Abstract
Background
Laparoscopic Roux-en-Y gastric bypass (RYGB) induces a more favorable metabolic profile than expected by weight loss alone. In this study, we investigated the effect of RYGB on serum bile acid levels and their relation to clinical outcomes.
Methods
We included 30 obese patients who underwent RYGB (BMI=46.1±5.9 kg/m2). Clinical measurements and laboratory determinations were performed before surgery and 1 year after surgery. Fasting serum bile acids were measured by an enzymatic method and individual bile acids were quantified by HLPC-tandem mass spectrometry. Indirect calorimetry was performed to measure the rates of energy expenditure and substrate oxidation.
Results
Fasting total serum bile acid levels increased twofold after RYGB (pre, 3.68±2.03 vs. post, 7.06± 9.65 μmol/l, +92 %, p=0.002). This increase in total bile acids was accompanied by a decrease in conjugated bile acids, which correlated with decreased glucose oxidation (r=0.571, p=0.002) and with increased lipid oxidation (r=−0.626, p=0.0004). The change in taurineconjugated bile acids correlated with altered DIO2 mRNA expression in adipose tissue (r=−0.498, p=0.013) potentially linking bile acid conjugation to substrate oxidation through DIO2.
Conclusions
Fasting serum bile acid levels increase after RYGB. More specifically, changes in bile acid conjugation after RYGB associate with altered energy metabolism.
Keywords: Obesity surgery, Bile acids, Glucose and lipid oxidation, DIO2, CYP27A1
Introduction
Obesity surgery has rapidly gained more interest because of its capability to induce sustained weight loss and resolution of obesity-related co-morbidities including type 2 diabetes (T2DM) [1, 2]. It has been shown that laparoscopic Rouxen-Y gastric bypass (RYGB) induces more weight loss than purely restrictive laparoscopic adjustable gastric banding (LAGB) [3, 4]. Furthermore, RYGB induces more favorable glucose and lipid metabolism than LAGB, which cannot be explained solely by additional weight loss [5].
The most common explanation for the more effective improvement in blood glucose levels after RYGB is altered secretion of gastrointestinal hormones, including GLP-1 and GIP, which stimulate insulin secretion [5–8]. It has been suggested that changes in bile acid metabolism could also contribute to improved metabolism [9–11]. Traditionally, bile acids are known to regulate lipid and glucose metabolism through the nuclear receptor FXR [12–15]. More recently, the G-protein family receptor TGR5 has been identified as a cell surface receptor for bile acids [16, 17]. Through this receptor, bile acids induce energy expenditure in mice by promoting FXR-independent intracellular thyroid hormone activation [18]. Furthermore, it has been shown that bile acids promote GLP-1 secretion through TGR5 activation in mouse models [19, 20]. Moreover, individual conjugated and unconjugated bile acids have different effects on their receptors between different tissues [11, 21].
Because serum bile acid levels are more than twofold higher in humans after RYGB compared to lean and obese individuals [22], RYGB forms an interesting human model to investigate interactions between serum bile acid levels and metabolic regulation. The purpose of this study was to investigate effects of RYGB on circulating levels of bile acids. We hypothesized that altered bile acid metabolism is a contributing factor to observed metabolic benefits after RYGB. We demonstrate in a longitudinal study that bile acid levels increase after RYGB surgery. Furthermore, we could link alterations in serum levels of conjugated bile acids after RYGB with the regulation of substrate oxidation.
Methods and Procedures
Subjects
All patients undergoing obesity surgery in Kuopio University Hospital are recruited into our ongoing study investigating metabolic consequences of obesity surgery (Kuopio Obesity Surgery Study) [23]. This study included 30 consecutive adult patients who were accepted for Roux-en-Y gastric bypass (RYGB) over the years 2005–2007 with the following criteria: (1) BMI>40 or 35 kg/m2 with significant co-morbidity, (2) failure of dietary and drug treatments, and (3) no contraindication for operation. Every participant had a 1-day visit to the hospital for screening eligibility for obesity surgery. Fasting blood samples were drawn after 12 h of fasting followed by oral glucose tolerance test (OGTT), if T2DM had not been previously diagnosed. There were no dropouts with patients enrolled in study. This study was approved by the Ethics committee of the Kuopio University Hospital, and it was in accordance with the Helsinki Declaration.
Clinical Measurements
Body mass index (BMI) was calculated as weight (kilogram) divided by height (meter) squared. Bioelectrical impedance analysis (RJL Systems, Clinton Township, MI, USA) was used to determine lean body mass (LBM). Indirect calorimetry was performed with a computerized flow-through canopy gas-analyzer system (Deltatrac, Datex, Helsinki, Finland) at baseline and 1 year after surgery. This device has precision of 2.5 % for O2 consumption and 1.0 % for CO2 production. Data were used for calculation of respiratory quotient (RQ) and resting energy expenditure (REE) [24]. RQ represents the ratio of CO2 (VCO2) exhaled to the amount of O2 (VO2) consumed by the subject. A Simplified Weir equation without urinary nitrogen was used to calculate 24-h resting energy expenditure (REE): REE(kcal/day)0[(3.941× VO2)+(1.106×VCO2)]1,440. The calorimetric values of VO2 and VCO2 were used to determine endogenous metabolism of lipids and carbohydrates in the fasting state [25]. The lean body mass (LBM)-corrected values of substrate oxidation were used in analysis. Macronutrient intake was estimated using the dietary records of 14 study subjects in the RYGB group at baseline and in follow-up.
Laboratory Determinations
Plasma glucose was measured by an enzymatic hexokinase photometric assay (Konelab Systems Reagents, Thermo Fischer Scientific, Vantaa, Finland). Insulin was determined by an immunoassay (ADVIA Centaur Insulin IRI, no 02230141, Siemens Medical Solutions Diagnostics, Tarrytown, NY). Serum free fatty acids (FFA) were analyzed by an enzymatic method (Wako Chemicals GmbH, Neuss, Germany). Insulin resistance and secretion indexes were calculated based on homeostasis model assessment (HOMA-IR and HOMA-IS).
Serum Bile Acid Measurements
Fasting levels of total serum bile acids were assayed by an enzymatic method (Fumouze Diagnostics, Levallois-Perret, France). Importantly, baseline and follow-up samples were paired for the analysis. Individual serum bile acids were measured by high-performance liquid chromatography (HPLC) tandem mass spectrometry (Waters™ S.A.S., St-Quentin En Yvelines, France) and quantified using deuterium-labeled internal standards [26]. The combined concentrations of primary, secondary, conjugated, and unconjugated bile acids were calculated from concentrations of individual bile acids.
Liver and Adipose Tissue Biopsies
Adipose tissues biopsies were taken during RYGB surgery and 1 year after surgery. Liver biopsies were obtained using Trucut needle (Radiplast AB, Uppsala, Sweden) during surgery. Eleven patients had clinical indication for a follow-up liver biopsy 1 year after surgery, and therefore, we could also obtain samples for gene expression analysis from these individuals. All samples for gene expression analysis were immediately frozen in liquid nitrogen.
RNA Extraction and Quantitative PCR Analysis
Total RNA from adipose tissue and liver was extracted using Tri-Reagent [Applied Biosystems (ABI) Foster City, CA, USA] and reverse transcribed using high capacity cDNA reverse transcription kit (ABI) according to manufacturer's protocol. Quantitative real-time PCR was carried out in the Applied Biosystems 7500 Real Time PCR System using KAPA SYBR FAST ABI Prism qPCR (Kapa Biosystems, Woburn, MA, USA). Reactions comprised of 1× qPCR Master Mix, 200 nM forward and reverse primers for DIO2 (forward 5′-AGAGGGACTGCGCTGCGTCT-3′, reverse 5′-CTGGAGACATGCACCACACTGGAA-3′), CYP7A1 (forward 5′-CGTGGTCCTCTGGGCATCGC-3′, reverse 5′-AGGCACTGGAAAGCCTCAGCG-3′), CYP27A1 (forward 5′-GGAGCTATGGAAGGAGCAC-3′, reverse 5′-AGCTGGTCCAGTCGAGTCAT-3′) and an endogenous control RPLP0 (forward 5′-GGCGACCTGGAAG TCCAACT-3′, reverse 5′-CCATCAGCACCACAGCCTTC-3′) and 5 ng RNA (adipose tissue) or 3 ng (liver tissue) equivalent of sample cDNA.
Statistical Analysis
Data are presented as mean±SD. Nonparametric Wilcoxon signed-rank test was used for comparisons of differences between baseline and follow-up measurements. Relationships among parameters were analyzed by Spearman's correlation test. All analyses were conducted with the SPSS v.17.0 for Windows (SPSS, IL, USA). P value <0.05 was considered statistically significant.
Results
Baseline and follow-up characteristics are presented in Table 1. At baseline BMI was 46.1±5.9 kg/m2 and 1 year after surgery BMI decreased to 34.3±5.9 kg/m2 (−26 %). This result, as well as RYGB's favorable effect on serum lipid profile, has previously been published in our report investigating effects of RYGB and LAGB on cholesterol metabolism [23]. Resting energy expenditure (REE) decreased after RYGB (32.1±3.6 vs. 29.7±3.1 kcal/day/kg LBM, p=0.001). In addition, RQ decreased after RYGB (0.86±0.05 vs. 0.83±0.06, p=0.021), suggesting an increase in the ratio of lipid and glucose oxidation (Table 1).
Table 1.
Characteristics of individuals who underwent RYGB before (baseline) and 12 months (follow-up) after obesity surgery
| Baseline | Follow-up | Change (%) | p | |
|---|---|---|---|---|
| Gender (male/female) | 3/27 | – | – | – |
| Age (years) | 45.2±7.9 | – | – | – |
| Weight (kg) | 129.9±19.9 | 96.7±18.9 | –26 % | <0.001 |
| Body mass index (kg/m2) | 46.1±5.9 | 34.3±5.9 | –26 % | <0.001 |
| Fasting glucose (mmol/L) | 6.58±2.07 | 5.39±0.71 | –18 % | <0.001 |
| HOMA-IR (mmol/l×mU/L) | 6.7±6.5 | 2.1±2.2 | –69 % | <0.001 |
| ALT (U/L) | 38.2±26.1 | 20.7±8.9 | –46 % | <0.001 |
| TSH (mU/L) | 1.75±0.88 | 1.64±0.74 | –6 % | 0.424 |
| Energy expenditure(kcal/day/kg LBM) | 32.1±3.6 | 29.7±3.1 | –7 % | 0.001 |
| Respiratory quotient | 0.86±0.05 | 0.83±0.06 | –3 % | 0.021 |
| Glucose oxidation(g/day/kg LBM) | 4.07±1.41 | 3.11±1.46 | –24 % | 0.002 |
| Lipid oxidation (g/day/kg LBM) | 1.70±0.63 | 1.88±0.71 | +11 % | 0.111 |
Mean±SD shown. Percentage change is the difference in percents between baseline and follow-up
Nonparametric Wilcoxon signed-rank test was used for paired difference between baseline and follow up (p=two-tailed significance)
HOMA-IR homeostasis model assessment of insulin resistance;
ALT serum alanine aminotransferase; TSH thyroid stimulating hormone; LBM lean body mass
Bile Acid Levels Increase after RYGB
Fasting total serum bile acid levels increased twofold after RYGB (3.68±2.03 vs. 7.06±9.65 μmol/L, p=0.002) (Fig. 1). This change in serum bile acid levels after surgery was not associated with weight loss (r=−0.109, p=0.453), and the levels after the surgery were not associated with the postsurgical BMI (r=−0.217, p=0.249).
Fig. 1.
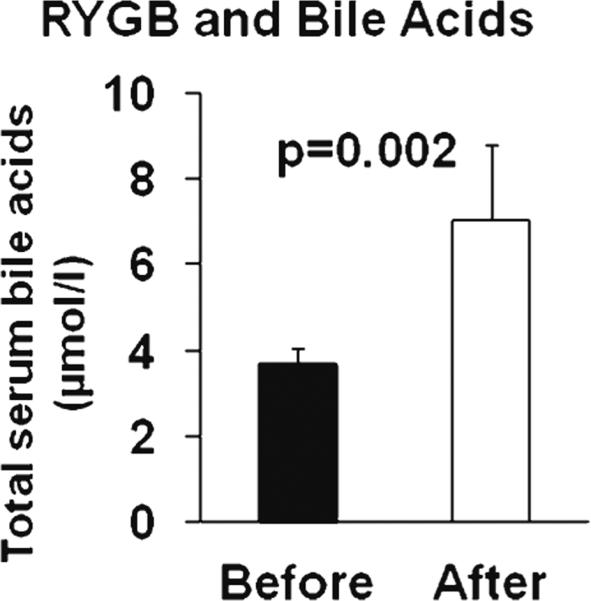
Fasting serum total bile acid levels before and 12 months after Roux-en-Y gastric bypass (RYGB). P indicates a difference between baseline and follow-up, mean±SEM shown
The mean levels of primary, secondary and tertiary bile acids increased three- to fourfold after RYGB (Table 2). However, the difference between baseline and 1-year follow-up was statistically significant only for secondary bile acids (p=0.012). Levels of all individual bile acids are shown in Table 3 demonstrating a statistically significant 2.9-fold increase in deoxycholic acid (p=0.012). Taurineconjugated bile acids were the only bile acid levels that tended to decrease after RYGB (Tables 2, 3), decreasing from 9.4 % to 4.0 % (proportion of all bile acids) in response to RYGB (p=0.035). The ratio of taurine/glycine conjugation decreased significantly (p=0.009, Table 2).
Table 2.
Changes in serum fasting bile acid levels (μmol/L, mean±SD shown) in response to Roux-en-Y gastric bypass
| Baseline | Follow-up | Change (%) | p | |
|---|---|---|---|---|
| Primary bile acids | 1.04±1.82 | 3.43±8.67 | +230 % | 0.185 |
| Secondary bile acids | 0.65±0.63 | 1.90±3.52 | +191 % | 0.012 |
| Tertiary bile acids | 0.10±0.12 | 0.43±1.83 | +318 % | 0.910 |
| Unconjugated bile acids | 1.79±2.27 | 5.75±13.88 | +221 % | 0.072 |
| Conjugated bile acids | 4.45±6.66 | 3.73±3.61 | –16 % | 0.877 |
| Glycine-conjugated | 3.06±2.88 | 3.49±3.39 | +14 % | 0.441 |
| Taurine-conjugated | 1.38±5.85 | 0.25±0.36 | –82 % | 0.153 |
| Taurine/glycine (ratio) | 0.44±1.80 | 0.07±0.49 | –84 % | 0.009 |
| Unconjugated/conjugated (ratio) | 0.81±1.06 | 1.85±3.28 | +128 % | 0.206 |
Percentage change is the difference in percents between baseline and follow-up. Non-parametric Wilcoxon signed-rank test was used for paired difference between baseline and follow up (p = two-tailed significance)
Table 3.
Fasting levels of individual serum bile acids (micromole per liter) before and 12 months after Roux-en-Y gastric bypass
| Baseline | Follow-up | Change (%) | p | |
|---|---|---|---|---|
| Primary bile acids | ||||
| Cholic acid | 0.321 ±0.735 | 1.513±4.306 | +371 % | 0.141 |
| Chenodeoxycholic acid | 0.720±1.207 | 1.919±4.397 | +167 % | 0.185 |
| Secondary bile acids | ||||
| Deoxycholic acid | 0.643±0.623 | 1.881±3.506 | +193 % | 0.012 |
| Lithocholic acid | 0.008±0.013 | 0.014±0.025 | +43 % | 0.155 |
| Tertiary bile acids | ||||
| Ursodeoxycholic acid | 0.102±0.116 | 0.426±1.833 | +318 % | 0.910 |
| Conjugated bile acids | ||||
| Glycocholic acid | 0.357±0.403 | 0.301±0.366 | –16 % | 0.267 |
| Glycochenooxycholid acid | 1.961±2.196 | 2.057±2.141 | +0.5 % | 0.309 |
| Glycodeoxycholic acid | 0.575±0.671 | 0.942±0.929 | +64 % | 0.072 |
| Glycolithocholic acid | 0.019±0.022 | 0.041±0.047 | +116 % | 0.009 |
| Glycoursodeoxycholic acid | 0.152±0.173 | 0.145±0.174 | –5 % | 0.644 |
| Taurocholic acid | 0.109±0.159 | 0.062±0.148 | –43 % | 0.043 |
| Taurochenodeoxycholic acid | 0.097±0.155 | 0.077±0.143 | –21 % | 0.496 |
| Taurodeoxycholic acid | 1.170 ±5.825 | 0.098±0.149 | –92 % | 0.888 |
| Taurolithocholic acid | 0.003±0.005 | 0.003±0.008 | +9 % | 0.638 |
| Tauroursodeoxycholic acid | 0.005±0.005 | 0.007±0.010 | +31 % | 0.841 |
Mean±SD shown. Percentage change is the difference in percents between baseline and follow-up
Nonparametric Wilcoxon signed-rank test was used for paired difference between baseline and follow up (p = two-tailed significance)
We next asked if increased bile acid levels after RYGB could be related to alterations in expression of genes regulating classical (CYP7A1) or acidic (CYP27A1) bile acid synthesis pathway. The latter has been suggested to be affected after obesity surgery in rodents [27]. We found that the increased bile acid levels after RYGB could be related to increased hepatic production of bile acids. CYP7A1 mRNA levels did not change in response to RYGB (0.10±0.08 at baseline and 0.11±0.09 at follow-up, p=0.657). However, CYP27A1 mRNA levels tended to increase after RYGB (1.09±0.23 vs.1.51±0.67, p=0.091) and the change in CYP27A1 mRNA expression correlated positively with the change in total bile acids levels (r=0.670, p=0.029). We acknowledge that this analysis has to be considered preliminary because of a limited number of liver biopsies at follow-up (n=11).
Decrease in Serum-Conjugated Bile Acids is Linked with Decreased RQ after RYGB
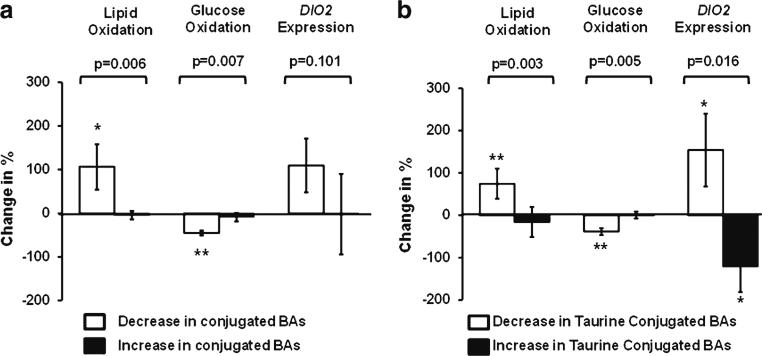
We next asked if the increase in serum total bile acids or the changes in conjugated bile acids correlated with changes in energy metabolism after RYGB. The increase in serum total bile acid levels did not correlate with the observed decreases in REE and RQ after RYGB (Table 4). However, the decrease in serum-conjugated bile acid levels correlated positively with the change in RQ (r=0.664, p=0.0001, Table 4) and glucose oxidation (r=0.571, p=0.002), and negatively with the change in lipid oxidation (r=−0.626, p=0.0004), suggesting that altered conjugation of bile acids may link with energy metabolism. Next, we divided subjects to those who demonstrated an increase or a decrease in serum levels of conjugated bile acids. Figure 2a demonstrates that the characteristic increase in lipid oxidation and decrease in glucose oxidation after RYGB (see Table 1) was statistically significant only in those individuals who had a decrease in serum-conjugated bile acid levels (Fig. 2a), more specifically in those who demonstrated a decrease in levels of taurine-conjugated bile acids (Fig. 2b). We did not observe any significant associations of total bile acid or conjugated bile acids with weight loss, plasma glucose, HOMA-IR, HOMA-IS, or serum free fatty acid levels (data not shown).
Table 4.
Spearman's correlations of changes (from baseline to follow-up) in serum bile acid levels with changes in resting energy expenditure (REE) and respiratory quotient (RQ) in subjects who underwent Roux-en-Y gastric bypass
| REE | RQ | |
|---|---|---|
| Total bile acids | –0.230 | 0.333 |
| Primary bile acids | 0.184 | 0.285 |
| CA | 0.160 | 0.217 |
| CDCA | 0.210 | 0.325 |
| Secondary bile acids | 0.136 | 0.019 |
| DCA | 0.145 | 0.024 |
| LCA | –0.014 | –0.215 |
| Tertiary bile acids | . | . |
| UDCA | 0.084 | 0.402a |
| Unconjugated bile acids | 0.260 | 0.135 |
| Conjugated bile acids | –0.131 | 0.664c |
| Glycine-conjugated | 0.065 | 0.545b |
| Taurine-conjugated | –0.267 | 0.559b |
| Unconjugated/conjugated | 0.192 | 0.053 |
p<0.05 (significance; two-tailed)
p<0.01(significance; two-tailed)
p<0.001 (significance; two-tailed)
CA cholic acid; CDCA chenodeoxycholic acid; DCA deoxycholic acid; LCA lithocholic acid; UDCA ursodeoxycholic acid
Fig. 2.
a Increased lipid oxidation and decreased glucose oxidation is observed in individuals demonstrating a decrease in levels of serum conjugated bile acids. b Increased lipid oxidation and DIO2 expression and decreased glucose oxidation is observed in individuals demonstrating a decrease in levels of serum taurine-conjugated bile acids. P shows the statistical significance between groups (decrease vs. increase bile acid levels). *p<.05 and **p<.01 show statistically significant changes inside the groups compared to baseline. Mean±SEM shown
Decrease in Serum Taurine-Conjugated Bile Acids Associates with an Increase in DIO2 Expression
To investigate potential mediators of the association between bile acid conjugation and energy metabolism, we tested the hypothesis that the link between serum taurineconjugated bile acids and energy metabolism is explained by the bile acid—TGR5—DIO2 cascade [18]. To this aim, we assessed DIO2 mRNA expression in adipose tissue. There was no correlation between changes in total serum bile acid levels and adipose tissue DIO2 mRNA expression (r=−0.066, p=0.759, n=24). However, the decrease in serum levels of taurine-conjugated bile acids associated with an increase in DIO2 mRNA expression in subcutaneous adipose tissue (r=−0.498, p=0.013, Fig. 2b). Accordingly, the change in DIO2 correlated negatively with the change in glucose oxidation (r=−0.465, p=0.025) and positively with the change in lipid oxidation (r=0.474, p=0.022) after RYGB. We did not observe any statistically significant correlations between the change in DIO2 mRNA levels and REE, weight loss, TSH, HOMA-IR, HOMA-IS, serum free fatty acids or plasma glucose levels (data not shown).
Discussion
In this 1-year prospective longitudinal study, we verified that serum bile acid levels increase approximately twofold after RYGB (Fig. 1). This increase could be related to a change in CYP27A1 mRNA expression, suggesting a link with acidic bile acid synthesis pathway. Interestingly, changes in the levels of conjugated bile acids linked with the characteristic decrease in RQ after RYGB [28, 29]. Overall, these results suggest that some of the beneficial effects of RYGB on energy metabolism are mediated by altered bile acid metabolism.
Our study confirms the earlier observations that the levels of serum total bile acids increase after RYGB [22, 30]. In our prior cross-sectional study, we demonstrated that bile acid levels are twofold higher in subjects who have undergone RYGB 2–4 years earlier compared to pre- and postoperatively weight-matched cohorts [22]. Later, Nakatani et al. [30] reported that serum total bile acids were already increased at 1 and 3 months after obesity surgery. Interestingly, it was reported recently that ileal interposition increases bile acid recycling in rats [27] and also increases liver bile acid synthesis through acidic bile acid synthesis pathway regulated by CYP27A1, but not through classical CYP7A1. In our study, there was no association between bile acid levels and CYP7A1 mRNA expression. However, increase in CYP27A1 mRNA expression was associated with an increase in total bile acid levels in response to surgery, suggesting that increased hepatic production of bile acids could partly explain increased serum levels of total bile acids. We acknowledge that this analysis has to be considered preliminary because of difficulties to obtain human liver samples at follow-up (n=11).
The novel important finding in our current study is that, not the increases in serum total bile acids after RYGB, but the decrease in serum levels of conjugated bile acids links with observed decrease in RQ after RYGB (Table 4; Fig. 2a). This leads to the hypothesis that altered conjugation of bile acids after RYGB is an important mediator of metabolic consequences after RYGB, independent of changes in total serum bile acids. Although the mean levels of taurine-conjugated bile acids did not decrease significantly (Table 2), their relative abundance dropped from 9.4 % to 4.0 % (of all bile acids) in response to RYGB (p=0.035). At the same time, levels of unconjugated bile acids tended to increase (p=0.072, Table 2), suggesting that an alteration in conjugation occurs after RYGB.
A potential explanation for the link between serum-conjugated bile acids and substrate oxidation is that both are associated with decreased lipid absorption after RYGB. Because we have reported that cholesterol absorption is reduced after RYGB [23], we asked if changes in serum bile acid levels or conjugation associate with markers of cholesterol absorption (serum plant sterols, n=25, data not shown). However, there was no association between bile acid levels or conjugation and markers of cholesterol absorption. Similar to our observation in response to RYGB (Table 2), the ratio of taurine/glycine conjugation decreases in response to treatment with bile acid sequestrants or external biliary drainage [11]. Future experiments directly measuring lipid and bile acid absorption will be required to solve the exact mechanisms of altered conjugation after RYGB.
Although the reason for altered levels of conjugated bile acids remains unknown, the levels seem to associate with known biological targets of bile acid metabolism. We investigated if the link between altered bile acid levels and altered substrate oxidation after RYGB is explained by bile acid—TGR5—DIO2 cascade [18] by assessing DIO2 gene expression in adipose tissue. Decreased levels of taurine-conjugated bile acids associated with increased expression of DIO2 in white adi-pose tissue (Fig. 2b). Consistently, DIO2 expression correlated negatively with glucose oxidation and positively with lipid oxidation supporting the hypothesis that altered bile acid metabolism could lead to altered substrate oxidation by modifying DIO2 activity. This explanation is supported by results showing that increased DIO2 expression is associated with increased lipid oxidation in the brown adipose tissue of mice [18]. In conclusion, we propose that altered bile acid metabolism, possibly decreased conjugation, affects TGR5-DIO2 signaling after RYGB.
One of the limitations in the current and prior studies [22, 30] is that the serum bile acid levels were measured in a fasting state. Serum bile acids can increase 4.5–6-fold during the first 30 min after a single oral glucose tolerance test [31]. However, it has been reported that bile acid levels in peripheral venous samples correlate with portal venous samples in fasting and postprandial state and also that measurement of peripheral serum bile acids give information about the status of the enterohepatic circulation [32]. Secondly, we did not investigate links between serum gut peptides and bile acids; measurement of postprandial levels of both will be done in our ongoing studies. Thirdly, we had limited number of food records (14 study subjects) to exclude the effect of dietary changes after RYGB. Our food records showed that intake of energy (p=0.003), protein (p=0.003), fat (p=0.026), and carbohydrates (p=0.003) decreased after RYGB, as expected. However, food records did not show any significant change in the ratio of carbohydrate to fat intake (p=0.826) that could have explained the observed change in substrate oxidation. Finally, due to the relatively low number of individuals these results need to be verified in larger studies, including also other types of surgery.
In summary, serum bile acids increase twofold after RYGB. Increased hepatic synthesis through CYP27A1 pathway needs to be studied as an explanation for the elevated total bile acid levels after RYGB. However, the altered substrate oxidation after RYGB is more associated with changes in conjugation of bile acids than with the observed increase in serum total bile acid levels. We suggest that the mechanism behind this interaction is related to differentially activated bile acid regulated pathways, including DIO2 [9]. Overall, these results support the concept that alterations in bile acid metabolism contribute to metabolic benefits after RYGB.
Acknowledgments
We thank Päivi Turunen and Tiina Sistonen for their careful work in patient recruitment and laboratory analyses. We also greatly thank greatly Carole Jamey for the technical assistance on bile acid measurements.
Funding This study was supported by the Finnish Diabetes Research Foundation (to JPI). JPI has an Academy of Finland Clinical Researcher fellowship (grant 120979 2008–2010 and 138006 2011– 2013). JA acknowledges grant support of the EU Ideas program (ERC-2008-AdG-23118), the Swiss National Science Foundation (FNS), and the Ecole Polytechnique Fédérale de Lausanne.
Abbreviations
- BMI
Body mass index
- DIO2
Type II iodothyronine deionidinase
- FXR
Farnesoid X-receptor
- GIP
Gastric inhibitory polypeptide
- GLP1
Glucagon-like peptide-1
- HOMA-IR
Homeostasis model of assessment-insulin resistance
- HOMA-IS
Homeostasis model of assessment-insulin sensitivity
- LAGB
Laparoscopic adjustable gastric banding
- LBM
Lean body mass
- OGTT
Oral glucose tolerance test
- REE
Resting energy expenditure
- RQ
Respiratory quotient
- RYGB
Laparoscopic Roux-en-Y gastric bypass
- TGR5
G protein-coupled bile acid receptor
- TSH
Thyroid stimulating hormone
Footnotes
Disclosure Statement All contributing authors (M Simonen, N Dali-Youcef, T Kuulasmaa, S Venesmaa, P Käkelä, M Pääkkönen, M Hallikainen, M Kolehmainen, M Uusitupa, L Moilanen, M Laakso, H Gylling, ME Patti, J Auwerx and J Pihlajamäki) declare that they have no conflicts of interests.
Conflict of Interest The authors have nothing to disclose.
Contributor Information
M. Simonen, Department of Medicine, University of Eastern Finland and Kuopio University Hospital, Kuopio, Finland
N. Dali-Youcef, Hôpitaux Universitaires de Strasbourg and Institut de Génétique et de Biologie Moléculaire et Cellulaire, Université de Strasbourg, Strasbourg, France
D. Kaminska, Institute of Public Health and Clinical Nutrition, University of Eastern Finland, Kuopio, Finland
S. Venesmaa, Department of Surgery, University of Eastern Finland and Kuopio University Hospital, Kuopio, Finland
P. Käkelä, Department of Surgery, University of Eastern Finland and Kuopio University Hospital, Kuopio, Finland
M. Pääkkönen, Department of Surgery, University of Eastern Finland and Kuopio University Hospital, Kuopio, Finland
M. Hallikainen, Institute of Public Health and Clinical Nutrition, University of Eastern Finland, Kuopio, Finland
M. Kolehmainen, Institute of Public Health and Clinical Nutrition, University of Eastern Finland, Kuopio, Finland
M. Uusitupa, Institute of Public Health and Clinical Nutrition, University of Eastern Finland, Kuopio, Finland
L. Moilanen, Department of Medicine, University of Eastern Finland and Kuopio University Hospital, Kuopio, Finland
M. Laakso, Department of Medicine, University of Eastern Finland and Kuopio University Hospital, Kuopio, Finland
H. Gylling, Institute of Public Health and Clinical Nutrition, University of Eastern Finland, Kuopio, Finland
M. E. Patti, Joslin Diabetes Center, Harvard Medical School, Boston, MA, USA
J. Auwerx, Laboratory of Integrative Systems Physiology, Ecole Polytechnique Fédérale de Lausanne, Lausanne, Switzerland
Jussi Pihlajamäki, Institute of Public Health and Clinical Nutrition, University of Eastern Finland, Kuopio, Finland; Department of Clinical Nutrition and Obesity Center, Kuopio University Hospital, Kuopio, Finland.
References
- 1.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 2.Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–93. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 3.Weber M, Muller MK, Bucher T, et al. Laparoscopic gastric bypass is superior to laparoscopic gastric banding for treatment of morbid obesity. Ann Surg. 2004;240:975–82. doi: 10.1097/01.sla.0000145924.64932.8f. discussion 982–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen NT, Slone JA, Nguyen XM, et al. A prospective randomized trial of laparoscopic gastric bypass versus laparoscopic adjustable gastric banding for the treatment of morbid obesity: outcomes, quality of life, and costs. Ann Surg. 2009;250:631–41. doi: 10.1097/SLA.0b013e3181b92480. [DOI] [PubMed] [Google Scholar]
- 5.Thaler JP, Cummings DE. Minireview: hormonal and metabolic mechanisms of diabetes remission after gastrointestinal surgery. Endocrinology. 2009;150:2518–25. doi: 10.1210/en.2009-0367. [DOI] [PubMed] [Google Scholar]
- 6.le Roux CW, Aylwin SJ, Batterham RL, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243:108–14. doi: 10.1097/01.sla.0000183349.16877.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.le Roux CW, Welbourn R, Werling M, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246:780–5. doi: 10.1097/SLA.0b013e3180caa3e3. [DOI] [PubMed] [Google Scholar]
- 8.Korner J, Bessler M, Inabnet W, et al. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis. 2007;3:597–601. doi: 10.1016/j.soard.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas C, Pellicciari R, Pruzanski M, et al. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7:678–93. doi: 10.1038/nrd2619. [DOI] [PubMed] [Google Scholar]
- 10.Hofmann AF, Hagey LR. Bile acids: chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cell Mol Life Sci. 2008;65:2461–83. doi: 10.1007/s00018-008-7568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lefebvre P, Cariou B, Lien F, et al. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89:147–91. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- 12.Bilz S, Samuel V, Morino K, et al. Activation of the farnesoid X receptor improves lipid metabolism in combined hyperlipidemic hamsters. Am J Physiol Endocrinol Metab. 2006;290:E716–22. doi: 10.1152/ajpendo.00355.2005. [DOI] [PubMed] [Google Scholar]
- 13.Cariou B, van Harmelen K, Duran-Sandoval D, et al. The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice. J Biol Chem. 2006;281:11039–49. doi: 10.1074/jbc.M510258200. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe M, Houten SM, Wang L, et al. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113:1408–18. doi: 10.1172/JCI21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma K, Saha PK, Chan L, et al. Farnesoid X receptor is essential for normal glucose homeostasis. J Clin Invest. 2006;116:1102–9. doi: 10.1172/JCI25604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maruyama T, Miyamoto Y, Nakamura T, et al. Identification of membrane-type receptor for bile acids (M-BAR). Biochem Biophys Res Commun. 2002;298:714–9. doi: 10.1016/s0006-291x(02)02550-0. [DOI] [PubMed] [Google Scholar]
- 17.Kawamata Y, Fujii R, Hosoya M, et al. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–40. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–9. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 19.Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun. 2005;329:386–90. doi: 10.1016/j.bbrc.2005.01.139. [DOI] [PubMed] [Google Scholar]
- 20.Thomas C, Gioiello A, Noriega L, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–77. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parks DJ, Blanchard SG, Bledsoe RK, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;21:1365–8. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 22.Patti ME, Houten SM, Bianco AC, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring) 2009;17:1671–7. doi: 10.1038/oby.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pihlajamaki J, Gronlund S, Simonen M, et al. Cholesterol absorption decreases after Roux-en-Y gastric bypass but not after gastric banding. Metabolism. 2010;59:866–72. doi: 10.1016/j.metabol.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Ferrannini E. The theoretical bases of indirect calorimetry: a review. Metabolism. 1988;37:287–301. doi: 10.1016/0026-0495(88)90110-2. [DOI] [PubMed] [Google Scholar]
- 25.da Rocha EE, Alves VG, da Fonseca RB. Indirect calorimetry: methodology, instruments and clinical application. Curr Opin Clin Nutr Metab Care. 2006;9:247–56. doi: 10.1097/01.mco.0000222107.15548.f5. [DOI] [PubMed] [Google Scholar]
- 26.Argmann CA, Houten SM, Champy MF, et al. Lipid and bile acid analysis. Curr Protoc Mol Biol. 2006;29:29B.2.1–29B.2.24. doi: 10.1002/0471142727.mb29b02s75. [DOI] [PubMed] [Google Scholar]
- 27.Kohli R, Kirby M, Setchell KD, et al. Intestinal adaptation after ileal interposition surgery increases bile acid recycling and protects against obesity-related comorbidities. Am J Physiol Gastrointest Liver Physiol. 2010;299:652–6. doi: 10.1152/ajpgi.00221.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Castro Cesar M, de Lima Montebelo MI, Rasera I, Jr, et al. Effects of Roux-en-Y gastric bypass on resting energy expenditure in women. Obes Surg. 2008;18:1376–80. doi: 10.1007/s11695-008-9460-8. [DOI] [PubMed] [Google Scholar]
- 29.Bobbioni-Harsch E, Morel P, Huber O, et al. Energy economy hampers body weight loss after gastric bypass. J Clin Endocrinol Metab. 2000;85:4695–700. doi: 10.1210/jcem.85.12.7083. [DOI] [PubMed] [Google Scholar]
- 30.Nakatani H, Kasama K, Oshiro T, et al. Serum bile acid along with plasma incretins and serum high-molecular weight adiponectin levels are increased after bariatric surgery. Metabolism. 2009;58:1400–7. doi: 10.1016/j.metabol.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Zhao X, Peter A, Fritsche J, et al. Changes of the plasma metabolome during an oral glucose tolerance test: is there more than glucose to look at? Am J Physiol Endocrinol Metab. 2009;296:E384–93. doi: 10.1152/ajpendo.90748.2008. [DOI] [PubMed] [Google Scholar]
- 32.Angelin B, Bjorkhem I, Einarsson K, et al. Hepatic uptake of bile acids in man. Fasting and postprandial concentrations of individual bile acids in portal venous and systemic blood serum. J Clin Invest. 1982;70:724–31. doi: 10.1172/JCI110668. [DOI] [PMC free article] [PubMed] [Google Scholar]