On April 10, 2010 the Institutes of Medicine (IOM) released its report, A National Cancer Clinical Trials System for the 21st Century: Reinvigorating the NCI Cooperative Group Program. The IOM report called for major efforts to improve the speed and efficiency of design, launch, and conduct of cancer clinical trials; to make optimal use of scientific innovations; to improve the selection, prioritization, support, and completion of trials; and to foster expanded participation of both patients and physicians [1].
Almost 2 years later, in the May 10 issue of New England Journal of Medicine, 2 original articles describing the beneficial effect of continuous lenalidomide therapy after autologous hematopoietic stem cell transplantation (HCT) for multiple myeloma were published [2,3]. These articles were cited as demonstrating major advances in cancer treatment in 2012 by the American Society of Clinical Oncology in its annual Report on Progress Against Cancer [4]. One of these articles [2] was noteworthy not only for its potentially practice-changing findings but also because it was the product of a rigorously conducted, large, cooperative group study performed in the United States.
The other study [3] was a product of the Intergroupe Francophone du Myelome, based in France, which has completed at least 5 definitive trials of HCT in myeloma since 1996. During that time, only 1 large cooperative group study of HCT for myeloma was completed in the United States. However, this paucity of US HCT trials seems to be changing. In 2011, the Blood and Marrow Transplantation Clinical Trials Network (BMT CTN) 0102 study was published [5]. BMT CTN 0102 enrolled 710 patients from 37 centers over 3 years to compare an allogeneic versus a second autologous HCT after autologous HCT done as consolidation therapy for the initial treatment of myeloma. It found no significant advantage for allogeneic HCT in patients with standard-risk disease, which is in itself an important observation, but the study also demonstrated that large multicenter HCT trials can be successfully performed in North America.
The BMT CTN was established in 2001 to “conduct scientifically meritorious multicenter trials in an efficient manner to improve transplantation outcomes.” It is funded by the National Heart, Lung, and Blood Institute and the National Cancer Institute (NCI) and has enrolled more than 4800 patients to 25 trials since 2003. It provides an important infrastructure to design, implement, and complete multicenter HCT trials in the United States. Although composed of 20 core centers and/or consortia, other US centers may access trials as affiliate centers through a simple application process.
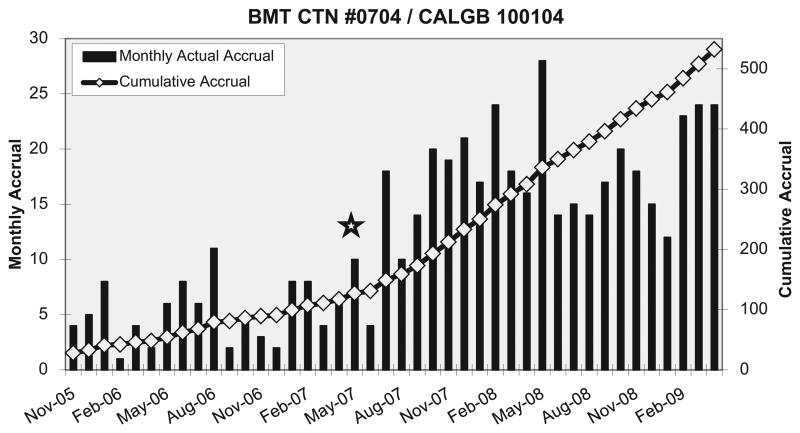
As accrual to BMT CTN 0102 neared completion, and realizing the importance of national collaboration in the success of that trial's accrual, the BMT CTN convened several meetings of experts in the US myeloma HCT community to consider a successor trial. These meetings included representatives from the BMT CTN and the NCI-funded cooperative trial groups. The first meeting of this Myeloma BMT CTN-Intergroup Committee was in Minneapolis in November 2006. There was consensus that successful accrual to multicenter trials required active collaboration to prioritize the questions of interest and coordination to avoid competing trials. The group also agreed that after accrual to BMT CTN 0102 was complete, the BMT CTN would endorse and help complete accrual to the ongoing but, at that time, slowly accruing Cancer and Leukemia Group B (CALGB) 100104 study, which was evaluating the use of lenalidomide maintenance therapy after HCT [2]. BMT CTN centers affiliated with CALGB or the Eastern Cooperative Oncology Group were encouraged to activate the trial through their cooperative group. An amendment was then added to allow another 5 BMT CTN centers to access the trial though the Clinical Trials Support Unit. Immediately after the BMT CTN endorsement, more than 20 additional centers activated the trial with minimal delay. At the end of 2006, only 17 centers had enrolled patients on this trial; by the end of 2007, 30 had enrolled patients and by the end of 2009, 46 had enrolled patients. The BMT CTN Data and Coordinating Center, in collaboration with the Myeloma BMT CTN-Intergroup Committee, initiated an intensive accrual effort, including webcasts, video presentation, e-mail broadcasts, informational materials, and a series of presentations at national meetings. The effect of these efforts on enrollment is shown in Figure 1, which illustrates accrual before and after May 2007, when BMT CTN officially endorsed the trial and implemented its accrual plan.
Figure 1.
Monthly and cumulative accrual to Cancer and Leukemia Group B 100104. The star indicates the date that Blood and Marrow Transplant Clinical Trials Network (BMT CTN) officially endorsed the trial and began intensive efforts at boosting accrual.
The trial completed accrual in 2009 and the results, published in New England Journal of Medicine [2], showed a survival advantage and a dramatic improvement in progression-free survival with use of maintenance therapy with lenalidomide compared with controls. This achievement was only possible with full support, not only from investigators in dozens of centers, but also from staff at the National Heart, Lung, and Blood Institute, NCI, the BMT CTN Data and Coordinating Center, and the Clinical Trials Support Unit. CALGB 100104 was truly a cooperative group effort involving multiple centers, multiple networks, and multiple institutes. This aspect of its successful completion is as important as any of the other “Methods” employed in its implementation and described in the manuscript.
The collaborative efforts that began with the first BMT CTN myeloma trial and continued with CALGB 100104 also resulted in the design and implementation of BMT CTN 0702 (the STaMINA trial), which explores the role of 3 different consolidation strategies in myeloma. BMT CTN 0702 has been endorsed by Eastern Cooperative Oncology Group, the Southwest Oncology Group (now SWOG), and CALGB (now the Alliance). This trial opened in June 2010 and has already accrued 469 patients (70 ahead of predicted) and is expected to reach its target of 750 patients by December 2013.
Our experience demonstrates that the recommendations of the IOM can be realized as long as our commitment to collaboration continues. The Myeloma BMT CTN-Intergroup Committee will meet this fall to prepare a follow-up study for BMT CTN 0702. Many questions remain to be addressed: Is there a better conditioning regimen than melphalan 200 mg/m2? What is the optimal duration of lenalidomide maintenance after transplantation? Will the addition of new agents, such as elotuzumab, oral proteasome inhibitors, or bortezomib, to post-transplantation lenalidomide result in better disease control? Our experience with CALGB 100104 tells us that these questions can be answered in a timely manner only if we truly work together and collaboratively select, design, and implement the critical trials needed to improve patient outcomes.
Acknowledgments
Financial disclosure: Cancer and Leukemia Group B 100104 was supported, in part, by grants from the National Cancer Institute and the National Heart, Lung, and Blood Institute to the Cancer and Leukemia Group B (CA31946, CA33601), to the Blood and Marrow Transplantation Clinical Trials Network (HL069294), and to the Eastern Cooperative Oncology Group (CA21115). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Heart, Lung, and Blood Institute.
Footnotes
Financial disclosure: See Acknowledgments on page 859.
References
- 1.Nass SJ, Moses HL, Mendelsohn J, editors. A National Cancer Clinical Trials System for the 2lst Century: Reinvigorating the NCI Cooperative Group Program. Washington, DC: The National Academies Press; 2010. [PubMed] [Google Scholar]
- 2.McCarthy P, Owzar K, Hofmeister C, et al. Lenalidomide after stem cell transplantation for multiple myeloma. N Engl J Med. 2012;366:1770–1781. doi: 10.1056/NEJMoa1114083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attal M, Lauwers-Cances V, Marit G, et al. Lenalidomide maintenance after stem cell transplantation for multiple myeloma. N Engl J Med. 2012;366:1782–1791. doi: 10.1056/NEJMoa1114138. [DOI] [PubMed] [Google Scholar]
- 4.Roth BJ, Krilov L, Adams S, et al. Clinical Cancer Advances 2012: annual report on progress against cancer from the American Society of Clinical Oncology. J Clin Oncol. 2013;31:131–161. doi: 10.1200/JCO.2012.47.1938. [DOI] [PubMed] [Google Scholar]
- 5.Krishnan A, Pasquini MC, Logan B, et al. Autologous haemopoietic stem-cell transplantation followed by allogeneic or autologous haemopoietic stem-cell transplantation in patients with multiple myeloma (BMT CTN 0102): a phase 3 biological assignment trial. Lancet Oncol. 2011;12:1195–1203. doi: 10.1016/S1470-2045(11)70243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]