7, 9, 11 and 14 days with WST-1 reagent (Roche, Mannheim, Germany). WST-1 reagent and media in a 1:10 ratio was added to each well in culture plate and incubated at 37°C in 5% CO2 and 100% humidity for 1 hour. The supernatants were then transferred to a 96 well plate (BD Falcon, San Jose, CA) and the absorbance (450mm–685mm) was determined in a microplate reader (Genius; Tecan).
Statistical Analysis
Four independent experiments were run in triplicates for each condition. The Statistical analysis was completed with the support of the University of Michigan’s Center for Statistical Consultation and Research (CSCAR). All data was analyzed using one-way ANOVA with a Bonferroni comparison and a P value less than or equal to 0.05 as statistically significant. The analysis was carried out using SigmaStat 2.0 Software (Systat Software, San Jose, CA, USA).
Results
SCAP morphology was not affected by the presence of WMTA
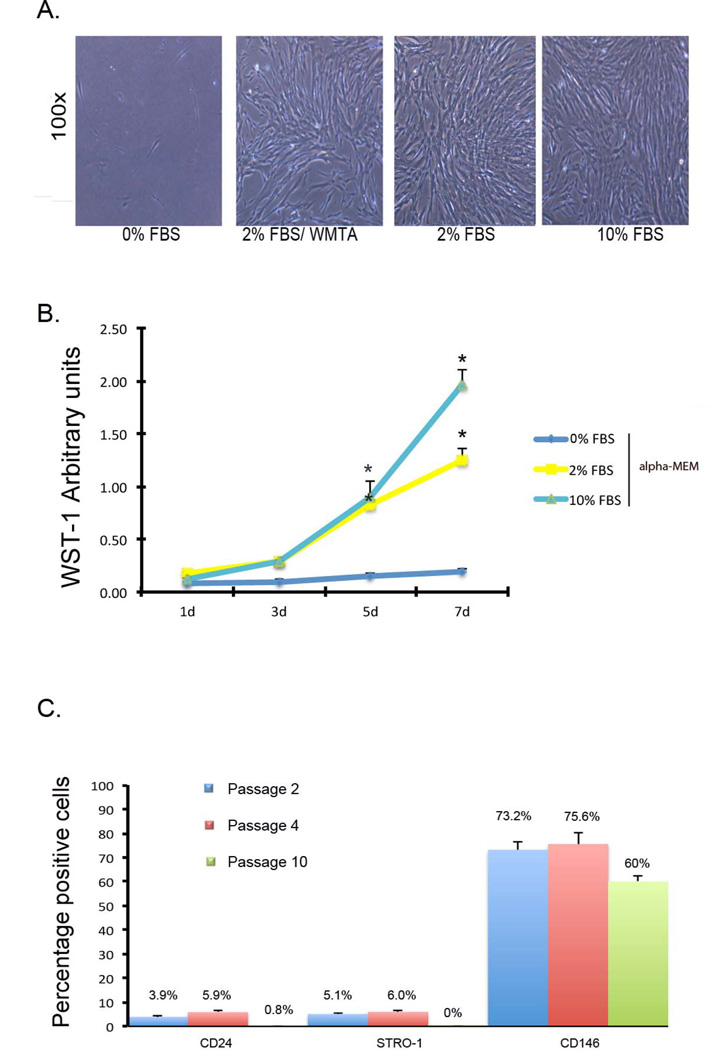
To select the conditions used for each test, the SCAP were cultured in plain α-MEM, 2% or 10% FBS α-MEM or 2% FBS α-MEM with WMTA. After 7 days, SCAP grown in plain α-MEM survived but did not show the confluency seen in SCAP grown in α-MEM with 2% FBS with or without WMTA or 10% FBS (Fig. 1A). SCAP grown with WMTA in α-MEM with 2% FBS showed similar morphological features as those cells grown in α-MEM with 2% or 10% FBS (Fig. 1A). A significant effect on SCAP proliferation was found by WST-1 assay after one week in culture with α-MEM and FBS at 2% or 10% as compared with plain α-MEM (Fig. 1B).
Figure 1. Morphology, proliferation and characterization of SCAP.
(A) Microphotographs showing SCAP at passage 4 after 7 days of culture in plain α-MEM, 2% FBS α-MEM plus 24 h set WMTA, 2% and 10% FBS α-MEM. Phase contrast microphotographs at 100X. FBS induces proliferation. (B) 2% and 10% FBS had a significant effect on SCAP proliferation as analyzed by WST-1. Stem cell markers decrease on SCAP after passage 4. (C) Flow-cytometry data expressed in percentage of the total cell population expressing CD24, CD146, and STRO-1 markers at passage 2, 4, and 10 in culture with 10% FBS α-MEM.
SCAP loose stemness by passage 10
To characterize the SCAP used in the experiments the expression of putative stem cell markers were evaluated by flow cytometry. SCAP were cultured in 10% FBS α-MEM up to 10 passages and evaluation of CD24, STRO-1 and CD146 positive cells were done at passages 2, 4 and 10. The percentage of CD24+ cells at passage 2 was 3.9%, at passage 4 was 5.9%, and decreased to 0% at passage 10. Only 5.1% of the cell population was STRO-1+ at passage 2, 6% at passage 4 and decreased to 0.8% at passage 10. At Passage 2, 73.2% of the cells were CD146+, 75.6% at passage 4 and decreasing to 60% of at passage 10 (Fig. 1C).
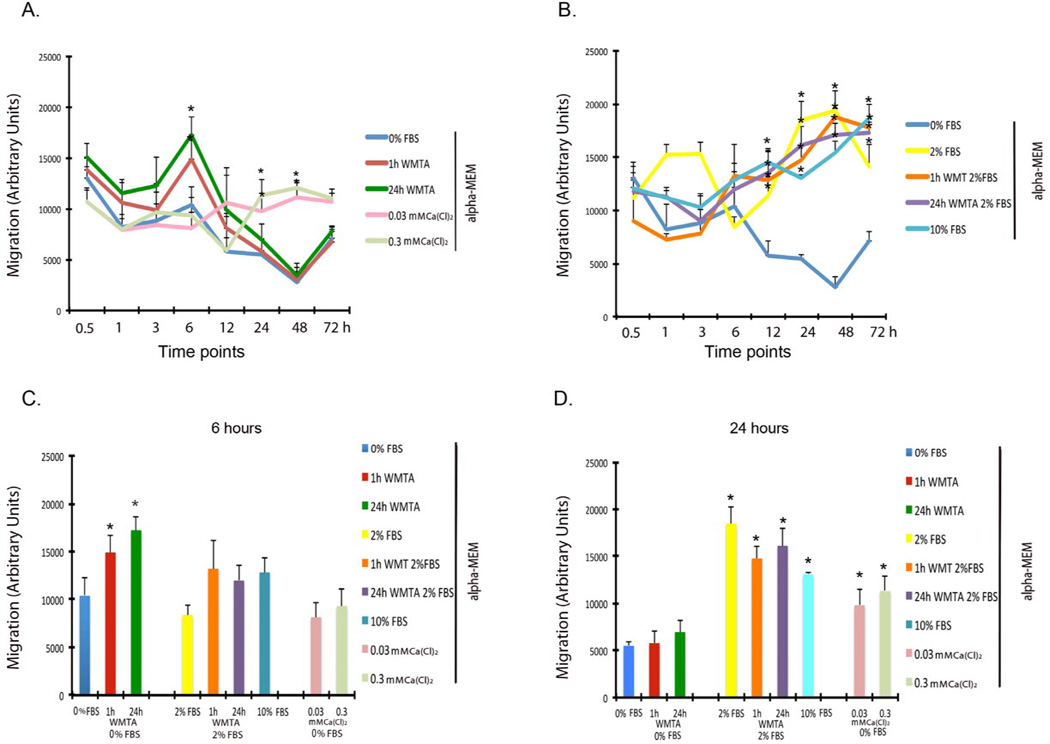
WMTA Increases early and short-term SCAP migration
The migration of SCAP started after 1 hour and continued up to 6 hours after exposure to 1h or 24 h set WMTA in plain α-MEM. There was a significant difference at 6 hours for both groups as compared with the plain α-MEM group (P = <0.038). In these groups the proliferation was reduced gradually after 12, 24 and 48 hours of exposure (Fig. 2A and C). Other time points of exposure did not show any significant differences (Fig. 2A). After 12 hours α-MEM containing both 2% and 10% FBS induced a significant increase in migration as compared to the plain α-MEM groups and the migration was maintained steady over the exposure time (P = <0.05,) (Fig. 2B and D).
Figure 2. WMTA induces short-term migration of SCAP.
(A) SCAP migration in plain α-MEM. The SCAP migration started after 3 hours and got a maximum level and significance at 6 h for all groups exposed to WMTA as compared to the plain α-MEM group (*P≤ 0.05). The migration was then gradually reduced from 12 to 48 h of exposure to WMTA and significantly induced in the calcium-chloride (0.3 and 0.03mmol) enriched groups (*P≤ 0.05). (B) SCAP migration in α- MEM with 2% or 10% FBS. The migration was induced after 12 h of exposure to α-MEM with 2% or 10% FBS and 1 or 24 h set WMTA in 2% FBS as compared with plain α-MEM groups (* P≤ 0.05). (C) Migration at 6 hours. (D) Migration at 24 hours. There was statistically significant difference when compared to the plain α-MEM group (* P≤ 0.05).
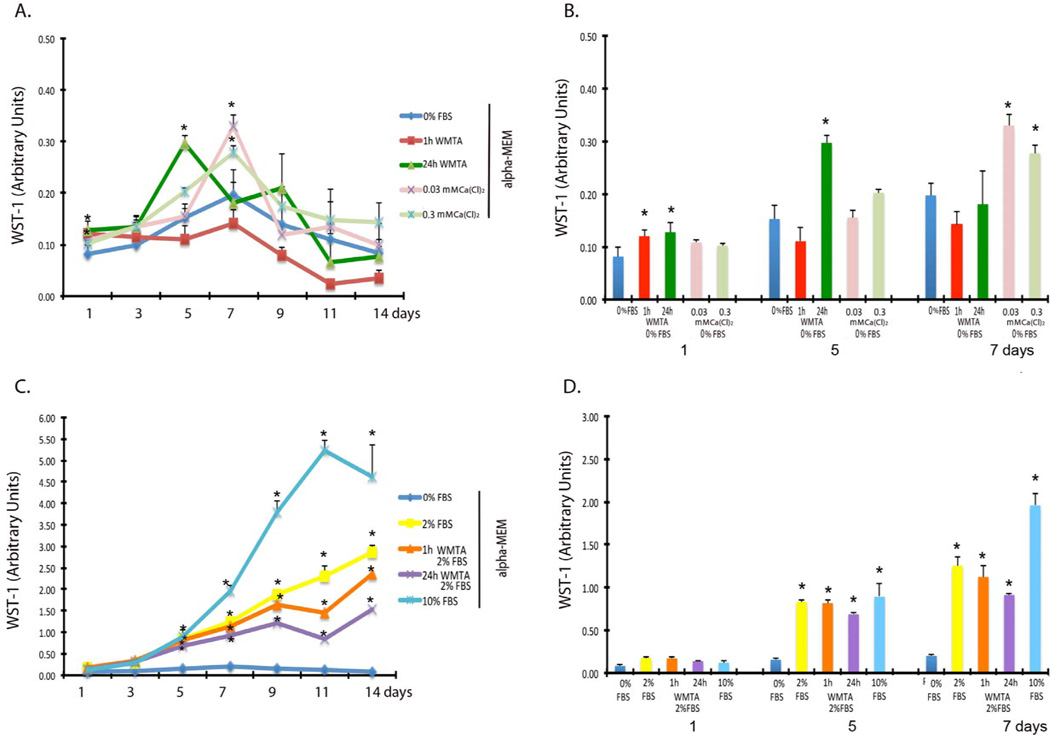
WMTA enhances early and short-term SCAP proliferation
SCAP exposed to 1 h set WMTA in plain α-MEM showed a statistically significant increase in proliferation after 1 day (P = 0.023) and to 24 h set WMTA in plain α-MEM after 1 and 5 days of exposure (P = 0.023 and P = 0.008, respectively). Other times of exposure did not show any significant differences and the proliferation was reduced after these time points. The 1 h set WMTA show levels of proliferation even lower than the plain α-MEM group (Fig 3. A and B). The 10% FBS group showed a significant increase in proliferation over all groups after 5 days of exposure (P = <0.002) (Fig. 3C and D).
Figure 3. WMTA induces short-term proliferation of SCAP.
(A) SCAP Proliferation in plain α-MEM from 1 to 14 days analyzed by WST-1 assay. Cells growing in plain α-MEM did no show any significant increase in proliferation after 14 days. 24 h set WMTA in plain α-MEM induced proliferation up to 5 days of exposure, but gradually was reduced to the level of plain α-MEM groups. 1 h set WMTA only induced proliferation after the first day and then the proliferation was below the levels of the plain α-MEM group. The calcium-chloride (0.3 and 0.03mmol) enriched groups induced proliferation at 7 days and gradually reduced to the level of the plain α-MEM group (* P≤ 0.05). (B) SCAP proliferation in plain α-MEM at 1, 5 and 7 days. There was SS difference in proliferation when the groups were compared to the plain α-MEM (* P≤ 0.05). (C) WMTA did not induce significant proliferation on SCAP grown in α-MEM with 2% FBS. There is SS difference when the cells were exposed to any of the conditions in 2% or 10% FBS when compared to plain α-MEM (* P≤ 0.05). Cells grown in WMTA in 2% FBS did no show induction in proliferation as compared to the 2% FBS α-MEM group. (D) SCAP proliferation in α-MEM with 2% or 10% FBS at 1, 5, 7 days. There was SS difference in proliferation when the groups were compared to the plain α-MEM groups (* P≤ 0.05).
Calcium Chloride induced later and long-term SCAP migration and proliferation
SCAP exposed to 0.3mmol or 0.03mmol calcium enriched α-MEM showed a statistically significant increase in migration after 24 hours and a steady migration continues up to 72 hours (P = <0.003 and P = <0.011, respectively) (Fig. 2A, C and D). SCAP exposed to 0.03mmol and 0.3mmol calcium enriched α-MEM showed a statistically significant increase in proliferation at 7 days and the proliferation decreases over time after this point (P = 0.006) (Fig. 3A and B). The 3.0 mmol calcium enriched α-MEM group was cytotoxic in all experiments and neither migration nor proliferation was seen (data not shown).
Discussion
With a mixed population of SCAP containing a low percentage of stem cells, we were able to show a significant early and shorter-term increase on SCAP migration. The same was shown for SCAP proliferation with a significant difference from controls at 1 and 5 days after exposure to 1 or 24h set WMTA. We were able to characterize the population of mixed cells used and found a low percentage of STRO-1 and CD24 positive cells and a progressive reduction of these markers as well as CD146 positive cells as the cells age.
The flow-cytometry results showed lower percentages of CD24+ and SRTO-1+ cells than the 7.56% and 18.12% respectively originally described by Sonoyama et al in 2006 (6). Only the percentage of CD146+ cells (73.2%) was in agreement with their report (72.3%). The CD24+ percentage dropped to zero by passage 10. This is crucial as CD24 is considered the identifying marker for SCAP (5, 6). Recent studies by Bakopoulou et al (28, 29) have shown that mixed population of SCAP with a percentage of STRO-1+ cells ranging from 1.7 to 19.51% have high proliferative features as compared to dental pulp stem cells. A selected population of STR-1+/CD146+ cells demonstrated better proliferation and differentiation potential when compared with the non sorted mix population of SCAP as used here (28, 29).
D'Antò et al (10) showed induction of migration of bone marrow mesenchymal stem cells following exposure to WMTA and measured at 18 h time point. In D'Antò’s study the migration was evaluated by MTT assay at only one time point and only 24 hours set MTA was used. Another study done in DPSC showed induction in genes related to cell migration after 1 and 3 days of exposure (27). In our study we found an early induction in migration up to 6 hours and this effect was gradually reduced. In the groups exposed to calcium chloride the migration was induced after 12 hours of exposure and gradually reduced but still high after 72 hours. Trans3 well migration assays were used in all these studies including ours, but we analyzed the migration by fluorescence. Although our data was reproducible, the differences in migration rates could be related to inherent differences in cell migration abilities, different stem cells and cell proportions, and/or a difference in laboratory technique. Our migration results using α-MEM containing FBS were consistent with the findings of others. The difference in response between the 1h, 24h set WMTA and calcium chloride groups can also be explained by the difference in calcium release (15, 17, 18). Han and Okiji (16) showed high levels of calcium ions release by set WMTA after 5 hours and maintained up to 24 hours. In our study under the experimental conditions used, the migration was induced after 6 and 12 hours by WMTA and Ca(Cl)2 respectively. Calcium hydroxide is a final product after WMTA sets and calcium chloride, Ca(Cl)2, has been used to accelerate the hydration reaction. It is hygroscopic and releases calcium (18, 28). Recent studies looking into the mechanisms of how calcium hydroxide modulates proliferation and migration have shown significant data on the EphB-EphrinB gene interaction (30). Calcium significantly increases chemotaxis and migration of hMSCs (21, 22). This allows the speculation that calcium hydroxide and the calcium released are highly involved in this process.
A faster and significant induction in proliferation was found when SCAP were exposed to aged mixed WMTA (24h set) as compared to freshly mixed WMTA (1h set) in plain α-MEM. Fresh mixed WMTA did not induced proliferation after the first day, instead a reduction in proliferation was observed as compared to the plain α-MEM groups. The freshly mixed calcium-silicate based cements can have continuous formation of calcium-silicates hydrates and precipitation of calcium phosphate and calcium carbonate as previously demonstrated by Gandolfi et al (31). It is speculated that this initial release of calcium could induce a toxic and inflammatory reaction. Genes related to inflammation are up-regulated 1 day after exposure to WMTA in dental pulp stem cells (27). Others have used GMTA instead of WMTA and showed initial toxicity and proliferation induction after 21 days (9, 20). The mineral composition or the difference in calcium ion release between the gray and white MTA could explain the difference in proliferation. Some results from our pilot studies demonstrated that GMTA significantly increased cell proliferation as compared to WMTA when in direct contact with SCAP (data not shown). The proliferation tested at longer periods than 14 days were unacceptable due to the confluency reach in the groups cultured with FBS. Some studies have evaluated the effect of WMTA on proliferation using medium containing 10% FBS in human dental pulp stem cells, human mesenchymal stem cells or human bone marrow stromal cells but no for SCAP (9, 10, 20, 27, 31, 32). Fetal bovine serum has by itself a potent effect on migration and proliferation as demonstrated here. Here 2% and 10% FBS were used as positive controls showing high levels of migration and higher levels of proliferation.
In this study the enriched calcium media was used to mimic the role of calcium ions as positive control and compared to WMTA. There are few studies on the effect of calcium ions on dental pulp stem cells. Only one study done on dental pulp cells has correlated the effect of calcium released from GMTA and proliferation (20). Our proliferation study partially agreed with this study showing proliferation after 7 days of exposure, whereas that study showed a concentration dependent increase in proliferation after 12 days. This may be explained by the fact that our calcium-enriched media was in plain α-MEM without FBS or other growth factors. To the best of our knowledge there are no previous reports of induction in proliferation and migration by calcium releasing materials on SCAP. More research is needed to determine the course of the release of the molecules implicated in this cellular stimulation.
In summary, under the conditions used in our study WMTA induce early and short-term SCAP migration (up to 6h) and proliferation (up to 5 days). Calcium-enriched α-MEM enhanced SCAP migration and proliferation at later time points; 12h and 7 days respectively. The pattern described by the flow-cytometry data confirmed that a mixed cell population of SCAP was used in our study with a low percentage of undifferentiated cells. Further research is needed to evaluate if the dentinogenic effect of calcium-releasing materials such as WMTA is mediated at least in part by their chemotactic and mitogenic capacity for the apical papilla stem cells.
Acknowledgments
We thank the American Association of Endodontics Foundation (AAEF), the NIH/NIDCR (grant R01-DE21410) and the Department of Cariology, Restorative Sciences and Endodontics, School of Dentistry, University of Michigan for the financial support for this project.
Footnotes
The authors declare no conflicts of interest related to this study.
References
- 1.Lovelace TW, Henry MA, Hargreaves KM, Diogenes A. Evaluation of the delivery of mesenchymal stem cells into the root canal space of necrotic immature teeth after clinical regenerative endodontic procedure. J Endod. 2011;37:133–138. doi: 10.1016/j.joen.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Jung IY, Lee SJ, Hargreaves KM. Biologically based treatment of immature permanent teeth with pulpal necrosis: a case series. J Endod. 2008;34:876–887. doi: 10.1016/j.joen.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 3.Bose R, Nummikoski P, Hargreaves K. A retrospective evaluation of radiographic outcomes in immature teeth with necrotic root canal systems treated with regenerative endodontic procedures. J Endod. 2009;35:1343–1349. doi: 10.1016/j.joen.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 4.Torabinejad M, Turman M. Revitalization of tooth with necrotic pulp and open apex by using platelet-rich plasma: a case report. J Endod. 2011;37:265–268. doi: 10.1016/j.joen.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Sonoyama W, Liu Y, Yamaza T, et al. Characterization of the Apical Papilla and Its Residing Stem Cells from Human Immature Permanent Teeth: A Pilot Study. J Endod. 2008;34:166–171. doi: 10.1016/j.joen.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sonoyama W, Liu Y, Fang D, et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One. 2006;1:e79. doi: 10.1371/journal.pone.0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tziafas D, Kodonas K. Differentiation potential of dental papilla, dental pulp, and apical papilla progenitor cells. J Endod. 2010;36:781–789. doi: 10.1016/j.joen.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Huang GTJ, Gronthos S, Shi S. Mesenchymal Stem Cells Derived from Dental Tissues vs. Those from Other Sources: Their Biology and Role in Regenerative Medicine. J Dent Res. 2009;88:792–806. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paranjpe A, Zhang H, Johnson JD. Effects of Mineral Trioxide Aggregate on Human Dental Pulp Cells after Pulp-capping Procedures. J Endod. 2010;36:1042–1047. doi: 10.1016/j.joen.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 10.D'Antò V, Di Caprio MP, Ametrano G, et al. Effect of Mineral Trioxide Aggregate on Mesenchymal Stem Cells. J Endod. 2010;36:1839–1843. doi: 10.1016/j.joen.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Kim Y-B, Shon W-J, Lee W, et al. Gene Expression Profiling Concerning Mineralization in Human Dental Pulp Cells Treated with Mineral Trioxide Aggregate. J Endod. 2010;36:1831–1838. doi: 10.1016/j.joen.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 12.Min K-S, Yang S-H, Kim E-C. The Combined Effect of Mineral Trioxide Aggregate and Enamel Matrix Derivative on Odontoblastic Differentiation in Human Dental Pulp Cells. J Endod. 2009;35:847–851. doi: 10.1016/j.joen.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 13.Min K, Park H, Lee S, et al. Effect of Mineral Trioxide Aggregate on Dentin Bridge Formation and Expression of Dentin Sialoprotein and Heme Oxygenase-1 in Human Dental Pulp. J Endod. 2008;34:666–670. doi: 10.1016/j.joen.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Chedella SCV, Berzins DW. A differential scanning calorimetry study of the setting reaction of MTA. Int Endod J. 2010;43:509–518. doi: 10.1111/j.1365-2591.2010.01708.x. [DOI] [PubMed] [Google Scholar]
- 15.Camilleri J, Montesin F, Brady K, et al. The constitution of mineral trioxide aggregate. Dent Mater. 2005;21:297–303. doi: 10.1016/j.dental.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Han L, Okiji T. Bioactivity evaluation of three calcium silicate-base endodontic materials. Int Endod J. 2013;46(9):808–814. doi: 10.1111/iej.12062. [DOI] [PubMed] [Google Scholar]
- 17.Camilleri J. Hydration mechanisms of mineral trioxide aggregate. Int Endod J. 2007;40:462–470. doi: 10.1111/j.1365-2591.2007.01248.x. [DOI] [PubMed] [Google Scholar]
- 18.Camilleri J. Characterization of hydration products of mineral trioxide aggregate. Int Endod J. 2008;41:408–417. doi: 10.1111/j.1365-2591.2007.01370.x. [DOI] [PubMed] [Google Scholar]
- 19.Felippe WT, Felippe MC, Rocha MJ. The effect of mineral trioxide aggregate on the apexification and periapical healing of teeth with incomplete root formation. Int Endod J. 2006;39:2–9. doi: 10.1111/j.1365-2591.2005.01037.x. [DOI] [PubMed] [Google Scholar]
- 20.Takita T, Hayashi M, Takeichi O, et al. Effect of mineral trioxide aggregate on proliferation of cultured human dental pulp cells. Int Endod J. 2006;39:415–422. doi: 10.1111/j.1365-2591.2006.01097.x. [DOI] [PubMed] [Google Scholar]
- 21.Aguirre A, Gonzalez A, Planell JA, Engel E. Extracellular calcium modulates in vitro bone marrow-derived Flk-1+ CD34+ progenitor cell chemotaxis and differentiation through a calcium14 sensing receptor. Biochem Biophys Res Commun. 2010;26:156–161. doi: 10.1016/j.bbrc.2010.01.109. [DOI] [PubMed] [Google Scholar]
- 22.Schraufstatter IU, DiScipio RG, Khaldoyanidi SK. Alpha 7 subunit of nAChR regulates migration of human mesenchymal stem cells. J Stem Cells. 2009;4:203–215. [PMC free article] [PubMed] [Google Scholar]
- 23.Wondergem R, Ecay TW, Mahieu F, et al. HGF/SF and menthol increase human glioblastoma cell calcium and migration. Biochem Biophys Res Commun. 2008;18:210–215. doi: 10.1016/j.bbrc.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 24.Choi HD, Noh WC, Park JW, et al. Analysis of gene expression during mineralization of cultured human periodontal ligament cells. J Perio Implant Sci. 2011;41:30–43. doi: 10.5051/jpis.2011.41.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCullen SD, Zhan J, Onorato ML, et al. Effect of varied ionic calcium on human adipose22 derived stem cell mineralization. Tissue Eng Part A. 2010;16:1971–1981. doi: 10.1089/ten.TEA.2009.0691. [DOI] [PubMed] [Google Scholar]
- 26.Kardos TB, Hunter AR, Hanlin SM, Kirk EE. Odontoblast differentiation: a response to environmental calcium? Endod Dent Traumatol. 1998;14:105–111. doi: 10.1111/j.1600-9657.1998.tb00821.x. [DOI] [PubMed] [Google Scholar]
- 27.Seo MS, Hwang KG, Lee J, et al. The effect of mineral trioxide aggregate on odontogenic differentiation in dental pulp stem cells. J Endod. 2013;39:242–248. doi: 10.1016/j.joen.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Bakopoulou A, Leyhausen G, Volk J, et al. Comparative analysis of in vitro osteo/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP) Arch Oral Biol. 2011;56:709–721. doi: 10.1016/j.archoralbio.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Bakopoulou A, Leyhausen G, Volk J, et al. Comparative characterization of STRO-1(neg)/CD146(pos) and STRO-1(pos)/CD146(pos) apical papilla stem cells enriched with flow cytometry. Arch Oral Biol. 2013;58:1556–1568. doi: 10.1016/j.archoralbio.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Jong G, Lin LM, et al. EphB-EphrinB interaction controls odontogenic/osteogenic differentiation with calcium hydroxide. J Endod. 2013;39:1256–1260. doi: 10.1016/j.joen.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 31.Gandolfi MG, Ciapetti G, Taddei P, et al. Apatite formation on bioactive calcium-silicate cements for dentistry affects surface topography and human marrow stromal cells proliferation. Dent Mater. 2010;26:974–992. doi: 10.1016/j.dental.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Moghaddame-Jafari S, Mantellini MG, Botero TM, et al. Effect of ProRoot MTA on pulp cell apoptosis and proliferation in vitro. J Endod. 2005;31:387–391. doi: 10.1097/01.don.0000145423.89539.d7. [DOI] [PubMed] [Google Scholar]