Abstract
Chemiluminescence (CL) is an important method for quantification and analysis of various macromolecules. A wide range of CL agents such as luminol, hydrogen peroxide, fluorescein, dioxetanes and derivatives of oxalate, and acridinium dyes are used according to their biological specificity and utility. This review describes the application of luminol chemiluminescence (LCL) in forensic, biomedical, and clinical sciences. LCL is a very useful detection method due to its selectivity, simplicity, low cost, and high sensitivity. LCL has a dynamic range of applications, including quantification and detection of macro and micromolecules such as proteins, carbohydrates, DNA, and RNA. Luminol-based methods are used in environmental monitoring as biosensors, in the pharmaceutical industry for cellular localization and as biological tracers, and in reporter gene-based assays and several other immunoassays. Here, we also provide information about different compounds that may enhance or inhibit the LCL along with the effect of pH and concentration on LCL. This review covers most of the significant information related to the applications of luminol in different fields.
Keywords: Luminol, Chemiluminescence, Electrochemiluminescence, Cellular localization, Reporter gene assay, Forensic science
Introduction
Luminol (5-amino-2,3-dihydro-1,4-phthalazinedione) is a yellow-colored crystalline solid powder and soluble in most polar organic solvents, but insoluble in water [1]. Luminol is a diprotic acid (denoted as LH2) with pKa values of 6.74 and 15.1. These two pKa values correspond to the loss of two acylhydrazide protons at (pKa1) and (pKa2), respectively [2–4]. Luminol exists mostly as LH, but in acidic solutions it becomes fully protonated (LH2), while in alkaline solutions, dissociation of luminol occurs to the monoanion (LH−) and dianion (L2−). Luminol solutions are highly sensitive to light and incompatible with strong oxidizing agents, strong acids, strong bases, and strong reducing agents [1]. Luminol solutions are thermally unstable; therefore, they should be protected from high temperatures [5].
Luminescence-based methods are widely accepted to study the biological system [6–11]. Luminol is one of the most widely used chemiluminescent compounds because of its availability and low cost. An alkaline solution of luminol oxidized by oxidizing agents, such as ozone, halogens, singlet oxygen, persulphates, hypochlorites [12], or H2O2, K3Fe(CN)6 [13] exhibits chemiluminescence (CL) at 425 nm λmax [14]. Luminol can also be oxidized by other oxidizing agents in the presence of catalysts, such as horseradish peroxidase, lactoperoxidase and myeloperoxidase, etc. Hydrogen peroxide is the most significant oxidizing agent, which increases the luminescent intensity of luminol. The most important oxidizing system is HRP-luminol-H2O2 that has been used in many CL assays [1]. Strong alkaline conditions may cause protein denaturation. Therefore, peroxidases are usually preferred because luminol can be oxidized in mild conditions. However, myeloperoxidase can be used to carry out the oxidation of luminol at high pH (10–13) [15, 16]. This CL system has been employed in the detection of antioxidants [17].
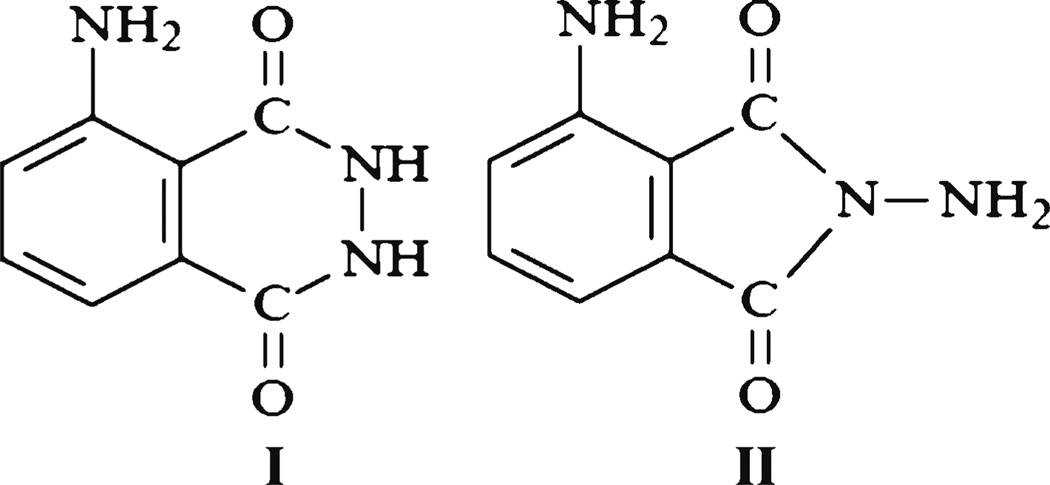
The CL property of luminol was first discovered by Albrecht in 1928 [18]. Since then, the luminescent properties of luminol have been extensively explored. Luminol exists in two isomers (Fig 1). Isomer I is more stable, exhibits CL in the presence of oxidizing agents, and also has fluorescence properties. However, isomer II neither exhibits CL nor fluorescence [19].
Fig. 1.
Chemical structure of luminol isomer
The CL properties of luminol are widely used as an analytical tool for environmental applications, immunoassays [20, 21], monitoring of metabolic pathways, detection of free radicals [22], analysis of a variety of trace metals, and forensic identification and detection of inorganic substances. Furthermore, luminol has been widely used in the area of pharmaceutical and life science industries. The toxicity of luminol has not been fully investigated in humans, although mucosa irritation has been described in eyes, skin, respiratory tract and gastrointestinal tract (with nausea, vomiting, and diarrhea) [1]. Due to its large number of applications, researchers have searched for some luminol derivatives to maximize the luminescence intensity and increase the range of emission wavelengths in the visible region [23].
Despite its various applications, an organized literature on luminol dealing with the basic features and clinical applications is still unavailable. In this review, our aim is to gather the most essential and recent information regarding the chemical features, CL, clinical and nonclinical applications and future uses of luminol.
Physical and Chemical Properties of Luminol
Luminol is a cyclic diacylhydrazide belonging to the class cyclic acylhydrazides and exhibits the typical properties of this class of compounds [24]. The important physical, chemical, and toxicological properties of luminol are briefly described in Table 1 [25]. Luminol solutions are sensitive to light and metal cations and are stable for 8–12 h. Keto-enol tautomerism is observed in luminol both in solution and solid state when fully protonated or mono deprotonated [25, 26]. Recently, luminol chemiluminescence (LCL) was reviewed by Barnett and Francis [27]. Oxidation of luminol in the presence of light is a complex, multistep process and depends on several factors such as pH, temperature, metal catalyst, hydroxide ions, ionic strength of the reaction medium and reactive species present in solution that interact with luminol [27].
Table. 1.
Physical and chemical properties of luminol
| S. no. | Property | Description |
|---|---|---|
| 1. | Name | 5-amino-2,3-dihydro-1,4-phthalazine-dione, o-aminophthalylhydrazide, 3-aminophthalic hydrazide |
| 2. | Molecular formula | C8H7N3O2 |
| 3. | Structural formula | 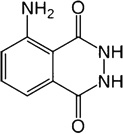 |
| 4. | Molecular mass | 177.16 amu |
| 5. | Melting point | 319–320 °C |
| 6. | pKa1 | 6.74 |
| 7. | pKa2 | 15.1 |
| 8. | Solubility in water | <0.1 g/100 mL at room temperature |
| 9. | Physical properties | Yellow crystalline solid (grainy crystals) |
| 10. | General properties | Stable at room temperature; sensitive to light; combustible; incompatible with strong oxidizing agents, strong acids, strong bases, strong reducing agents; emits light on reaction with oxidizers (CL). |
| 11. | Safety information and potential health effects | The toxicological properties have not been fully investigated in humans; mucosa irritation has been described in eyes, skin, respiratory tract, and gastrointestinal tract (with nausea, vomiting, and diarrhea). No data available about chronic effects. More information available at The National Toxicology Program (The National Institute of Environmental Health Sciences, NC, USA; website: http://ntp.niehs.nih.gov/index.cfm) |
White et al. [28] demonstrated that the fluorescence spectrum of the intermediate molecule (3-aminophthalate) in an electronically excited intermediate state (3-APA*) perfectly matches the CL spectra of luminol. The excited intermediate state is considered a light-emitting species upon de-excitation to ground state (3-APA). For example, it was found that in dipolar aprotic solvents, like dimethylsulfoxide (DMSO), that contains oxygen, in moderately strong alkaline protic solvents (pH 8–11), like water or lower alcohols, in the presence of a strong-mild oxidant (in most cases H2O2) and in the presence of a suitable catalyst, such as a metal ion or some kind of oxidoreductase enzyme, the excited 3-aminophthalate dianion (3-APA*) returns to the ground state (3-APA) by releasing energy in the form of light. In protic media, the 3-aminophthalate dianion is produced in an almost quantitative fashion [28, 29].
The spectral emission range of luminol is broader, but in aqueous solution it ranges between blue-violet and blue-green. The observed maxima is dependent on several reaction parameters [30, 31] such as the presence of iron, which strongly absorbs at 420 nm. As a result, a shift was observed in the maximum emission of LCL to 455 nm [32]. Awide range of transition metals and metal complexes are available that catalyze this reaction by working at a varied range of pH from 8–11. The optimum pH conditions for the reaction depend on the identity of the catalyst used. Thus, the multiplicity of potential catalysis mechanisms is observed while working in basic medium [33].
Effect of Interfering Substances on LCL
A wide range of environmental, pharmaceutical, domestic, and industrial substances affect luminol-induced CL. This may be due to an alteration in the catalytic activity, redox properties, or chemical reactivity with the luminol mixture. Here, we describe various reagents that affect LCL.
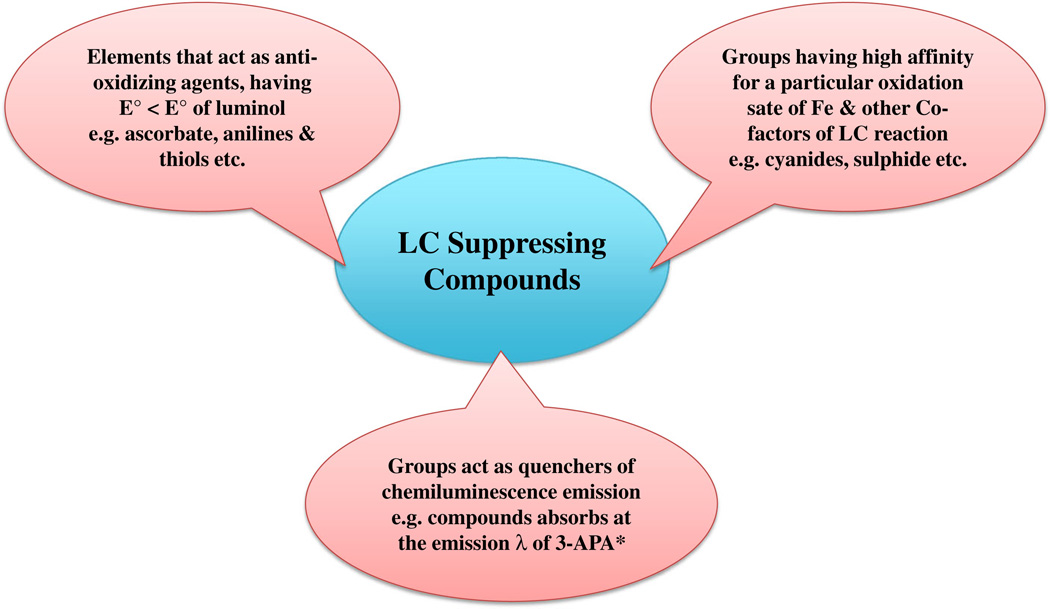
Ligands with a high affinity/reactivity for a specific oxidation state of iron or cyanide and compounds acting as anti-oxidizing species (standard reduction potential, E0 ′ <E0 ′luminol), such as ascorbate, phenolics, anilines, and thiols [34], act as molecular traps and reduce luminol intensity significantly. Quenching (intermolecular electronic energy transfer) and inner-filter effects (molecule absorbing at the emission wavelength of the emitter) are also likely to be possible interfering conditions that can decrease the observed CL intensity [35]. A list of compounds that suppress LCL are summarized in Fig 2.
Fig. 2.
Classification of LCL suppressing compounds
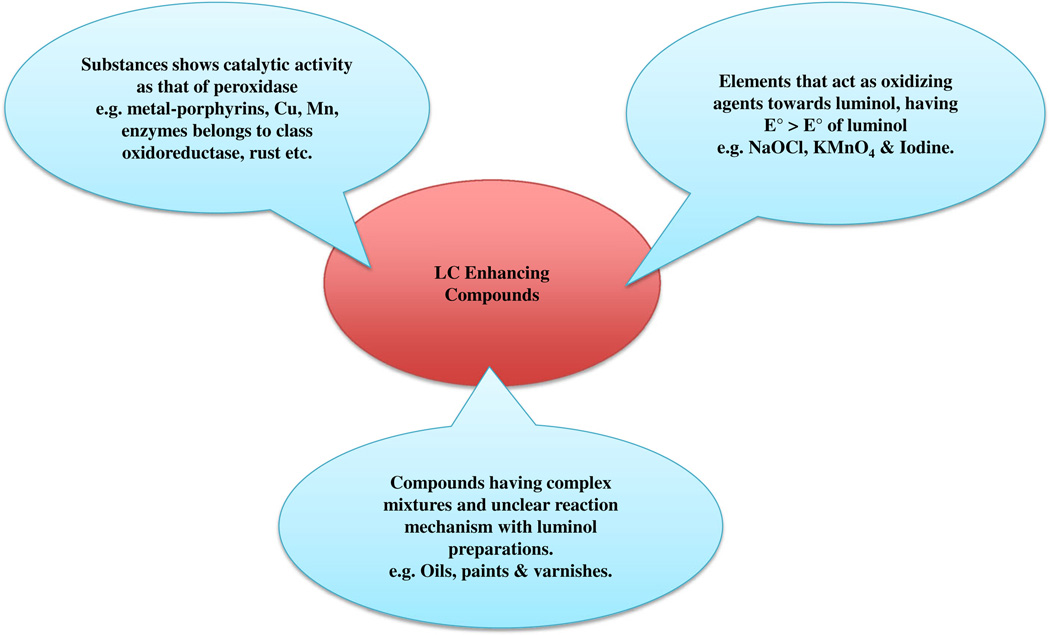
Compounds that generate or enhance LCL are divided into three major categories: (1) compounds showing a catalytic true peroxidase or peroxidase-like activity, (2) compounds with a high oxidizing capacity towards luminol and (3) compounds with a complex chemical composition with an undefined action (Fig 3). The first group includes inorganic or bioinorganic species that are a major source of luminol interferences. These compounds often show excellent catalyzing properties in redox reactions. These compounds are free metal ions, biological complexes between metal ions and organic components and enzymes belonging to the oxidoreductase class, especially horseradish peroxidase. Ferric or ferrous ions and other metals ions, especially cobalt, copper, and manganese that are present in biological molecules as prosthetic groups mainly enhance LCL [36, 37]. Several metal ions, such as cobalt, chromium, nickel, copper, and manganese, which are present in soils or chemical products, are capable of producing visible CL when exposed to the luminol solution [38–40].
Fig. 3.
Classification of LCL enhancing compounds
The second category of LCL-interfering agents includes compounds that show strong oxidizing properties for luminol, e.g., sodium hypochlorite, potassium permanganate, and iodine. These species are present in industrial and household chemical solutions, such as insecticides, cleaning agents, disinfectants, and antiseptics [41]. Hypohalites of chlorine and bromine (hypochlorite, hypobromite) and related oxidants, such as N-bromosuccinimide, 1,3-dibromo-5,5-dimethylhydantoin and chloramine-T, are well-known oxidants in CL reactions. Hypochlorite (OCl−) is used to induce a brilliant blue emission by oxidation of luminol [42, 43]. Hypochlorite has a reduction potential (E0) of 0.841 Vand thus acts as a medium-strong oxidant [44].Therefore, it amplifies the CL emission in luminol oxidation by hydrogen peroxide when both are present in the reaction medium [44].
Arnhold et al. [45] gave a linear relationship between the concentration of H2O2 and light intensity. The concentration range was 5×10−8 to 7.5×10−6 mol/L with a 550-fold amplification level at a range of 5×10−8 to 7.5×10−6 mol/L H2O2. Hypochlorite can generate the diazaquinone intermediate efficiently followed by rapid reaction with peroxide. Due to this property, luminol shows increased CL in the presence of H2O2 and NaOCl (sodium hypochlorite). It has been observed that the wavelength maxima at 431 nm is independent of the concentration of H2O2 in the CL spectra of their reaction. Thus, H2O2 is a necessary component of the chemiluminescent oxidation of luminol by sodium hypochlorite [31]. The activity of these compounds as oxidants in CL reactions has been comprehensively reviewed by Francis and coworkers [46]. They suggested that these reagents can be used in a wide range of applications and are useful analytically due to their potential oxidizing properties.
The last category of interfering substances covers a range of compounds that are present in materials such as domestic and commercial oils, glues, carpets, sinks, automobile seats, paints and varnishes, and soils [47–49]. These substances often catalyze CL as effectively as blood due to their complex chemical composition. The exact mechanism underlying these interferences is not yet completely understood.
Effects of Experimental Conditions on LCL
Besides the common factors discussed before, there are some specific compounds and conditions that can either enhance or can suppress LCL according to experimental conditions.
Effect of pH on Luminol Intensity
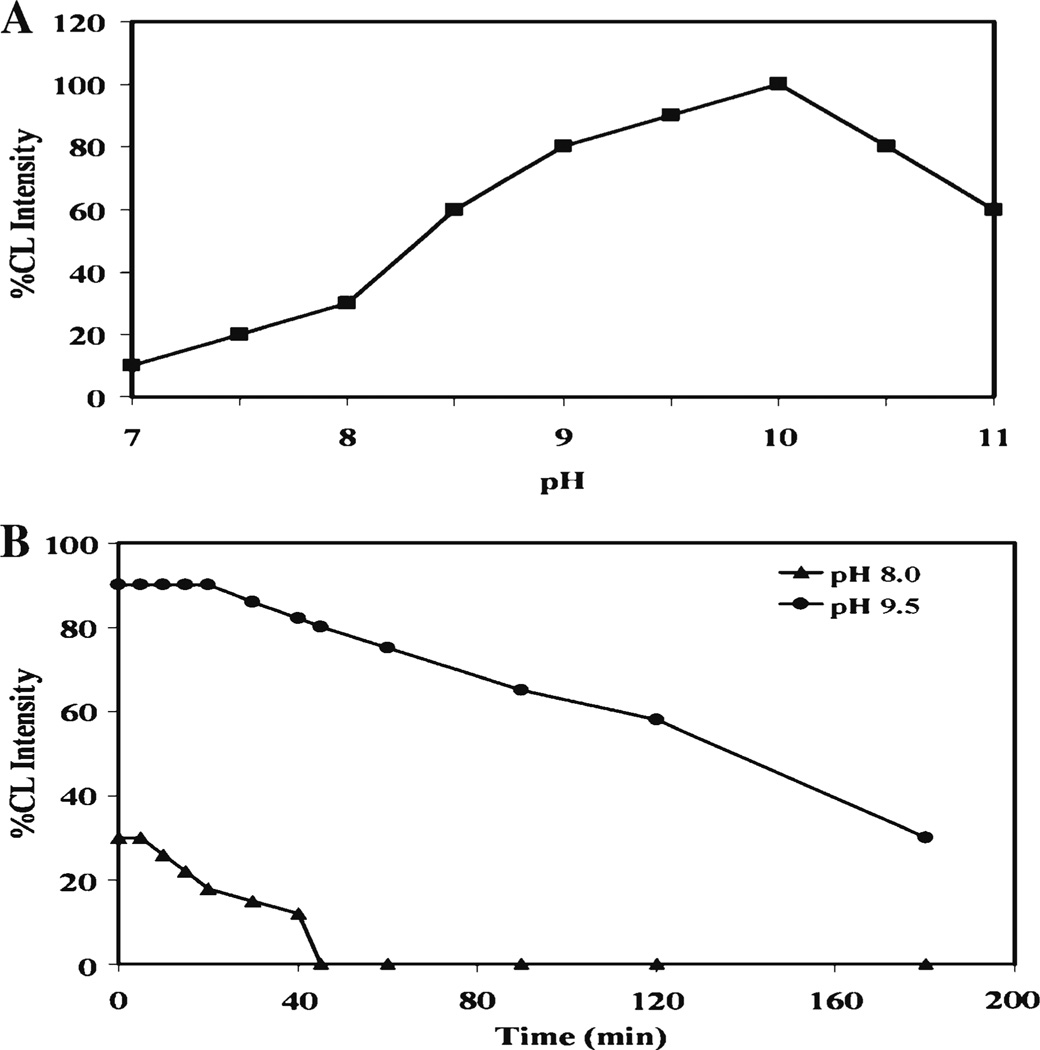
pH plays vital role on the CL of the luminol CL system. At lower pH, the intensity of luminol was drastically reduced, while at higher pH, a significant increase in the luminol intensity was observed [50]. Some organic molecules, such as phenolic compounds and amino acids, have been tested to investigate their effect on luminol intensity at different pH. At lower pH, these compounds showed inhibitory effects on luminol intensity, while at higher pH, a significant increase in luminol intensity was observed (Fig 4).
Fig. 4.
Chemiluminescent intensity of luminol. a. CL intensity at different pHs. The maximum CL intensity was between pH 9.5 and pH 10.0. b. CL intensity and stability at pH 8.0 and pH 9.5 were determined at different times. CL intensity at pH 8.0 was lower than at pH 9.5, and CL intensity at pH 8.0 decreased more rapidly than at pH 9.5
The maximum CL intensity was measured in the range of pH 8.0 and pH 9.5. In this range of pH, both inhibiting and enhancing signal were simultaneously observed. When we compared the CL intensity at pH 8.0 and pH 9.5, the relative CL intensity at pH 8.0 was lower and decreased with a half-life of 20 min. However, the relative CL intensity at pH 9.5 was high and lasted for a longer time, then decreased slowly so 30 % of the CL intensity was recorded even after 3 h. We observed from our study that the chemiluminescent signal of luminol at alkaline pH is strong and stable (Fig 4).
The most important variable of peroxide-mediated CL is pH. It is necessary to compromise between the optimum pH for peroxidase activity and the optimum pH for maximum CL intensity from luminol. At pH 8, the CL intensity reaches steady state within a few seconds, reflecting high peroxidase activity; however, sensitivity is low because the CL efficiency of luminol is low at this pH. At pH 9.5, on the other hand, sensitivity is good but peroxidase activity is low (Fig 5). As a consequence, peroxide diffuses further into the gel before it is completely reacted, and it takes much longer to reach steady state. The best compromise is to work at a pH where steady state is still reached in about 30 s, and sensitivity is still satisfactory.
Fig. 5.
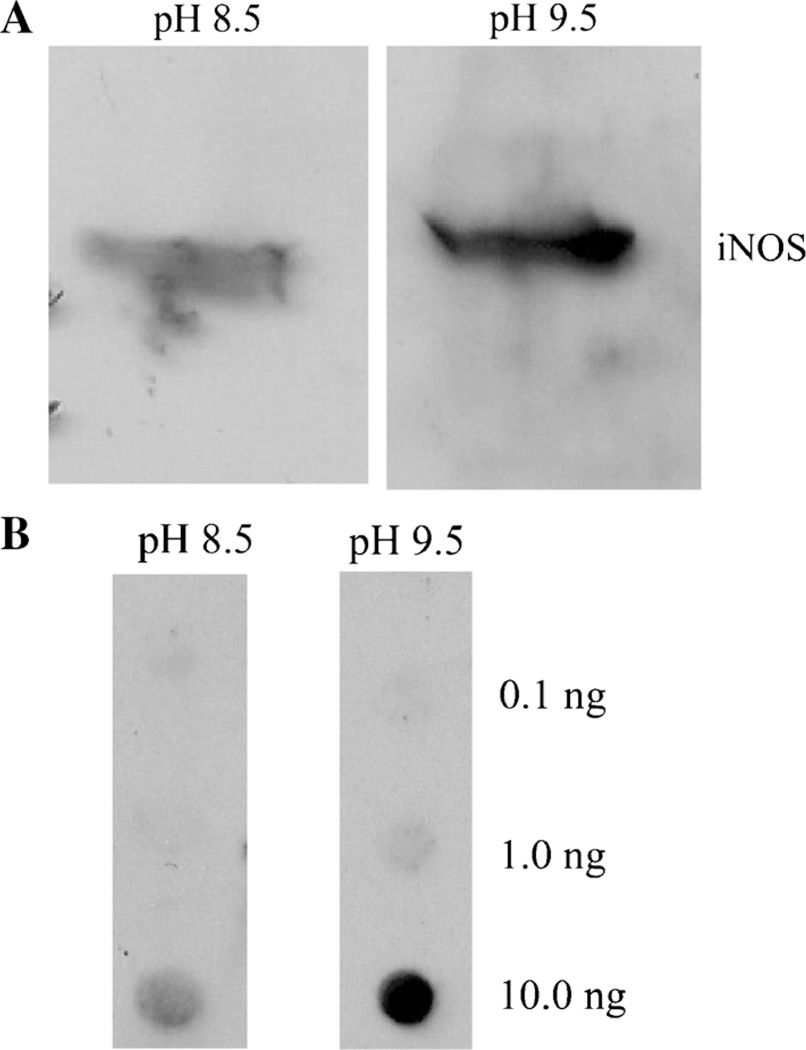
Western blot of iNOS and dot blot of biotinylated DNA. a. RAW 264.7 cells (4×105/400 µl) were incubated for 24 h in the presence of 10 µg/mL LPS. Equal amounts of SDS lysate of the cells were subjected to SDS-PAGE and transferred to a nitrocellulose membrane. After blocking, the blot was then incubated overnight with rabbit anti-iNOS (1:1,000 dilution). The blot was washed three times and incubated for 1 h with donkey anti-rabbit IgG-peroxidase conjugate. The membrane was washed and divided. Half the membrane was stained at pH 8.5 and the other half at pH 9.5, as described in the text. b. Increasing amounts of biotinylated DNA were applied to a nitrocellulose membrane in duplicate. After blocking, the dot blot was incubated with 0.1 µg/mL streptavidin-peroxidase conjugate for 30 min. The membrane was washed and divided. Half the blot was stained with the described ECL reagent at pH 8.5 and the other half stained at pH 9.5
When potassium permanganate (KMnO4) was used in the system in the presence of Al(III) at different pH, it was observed that the CL signal at pH<10.2 was weak. However, the CL signal increased significantly at pH>11.45. This increase was due to the fact that Al(III) forms hydroxide precipitate in mildly alkaline conditions, creating a turbid solution and a decrease in the CL signal. On the other hand, in strong alkaline conditions, hydroxide precipitate dissolved and the CL signal was increased [51, 52].
Effect of Protein Concentration
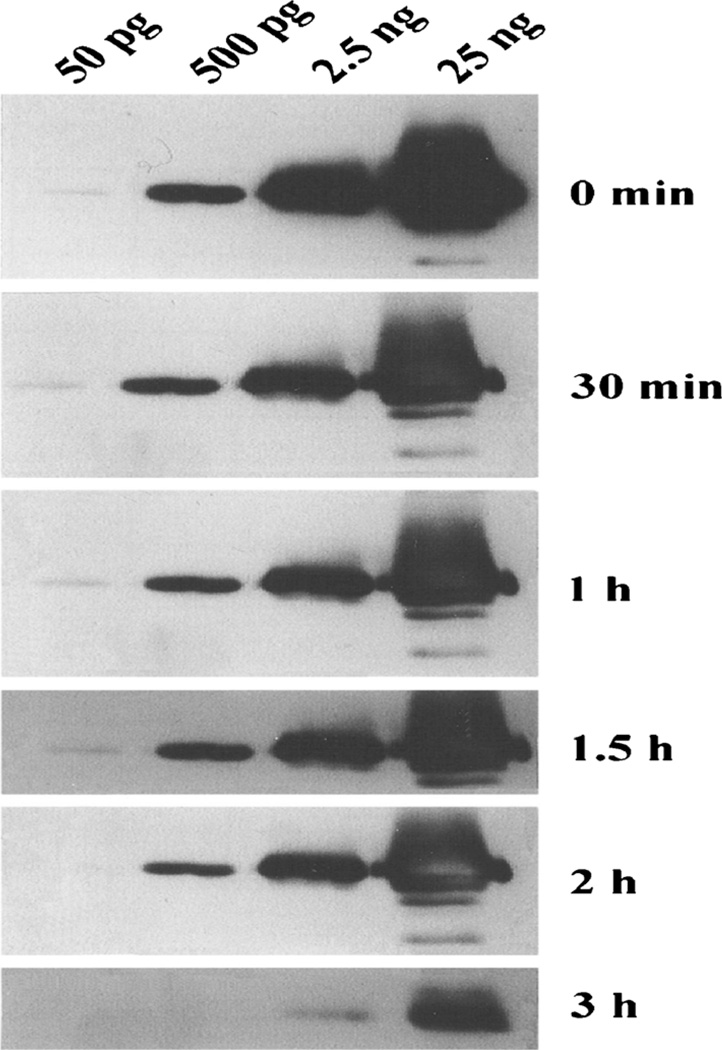
We also checked the sensitivity and stability of the luminol reaction at pH 9.5 in Western blot analysis [53, 54]. After separation of carbonic anhydrase IV (CAIV) on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), the polypeptides were electrophoretically transferred to polyvinylidene difluoride (PVDF) membrane using transblot [55]. After successful development of the blot on PVDF membranes, membranes were washed with TBST 5 times for 5 min each and incubated with 25 µg/mL luminol or isoluminol, 25 µg/mL 4-iodophenol, and 0.01 % H2O2 in pH 9.5 luminol buffer for 5 min. For zero time storage, one membrane was exposed immediately to X-ray film for 1 min. Other membranes were stored in the dark for 30–180 min at RT before exposing to X-ray film for 1 min. The results of such an experiment are summarized in Fig 6. At zero time at all concentrations of CA IV from 50 pg to 25 ng, the polypeptides for CA IV were visualized. After 30–90 min of storage, the apparent CL intensity of the polypeptide was reduced. However, the polypeptides at 50 pg were still visible. After 120 min, the CL intensity of the 50-pg polypeptide was hard to see, but the polypeptide intensity for 500 pg was still very strong. After180 min of storage, only 2.5 and 25-ng protein polypeptides were visible with reduced intensity. We used different concentrations of CAIV protein from 0.1 to 10 ng at pH 8.0 and 9.5, respectively. The LCL signal of 10 ng of CAIV was prominent at pH 9.5, while at pH 8.5, the band was only slightly visible (Fig 6).
Fig. 6.
Western blot analyses of CAIV. Recombinant human CAIV enzyme at 50 pg–25 ng were analyzed by SDS-PAGE and electrophoretically transferred to PVDF membranes. The polypeptides for CAIV were immunostained using luminol and H2O2 substrate for peroxidase at pH 9.5. The membranes were stored for zero to 3 h before exposing to X-ray film. The polypeptide intensities at zero time without any storage were highest and, with increasing storage time, intensities were decreased. CAIV polypeptides at 50 pg were visible until 90 min. After 2 h of storage, the polypeptides from 2.5 ng protein were still visible
Effect of Luminol Concentrations
Luminol concentration is an important factor that affects CL intensity. The intensity of LCL does not depend on the concentration of luminol alone, but also on other factors like concentration of oxidizing agents, enzymes, and pH. Chenet et al. [56] investigated the effect of luminol concentration by keeping the concentration of H2O2 and silver nanoparticle (AgNPs) constant. They used concentrations of luminol in the range of 0.01–1 mmol/L. Maximum recorded CL intensity was at a luminol concentration of 0.3 mmol/L. CL intensity increased linearly with increasing concentrations of luminol in the range of 0.01 to 0.3 mmol/L. However, further increases of luminol concentration caused a decrease in CL intensity [56, 57]. Several studies suggested that the CL signal increased linearly with an increase in luminol concentration, which reaches its maximum intensity at a particular concentration. Above this concentration of luminol, CL signals decreased.
Applications of Luminol in the Clinical Laboratory
Luminol and its derivatives are widely used in different fields, such as routine clinical experiments, clinical research, forensic sciences, and other biological and chemical assays. Here, we describe all applications in detail.
The most important routine applications of CL in the clinical laboratory are immunoassays and nucleic acid assays. CL immunoassay test kits and automated immunoassay analyzers have been developed and commercialized by several diagnostics companies. The comprehensive test menus cover analytes routinely measured in clinical chemistry, immunology, toxicology, virology, and endocrinology laboratories for the assessment of thyroid function, fertility, myocardial damage, anemia, therapeutic drug levels and diagnosing and monitoring drug abuse, cancer, and infectious diseases (e.g., hepatitis, HIV).
The cyclic diacylhydrazides, luminol, isoluminol, and analogs, such as naphthalene-1,2-dicarboxylic acid hydrazide, are co-substrates for horseradish peroxidase [58] and undergo a peroxidase-catalyzed oxidation in the presence of a suitable oxidant, such as hydrogen peroxide or sodium perborate. This reaction was made commercially viable as an assay for peroxidase labels in enzyme immunoassay by the discovery that certain compounds increased and prolonged the signal in the presence of peroxidase and, at the same time, decreased background light emission. The intensity of the glow-type light emission (centered at 425 nm for luminol) is proportional to peroxidase activity. Kinetic studies have indicated that preferential oxidation of enhancers by peroxidase intermediates (compounds I and II) and a rapid formation of enhancer radicals are most likely the mechanisms of this enhancement effect. Many types of molecules have been shown to act as enhancers, including firefly luciferin, naphthols, 6-hydroxybenzothiazoles, and aromatic amines [58]. A large number of nonradioactive alternatives are now available, which utilize an enzyme label for detection. One such method is luminol chemiluminescence (LCL); in this method, the probe is first labeled with biotin and then the labeled probe can be detected using an HRP-luminol-H2O2 system [59].
A CL end point is also used in the branched chain DNA assay for hepatitis C virus RNA [60]. Patolsky et al. [61] reported magnetically amplified DNA assays for viral DNA and of single-base mismatches using nucleic acid functionalized magnetite particles and electrochemiluminescence (ECL). The HRP-catalyzed oxidation of luminol by the electro-generated H2O2 results in CL. CL assays for different enzymes have been developed using indoxyl derivatives as substrates. Alkaline phosphatase-β-d-galactosidase and β-glucosidase were assayed by this method. A novel LCL imaging strategy has been developed for discrimination of single-base mismatches and for sensitive target DNA detection by designing an amplified synthesis of horseradish peroxidase mimicking DNAzyme. The amplification strategy significantly enhanced the CL emission of the luminol-H2O2 system [62]. Lin et al. [63] describe a highly sensitive, capillary electrophoresis with CL detection (CE-CL) method for the determination of four heme proteins: horseradish peroxidase (HRP), catalase (Cat), myoglobin (Mb) and cytochrome c. This method is based on the LCL reaction catalyzed by metalloproteinase in alkaline medium. This proposed CE-CL method has great potential for Mb determination in clinical diagnosis. In a similar manner, with the help of LCL, the specific reaction between aminoacyl-tRNA synthetase and its corresponding amino acid was used to measure amino acid concentrations [64]. This method of free amino acid analysis in urine and plasma is very useful for estimating disease status in clinical diagnosis.
Applications of Luminol in Clinical Research
In clinical research, the high sensitivity, specificity, broad range, and diversity of CL assays made luminol a remarkable CL reagent with a dynamic range of applications. Luminol, isoluminol, and their derivatives are currently used in blotting, immunoassays, microarray-based assays, monitoring reactive oxygen species, detection of reaction intermediates, capillary electrophoresis, and flow-injection analysis.
Immunoassay
LCL detection has been applied in various immunoassays because oxidation of luminol derivatives led to the identification of labeled antigen or antibody. In an excellent review, Rongen et al. [65] suggested more than 60 immunoassays from the different classes of luminol derivatives. Luminol is more efficient in the free state and is used mostly in enzyme-labeled immunoassays. Furthermore, enzyme-labeled immunoassays using enhanced luminol detection have been proposed for the detection and quantification of estrone and testosterone via biotinylated steroid and HRP avidin conjugate [66]. A large number of emerging clinical tests have been developed using LCL, e.g., cytokines (IL-4, IL-5, IL-6, IL-10) [67], vascular endothelial growth factor-C [68], epidermal growth factor, and benzo[a]pyrene-DNA adducts [69].
Development of a sensitive non-separation measure assay called the luminescent oxygen channeling immunoassay (LOCI) [70] has been commercialized. This technique exploits in situ production of a CL compound due to singlet oxygen transfer between a donor and an acceptor antibody-coated microbead (250-nm diameter) brought into contact as a result of specific binding with the test antigen. The assay has been adapted for competition assays (cAMP assay), interaction assays, enzyme assays (e.g., protease, kinase, helicase), and immunoassays (thyrotropin, hepatitis B surface antigen, digoxin). The LOCI-type assay described here has also been adapted for detection of single-nucleotide polymorphism typing [71]. A rapid, facile, and enzyme-amplified CL immunoassay (CLIA) was developed for the detection of porcine parvovirus infection by measuring the LCL [72].
Non-immunoassay Applications
Apart from their use in immunoassays, luminol derivatives have been extensively employed for direct monitoring of metabolic pathways. Lipid hydroperoxides have been quantified in biological media by direct luminescence measurements. The high sensitivity achieved in these assays has allowed for direct measurements in plasma using the luminol hemin reaction [73]. CL-based assays and protocols are extensively studied and applied in various clinical industries, such as urinary N-acetyl-β-d-glucosaminidase, vitamin C in seminal plasma, and blood spot screening tests for phenylketonuria, galactosemia, and maple syrup urine disease [74]. Shkapova et al. [75] observed that there is high basal production of primary active oxygen species in the peripheral blood neutrophils of patients with renal cancer. Based on this observation, they reported luminol-dependent CL of blood neutrophils in patients with renal cancer. The CL detection system also allows the determination of molecules, such as choline containing phospholipids, acetylcholine, and amikacin antibiotic [76].
It is reported that isoluminol derivatives are responsible for the release of oxygen metabolites into the extracellular medium [77]. This study of neutrophil metabolism by CL reports on predicting the course of infectious diseases and studying the antimicrobial activity of the neutrophil peroxidase system. Intensity of luminescence has been correlated to acute and chronic diseases. Studies of leukocyte CL have led to a better understanding of inflammatory processes and oxygen metabolite-related diseases. Luminol-amplified CL was used to measure the transient tumor necrosis factor alpha (TNF-α)-mediated increase in reactive oxygen species production. This measurement was helpful in the study of chronic liver inflammations and TNF-α-induced mtDNA depletions [78].
LCL-based high-performance liquid chromatography (HPLC) post-column-type reactions are widely used in clinical analysis for the determination of endogenous lipid hydroperoxides (LOOHs). Avariety of free radicals and reactive oxygen species are generated in living bodies as a result of lipid peroxidation that destroy biologically important living body constituents, such as proteins, lipids, carbohydrates, and DNA. The reaction of LOOHs with cytochrome-c is very specific, which results in the formation of lipid alkoxl radicals that further react with luminol to emit CL. The LCL reaction with cytochrome-c has also been used for different types of HPLC-CL studies related to the analysis of LOOHs, such as the determination of oxidative stress in human beings after paraquat ingestion by measuring 7α- and 7β- hydropyroxylcholest-5-en-3β-ol (these are pathogenic markers of reactive oxygen species) [79]. Other studies include simultaneous analysis of hydroperoxides of cholesterol esters as an indication of liver failure [80] and understanding the plasma phosphatidyl choline hydroperoxides pathway by using synthetic 1-stearoyl-2-erucoyl-phosphatidylcholine monohydroperoxide as an internal standard [81].
Biosensors
The nanomaterial-based ECL detection system has become an important subject in biosensor applications. Chai et al. [82] reported a novel strategy for the ECL detection of specific DNA sequences by combining luminol-functionalized gold nanoparticle (luminol-AuNPs) labeling and amplification of AuNPs with the biotin–streptavidin (SA) system. With the help of a novel approach, networked gold nanoparticles were prepared, along with nonionic fluoro surfactant assistance. A strong oxidizing agent is attached to the surface of the networked gold nanoparticles that causes emission of CL from luminol without the addition of H2O2. These networked gold nanoparticles have been used for ultrasensitive detection of aminthiols in human plasma and urine samples [83]. A very sensitive and simple LCL method using DNA-stabilized gold nanoparticle has been used for the detection of Hg2+ ions in aqueous solutions [84]. Non-enzymatic determination of sugars like glucose, fructose, and other hydrolysable sugars was possible by analyzing CL emission from a luminol-tetrachloroaurate system on a microfluidic chip [85].
Another novel strategy was developed by Guan et al. [86] for chemo/biodetection. They developed intrinsically selective CL switching at the surface of Fe3O4 nanoparticles. Fe3O4 nanoparticles possess peroxidase-like activity and catalyze the decomposition of dissolved oxygen to generate superoxide anions, which resulted in at least a 20-times amplification of the LCL intensity. The CL signals can be quenched by the addition of ethanol because ethanol reacts readily with superoxide anions as a radical scavenger. This LCL-based method was used for simultaneous determination of various pesticides [86]. A molecularly imprinted electrochemical luminescence (MIP-ECL) sensor was developed for Gibberellin-A3 (GA3) determination. This method was based on the difference in binding affinities between GA3 and rhodamine-B-labeled GA3 for MIP film. After binding to MIP, rhodamine-B-labeled GA3 was oxidized, which further amplified the ECL of luminol [87].
A selective and specific assay was developed by Giokas et al. [88] for the determination of an important class of phenolic compounds named trihydroxybenzoates, based on the fact that they enhance CL between gold ions and luminol. Recently, Hong et al. [89] described a chemiluminescent cholesterol sensor with good selectivity and enhanced sensitivity based on the peroxidase-like activity of cupric oxide nanoparticles. A homogeneous CL immunoassay for thyroxine (T4) present in serum samples was also described [90]. A very sensitive LCL enhancement-based detection method was developed for hydrogen peroxide and glucose using cupric oxide nanoparticles as enhancers for LCL [91].
A unique CL sensor array based on CeO2 nanoparticle membranes was developed for the detection of triacetone triperoxide (TATP). This sensor had an excellent catalytic affect on the luminol–H2O2 CL reaction in alkaline medium [92]. This aptasensor was based on CL resonance energy transfer and was used for the highly sensitive and selective detection of riboflavin [93], ATP [94], and thrombin [95]. A cholesterol biosensor was also prepared by another group based on gold nanoparticle-catalyzed luminol ECL [96]. A sodium dodecylsulfate-assisted zirconia nanoparticle-enhanced copper (II)-catalyzed luminolhydrogen peroxide CL method was used for the sensitive and selective determination of heme proteins [97]. Hao et al. [98] found that graphene oxide (GO) catalyzed the luminol-O2 reaction, which yielded a novel CL. Based on this CL, a new LCL sensor for glucose, based on pH-dependent graphene oxide, was constructed.
Oncology
Recently, a novel CL-based sensitive detection method for p53 protein was proposed. The p53 protein was captured in solution by a highly specific, consensus double-stranded (ds) oligonucleotide immobilized on gold plate. The cysteine residues present on the exterior side of the p53 molecules were derivatized with N-biotinoyl-N′-(6-maleimidohex-anoyl) hydrazide (biotin-Mi) to attach the streptavidin-HRP complex. The attached HRP molecules catalyzed the CL reaction between luminol and H2O2 to produce the enhanced CL signal. The CL intensity was dependent on the surface coverage of HRP molecules (related to the concentration of p53 protein) and increased linearly with the concentration of p53 protein that ranged from 0.01–0.5 nM under optimal conditions. The estimated detection limit was 3.8 pM. This assay was a successful method for the detection of p53 protein in normal and cancer cell lysates. The sensing protocol used in this study is very sensitive, cost-effective, and holds great importance for clinical diagnosis [99].
Huang et al. [100] reported a highly sensitive CL reaction of luminol and H2O2 catalyzed by HRP to check the presence of alpha fetoprotein, a cancer biomarker. This technique was used successfully for the determination of AFP levels in sera from cancer patients. An ultrasensitive ECL immunosensor was constructed by Cao et al. [101] for detection of carcinoembryonic antigen (CEA) based on an amplified cathodic ECL of luminol. Another group developed a detection method for CEA by using ZnO nanoparticles and a glucose oxidase-based immunosensor with the help of the ECL reaction of the luminol-H2O2 system [102].
Nucleic Acid Assay
The LOCI-type assay described in the previous section has also been adapted for detection of single-nucleotide polymorphism typing [71]. Other CL nucleic acid-based assays include infectious disease testing in combination with nucleic acid amplification techniques, such as the polymerase chain reaction, e.g., telomere DNA [103], herpes simplex [104], lassa fever virus [105], and Trichomonas vaginalis [106].
Reporter Gene-based Assays
Another successful research application for CL is detecting the expressed products of reporter genes developed as alternatives to the traditional chloramphenicol acetyl transferase gene [107]. Chemiluminescent dioxetane, luminol derivative-type substrates are also available to detect and quantitate expression products of genes for placental alkaline phosphatase, β-galactosidase, and β-glucuronidase [108]. These assays are sensitive and have a linear range over several orders of magnitude.
Cellular Chemiluminescense
The available methods for cellular localization is not sufficient [109]. Hence, luminol or lucigenin-enhanced light emission from polymorphonuclear neutrophils or phagocytes, or biological fluids (e.g., whole blood) is an important tool for cell-based studies, including investigations of respiratory burst. A large proportion of publications on CL are related to cellular CL [110–114]. The scope of cellular CL studies is large and includes investigations of reactive oxygen species produced by human spermatozoa [115], defects in the production of reactive oxygen intermediates [116], responses of cells (e.g., PMN-leukocytes, neutrophils) to drugs [117] and different agents, such as H1-antagonists [118], Fc gamma and complement receptors [119], polyunsaturated fatty acids [120], lectins [121], lysophosphatidic acid [122], Helicobacter pylori lipopolysaccharide [123], and endotoxins [124]. A noninvasive luminol bioluminescence imaging method was developed to distinguish chronic and acute inflammation in animal models. In this method, systemic delivery of luminol allows detection of acute inflammation, which is largely mediated by tissue-infiltrating neutrophils whose myeloperoxidase (MPO) activity is responsible for luminol bioluminescence. With the help of these noninvasive imaging methods, we can further elucidate the role of neutrophils and macrophages in a variety of pathological conditions [125]. Similarly, an improved CL assay was developed that utilizes HRP conjugate and luminol for generating a cell-free oxygen radical system [126].
Quantification of Protein
In routine laboratory research experiments, quantification of proteins is performed using Western blotting. Western blots are widely used to study steady-state protein levels, biosynthesis and turnover, effects of mutations on protein stability, and effects of pharmacological compounds on protein expression [127, 128]. Following SDS-PAGE of protein samples and electrophoretic transfer of protein polypeptides from the gel to PVDF or nitrocellulose membranes, polypeptides are identified using protein-specific primary antibodies, secondary antibody conjugates of peroxidase or alkaline phosphatase, and chromogenic or chemiluminescent substrates of peroxidase or alkaline phosphatase [53, 54, 129]. A stable and strong CL in Western blots is desirable for identification of proteins. We also described a new luminol-containing buffer of very alkaline pH (9.5) that provided a strong and stable chemiluminescent signal in Western and dot blots. With the help of this strong and stable LCL signal, we could easily analyze different aspects of proteins. Application of luminol in these methods further improved the quality of detection and measurements [130].
Environmental Monitoring
Highly sensitive, CL-based analytical techniques have received considerable attention from researchers. The main characteristics that make the methods attractive are low detection limits, short analysis time, simplicity in measurement, and availability of large calibration ranges. There have been few reports on the analysis of surfactants in environmental samples through CL [131, 132]. Liu et al. [133] developed a simple, rapid, and sensitive CL method for determination of the nonionic surfactant, Triton X-100. The study highlighted Triton X-100-enhanced CL emission resulting from the reaction of luminol with H2O2 and reported successful detection of Triton X-100 in environmental water samples. A simple and sensitive flow-injection (FI) method for the determination of nitrate and nitrite in natural waters, based on LC detection, was also reported [134].
Inhibited LCL emission has been also used for the direct analysis of aminoglycoside antibiotics in water samples. The method was based on the inhibitory effect of AGs on the CL signal provided by the Cu(II)-catalyzed luminol-hydrogen peroxide reaction, due to the formation of a Cu(II)-AG complex [135]. With the help of LCL, detection of Cu2+ ions, Hg2+ ions, and cocaine was achieved by implementing Cu2+-dependent ligation DNAzyme [136]. The flow injection CL (FI-CL) method was used for the determination of the fungicide, azoxystrobin. CL emission was observed when azoxystrobin was injected into a mixture of luminol and KMnO4 [137]. A method named ion chromatography equipped with CL detection (IC-CLD) method was used for the speciation of iron in aqueous samples by the simultaneous analysis of divalent and trivalent iron ions [138].
General Applications of Luminol
Another technique based on CL, optically pumped CL, was used in clinical laboratories for diagnostic purposes. In this technique, luminol was oxidized by pulsed laser light in an excited state with or without the participation of oxygen [139]. A relatively simple CL assay was also developed for antioxidants (e.g., vitamins C and E and proteins) [140] based on the abolition of light emission from the enhanced CL reaction between HRP– luminol and 4-iodophenol caused by an antioxidant. The CL emission resumed after consumption of the antioxidant and duration of delay was proportional to the antioxidant [141]. This assay was adopted as part of public health measures to test water quality (http://www.severn-trent.com).
CL reactions are also useful as detection reactions for components of mixtures separated by HPLC [142], capillary electrophoresis [143], and flow injection [144]. These techniques are useful in the detection of polymerase chain amplified hepatitis C DNA [145] separated by capillary electrophoresis and post-column detection (peroxyoxalate reaction) of phosphatidylcholine and phosphatidyl ethanolamine hydroperoxides in red blood cells [146]. A method for simultaneous monitoring and identification of antioxidants in Fructus sp. has been used by coupling high-performance liquid chromatography-diode array detector-electrospray ionization-ion trap-time of flight-mass spectrometry with post-column derivatization and luminol-potassium ferricyanide CL. HPLC fingerprint, structural identification, and radical scavenging profile were rapidly obtained by online assay using ultraviolet absorption, mass spectrometry, and luminol-potassium ferricyanide CL. This method is precise, rapid, sensitive, and effective for quality analysis of medical samples and foods [147].
Nucleic acid and protein microarrays are important tools in genomic and proteomic investigations, respectively. Although fluorescence is the dominant detection technique, CL imaging with charged, coupled detection device cameras could be used to detect molecules bound to arrays [148–152]. This technology is used for the detection of allergen-specific IgE [148], cytokines [153], and differential gene expression profiling [154]. Another emerging application of imaging coupled with CL is in microscopy [155] and monitoring assays in high-density microwell plates for drug assays [156]. A new nanoparticle-based niobate semiconductor photocatalyst, Sr(0.4)H(1.2)Nb(2) O(6) H(2)O(HSN), was used to determine the cathodic ECL of luminol. It was noted that HSN can be excited electrically, and this electrode, under a low potential, injected electrons into the aqueous solution of electrolytes and that these electrons only excited luminol. This luminol-ECL method has a lot of advantages, such as high sensitivity response, simple instrumentation, high stability, a less time-consuming procedure, and a high interference ability [157].
Forensic Science
Luminol is commonly used in forensics as a diagnostic tool for the detection of blood stains. Most crime scene investigation, known as criminalistics, is based on the fact that nothing vanishes without a trace and minute particles of blood will adhere to most surfaces for years. The basic idea of luminol is to reveal these traces with a light-producing chemical reaction between several chemicals and hemoglobin, an oxygen-carrying protein in the blood. The presence of unnoticed, minute, or hidden blood stains diluted to a level of 1×106 (1 µL of blood in 1 L of solution) can be detected using luminol [47, 158], disclosing the distribution of bloodstains and allowing easy evaluation of their patterns. Investigators are then able to reconstruct the source of the events of a crime by visualizing and analyzing these patterns [159, 160]. Other chemical-based tests have also been widely employed, such as fluorescein, phenolphthalein (Kastle–Meyer reaction), leucomalachite green (Medinger reaction), and tetramethylbenzidine. Physical techniques, like the use of the Polilight® (Rofin, Dingley, Australia) light source in the forensic detection of blood, are useful under specific conditions, but they do not possess the high sensitivity of luminol [161–163].
Patented luminol molecules or luminol blood-dependent CL enhancers are used in several luminol preparations [142, 164]. They are thought to improve sensitivity, specificity, and duration of the emission of luminescence. This formulation is used by forensic investigators due to their good performance, low cost, easy availability of ingredients, and simplicity of preparation. Luminol solution is usually sprayed directly in a completely dark environment, regardless of preparation. The luminescent areas are marked for their detection once the light emission has faded, and the light obtained can be photographed or filmed [47, 165].
DNA Detection
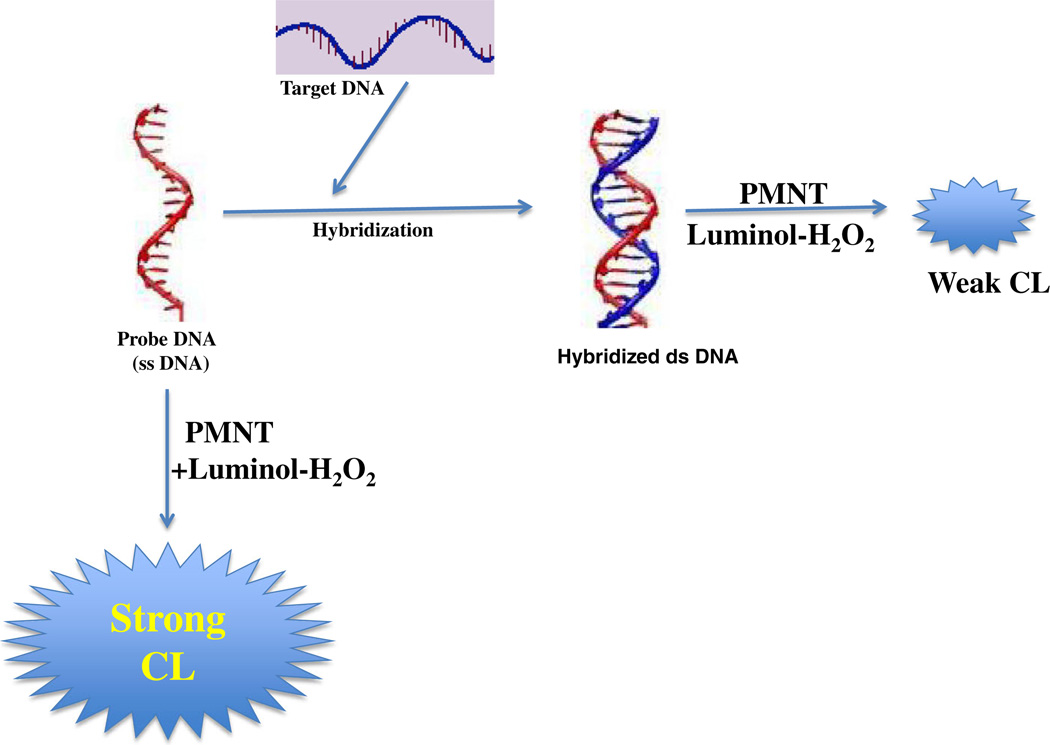
Recently, conjugated polyelectrolytes (CPEs) were shown to initiate strong LCL emission in weakly basic medium. Liu et al. [166] explored the effects of CPEs on LCL by taking the luminol-H2O2 reaction as a model. The polyelectrolyte conjugate, named poly[3–(3′-N, N, N-triethylamino-1′-propyloxy)-4-methyl-2,5-thiophenehydrochloride] (PMNT), is a cationic CPE and can significantly enhance luminol–H2O2 CL emission. The enhancement property of PMNT on the LC system was found to be closely related to the conformation of PMNT. LCL intensity of the luminol-H2O2–PMNT system was much greater in the presence of single-stranded DNA (ssDNA) than that of double-stranded DNA (dsDNA). Using this technique, a new label-free and homogeneous CL-based detection method for DNA hybridization was proposed (Fig 7). In this method, DNA strands do not require a labeling or modification step and hybridization was completely separate from detection, so it could be done under optimal conditions in solution without the steric constraints of surface-bound probes. The detection limit of target DNA was as low as 3.7×10−13 M. Therefore, the CPE-based LCL method opens a new pathway for highly sensitive detection of DNA hybridization.
Fig. 7.
Label-free CL detection of DNA hybridization using cationic-conjugated polyelectrolyte as the catalyst under different conformations produced with nucleic acid
Pharmaceutical Analysis
Recently, Shao et al. [167] developed an LCL-based flow injection method where a myoglobin-luminol system was used for the detection of the drug, aniracetam, which is used for treatment of the central nervous system. The myoglobin-conjugated complex of aniracetam catalyzed the CL reaction of luminol, leading to an enhanced CL compared to that without aniracetam. This method was also employed for determination of cefazolin sodium in injectable powder preparations and human urine [168]. Similarly, Huet al. [169] developed a new LCL-based method for the quantification of lincomycin hydrochloride in human serum. A highly sensitive and simple method based on increased CL intensity of a luminol–H2O2 system has been used for determination of sulpiride content in drugs and biological fluids [170].
A luminol-periodate potassium CL system was used to analyze isoniazid, most frequently used as an antituberculosis drug [171]. Tanshinol-borneol ester is a chemical combination of danshensu and borneol used as an experimental drug that exhibits efficacious anti-ischemic activity. An ultrasensitive LCL method for the determination of tanshinol was established based on the inhibitory effect of tanshinol on the CL signal produced from the reaction between potassium permanganate and luminol [172]. Another simple and selectivemethod was developed for the detection of quinones. This method employed time-resolved fluorescence microplate reader-based CL detection for measurement. When quinones are photo-irradiated, they liberate reactive oxygen species, which further react with photosensitized CL reagents. This method was successfully applied for determination of menadione in spiked human serum samples [173].
A rapid CL system coupled with a microfluidic chip was developed to determine vitamin B12 based on the reaction of luminol and silver nitrate in the presence of gold nanoparticles. Ag+ was used as a chemiluminogenic oxidant in this CL reaction that oxidizes luminol to produce a strong CL signal in the presence of gold nanoparticles. Luminol reacts with silver nitrate by using gold nanoparticles as a catalyst to produce luminol radicals, which react with dissolved oxygen and emit CL light. This CL method was used to determine the amount of vitamin B12 in tablets and multivitamin preparations [174].
Another luminol reagent, 4-(6,7-dihydro-5,8-dioxothiazolo[4,5–g]phthalazin-2-yl) benzoic acid N-hydroxysuccinimide ester, was synthesized as a CL-emitting source for some amines. This reagent reacts with amines in the presence of triethylamine to produce derivatives that show CL emission in the presence of potassium hexacyanoferrate (III) and H2O2 [175]. This CL method has been used to determine the concentration of amantadine hydrochloride in humans. The concentration of levodopa [(−)-(3)-(3,4-dihydroxylphenyl)-l-alanine], which is a very important neurotransmitter used for the treatment of Parkinson’s disease, has been determined based on the luminol-potassium ferricyanide reaction. This LCL-based HPLC detection method with microdialysis sampling allowed for the study of the pharmacokinetics of levodopa [176]. LCL methodology was also reported for the determination of unbound oxacillin and for the study of its interactions with cefoperazone [177, 178]. Post-column photolysis, including derivatization and CL detection by means of luminol, has been used for the determination of busulfan (drug used for leukemia treatment) in serum. Other drugs, like amikacin, a water soluble, semisynthetic, broad spectrum aminoglycoside antibiotic, were measured in body fluids by an LCL-based HPLC system [76]. With the help of a highly selective flow injection CL method, fenvalerate has been detected in renal samples with satisfactory results [179].
Catecholamines can be quantified with the help of LCL using potassium ferricyanide as an oxidant, which enhances LCL emission. This rapid, accurate, and simple reverse phase HPLC-CL method has been developed for the determination of epinephrine, norepinephrine, and dopamine [180] and has been applied successfully in spiked human serum samples. These compounds inhibit LCL in the luminol-I2 system, and the inhibitory property of these catecholamines has been applied in HPLC-CL-based applications for the quantification of dopamine, epinephrine, and norepinephrine [181] and also for the analysis of catecholamines in urine samples. Another luminol derivative, 6-aminomethylphthalhydrazide (6-AMP), has been used as a CL agent for the quantification of 5-hydroxyindole-3-acetic acid in human urine, an abundant end product for peripheral and central serotonin metabolism. The luminol-related CL reagents have a benzylamine skeleton that reacts with 5-hydroxyindole and produces a benzoxazole derivative, which further reacts with H2O2 in the presence of hexacyanoferrate(III) and produces CL [182]. Another similar method has been developed to compare the levels of epinephrine hormone in urine from smokers and nonsmokers. In this sensitive CL system, the production of luminol-diperiodatocuprate (III) (K5[Cu(HIO6)2], DPC) can be used to measure epinephrine hormone [183].
Advantages of Luminal and Concluding Remarks
Luminol, fluorescein (hemascein), hydrogen peroxide, acridinium derivatives, dioxetanes, coelenterazines, and peroxyoxalic derivatives are currently used in CL reactions for the detection and analysis of different types of compounds or molecules. Each has its own advantages and disadvantages. Since the report that hemascein produced background fluorescence and also appeared to give a negative result, it was no longer considered a viable option. Both fluorescein and hemascein were disregarded as potential methods due to excessive background staining and the inability of hemascein to react with neat blood.
Hydrogen peroxide was a less sensitive method compared to luminol. Hydrogen peroxide can cause irritation of the skin, eyes, throat, or lungs. Oxalate derivatives were not stable in water or in moist solvents. After partial water hydrolysis, the monosubstituted derivative was further decomposed by decarboxylation and decarbonylation [184]. This instability strongly limited their applications in the diagnostic field. Another problem was the low water solubility of both partners of this CL reaction, which made them unsuitable for protein coupling. The unresolved water solubility and stability problems, in addition to a loss of efficiency in water, certainly explained the limited success of peroxyoxalate CL in immunoassays and biomedical applications.
The derivatives of acridinium had high quantum yields. Background signals were low and high sensitivities were frequently obtained, as catalysts were not needed. But, in this method, instantaneous light emission took place, which was considered a major disadvantage, as it creates measuring problems in high-rated automated analyzers. As was the case for acridinium derivatives, low background signals were observed. Although dioxetanes showed prolonged light emission, they needed a longer time period before a constant signal was achieved. This delay preceding the steady-state CL, which was directly related to the alkaline phosphatase concentration, represented an unnecessary addition of time in the incubation periods of immunoassays.
Luminol, isoluminol, and their derivatives have been applied in a broad field, including immunoassays or non-immunoassay diagnostics, in monitoring techniques and in biosensors. The sensitivity, specificity, broad range, and diversity of LCL have a dynamic range of applications. Luminol is the most frequently used CL compound. It requires a catalyst for the emission of CL and an enhancer to be competitive in terms of sensitivity, which can result in higher background signals. However, this problem can be overcome by using optimized luminol preparations. Another advantage of the luminol test is that it cannot cause much damage to genetic material, especially when the sample may be used for further experiments, like the use of modern PCR techniques to analyze DNA.
Future Prospective
Luminol-based detection methods can be further standardized and developed for the ultra sensitive, high-throughput detection of pathogens capable of causing infectious diseases. Some groups tried to develop such detection methods by using luminol and the specific hybridization of DNA sequences. This method was validated by the successful identification of pathogens that associated with infectious diarrheal diseases, such as Vibrio cholerae, Shigella, and Salmonella [185]. Such method should be extended to other diseases by immobilizing corresponding probe associated with those diseases. By the development of luminol-based ultra sensitive assays, we can get success in disease diagnosis and prevention.
Other scenario of future applications of luminol is in the field of flow detection methods, and as described earlier, luminol-coupled flow injection CL has been used in various detection and purification methods. We require further improvements and optimization in this field, so that LCL-based flow injection methods can be used widely and with high efficiency.
We can also use LCL in controlling infections born by infected blood and blood-related instruments in hospitals; luminol test provides a suitable method to identify blood contaminations in hospital environments. We can check decontamination procedures and efficiency of cleaning methods by LC test. Luminol-H2O2-HRP system can also be used for the imaging purposes such as in cancerous cell imaging but it requires further improvements so that it can be used with equal efficiency in other cell or tissue imaging or in metabolic pathway studies [186]. LC-based techniques can be used as valuable tools in neutrophil-related research on innate immunity mechanisms; as with the help of LCL, we can study ROS level during infections which is a very important information for the study of innate immunity and inflammation. In this way, LCL can be further exploited in the future for research purposes. In the future, LCL can also be used in more clinical and non-clinical research applications by developing novel luminol preparations that enhance the CL for use in more sensitive and reliable CL assays.
Acknowledgments
This work was supported by National Institutes of Health grants DK040163 to W.S.S. and DK52194, DK068839 and AI44458 to J.A.C. We thank Tracey Baird and Shirley Bratcher for the editorial assistance in preparing this manuscript. MIH is thankful to the Indo-US Science and Technology Forum (IUSTF) for the Award of an IUSTF fellowship. PK and DI thank the Indian Council of Medical Research and University Grants commissions, respectively, for the award of fellowship.
Footnotes
Patent information Waheed et al., Patent NO: US 7674629 B2, Dated: March 9, 2010.
Contributor Information
Parvez Khan, Centre for Interdisciplinary Research in Basic Sciences, Jamia Millia Islamia, Jamia Nagar, New Delhi 110025, India.
Danish Idrees, Centre for Interdisciplinary Research in Basic Sciences, Jamia Millia Islamia, Jamia Nagar, New Delhi 110025, India.
Michael A. Moxley, Edward A. Doisy Department of Biochemistry and Molecular Biology, Saint Louis University School of Medicine, 1100 S. Grand Blvd., DRC Room 615, St. Louis, MO, USA
John A. Corbett, Department of Biochemistry, Medical College of Wisconsin, Milwaukee, WI, USA
Faizan Ahmad, Centre for Interdisciplinary Research in Basic Sciences, Jamia Millia Islamia, Jamia Nagar, New Delhi 110025, India.
Guido von Figura, Department of Internal Medicine, Klinikum Rechts der Isar, Technical University of Munich, Munich, Germany.
William S. Sly, Edward A. Doisy Department of Biochemistry and Molecular Biology, Saint Louis University School of Medicine, 1100 S. Grand Blvd., DRC Room 615, St. Louis, MO, USA
Abdul Waheed, Email: waheeda@slu.edu, Edward A. Doisy Department of Biochemistry and Molecular Biology, Saint Louis University School of Medicine, 1100 S. Grand Blvd., DRC Room 615, St. Louis, MO, USA.
Md. Imtaiyaz Hassan, Email: mihassan@jmi.ac.in, Centre for Interdisciplinary Research in Basic Sciences, Jamia Millia Islamia, Jamia Nagar, New Delhi 110025, India.
References
- 1.Barni F, Lewis SW, Berti A, Miskelly GM, Lago G. Talanta. 2007;72(3):896–913. doi: 10.1016/j.talanta.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 2.Isacson U, Kowalewska Wettermark JG. Journal of Inorganic and Nuclear Chemistry. 1978;40:1653–1656. 40, 1653–1656. [Google Scholar]
- 3.Haapakka KE, Kankare JJ, Linke JA. Analytica Chimica Acta. 1982;139:379–382. [Google Scholar]
- 4.Merenyi G, Lind J, Eriksen TE. Journal of Bioluminescence and Chemiluminescence. 1990;5(1):53–56. doi: 10.1002/bio.1170050111. [DOI] [PubMed] [Google Scholar]
- 5.Kricka LJ, Whitehead TP. Journal of Pharmaceutical and Biomedical Analysis. 1987;5(8):829–833. doi: 10.1016/0731-7085(87)80101-2. [DOI] [PubMed] [Google Scholar]
- 6.Tan X, Song Z. Applied Biochemistry and Biotechnology. 2014;172(3):1320–1331. doi: 10.1007/s12010-013-0605-4. [DOI] [PubMed] [Google Scholar]
- 7.Li X, Zhang Z, Tao L, Li Y, Li YY. Applied Biochemistry and Biotechnology. 2013;171(1):63–71. doi: 10.1007/s12010-013-0345-5. [DOI] [PubMed] [Google Scholar]
- 8.Yu L, Li Q, Gai H, Wang Z. Applied Biochemistry and Biotechnology. 2012;166(3):786–795. doi: 10.1007/s12010-011-9467-9. [DOI] [PubMed] [Google Scholar]
- 9.Li X, Liu H, He X, Song Z. Applied Biochemistry and Biotechnology. 2010;160(4):1065–1073. doi: 10.1007/s12010-009-8598-8. [DOI] [PubMed] [Google Scholar]
- 10.Xin TB, Wang X, Jin H, Liang SX, Lin JM, Li ZJ. Applied Biochemistry and Biotechnology. 2009;158(3):582–594. doi: 10.1007/s12010-008-8356-3. [DOI] [PubMed] [Google Scholar]
- 11.Lin J, Yan F, Ju H. Applied Biochemistry and Biotechnology. 2004;117(2):93–102. doi: 10.1385/abab:117:2:093. [DOI] [PubMed] [Google Scholar]
- 12.Francis PS, Barnett NW, Lewis SW, Lim KF. 2004;(19):94–115. doi: 10.1002/bio.756. [DOI] [PubMed] [Google Scholar]
- 13.Wang T, Xue BC, Liu EB. Guang Pu Xue Yu Guang Pu Fen Xi. 2008;28(5):1026–1029. [PubMed] [Google Scholar]
- 14.White EH, Roswell DF. J. G. Burr, ed. 1985:215. [Google Scholar]
- 15.Hui-Chun Y, Wann-Yin L. Analytica Chimica Acta. 2001;442:71–77. [Google Scholar]
- 16.Yeh HC, Lin WY. Talanta. 2003;59(5):1029–1038. doi: 10.1016/S0039-9140(03)00003-1. [DOI] [PubMed] [Google Scholar]
- 17.Hirayama O, Takagi M, Hukumoto K, Katoh S. Analytical Biochemistry. 1997;247(2):237–241. doi: 10.1006/abio.1997.2053. [DOI] [PubMed] [Google Scholar]
- 18.Albrecht HO. Chemical. 1928;136:321–330. [Google Scholar]
- 19.Petre R, Gheorghe Hubca G. U.PB. Sciences. Bulletin, Series B. 2013;75:23–34. [Google Scholar]
- 20.Zhang C, Qi H, Zhang M. Luminescence. 2007;22(1):53–59. doi: 10.1002/bio.926. [DOI] [PubMed] [Google Scholar]
- 21.Neupert W, Oelkers R, Brune K, Geisslinger G. Prostaglandins. 1996;52(5):385–401. doi: 10.1016/s0090-6980(96)00103-7. [DOI] [PubMed] [Google Scholar]
- 22.Rose AL, Waite TD. Analytical Chemistry. 2001;73(24):5909–5920. doi: 10.1021/ac015547q. [DOI] [PubMed] [Google Scholar]
- 23.Jiao TF, Xing YY, Zhou JX. Materials Science Forum. 2011;694:565–569. [Google Scholar]
- 24.Paradies HH. Berichte Der Bunsen-Gesellschaft-Phys. Chemistry Chemical Physics. 1992;96:1027–1031. [Google Scholar]
- 25.Kricka LJ. Analytical Chemistry. 1995;67(12):499R–502R. doi: 10.1021/ac00108a035. [DOI] [PubMed] [Google Scholar]
- 26.Isaction U. Journal of Inorganic and Nuclear Chemistry. 1978;40:1653–1656. [Google Scholar]
- 27.Barnett NW. Encyclopedia of Analytical Science, Second Edition. Elsevier: Oxford; 2005. pp. 511–521. [Google Scholar]
- 28.White EH ZO, Kagi HH, Hill JHM. Journal of the American Chemical Society. 1964;86:940–941. [Google Scholar]
- 29.Rauhut MM, Grayson M. New York: third ed. John Wiley and Sons, Inc.; 1985. pp. 247–248. [Google Scholar]
- 30.White EH, Bursey MM. Journal of the American Chemical Society. 1964:941–942. [Google Scholar]
- 31.Roswell DF, White EH. Methods in Enzymology. 1978;57:409–483. doi: 10.1016/s0076-6879(77)46022-1. [DOI] [PubMed] [Google Scholar]
- 32.King R, Miskelly GM. Talanta. 2005;67(2):345–353. doi: 10.1016/j.talanta.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 33.Larena A, Bernabeu MVE. Optical Pura y Aplicada. 1983;16(2):91–96. [Google Scholar]
- 34.Cui H, Meng R, Jiang H, Sun Y, Lin X. Luminescence: the Journal of Biological and Chemical Luminescence. 1999;14(3):175–182. doi: 10.1002/(SICI)1522-7243(199905/06)14:3<175::AID-BIO540>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 35.Bottu G. Journal of Bioluminescence and Chemiluminescence. 1989;3(2):59–65. doi: 10.1002/bio.1170030205. [DOI] [PubMed] [Google Scholar]
- 36.Lindley PF. Reports on Progress in Physics. 1996;59:867–933. [Google Scholar]
- 37.Wilson R, Schiffrin DJ. Analytical Chemistry. 1996;68(7):1254–1257. doi: 10.1021/ac951023c. [DOI] [PubMed] [Google Scholar]
- 38.Burgoyne LA. U.S. Patent. 1996;5:496–562. [Google Scholar]
- 39.Seitz WR, Hercules DM. Analytical Chemistry. 1972;44:2143–2149. [Google Scholar]
- 40.Lin JM, Shan X, Hanaoka S, Yamada M. Analytical Chemistry. 2001;73(21):5043–5051. doi: 10.1021/ac010573+. [DOI] [PubMed] [Google Scholar]
- 41.Shen J, Chen L, Gao W, Zhou Q. Lihua Jianyan, Huaxue Fence. 1998;34:132–134. [Google Scholar]
- 42.Seitz WR. The Journal of Physical Chemistry. 1975;79:101–106. [Google Scholar]
- 43.Vorobeva TP, et al. Moscow: Bulletin. Academia. Science. USSR Individual. Chemical Science. 1978:474–478. [Google Scholar]
- 44.Lide DR. Boca Raton: CRC Press. (80th ed.) 2000:8–27. [Google Scholar]
- 45.Arnhold J, Mueller S, Arnold K, Grimm E. Journal of Bioluminescence and Chemiluminescence. 1991;6(3):189–192. doi: 10.1002/bio.1170060309. [DOI] [PubMed] [Google Scholar]
- 46.Francis PS, Barnett NW, Lewis SW, Lim KF. Luminescence : the Journal of Biological and Chemical Luminescence. 2004;19(2):94–115. doi: 10.1002/bio.756. [DOI] [PubMed] [Google Scholar]
- 47.Laux DL, James S, Kish PE, Sutton TP. Boca Raton: CRC Press. 2005:369–389. [Google Scholar]
- 48.Quickenden TI, Creamer JI. Luminescence : the Journal of Biological and Chemical Luminescence. 2001;16(4):295–298. doi: 10.1002/bio.657. [DOI] [PubMed] [Google Scholar]
- 49.Creamer JI, Quickenden TI, Crichton LB, Robertson P, Ruhayel RA. Luminescence: the Journal of Biological and Chemical Luminescence. 2005;20(6):411–413. doi: 10.1002/bio.865. [DOI] [PubMed] [Google Scholar]
- 50.Cui H, Shi MJ, Meng R, Zhou J, Lai CZ, Lin XQ. Photochemistry and Photobiology. 2004;79(3):233–241. doi: 10.1562/be-03-28.1. [DOI] [PubMed] [Google Scholar]
- 51.Pan J, Huang Y, Shu W, Cao J. Talanta. 2007;71(5):1861–1866. doi: 10.1016/j.talanta.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 52.Yun Luo YL, Baoqiang Lv, Zhou Z, Xiao D, Choi MMF. Microchim Acta. 2009;164:411–417. [Google Scholar]
- 53.Liu RH, Jacob J, Tennant B. BioTechniques. 1997;22(4):594–595. doi: 10.2144/97224bm01. [DOI] [PubMed] [Google Scholar]
- 54.Vachereau A. Analytical Biochemistry. 1989;179(1):206–208. doi: 10.1016/0003-2697(89)90227-3. [DOI] [PubMed] [Google Scholar]
- 55.Zhu XL, Sly WS. The Journal of Biological Chemistry. 1990;265(15):8795–8801. [PubMed] [Google Scholar]
- 56.Chen H, Gao F, He R, Cui D. Journal of Colloid and Interface Science. 2007;315(1):158–163. doi: 10.1016/j.jcis.2007.06.052. [DOI] [PubMed] [Google Scholar]
- 57.Yaoa HCHC, Yang XF, Lia H. Journal of the Chinese Chemical Society. 2007;54:949–956. [Google Scholar]
- 58.Thorpe GH, Kricka LJ. Methods in Enzymology. 1986;133:331–353. doi: 10.1016/0076-6879(86)33078-7. [DOI] [PubMed] [Google Scholar]
- 59.Matthews JA, Batki A, Hynds C, Kricka LJ. Analytical Biochemistry. 1985;151(1):205–209. doi: 10.1016/0003-2697(85)90073-9. [DOI] [PubMed] [Google Scholar]
- 60.Urdea MS, Horn T, Fultz TJ, Anderson M, Running JA, Hamren S, Ahle D, Chang CA. Nucleic Acids Symposium Series. 1991;24:197–200. [PubMed] [Google Scholar]
- 61.Patolsky F, Weizmann Y, Katz E, Willner I. Angewandte Chemie (International Ed. in English) 2003;42(21):2372–2376. doi: 10.1002/anie.200250379. [DOI] [PubMed] [Google Scholar]
- 62.Dong H, Wang C, Xiong Y, Lu H, Ju H, Zhang X. Biosensors & Bioelectronics. 2013;41:348–353. doi: 10.1016/j.bios.2012.08.050. [DOI] [PubMed] [Google Scholar]
- 63.Lin Z, Sun X, Lin Y, Chen G. The Analyst. 2013;138(8):2269–2278. doi: 10.1039/c3an36503d. [DOI] [PubMed] [Google Scholar]
- 64.Kugimiya A, Fukada R, Funamoto D. Analytical biochemistry. 2013;443(1):22–26. doi: 10.1016/j.ab.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 65.Rongen HA, Hoetelmans RM, Bult A, van Bennekom WP. Journal of Pharmaceutical and Biomedical analysis. 1994;12(4):433–462. doi: 10.1016/0731-7085(94)80027-8. [DOI] [PubMed] [Google Scholar]
- 66.Luppa P, Bruckner C, Schwab I, Hauck S, Schmidmayr S, Birkmayer C, Paulus B, Hauptmann H. Clinical Chemistry. 1997;43(12):2345–2352. [PubMed] [Google Scholar]
- 67.Satoh T, Tollerud DJ, Guevarra L, Rakue Y, Nakadate T, Kagawa J. Arerugi = [Allergy] 1995;44(7):661–669. [PubMed] [Google Scholar]
- 68.Duff SE, Li C, Renehan A, O'Dwyer ST, Kumar S. International Journal of Oncology. 2003;22(2):339–343. doi: 10.3892/ijo.22.2.339. [DOI] [PubMed] [Google Scholar]
- 69.Divi RL, Beland FA, Fu PP, Von Tungeln LS, Schoket B, Camara JE, Ghei M, Rothman N, Sinha R, Poirier MC. Carcinogenesis. 2002;23(12):2043–2049. doi: 10.1093/carcin/23.12.2043. [DOI] [PubMed] [Google Scholar]
- 70.Ullman EF, Kirakossian H, Switchenko AC, Ishkanian J, Ericson M, Wartchow CA, Pirio M, Pease J, Irvin BR, Singh S, Singh R, Patel R, Dafforn A, Davalian D, Skold C, Kurn N, Wagner DB. Clinical chemistry. 1996;42(9):1518–1526. [PubMed] [Google Scholar]
- 71.Beaudet L, Bedard J, Breton B, Mercuri RJ, Budarf ML. Genome Research. 2001;11(4):600–608. doi: 10.1101/gr.1725501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou Y, Zhou T, Zhou R, Hu Y. Luminescence : the journal of biological and chemical luminescence. 2013 [Google Scholar]
- 73.Zamburlini A, Maiorino M, Barbera P, Pastorino AM, Roveri A, Cominacini L, Ursini F. Biochimica et Biophysica acta. 1995;1256(2):233–240. doi: 10.1016/0005-2760(95)00025-8. [DOI] [PubMed] [Google Scholar]
- 74.Mahant VK, Gabardy RA. In: Campbell AK, Kricka LJ, Stanley PE, editors. Chichester: Wiley; 1994. p. 257. [Google Scholar]
- 75.Shkapova EA, Kurtasova LM, Savchenko AA. Bulletin of Experimental Biology and Medicine. 2010;149(2):239–241. doi: 10.1007/s10517-010-0916-1. [DOI] [PubMed] [Google Scholar]
- 76.Serrano JM, Silva M. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences. 2006;843(1):20–24. doi: 10.1016/j.jchromb.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 77.Lundqvist H, Dahlgren C. Free Radical Biology & Medicine. 1996;20(6):785–792. doi: 10.1016/0891-5849(95)02189-2. [DOI] [PubMed] [Google Scholar]
- 78.Vadrot N, Ghanem S, Braut F, Gavrilescu L, Pilard N, Mansouri A, Moreau R, Reyl-Desmars F. PLoS One. 2012;77:e40879. doi: 10.1371/journal.pone.0040879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Adachi J, Kudo R, Ueno Y, Hunter R, Rajendram R, Want E, Preedy VR. The Journal of Nutrition. 2001;131(11):2916–2920. doi: 10.1093/jn/131.11.2916. [DOI] [PubMed] [Google Scholar]
- 80.Hui SP, Murai T, Yoshimura T, Chiba H, Nagasaka H, Kurosawa T. Lipids. 2005;40(5):515–522. doi: 10.1007/s11745-005-1412-2. [DOI] [PubMed] [Google Scholar]
- 81.Hui SP, Chiba H, Sakurai T, Asakawa C, Nagasaka H, Murai T, Ide H, Kurosawa T. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences. 2007;857(1):158–163. doi: 10.1016/j.jchromb.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 82.Chai Y, Tian D, Wang W, Cui H. Chemical Communications (Cambridge, England) 2010;46(40):7560–7562. doi: 10.1039/c0cc02356f. [DOI] [PubMed] [Google Scholar]
- 83.Zhang L, Lu B, Lu C. The Analyst. 2013;138(3):850–855. doi: 10.1039/c2an36510c. [DOI] [PubMed] [Google Scholar]
- 84.Yu X, Liu X, Mou C, Wang Z. Luminescence: the journal of biological and chemical luminescence. 2012;28(6):847–852. doi: 10.1002/bio.2444. [DOI] [PubMed] [Google Scholar]
- 85.Alam AM, Kamruzzaman M, Dang TD, Lee SH, Kim YH, Kim GM. Analytical and Bioanalytical Chemistry. 2012;404(10):3165–3173. doi: 10.1007/s00216-012-6429-1. [DOI] [PubMed] [Google Scholar]
- 86.Guan G, Yang L, Mei Q, Zhang K, Zhang Z, Han MY, Guan G, Yang L, Mei Q, Zhang K, Zhang Z, Han MY. Analytical Chemistry. 2012;84(21):9492–9497. doi: 10.1021/ac302341b. [DOI] [PubMed] [Google Scholar]
- 87.Li J, Li S, Wei X, Tao H, Pan H. Analytical chemistry. 2012;84(22):9951–9955. doi: 10.1021/ac302401s. [DOI] [PubMed] [Google Scholar]
- 88.Giokas DL, Christodouleas DC, Vlachou I, Vlessidis AG, Calokerinos AC. Analytica Chimica Acta. 2013;764:70–77. doi: 10.1016/j.aca.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 89.Hong L, Liu AL, Li GW, Chen W, Lin XH. Biosensors & Bioelectronics. 2013;43:1–5. doi: 10.1016/j.bios.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 90.Zhao S, Liu YM. Methods in Molecular Biology. 2013;919:79–85. doi: 10.1007/978-1-62703-029-8_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen W, Hong L, Liu AL, Liu JQ, Lin XH, Xia XH. Talanta. 2012;99:643–648. doi: 10.1016/j.talanta.2012.06.061. [DOI] [PubMed] [Google Scholar]
- 92.Li X, Zhang Z, Tao L. Biosensors & Bioelectronics. 2013;47:356–360. doi: 10.1016/j.bios.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 93.Xu S, Zhang X, Liu W, Sun Y, Zhang H. Biosensors & Bioelectronics. 2013;43:160–164. doi: 10.1016/j.bios.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 94.Zhou ZM, Yu Y, Zhao YD. The Analyst. 2012;137(18):4262–4266. doi: 10.1039/c2an35520e. [DOI] [PubMed] [Google Scholar]
- 95.Li F, Cui H. Biosensors & Bioelectronics. 2013;39(1):261–267. doi: 10.1016/j.bios.2012.07.060. [DOI] [PubMed] [Google Scholar]
- 96.Zhang M, Yuan R, Chai Y, Chen S, Zhong H, Wang C, Cheng Y. Biosensors & Bioelectronics. 2012;32(1):288–292. doi: 10.1016/j.bios.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 97.Liu Q, Wu J, Tian J, Zhang C, Gao J, Latep N, Ge Y, Qin W. Talanta. 2012;97:193–198. doi: 10.1016/j.talanta.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 98.Hao M, Liu N, Ma Z. The Analyst. 2013;138(15):4393–4397. doi: 10.1039/c3an00451a. [DOI] [PubMed] [Google Scholar]
- 99.Xiaolan C, Chuhua H, Zhifan Z, Jianxiu W. Biosensors and Bioelectronics. 2013;47:335–339. [Google Scholar]
- 100.Huang X, Ren J. Analytica Chimica Acta. 2011;686(1–2):115–120. doi: 10.1016/j.aca.2010.11.043. [DOI] [PubMed] [Google Scholar]
- 101.Cao Y, Yuan R, Chai Y, Liu H, Liao Y, Zhuo Y. Talanta. 2013;113:106–112. doi: 10.1016/j.talanta.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 102.Cheng Y, Yuan R, Chai Y, Niu H, Cao Y, Liu H, Bai L, Yuan Y. Analytica Chimica Acta. 2012;745:137–142. doi: 10.1016/j.aca.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 103.Fordyce CA, Heaphy CM, Griffith JK. BioTechniques. 2002;33(1):144–146. 148. doi: 10.2144/02331md02. [DOI] [PubMed] [Google Scholar]
- 104.Vesanen M, Piiparinen H, Kallio A, Vaheri A. Journal of Virological Methods. 1996;59(1–2):1–11. doi: 10.1016/0166-0934(95)01991-x. [DOI] [PubMed] [Google Scholar]
- 105.Demby AH, Chamberlain J, Brown DW, Clegg CS. Journal of Clinical Microbiology. 1994;32(12):2898–2903. doi: 10.1128/jcm.32.12.2898-2903.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.JU’Ren JV. In: Campbell AK, Kricka LJ, Stanley PE, editors. Chichester: Wiley; 1994. p. 60. [Google Scholar]
- 107.Bronstein I, Fortin J, Stanley PE, Stewart GS, Kricka LJ. Analytical Biochemistry. 1994;219:169–181. doi: 10.1006/abio.1994.1254. [DOI] [PubMed] [Google Scholar]
- 108.Bronstein I, Martin CS, Fortin JJ, Olesen CE, Voyta JC. Clinical Chemistry. 1996;42(9):1542–1546. [PubMed] [Google Scholar]
- 109.Hassan MI, Waheed A, Ahmad F, Van Etten RL. Applied Biochemistry and Biotechnology. 2013;170(4):972–979. doi: 10.1007/s12010-013-0242-y. [DOI] [PubMed] [Google Scholar]
- 110.Aoki M, Ono Y, Kunii O, Goldstein E. The Journal of Antimicrobial Chemotherapy. 1994;34(3):383–390. doi: 10.1093/jac/34.3.383. [DOI] [PubMed] [Google Scholar]
- 111.Hawkins E, Cumming R. The Journal of Histochemistry and Cytochemistry. 1990;38(3):415–419. doi: 10.1177/38.3.1689340. [DOI] [PubMed] [Google Scholar]
- 112.Mavri-Damelin D, Wilden J, Mani AR, Selden C, Hodgson HJ, Damelin LH. Bioconjugate Chemistry. 2009;20(2):266–273. doi: 10.1021/bc800361r. [DOI] [PubMed] [Google Scholar]
- 113.Tsai JJ, Yu LN, Wang SR. Zhonghua Minguo Guo Wei Sheng Wu Ji Mian Yi Xue Za Zhi. 1992;25(2):78–90. [PubMed] [Google Scholar]
- 114.Van Dyke K, Van Dyke C. Methods in Enzymology. 1986;133:493–507. doi: 10.1016/0076-6879(86)33086-6. [DOI] [PubMed] [Google Scholar]
- 115.Saleh RA, Agarwal A, Kandirali E, Sharma RK, Thomas AJ, Nada EA, Evenson DP, Alvarez JG. Fertility and Sterility. 2002;78(6):1215–1224. doi: 10.1016/s0015-0282(02)04237-1. [DOI] [PubMed] [Google Scholar]
- 116.Moran P, Rico G, Ramiro M, Olvera H, Ramos F, Gonzalez E, Valadez A, Curiel O, Melendro EI, Ximenez C. The American Journal of Tropical Medicine and Hygiene. 2002;67(6):632–635. doi: 10.4269/ajtmh.2002.67.632. [DOI] [PubMed] [Google Scholar]
- 117.Braga PC, Dal Sasso M, Dal Negro R. Drugs under Experimental and Clinical Research. 2002;28(4):133–145. [PubMed] [Google Scholar]
- 118.Nosal R, Drabikova K, Ciz M, Lojek A, Danihelova E, et al. Inflammation Research: Official Journal of the European Histamine Research Society. 2002;51(11):557–562. doi: 10.1007/pl00012427. [DOI] [PubMed] [Google Scholar]
- 119.Marzocchi-Machado CM, Alves CM, Azzolini AE, Polizello AC, Carvalho IF, Lucisano-Valim YM. Lupus. 2002;11(4):240–248. doi: 10.1191/0961203302lu172oa. [DOI] [PubMed] [Google Scholar]
- 120.Huang ZH, Hii CS, Rathjen DA, Poulos A, Murray AW, Ferrante A. The Biochemical Journal. 1997;325(Pt 2):553–557. doi: 10.1042/bj3250553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Braun JM, Gemmell CG, Beuth J, Ko HL, Pulverer G. Zentralblatt fur Bakteriologie: International Journal of Medical Microbiology. 1995;283(1):90–94. doi: 10.1016/s0934-8840(11)80894-7. [DOI] [PubMed] [Google Scholar]
- 122.Chettibi S, Lawrence AJ, Stevenson RD, Young JD. FEMS immunology and Medical Microbiology. 1994;8(3):271–281. doi: 10.1111/j.1574-695X.1994.tb00452.x. [DOI] [PubMed] [Google Scholar]
- 123.Hansen PS, Petersen SB, Varning K, Nielsen H. Scandinavian Journal of Gastroenterology. 2002;37(7):765–771. doi: 10.1080/00365520213255. [DOI] [PubMed] [Google Scholar]
- 124.Moriwaki Y, Sugiyama M, Ozawa Y, Mochizuki Y, Kunisaki C, Kamiya N, Yamazaki Y, Suda T. World Journal of Surgery. 2002;26(5):521–526. doi: 10.1007/s00268-001-0293-z. [DOI] [PubMed] [Google Scholar]
- 125.Tseng JC, Kung AL. Chemistry & Biology. 2012;19(9):1199–1209. doi: 10.1016/j.chembiol.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 126.Muller CH, Lee TK, Montano MA. Methods in Molecular Biology. 2013;927:363–376. doi: 10.1007/978-1-62703-038-0_31. [DOI] [PubMed] [Google Scholar]
- 127.Towbin H, Staehelin T, Gordon J. Proceedings of the National Academy of Sciences of the United States of America. 1979;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bonapace G, Waheed A, Shah GN, Sly WS. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(33):12300–12305. doi: 10.1073/pnas.0404764101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Waheed A, Zhu XL, Sly WS. The Journal of Biological Chemistry. 1992;267(5):3308–3311. [PubMed] [Google Scholar]
- 130.Khan I, Siddique I, Al-Awadi FM, Mohan K. Canadian journal of gastroenterology = Journal canadien de gastroenterologie. 2003;17(1):31–36. doi: 10.1155/2003/673819. [DOI] [PubMed] [Google Scholar]
- 131.Guo Fang ZHC. Analytica Chimica Acta. 2000;409:75–81. [Google Scholar]
- 132.Afsaneh SMAK. Analytica Chimica Acta. 2002;468:53–63. [Google Scholar]
- 133.Xiaoyu Liu AL, Zhou B, Qiu C, Ren H. Chemistry Central Journal. 2009;3:7. doi: 10.1186/1752-153X-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yaqoob M, Folgado Biot B, Nabi A, Worsfold PJ. Luminescence: the Journal of Biological and Chemical Luminescence. 2012;27(5):419–425. doi: 10.1002/bio.1366. [DOI] [PubMed] [Google Scholar]
- 135.Serrano JM, Silva M. Journal of Chromatography A. 2006;1117(2):176–183. doi: 10.1016/j.chroma.2006.03.086. [DOI] [PubMed] [Google Scholar]
- 136.Wang F, Orbach R, Willner I. Chemistry. 2012;18(50):16030–16036. doi: 10.1002/chem.201201479. [DOI] [PubMed] [Google Scholar]
- 137.Yang XA, Zhang WB. Luminescence: the journal of biological and chemical luminescence. 2013;28(5):641–647. doi: 10.1002/bio.2409. [DOI] [PubMed] [Google Scholar]
- 138.Chen YC, Jian YL, Chiu KH, Yak HK. Analytical Sciences. 2012;28(8):795–799. doi: 10.2116/analsci.28.795. [DOI] [PubMed] [Google Scholar]
- 139.Motsenbocker M, Sugawara T, Shintani M, Masuya H, Ichimori Y, Kondo K. Analytical Chemistry. 1993;65:403–408. [Google Scholar]
- 140.Kikuchi K, Nagano T, Hayakawa H, Hirata Y, Hirobe M. Analytical Chemistry. 1993;65(13):1794–1799. doi: 10.1021/ac00061a025. [DOI] [PubMed] [Google Scholar]
- 141.Whitehead TP, Thorpe GHG, Maxwell SRJ. Analytica Chimica Acta. 1992;266:265–277. [Google Scholar]
- 142.Yamaguchi M, Yoshida H, Nohta H. Journal of Chromatography A. 2002;950(1–2):1–19. doi: 10.1016/s0021-9673(02)00004-3. [DOI] [PubMed] [Google Scholar]
- 143.Zhu R, Kok WT. Journal of Pharmaceutical and Biomedical Analysis. 1998;17(6–7):985–999. doi: 10.1016/s0731-7085(98)00065-x. [DOI] [PubMed] [Google Scholar]
- 144.Fletcher P, Andrew KN, Calokerinos AC, Forbes S, Worsfold PJ. Luminescence: the Journal of Biological and Chemical Luminescence. 2001;16(1):1–23. doi: 10.1002/bio.607. [DOI] [PubMed] [Google Scholar]
- 145.Felmlee TA, Mitchell PS, Ulfelder KJ, Persing DH, Landers JP. Journal. Capital. Electron. 1995;2:125–130. [Google Scholar]
- 146.Miyazawa T, Suzuki T, Fujimoto K, Kinoshita M. Mechanisms of Ageing and Development. 1996;86(3):145–150. doi: 10.1016/0047-6374(95)01687-2. [DOI] [PubMed] [Google Scholar]
- 147.Lin Z, Wang H, Xu Y, Dong J, Hashi Y, Chen S. Food Chemistry. 2012;134(2):1181–1191. doi: 10.1016/j.foodchem.2012.02.161. [DOI] [PubMed] [Google Scholar]
- 148.Fall BI, Eberlein-Konig B, Behrendt H, Niessner R, Ring J, Weller MG. Analytical Chemistry. 2003;75(3):556–562. doi: 10.1021/ac026016k. [DOI] [PubMed] [Google Scholar]
- 149.Kricka LJ. Annals of Clinical Biochemistry. 2002;39(Pt 2):114–129. doi: 10.1258/0004563021901865. [DOI] [PubMed] [Google Scholar]
- 150.Cheek BJ, Steel AB, Torres MP, Yu YY, Yang H. Analytical Chemistry. 2001;73(24):5777–5783. doi: 10.1021/ac0108616. [DOI] [PubMed] [Google Scholar]
- 151.Huang RP. Journal of Immunological Methods. 2001;255(1–2):1–13. doi: 10.1016/s0022-1759(01)00394-5. [DOI] [PubMed] [Google Scholar]
- 152.Roda A, Guardigli M, Russo C, Pasini P, Baraldini M. BioTechniques. 2000;28(3):492–496. doi: 10.2144/00283st07. [DOI] [PubMed] [Google Scholar]
- 153.Huang RP, Huang R, Fan Y, Lin Y. Analytical Biochemistry. 2001;294(1):55–62. doi: 10.1006/abio.2001.5156. [DOI] [PubMed] [Google Scholar]
- 154.Beck MT, Holle L, Chen WY. BioTechniques. 2001;31(4):782–784. doi: 10.2144/01314st04. [DOI] [PubMed] [Google Scholar]
- 155.Creton R, Jaffe LF. BioTechniques. 2001;31(5):1098–1100. doi: 10.2144/01315rv01. [DOI] [PubMed] [Google Scholar]
- 156.Pasini P, Musiani M, Russo C, Valenti P, Aicardi G, Crabtree JE, Baraldini M, Roda A. Journal of Pharmaceutical and Biomedical Analysis. 1998;18(4–5):555–564. doi: 10.1016/s0731-7085(98)00209-x. [DOI] [PubMed] [Google Scholar]
- 157.Xu H, Ye H, Zhu X, Liang S, Guo L, Lin Z, Liu X, Chen G. The Analyst. 2013;138(1):234–239. doi: 10.1039/c2an36009h. [DOI] [PubMed] [Google Scholar]
- 158.Fregeau CJ, Germain O, Fourney RM. Journal of Forensic Sciences. 2000;45(2):354–380. [PubMed] [Google Scholar]
- 159.Lytle LT, Hedgecock DG. Journal of Forensic Sciences. 1978;23(3):550–562. [PubMed] [Google Scholar]
- 160.Pex OJ. International Association. Bloodstain Pattern Analysis. Newslett. 2005:11–15. [Google Scholar]
- 161.Budowle B, Leggitt JL, Defenbaugh DA, Keys KM, Malkiewicz SF. Journal of Forensic Sciences. 2000;45(5):1090–1092. [PubMed] [Google Scholar]
- 162.Vandenberg N, van Oorschot RA. Journal of Forensic Sciences. 2006;51(2):361–370. doi: 10.1111/j.1556-4029.2006.00065.x. [DOI] [PubMed] [Google Scholar]
- 163.Webb JL, Creamer JI, Quickenden TI. Luminescence: the Journal of Biological and Chemical Luminescence. 2006;21(4):214–220. doi: 10.1002/bio.908. [DOI] [PubMed] [Google Scholar]
- 164.Thornton JI, Maloney RS. Calif. Assoc. Crim. Newslett. 1985:9–16. [Google Scholar]
- 165.Lefebvre G. International Association. Bloodstain Pattern Analysis. Newsletter. 2005:4–7. [Google Scholar]
- 166.Liu M, Li B, Cui X. Biosensors & Bioelectronics. 2013;47:26–31. doi: 10.1016/j.bios.2013.02.047. [DOI] [PubMed] [Google Scholar]
- 167.Shao X, Li Y, Li F, Liu Y, Song Z. Journal of AOAC International. 2011;94(5):1461–1466. doi: 10.5740/jaoacint.10-175. [DOI] [PubMed] [Google Scholar]
- 168.Sun H, Wang J, Wang T. Luminescence: the journal of biological and chemical luminescence. 2013;28(4):592–596. doi: 10.1002/bio.2398. [DOI] [PubMed] [Google Scholar]
- 169.Hu Y, Li G, Zhang Z. Luminescence: the Journal of Biological and Chemical Luminescence. 2011;26(5):313–318. doi: 10.1002/bio.1230. [DOI] [PubMed] [Google Scholar]
- 170.Khan MN, Jan MR, Shah J, Lee SH, Kim YH. Luminescence: the journal of biological and chemical luminescence. 2013;28(6):915–21. doi: 10.1002/bio.2458. [DOI] [PubMed] [Google Scholar]
- 171.Liu Y, Fu Z, Wang L. Luminescence: the Journal of Biological and Chemical Luminescence. 2011;26(6):397–402. doi: 10.1002/bio.1243. [DOI] [PubMed] [Google Scholar]
- 172.Nie F, Bu M, Wu L, Zheng J. Luminescence: the journal of biological and chemical luminescence. 2014;29(2):147–50. doi: 10.1002/bio.2519. [DOI] [PubMed] [Google Scholar]
- 173.Elgawish MS, Shimomai C, Kishikawa N, Ohyama K, Nakashima K, Kuroda N. The Analyst. 2012;137(20):4802–4808. doi: 10.1039/c2an35353a. [DOI] [PubMed] [Google Scholar]
- 174.Kamruzzaman M, Alam AM, Kim KM, Lee SH, Kim YH, Kabir AN, Kim GM, Dang TD. Biomedical Microdevices. 2013;15(1):195–202. doi: 10.1007/s10544-012-9716-x. [DOI] [PubMed] [Google Scholar]
- 175.Yoshida H, Nakao R, Matsuo T, Nohta H, Yamaguchi M. Journal of Chromatography A. 2001;907(1–2):39–46. doi: 10.1016/s0021-9673(00)01059-1. [DOI] [PubMed] [Google Scholar]
- 176.Chen F, Zhang Z, Zhang Y, He D. Analytical and Bioanalytical Chemistry. 2005;382:211. [Google Scholar]
- 177.Qiao M, Guo X, Li F. Journal of Chromatography A. 2002;952(1–2):131–138. doi: 10.1016/s0021-9673(02)00086-9. [DOI] [PubMed] [Google Scholar]
- 178.Li F, Guo X, Qiao M, Xiong Z, Zhou D. Se Pu. 2004;22(4):349–353. [PubMed] [Google Scholar]
- 179.Zang D, Yan M, Zhao P, Ge L, Liu S, Yu J. The Analyst. 2012;137(18):4247–4253. doi: 10.1039/c2an35738k. [DOI] [PubMed] [Google Scholar]
- 180.Fu-Nan Chen Y-XZ, Zhang Z-J. Chinese Journal of Chemistry. 2007;25(7):942–947. [Google Scholar]
- 181.Nalewajko E, Wiszowata A, Kojlo A. Journal of Pharmaceutical and Biomedical Analysis. 2007;43(5):1673–1681. doi: 10.1016/j.jpba.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 182.Yakabe T, Ishida J, Yoshida H, Nohta H, Yamaguchi M. Analytical Sciences. 2000;16:545. [Google Scholar]
- 183.Li T, Wang Z, Xie H, Fu Z. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences. 2012;911:1–5. doi: 10.1016/j.jchromb.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 184.Neuvonen H. Journal of the Chemical Society, Perkin Transactions. 1994;2:89–95. [Google Scholar]
- 185.Wang C, Xiao R, Dong P, Wu X, Rong Z, Xin L, Tang J, Wang S. Biosensors & Bioelectronics. 2014;57C:36–40. doi: 10.1016/j.bios.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 186.Zhang Y, Pang L, Ma C, Tu Q, Zhang R, Saeed E, Mahmoud AE, Wang J. Analysis Chemistry. 2014;86(6):3092–3099. doi: 10.1021/ac404201s. [DOI] [PubMed] [Google Scholar]