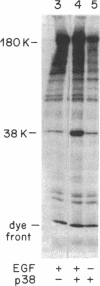
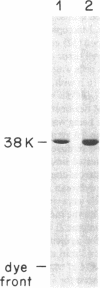
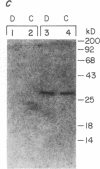
Abstract
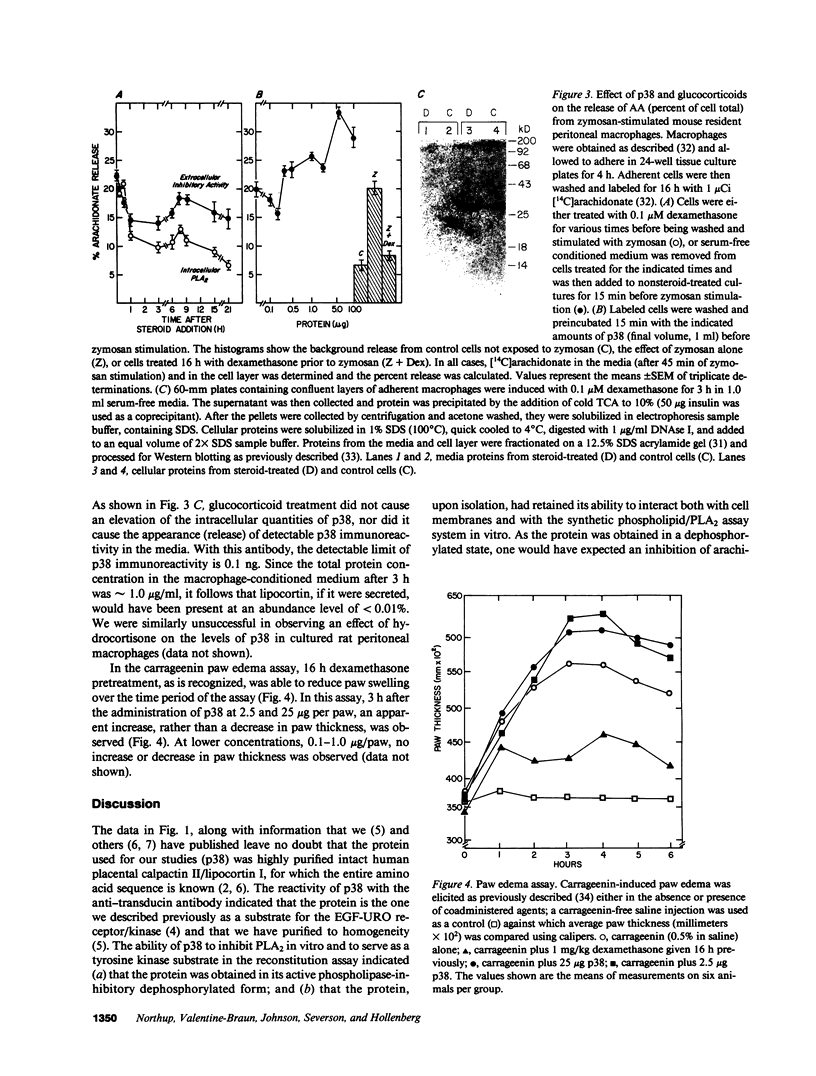
We have examined the ability of a highly purified 38-kD phospholipase-inhibitory protein (p38) isolated from human placental membranes that is also a preferred substrate for the epidermal growth factor-urogastrone (EGF-URO) receptor/kinase, to block the release of arachidonate from zymosan-stimulated murine peritoneal macrophages in vitro and to exhibit antiinflammatory activity in a carrageenin rat paw edema test in vivo. The ability of glucocorticoids to increase the amounts of this protein in macrophage cultures was also examined. p38 represents the naturally occurring, intact, NH2-terminally blocked human placental form of the protein termed calpactin II (or lipocortin I), for which partial amino acid sequence data and a complete amino acid sequence deduced from cDNA analysis have been reported. Our data demonstrated that, whereas p38 was an effective inhibitor of pancreatic phospholipase A2 in vitro, it was unable to inhibit either the release of arachidonate from cultured zymosan-stimulated mouse peritoneal macrophages or inflammation in a rat paw edema test. At comparatively high protein concentrations, p38 enhanced either arachidonate release from intact macrophages in vitro (0.5-10 micrograms/ml) or carrageenin-induced paw swelling in vivo (2.5 or 25 micrograms per injection). Furthermore, we were unable to detect induced amounts of p38 in cultures of glucocorticoid-treated peritoneal macrophages obtained from either mice or rats. Our data indicate that the antiphospholipase activity of p38 in vitro and the ability of p38 to serve as a receptor/kinase substrate may in no way relate to the putative ability of the protein to modify eicosanoid release from macrophages in vivo, so as to modulate the inflammatory process. Our data also raise the possibility that p38 (calpactin II) may not be a true representative of the lipocortin family of glucocorticoid-inducible antiinflammatory proteins, despite its ability to inhibit phospholipase A2 in vitro.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderem A. A., Scott W. A., Cohn Z. A. Evidence for sequential signals in the induction of the arachidonic acid cascade in macrophages. J Exp Med. 1986 Jan 1;163(1):139–154. doi: 10.1084/jem.163.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell G. J., Carnuccio R., Di Rosa M., Flower R. J., Ivanyi J., Langham C. S., Parente L., Persico P., Wood J. Suppression of arachidonate oxidation by glucocorticoid-induced antiphospholipase peptides. Adv Prostaglandin Thromboxane Leukot Res. 1983;11:65–71. [PubMed] [Google Scholar]
- Blackwell G. J., Carnuccio R., Di Rosa M., Flower R. J., Parente L., Persico P. Macrocortin: a polypeptide causing the anti-phospholipase effect of glucocorticoids. Nature. 1980 Sep 11;287(5778):147–149. doi: 10.1038/287147a0. [DOI] [PubMed] [Google Scholar]
- Carnuccio R., Di Rosa M., Persico P. Hydrocortisone-induced inhibitor of prostaglandin biosynthesis in rat leucocytes. Br J Pharmacol. 1980 Jan;68(1):14–16. doi: 10.1111/j.1476-5381.1980.tb10691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirino G., Flower R. J., Browning J. L., Sinclair L. K., Pepinsky R. B. Recombinant human lipocortin 1 inhibits thromboxane release from guinea-pig isolated perfused lung. Nature. 1987 Jul 16;328(6127):270–272. doi: 10.1038/328270a0. [DOI] [PubMed] [Google Scholar]
- Cirino G., Flower R. J. Human recombinant lipocortin 1 inhibits prostacyclin production by human umbilical artery in vitro. Prostaglandins. 1987 Jul;34(1):59–62. doi: 10.1016/0090-6980(87)90262-0. [DOI] [PubMed] [Google Scholar]
- Cloix J. F., Colard O., Rothhut B., Russo-Marie F. Characterization and partial purification of 'renocortins': two polypeptides formed in renal cells causing the anti-phospholipase-like action of glucocorticoids. Br J Pharmacol. 1983 May;79(1):313–321. doi: 10.1111/j.1476-5381.1983.tb10526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson F. F., Dennis E. A., Powell M., Glenney J. R., Jr Inhibition of phospholipase A2 by "lipocortins" and calpactins. An effect of binding to substrate phospholipids. J Biol Chem. 1987 Feb 5;262(4):1698–1705. [PubMed] [Google Scholar]
- De B. K., Misono K. S., Lukas T. J., Mroczkowski B., Cohen S. A calcium-dependent 35-kilodalton substrate for epidermal growth factor receptor/kinase isolated from normal tissue. J Biol Chem. 1986 Oct 15;261(29):13784–13792. [PubMed] [Google Scholar]
- Di Rosa M., Flower R. J., Hirata F., Parente L., Russo-Marie F. Anti-phospholipase proteins. Prostaglandins. 1984 Oct;28(4):441–442. doi: 10.1016/0090-6980(84)90232-6. [DOI] [PubMed] [Google Scholar]
- Fava R. A., Cohen S. Isolation of a calcium-dependent 35-kilodalton substrate for the epidermal growth factor receptor/kinase from A-431 cells. J Biol Chem. 1984 Feb 25;259(4):2636–2645. [PubMed] [Google Scholar]
- Flower R. J. Background and discovery of lipocortins. Agents Actions. 1986 Jan;17(3-4):255–262. doi: 10.1007/BF01982616. [DOI] [PubMed] [Google Scholar]
- Geisow M. J., Walker J. H., Boustead C., Taylor W. Annexins--new family of Ca2+-regulated-phospholipid binding protein. Biosci Rep. 1987 Apr;7(4):289–298. doi: 10.1007/BF01121450. [DOI] [PubMed] [Google Scholar]
- Haigler H. T., Schlaepfer D. D., Burgess W. H. Characterization of lipocortin I and an immunologically unrelated 33-kDa protein as epidermal growth factor receptor/kinase substrates and phospholipase A2 inhibitors. J Biol Chem. 1987 May 15;262(14):6921–6930. [PubMed] [Google Scholar]
- Hee-Cheong M., Fletcher T., Kryski S. K., Severson D. L. Diacylglycerol lipase and kinase activities in rat brain microvessels. Biochim Biophys Acta. 1985 Jan 9;833(1):59–68. doi: 10.1016/0005-2760(85)90253-x. [DOI] [PubMed] [Google Scholar]
- Hirata F., Matsuda K., Notsu Y., Hattori T., del Carmine R. Phosphorylation at a tyrosine residue of lipomodulin in mitogen-stimulated murine thymocytes. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4717–4721. doi: 10.1073/pnas.81.15.4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata F., Schiffmann E., Venkatasubramanian K., Salomon D., Axelrod J. A phospholipase A2 inhibitory protein in rabbit neutrophils induced by glucocorticoids. Proc Natl Acad Sci U S A. 1980 May;77(5):2533–2536. doi: 10.1073/pnas.77.5.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata F. The regulation of lipomodulin, a phospholipase inhibitory protein, in rabbit neutrophils by phosphorylation. J Biol Chem. 1981 Aug 10;256(15):7730–7733. [PubMed] [Google Scholar]
- Hock R. A., Hollenberg M. D. Characterization of the receptor for epidermal growth factor-urogastrone in human placenta membranes. J Biol Chem. 1980 Nov 25;255(22):10731–10736. [PubMed] [Google Scholar]
- Hollenberg M. D., Valentine-Braun K. A., Northup J. K. Protein tyrosine kinase substrates: Rosetta stones or simply structural elements? Trends Pharmacol Sci. 1988 Feb;9(2):63–66. doi: 10.1016/0165-6147(88)90119-8. [DOI] [PubMed] [Google Scholar]
- Huang K. S., Wallner B. P., Mattaliano R. J., Tizard R., Burne C., Frey A., Hession C., McGray P., Sinclair L. K., Chow E. P. Two human 35 kd inhibitors of phospholipase A2 are related to substrates of pp60v-src and of the epidermal growth factor receptor/kinase. Cell. 1986 Jul 18;46(2):191–199. doi: 10.1016/0092-8674(86)90736-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Patriarca P., Beckerdite S., Elsbach P. Phospholipases and phospholipid turnover in Escherichia coli spheroplasts. Biochim Biophys Acta. 1972 Apr 18;260(4):593–600. doi: 10.1016/0005-2760(72)90008-2. [DOI] [PubMed] [Google Scholar]
- Pepinsky R. B., Sinclair L. K. Epidermal growth factor-dependent phosphorylation of lipocortin. Nature. 1986 May 1;321(6065):81–84. doi: 10.1038/321081a0. [DOI] [PubMed] [Google Scholar]
- Rothhut B., Russo-Marie F., Wood J., DiRosa M., Flower R. J. Further characterization of the glucocorticoid-induced antiphospholipase protein "renocortin". Biochem Biophys Res Commun. 1983 Dec 28;117(3):878–884. doi: 10.1016/0006-291x(83)91678-9. [DOI] [PubMed] [Google Scholar]
- Saris C. J., Tack B. F., Kristensen T., Glenney J. R., Jr, Hunter T. The cDNA sequence for the protein-tyrosine kinase substrate p36 (calpactin I heavy chain) reveals a multidomain protein with internal repeats. Cell. 1986 Jul 18;46(2):201–212. doi: 10.1016/0092-8674(86)90737-3. [DOI] [PubMed] [Google Scholar]
- Valentine-Braun K. A., Hollenberg M. D., Fraser E., Northup J. K. Isolation of a major human placental substrate for the epidermal growth factor (urogastrone) receptor kinase: immunological cross-reactivity with transducin and sequence homology with lipocortin. Arch Biochem Biophys. 1987 Dec;259(2):262–282. doi: 10.1016/0003-9861(87)90494-2. [DOI] [PubMed] [Google Scholar]
- Valentine-Braun K. A., Northup J. K., Hollenberg M. D. Epidermal growth factor (urogastrone)-mediated phosphorylation of a 35-kDa substrate in human placental membranes: relationship to the beta subunit of the guanine nucleotide regulatory complex. Proc Natl Acad Sci U S A. 1986 Jan;83(2):236–240. doi: 10.1073/pnas.83.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner B. P., Mattaliano R. J., Hession C., Cate R. L., Tizard R., Sinclair L. K., Foeller C., Chow E. P., Browing J. L., Ramachandran K. L. Cloning and expression of human lipocortin, a phospholipase A2 inhibitor with potential anti-inflammatory activity. Nature. 1986 Mar 6;320(6057):77–81. doi: 10.1038/320077a0. [DOI] [PubMed] [Google Scholar]