INTRODUCTION
The Blood and Marrow Transplant Clinical Trials Network (BMT CTN) was chartered by the National Heart, Lung, and Blood Institute and the National Cancer Institute(NCI) in 2001 to conduct clinical trials aimed at improving the outcome of patients undergoing hematopoietic cell transplantation (HCT). Since its inception, activities of the BMT CTN have been guided by a series of State of the Science Symposia (SOSS), conducted to determine the most important and clinically relevant questions to be addressed by the cooperative activities of the Network. The first State of the Science Symposium identified 6 major questions that the BMT CTN should consider (see Table 1). Over the following 6 years, the BMT CTN activated 12 trials that addressed most of these questions, as well as others, and accrued more than 2000 patients to these trials. In 2007, a second SOSS (SOSS2) identified a new series of 11 clinically important questions (see Table 1) [1]. Since SOSS2, the BMT CTN has developed and activated 7 studies addressing these issues, 6 of which have completed accrual; accrual continues to the remaining study. The NCI cancer cooperative groups developed and activated 2 additional trials endorsed by the BMT CTN addressing these questions,1 of which has completed accrual with 1 ongoing. Studies addressing the final 2 SOSS2 questions were not initiated after further analysis determined that they were likely not feasible at this time. Overall, the BMT CTN has activated 33 trials addressing many of the most pressing questions facing the HCT community, has accrued >6700 patients to trials, and has published results in 37 manuscripts, including many high-impact, practice-changing papers [2].
Table 1.
Clinical Research Questions Identified at SOSS
First SOSS
|
SOSS2
|
Ph+ indicates philadelphia chromosome positive; CLL, chronic lymphocytic leukemia.
The BMT CTN held its third SOSS meeting in February 2014 to set a scientific agenda for the coming half decade. Given the success of the previous 2 SOSS meetings, the 2014 SOSS followed a similar format. Briefly, approximately 9 months before the meeting, a BMT CTN planning group formed 13 committees (similar to those in SOSS2) addressing 13 major topics in HCT, and the planning group named committee chairs and members for each committee. Committee members included cooperative group leaders, representatives from specialized programs of research excellence, individual cancer center leaders, and laboratory-oriented investigators and clinical trialists. To encourage diverse views and gain the broadest possible perspective, no individual was permitted to serve on more than 1 committee. Additionally, 2 external reviewers, who were not active participants in BMT CTN activities or centers, were identified for each committee. The planning group, committee chairs, members, and external reviewers are listed in Table 2. Each committee was charged with identifying up to 3 of the most important clinical questions in their area that could be addressed by the BMT CTN in the next few years. The committees met multiple times over the ensuing 6 months to develop their list and to create brief documents describing the outcomes of their deliberations. These reports were circulated to the SOSS planning group, the other committee chairs, and the external reviewers before the SOSS meeting. Participation in the SOSS meeting was open to the public and approximately 350 individuals attended. At the meeting, each committee chair presented his or her group’s report, following which the external reviewers presented their views. A discussion period followed each presentation; these discussions were open to all in attendance. At the conclusion of the public meeting, the planning committee, committee chairs, and external reviewers met, modified, and prioritized the study concepts, based on the SOSS meeting discussions. This article summarizes the individual committee reports and a list of those trials most enthusiastically endorsed by the symposium leadership.
Table 2.
BMT CTN SOSS Committees and Reviewers
| Committee/Position | Reviewers |
|---|---|
| Committee 1: Leukemia | |
| Chair: | Steven Devine, Ohio State University Medical Center, Columbus |
| Members: | Frederick Appelbaum, Fred Hutchinson Cancer Research Center, Seattle Richard Champlin, MD Anderson Cancer Center, Houston Stephen Couban, Queen Elizabeth II Health Science Center, Halifax Marcos de Lima, Case Western Reserve University, Cleveland John DiPersio, Washington University, St. Louis Harry Erba, University of Alabama at Birmingham Timothy Graubert, Massachusetts General Hospital, Boston Guido Marcucci, Ohio State University Medical Center, Columbus Richard Stone, Dana-Farber Cancer Institute, Boston Martin Tallman, Memorial Sloan-Kettering Cancer Center, New York |
| Outside reviewers: | Donald Bunjes, University of Ulm Vanderson Rocha, Hôpital Saint-Louis, Paris |
| Committee 2: Lymphoma | |
| Chair: | Ginna Laport, Stanford University, Palo Alto |
| Members: | Richard Ambinder, Johns Hopkins University, Baltimore Timothy Fenske, Medical College of Wisconsin, Milwaukee Richard Fisher, Fox Chase Cancer Center, Philadelphia Brad Kahl, University of Wisconsin, Madison John Leonard, Weill Cornell Medical College, New York Thomas Shea, University of North Carolina, Chapel Hill Julie Vose, University of Nebraska, Omaha Wyndham Wilson, National Institutes of Health, Bethesda |
| Outside reviewers: | Silvia Montoto, St. Bartholomew’s Hospital, London Eileen Smith, City of Hope, Duarte |
| Committee 3: Multiple Myeloma | |
| Chair: | Sergio Giralt, Memorial Sloan-Kettering Cancer Center, New York |
| Members: | Kenneth Anderson, Dana-Farber Cancer Institute, Boston William Bensinger, Fred Hutchinson Cancer Research Center, Seattle Parameswaran Hari, Medical College of Wisconsin, Milwaukee Amrita Krishnan, City of Hope, Duarte Carl Ola Landgren, National Institutes of Health, Bethesda Sagar Lonial, Emory University, Atlanta Philip McCarthy, Roswell Park Cancer Institute, Buffalo Robert Orlowski, MD Anderson Cancer Center, Houston Vincent Rajkumar, Mayo Clinic, Rochester Keith Stewart, Mayo Clinic, Rochester |
| Outside reviewers: | Nicolaus Kroeger, University Medical Center Hamburg-Eppendorf Maria-Victoria Mateos, Salamanca University Hospital |
| Committee 4: Nonmalignant Disease | |
| Chair: | Harold Atkins, Ottawa Hospital Research Institute |
| Members: | Joachim Deeg, Fred Hutchinson Cancer Research Center, Seattle George Georges, Fred Hutchinson Cancer Research Center, Seattle Linda Griffith, National Institute of Allergy & Infectious Diseases, Bethesda Carolyn Keever-Taylor, Medical College of Wisconsin, Milwaukee Richard Nash, Colorado Blood Cancer Institute, Denver Steven Pavletic, National Institutes of Health, Bethesda Michael Racke, Ohio State University Medical Center, Columbus Keith Sullivan, Duke University, Durham |
| Outside reviewers: | Harry Malech, National Institute of Allergy & Infectious Diseases, Bethesda Paolo Muraro, Imperial College/Hammersmith Hospital, London |
| Committee 5: Pediatric Transplantation – Indications/Approaches | |
| Chair: | Michael Pulsipher, University of Utah, Salt Lake City |
| Members: | Stephan Grupp, Children’s Hospital of Philadelphia Robert Krance, Baylor College of Medicine, Houston Joanne Kurtzberg, Duke University, Durham John Levine, University of Michigan, Ann Arbor Parinda Mehta, Cincinnati’s Children’s Hospital Sung-Yun Pai, Dana-Farber Cancer Institute, Boston Kirk Schultz, University of British Columbia, Vancouver Shalini Shenoy, Washington University, St. Louis Michael Verneris, University of Minnesota, Minneapolis Donna Wall, Manitoba Institute of Child Health, Winnipeg |
| Outside reviewers: | Adriana Seber, Instituto de Oncologia Pediátrica, Sao Paolo Paul Veys, Great Ormond Street Hospital for Children, London |
| Committee 6: Pediatric Transplantation – Outcomes/Late Effects | |
| Chair: | Stella Davies, Cincinnati’s Children’s Hospital Medical Center |
| Members: | Scott Baker, Fred Hutchinson Cancer Research Center, Seattle Farid Boulad, Memorial Sloan-Kettering Cancer Center, New York Paul Carpenter, Fred Hutchinson Cancer Research Center, Seattle Christine Duncan, Dana-Farber Cancer Institute, Boston Mary Eapen, Medical College of Wisconsin, Milwaukee David Jacobsohn, Children’s National Medical Center, Washington, DC Amy Keating, University of Colorado, Denver Carrie Kitko, University of Michigan, Ann Arbor Margaret MacMillan, University of Minnesota, Minneapolis |
| Outside reviewers: | Adriana Seber, Instituto de Oncologia Pediátrica. Sao Paolo Paul Veys, Great Ormond Street Hospital for Children, London |
| Committee 7: Optimal Donor and Graft Source | |
| Chair: | Claudio Anasetti, H. Lee Moffitt Cancer Center, Tampa |
| Members: | Juliet Barker, Memorial Sloan-Kettering Cancer Center, New York Asad Bashey, Northside Hospital, Atlanta Claudio Brunstein, University of Minnesota, Minneapolis Dennis Confer, National Marrow Donor Program, Minneapolis Sarah Cooley, University of Minnesota, Minneapolis Corey Cutler, Dana-Farber Cancer Institute, Boston Ephraim Fuchs, Johns Hopkins University, Baltimore John Hansen, Fred Hutchinson Cancer Research Center, Seattle Elizabeth Shpall, MD Anderson Cancer Center, Houston |
| Outside reviewers: | Bronwen Shaw, Anthony Nolan Research Institute, London Gerard Socie, Hôpital Saint-Louis, Paris |
| Committee 8: GVHD | |
| Chair: | Joseph Antin, Dana-Farber Cancer Institute, Boston |
| Members: | Amin Alousi, MD Anderson Cancer Center, Houston James Ferrara, University of Michigan, Ann Arbor Mary Flowers, Fred Hutchinson Cancer Research Center, Seattle Richard Jones, Johns Hopkins University, Baltimore Leslie Kean, Seattle Children’s Hospital Paul Martin, Fred Hutchinson Cancer Research Center, Seattle Richard Maziarz, Oregon Health & Science University, Portland David Porter, University of Pennsylvania, Philadelphia Daniel Weisdorf, University of Minnesota, Minneapolis |
| Outside reviewers: | Andrea Bacigalupo, San Martino Hospital, Genoa Ernst Holler, University Hospital Regensburg |
| Committee 9: Gene and Cell Therapy | |
| Chair: | Helen Heslop, Baylor College of Medicine, Houston |
| Members: | Catherine Bollard, Children’s National Medical Center, Washington, DC Steve Forman, City of Hope, Duarte Edwin Horwitz, Children’s Hospital of Philadelphia Michael Jensen, Seattle Children’s Hospital Donald Kohn, University of California- Los Angeles Marcela Maus, University of Pennsylvania, Philadelphia Jeffery Miller, University of Minnesota, Minneapolis Katy Rezvani, MD Anderson Cancer Center, Houston Jerry Ritz, Dana-Farber Cancer Institute, Boston Michel Sadelain, Memorial Sloan-Kettering Cancer Center, New York |
| Outside reviewers: | Rupert Handgretinger, University of Tübingen Armand Keating, Princess Margaret Hospital, Toronto |
| Committee 10: Comorbidity and RRT | |
| Chair: | Edward Stadtmauer, University of Pennsylvania, Philadelphia |
| Members: | Andrew Artz, University of Chicago Ami Bhatt, Dana-Farber Cancer Institute, Boston Guang-Shing Cheng, University of Washington, Seattle Kenneth Cooke, Johns Hopkins University, Baltimore Vincent Ho, Dana-Farber Cancer Institute, Boston John McCarty, Virginia Commonwealth University, Richmond Robert Soiffer, Dana-Farber Cancer Institute, Boston Mohamed Sorror, Fred Hutchinson Cancer Research Center, Seattle Greg Yanik, University of Michigan, Ann Arbor |
| Outside reviewers: | Jane Apperley, Imperial College, London Mohamad Mohty, Hotel-Dieu, Université de Nantes |
| Committee 11: Infection/Immune Reconstitution | |
| Chair: | John Wingard, University of Florida, Gainesville |
| Members: | Michael Boeckh, Fred Hutchinson Cancer Research Center, Seattle Nelson Chao, Duke University, Durham Mitchell Horwitz, Duke University, Durham Kieren Marr, Johns Hopkins University, Baltimore Richard O’Reilly, Memorial Sloan-Kettering Cancer Center, New York Stan Riddell, Fred Hutchinson Cancer Research Center, Seattle Marcel van den Brink, Memorial Sloan-Kettering Cancer Center, New York Edmund Waller, Emory University, Atlanta |
| Outside reviewers: | Per Ljungman, Karolinska University Hospital, Huddinge John Zaia, City of Hope, Duarte |
| Committee 12: Late Effects/QOL/Economics | |
| Chair: | Stephanie Lee, Fred Hutchinson Cancer Research Center, Seattle |
| Members: | Saro Armenian, City of Hope, Duarte Heather Jim, H. Lee Moffitt Cancer Center, Tampa Nandita Khera, Mayo Clinic, Rochester Navneet Majhail, National Marrow Donor Program, Minneapolis J. Douglas Rizzo, Medical College of Wisconsin, Milwaukee Bipin Savani, Vanderbilt University, Nashville Karen Syrjala, Fred Hutchinson Cancer Research Center, Seattle |
| Outside reviewers: | Jane Apperley, Imperial College, London Gerard Socie, Hôpital Saint-Louis, Paris |
| Committee 13: Clinical Trial Design | |
| Chairs: | Brent Logan, Medical College of Wisconsin, Milwaukee Liaison to Comorbidity/RRT and Pediatric Outcomes/Late Effects Committee Marcelo Pasquini, Medical College of Wisconsin, Milwaukee Liaison to Gene/Cell Therapy and Multiple Myeloma Committees |
| Members: | Tom Braun, University of Michigan, Ann Arbor Liaison to Gene/Cell Therapy and GVHD Committees Nancy DiFronzo, National Heart, Lung, and Blood Institute, Bethesda Liaison to Nonmalignant Disease and Pediatric Outcomes/Late Effects Committees Elihu Estey, Fred Hutchinson Cancer Research Center, Seattle Liaison to Comorbidity/RRT and Leukemia Committees Nancy Geller, National Heart, Lung, and Blood Institute, Bethesda Liaison to GVHD and Multiple Myeloma Committees Ted Gooley, Fred Hutchinson Cancer Research Center, Seattle Liaison to Late Effect/QOL/Economics and Leukemia Committees Mary Horowitz, Medical College of Wisconsin, Milwaukee Liaison to Lymphoma and Pediatric Indications/Approaches Committees Eric Leifer, National Heart, Lung, and Blood Institute, Bethesda Liaison to Late Effects/QOL/Economic and Pediatric Outcomes/Late Effects Committees Leo Luznik, Johns Hopkins University, Baltimore Liaison to Infection/Immune Reconstitution and Optimal Donor/Graft Source Committees Adam Mendizabal, The EMMES Corporation, Rockville Liaison to Nonmalignant Diseases and Pediatric Indications/Approaches Committees Joycelynne Palmer, City of Hope, Duarte Liaison to Infection/Immune Reconstitution and Lymphoma Committees |
| Outside reviewers: | N/A |
COMMITTEE 1: LEUKEMIA
Current State of the Science
Leukemia is the most common indication for allogeneic HCT and disease recurrence is the most common reason for transplantation failure. Relapse occurs most frequently early after transplantation before full donor immune reactivity has occurred. Accordingly, this committee chose to focus primarily on strategies to mitigate the risk of relapse in acute myeloid leukemia (AML) after HCT based on the availability of new agents, encouraging preliminary data, and trial feasibility. The committee also noted that the role of allogeneic HCT in older patients remains unsettled.
Strategy 1: A Randomized, Double-blind, Phase III Study of Fms-like tyrosine Kinase 3 (FLT3) Inhibition Compared with Placebo as Maintenance Therapy in Subjects with FLT3–internal Tandem Duplication (ITD)+ AML Who Are in Remission after Allogeneic HCT
Hypothesis
The continued administration of FLT3 inhibition in patients with FLT3-ITD+ AML in remission after HCT is feasible and will prevent early relapse leading to improved leukemia-free survival compared with placebo.
Background
Approximately 20% to 30% of patients with AML harbor an ITD mutation in the FLT3 receptor that results in a high risk of relapse after conventional chemotherapy [3]. Retrospective data suggest such patients may benefit from HCT, yet the risk of relapse after HCT is still high [4]. Agents that inhibit FLT3 signaling are available and have been tested in clinical trials [5].
Trial design
The committee proposed a phase III, randomized, double-blind, 2-arm study to determine the clinical benefit of FLT3 inhibitor monotherapy compared with placebo for patients with FLT3-ITD+ AML who are in remission after HCT. The primary endpoint would be leukemia-free survival with a sample size based on a comparison of the 2 arms. A hazard ratio of .6 was suggested.
Feasibility and logistics
This trial design would be definitive but would require a large sample size (~500 patients) and thus necessitate a multicenter and, possibly, multinational effort with support from 1 of the drug manufacturers. At this time, quizartinib appears to be the most promising agent, based on preliminary efficacy data [5].
Strategy 2: A Randomized, Phase III Study of Low-dose Azacitidine Maintenance Compared with no Maintenance in Patients with AML or Myelodysplastic Syndromes at High Risk of Relapse after HCT
Hypothesis
Post-transplantation low-dose azacitidine maintenance will decrease the risk of relapse after allogeneic HCT for AML and or myelodysplastic syndromes (MDS).
Background
The hypomethylating agents 5-azacitidine (AZA) and decitabine are clinically active against both MDS and AML [6]. In particular, AZA prolongs survival compared with supportive care in patients with MDS and is feasible to administer after HCT [7,8]. A phase I trial established a safe dose after HCT and the Alliance (formerly Cancer and Leukemia Group B) recently completed a 64-patient phase II study using that dose after reduced-intensity conditioning (RIC) HCT in patients with AML and MDS. A single-center phase III study is ongoing at MD Anderson.
Trial design
The committee proposed a randomized phase III study comparing subcutaneous AZA starting on post-transplantation day 40 to 100, given in 30-day cycles for 1 year, or approximately 12 cycles, compared with no maintenance. Event-free survival would be the primary endpoint. Patients who are FLT3 ITD+ would be excluded to avoid overlap with strategy 1. A hazard ratio of .6 was suggested.
Feasibility and logistics
Sample size would depend on the magnitude of the benefit postulated, but the trial would likely require 250 to 350 patients. There are now oral hypomethylating agents available that may increase trial feasibility and facilitate use of a placebo control.
Strategy 3: Prospective Comparative Trial Evaluating Postremission HCT versus Consolidation Chemotherapy in Older Patients with AML
Hypothesis
Patients with AML in first complete remission (CR1) who are 60 years or older will have prolonged survival after HCT compared with other consolidation strategies.
Background
Multiple retrospective series, as well as smaller prospective studies, suggest patients 60 years or older with AML in CR1 undergoing HCT may have superior outcomes compared with counterparts receiving nontransplantation-based consolidation [9,10]. These studies are confounded by differences in selection practices for HCT and non-HCT therapies. A definitive advantage with transplantation, demonstrated in a large prospective trial, would change the standard of therapy for this group of individuals.
Trial design
The committee proposed a phase III trial with 2 arms, ancillary to any active North American cooperative group trial for initial therapy in elderly patients (60 to 75 years old) with newly diagnosed AML. The trial would use a biologic assignment design. Patients with a suitable donor would be assigned to the HCT arm. Overall survival would be compared between those with versus those without a suitable donor.
Feasibility and logistics
This trial design would require 500 to 600 patients at initial diagnosis based on anticipated dropout because of failure to respond to initial therapy and other logistical issues that are not insignificant. The latter include the relatively low number of older AML patients with an available donor who actually proceed to HCT (<50%) and the recent availability of alternative donor transplants (eg, umbilical cord blood, haplo-identical relative), making transplantation an option for virtually all patients.
Summary of Discussion
There was significant enthusiasm for the concept of interventions to mitigate the risk of relapse in high-risk AML after allogeneic HCT. Both Strategies 1 and 2 were considered meritorious and should be high priority for the network in the near future. Concerns were raised regarding the current paucity of data regarding the safety and feasibility of administering FLT3 inhibitors after HCT, though phase II studies are ongoing that should help to inform trial design. Regarding Strategy 2, concerns were raised about the dose and schedule as well as route of administration (oral versus parenteral) of hypomethylating agents, but data from ongoing and recently completed phase II studies should be available to aid in trial design. Strategy 3 was given a lower priority because of an inability to resolve the substantial biases inherent in a trial design that does not allow randomization.
COMMITTEE 2: LYMPHOMA
Current State of the Science
HCT cures a subset of patients with relapsed/refractory non-Hodgkin lymphoma and diffuse large B cell lymphoma (DLBCL) is the second-most common indication for autologous HCT (AuHCT) worldwide. Efforts to reduce relapse rates after AuHCT for lymphoma include offering HCT earlier in the disease course. A recently published large randomized trial provides evidence, via a subset analysis, for the efficacy of AuHCT in CR1 for high-risk DLBCL [11]. High-risk DLBCL, such as “double hit” (DH) lymphoma and the activated B cell–type lymphoma (ABC), is being identified earlier [12]. AuHCT is offered in CR1 to younger patients with mantle cell lymphoma (MCL), but is not curative.
Strategy 1: A Randomized Trial of Ibrutinib during and after AuHCT in Patients with Relapsed and Refractory DLBCL of the ABC Subtype
Hypothesis
Ibrutinib will improve progression-free survival (PFS) after AuHCT for patients with relapsed or refractory ABC-subtype DLBCL.
Background
The 2 distinct subtypes of DLBCL, germinal center B cell–like and ABC [13], have significantly different 5-year survival rates of 60% versus 35%, respectively, with frontline chemotherapy with rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone (R-CHOP). AuHCT for relapsed and refractory DLBCL yields 2-year PFS and overall survival rates of 48% and 65%, respectively, for patients with chemotherapy-responsive disease [14]. Disease progression after AuHCT remains the primary cause of failure. Ibrutinib is a selective inhibitor of Bruton’s tyrosine kinase that achieves responses in patients with lymphoid malignancies, including those with heavily pretreated ABC-type DLBCL [15–17].
Trial design
The committee proposed a randomized, placebocontrolled phase III study in patients receiving AuHCT for ABC-type DLBCL that is chemosensitive to salvage therapy. Patients would be randomized to receive ibrutinib or placebo. Ibrutinib would start during the pretransplantation conditioning period and continue through 12 months after HCT. Patients who progressed on the placebo arm would be allowed to receive ibrutinib. The methodology to be used for determining the cell-of-origin (gene expression profiling, immunohistochemistry algorithms, or nanostring technologies) would be specified before trial initiation.
Feasibility and logistics
A Center for International Blood and Marrow Transplant Research (CIBMTR) query found that between 2009 and 2012, 770 patients per year with relapsed DLBCL underwent AuHCT. Assuming that 50% of these patients are of ABC subtype, approximately 335 patients per year will be eligible. Assuming that 1 of 3 patients is eligible and will participate, accrual of 100 patients per year is feasible. A sample size of 300 patients accrued over 3 years, with 2 years of follow-up, would provide 85% power to detect a clinically meaningful increase in PFS (hazard ratio of 1.6), assuming a median PFS of ~24 months in the placebo arm [18,19].
Strategy 2: A Phase II Trial for Previously Untreated DH DLBCL Patients: rituximab, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin (R-EPOCH) Induction Followed by AuHCT during First Partial Remission (PR1) or CR1
Hypothesis
AuHCT for previously untreated patients with DH DLBCL during first response (CR1 or PR1) will have improved 2-year PFS compared with DH DLBCL patients who receive conventional therapy only.
Background
DLBCL patients who express concurrent MYC and BCL2 translocation or DH lymphoma have a dismal outcome. Reported 3-year PFS rates range from 39% to 45%, with a 5-year PFS of 18% [20,21]. There are no controlled trials evaluating the impact of AuHCT early in the disease course. In a multicenter retrospective study of >100 DH lymphoma patients, R-EPOCH was superior to R-CHOP in achieving CR1. Additionally, AuHCT in CR1 was associated with improved survival [22]. A prospective study evaluating dose-adjusted R-EPOCH followed by AuHCT in first response would help define optimal treatment.
Trial design
The committee proposed a single-arm phase II study. The primary endpoint would be 2-year PFS. Eligibility would include patients with newly diagnosed DH DLBCL, identified by fluorescein in situ hybridization. Confirmation of the dual rearrangement of MYC and BCL2 would be done via a central lab performing fluorescein in situ hybridization analyses. One cycle of chemotherapy would be allowed before starting R-EPOCH induction. After induction and up to 6 cycles of consolidation, patients in PR1 or CR1 would proceed to AuHCT.
Feasibility and logistics
In 2013, ~21,000 cases of DLBCL were diagnosed. Approximately 25% of these cases carried DH mutations. Assuming a baseline 2-year PFS of ~ 38%, a sample size of 46 patients would allow detection of improvement to 58% with 90% power and an α of .05. It is estimated that accrual could be completed in less than 2 years.
Strategy 3: Therapeutic Alternatives for Mantle Cell Lymphoma
Hypothesis
See below.
Background
Median overall survival of patients with newly diagnosed MCL is 4 to 5 years with conventional chemotherapy [23]. Common induction regimens include R-CHOP or cytarabine-containing regimens, with younger patients often offered AuHCT in CR1 [24,25]. Recent reports show that maintenance rituximab after R-CHOP prolongs remission and reduces the risk of death in older patients [26]. Additionally, maintenance rituximab prolongs PFS after AuHCT, with 2-year PFS rates approaching 90% [27]. Bendamustine/rituximab (BR) is an increasingly used regimen conferring CR rates of ~50% with less toxicity than R-CHOP in newly diagnosed MCL [28]. The most common postremission approaches after BR induction consist of maintenance rituximab and/or AuHCT. There are no prospective studies of either approach (after BR) and no randomized comparison of maintenance rituximab after conventional therapy versus maintenance rituximab after AuHCT in patients who achieve CR1. Ibrutinib is also highly active in patients with relapsed/refractory MCL with response rates of approximately 70% [29].
Potential trial designs
The committee discussed several possible study designs. One concept involved performing AuHCT for MCL in CR1 and then randomizing patients to maintenance rituximab versus maintenance ibrutinib. The induction regimen would not be specified. The hypothesis is that maintenance ibrutinib would significantly extend 2-year PFS over maintenance rituximab. Another concept involved giving previously untreated MCL patients 6 cycles of BR induction followed by randomization to either AuHCT after by maintenance rituximab or maintenance rituximab alone. The hypothesis is that outcomes will be equivalent between AuHCT with maintenance rituximab versus maintenance rituximab alone. Potential pitfalls of this concept include the requirement for patients to enroll at the time of diagnosis, at which time they may not be at a transplantation center. Also, high-dose cytarabine is not part of the planned induction and some investigators may not feel comfortable offering BR as induction therapy. Although the above 2 concepts attempt to answer relevant questions, robust point estimates are currently not available in the literature to accurately project effect sizes.
Feasibility and logistics
A CIBMTR query showed that the numbers of MCL patients who underwent AuHCT in CR1 in 2011 and 2012 were 412 and 397, respectively. Thus, depending on the sample size of a proposed trial, a sufficient number of CR1 MCL patients may be eligible for such a trial. Both HCT physicians and lymphoma physicians would need to reach consensus regarding specific populations, indications, and timing of AuHCT and the potential role of maintenance therapy after AuHCT before a large MCL trial involving AuHCT could be successfully launched.
Summary of Discussion
There was considerable enthusiasm for Strategy 1, evaluating the role of ibrutinib added to AuHCT for patients with ABC DLBCL. Strategy 2 also was felt to be meritorious. There was less enthusiasm for Strategy 3, for reasons described in the sections on trial design and feasibility.
COMMITTEE 3: MULTIPLE MYELOMA
Current State of the Science
Background
The most compelling clinical question to be addressed today in multiple myeloma (MM) is the role of early versus late AuHCT. The ongoing Intergroupe Francophone du Myelome/ Dana-Farber Cancer Institute randomized trial (BMT CTN 1304) is addressing this issue and is the highest priority study identified by the BMT CTN Myeloma Intergroup Committee. The Myeloma SOSS committee also felt that defining whether risk-adapted therapy (prognostic index + response) can be used to guide therapy is an important goal. Studies might address whether “depth of response” based on multiparameter flow cytometry or polymerase chain reaction (PCR) predicts outcome, and whether a myeloma risk profile + “depth of response” can guide subsequent therapy (ie, need for high-dose consolidation or continued maintenance) [30,31]. Finally, the committee noted that disease recurrence/progression is the single most important cause of treatment failure and continued development of post-HCT therapies that reduce the risk of progression should be the focus of future research. Among the strategies being considered are new agents for post-HCT maintenance, new strategies for allogeneic HCT, and novel antimyeloma vaccines.
Strategy 1: A Multicenter Phase II, Placebo-Controlled Trial of Maintenance Ixazomib after Allogeneic Hematopoietic Cell Transplantation for High-Risk MM (BMT CTN 1302)
Hypothesis
RIC allogeneic HCT incorporating proteosome inhibitors in the preparative regimen and as post-transplantation maintenance will improve PFS in patients with high-risk multiple myeloma.
Background
Although existence of a graft-versus-myeloma effect is well documented, the role of allogeneic HCT in MM remains to be defined. In the upfront setting, most prospective trials failed to show a benefit for allografting as consolidation of an initial remission including the BMTCTN trial, 0102, which compared a tandem AuHCT approach with AuHCT followed by allogeneic HCT. Notwithstanding, long-term disease control and cures can be obtained with this approach, even in patients who have failed primary therapy [32]. Moreover, long-term follow-up of BMT CTN 0102 suggests that relapse rates may be decreased after a RIC allograft in patients with high-risk disease. Bortezomib is a proteosome inhibitor that has significant anti-myeloma and immune modulatory activity. In phase II trials, bortezomib can reduce the risk of grades 2 to 4 graft-versus-host disease (GVHD) in the HLA-mismatched setting [33]. Ixazomib (MLN9708) is a next-generation, small-molecule, boronate proteasome inhibitor that can be given orally once a week.
Trial design
The committee endorsed a randomized phase II trial, already in development by BMT CTN, that will explore the role of 2 proteosome inhibitors (bortezomib and ixazomib) in preventing disease recurrence after allogeneic HCT in patient with high-risk MM [34]. This trial incorporates bortezomib in a RIC regimen to reduce the risk of GVHD and improve myeloma control. After allogeneic HCT, patients are randomized to receive either placebo or the novel oral proteasome inhibitor ixazomib as maintenance therapy. The primary objective is to improve 18-month PFS in patients with high-risk myeloma (high-risk cytogenetics or first relapse after AuHCT) from 50% to 75%.
Feasibility and logistics
High-risk MM patients with an early relapse after autografts form a group for which all current therapies are suboptimal. Approximately 20% to 25% of AuHCT recipients will relapse within 2 years. A subset of these patients (based on age, performance status, effectiveness of second-line therapy) will be eligible for the proposed trial. Because there are > 6000 AuHCTs for MM annually in the United States, we expect there will be at least 500 post-AuHCT patients eligible each year. High-risk patients identified at diagnosis (by genetic tests) account for 20% of all patients with MM and another group eligible for this trial. Although this trial will compete with several non-HCT trials, we expect that at least 30% of newly diagnosed high-risk MM patients will be offered this trial. Patients enrolled in alternative nontransplantation trials would also be eligible for the 1302 trial at progression. Allogeneic HCT is not commonly performed for treatment of myeloma with approximately 100 transplantations per year, according to the CIBMTR. BMT CTN centers were surveyed to understand the challenges and local practices. The 5 centers with the highest transplantation activity for this indication were included in the survey. The 2 most common reasons for not proceeding to transplantation were insurance denials and lack of a clinical trial. Twenty-nine centers confirmed interest in participating in BMT CTN 1302, with a total estimated accrual rate of 100 patients per year.
Strategy 2: Multicenter Trial Exploring the Safety and Efficacy of a Plasma Cell–Dendritic Cell Fusion Vaccine
Hypothesis
Dendritic cell–plasma cell fusion vaccination in the post-HCT setting will enhance antimyeloma immunity, producing higher response rates and improved disease control.
Background
The immune system is known to play an important role in myeloma progression and control. Recently some myeloma vaccine trials have shown promising clinical results. Particularly encouraging is the approach described by Avigan et al. utilizing a plasma cell–dendritic cell fusion product [35].
Trial design
The committee endorsed a randomized phase II study of post-HCT vaccination. This trial is already in active development and is proposed to be performed in 2 stages: (1) screening to collect plasma cells for vaccine production, and (2) randomization to either post-HCT lenalidomide versus post-HCT lenalidomide + fusion vaccine administered 30 days after HCT and after the second, third, fourth, and 12th lenalidomide maintenance cycles. The primary end-point is an increase in CR rates from 40% to 60% at 18 months (20% effect size). To detect this effect size with a type I error of .1, the trial will need 120 randomized patients. A 30% drop out rate at the screening stage is expected, requiring enrollment of 170 patients.
Feasibility and logistics
Standardized vaccine production is the main barrier to be overcome for successful implementation of this protocol. Avigan et al. have shown that it is possible to export the vaccine production technology to other sites with Good Manufacturing Practice capabilities. Thus, this protocol will be limited to sites with Good Manufacturing Practice facilities that have the capacity to produce the vaccine. Likewise, vaccine production requires at least 20% plasma cells in the marrow aspirate. This means that many patients referred for transplantation will not be eligible. Proactive screening and recruitment of all newly diagnosed MM patients will be essential for successful protocol completion.
Strategy 3: Evaluating Risk-Adaptive Therapy for Multiple Myeloma
The committee discussed the design of a definitive trial addressing the role of risk-adaptive therapy for myeloma but concluded that this should be deferred until the current early versus late HCT trial completes accrual and the results of BMT CTN 0702 (single AuHCT with/without revlimid, vel-cade, dexamethasone (RVD) consolidation versus tandem AuHCT with maintenance therapy) and the long-term follow-up data of patients on lenalidomide maintenance in Cancer and Leukemia Group B 100104 are available.
Summary of Discussion
The BMT CTN has a robust myeloma portfolio at this time and is addressing the compelling question of optimal timing of AuHCT for patients with standard-risk disease. There was strong enthusiasm for Strategies 1 and 2, which will address important issues such as the role of allogeneic HCT and the use of a novel immune-therapeutic strategy to ameliorate progression after AuHCT. The committee endorsed the concept that follow on studies to the current BMT CTN portfolio should explore the concepts of risk-adapted therapy and further development of novel immune-therapeutic strategies. Future protocol development should take into consideration results from currently active clinical trials being conducted worldwide.
COMMITTEE 4: NONMALIGNANT DISEASES IN ADULTS
Current State of the Science
Nonmalignant diseases account for just 5% of HCT activity. One half to two thirds of the procedures are performed for a broad range of inherited diseases of the blood and immune system in children. Most of the remaining are performed for marrow failure, about one half in children. Over the last 15 years, through numerous early-phase observational or small randomized clinical trials, HCT has proven a feasible and powerful tool for repairing diseases characterized by dysfunctional hematopoiesis and immunity. Despite the potential to mitigate these often highly morbid or severe life-threatening conditions, they remain orphan HCT indications. Well-designed, randomized trials could help establish the role of HCT in mainstream clinical practice, to the benefit of patients. This committee examined adult nonmalignant disease indications that would be appropriate for multicenter HCT clinical trials.
Strategy 1: HCT for Multiple Sclerosis (MS)
Hypothesis
High-dose cytotoxic therapy with AuHCT will improve control of relapsing-remitting MS.
Background
Patients with highly active MS achieve long-term freedom from inflammatory activity and sustained accumulation of disability (SAD) after AuHCT without the need for ongoing immunotherapy. Despite evidence of effectiveness provided by the outcome of a combined CIBMTR and European Society for Blood and Marrow Transplantation registry follow-up study [36,37] and numerous phase II clinical trials [38], wide-spread use of HCT in MS is hindered by the lack of comparison to current costly immunosup-pressive agents.
Trial design
The committee proposed a randomized phase III trial in patients with highly active, relapsing-remitting MS and who have moderate disability and have failed at least 1 conventional disease-modifying drug to test whether ablative conditioning followed by AuHCT will result in better outcomes than the best available nontransplantation therapy. The primary endpoint would be 3-year inflammatory disease-free survival (DFS) (absence of clinical relapses, absence of gadolinium-enhancing lesions, and absence of new T2 lesions on magnetic resonance imaging scan). Secondary endpoints would include freedom from SAD, sustained improvement in disability, quality of life (QOL), and cost-effectiveness. Sixty patients per arm are required to detect absolute improvement in inflammatory DFS from 60% to 80%, assuming 90% power with P = .05. An extension study would examine 5 to 7 year freedom from SAD and responses to subsequent treatment for patients failing the study treatment.
Feasibility and logistics
Although there are currently more than 300,000 patients with MS in the United States, fewer than 50 each year undergo AuHCT. Patient recruitment could be an issue, highlighting the importance of developing a referral network whereby MS patients are sent to a defined number of study centers with expertise in both MS and HCT.
Strategy 2: Alternative Donor HCT for Aplastic Anemia
Hypothesis
Optimizing the conditioning regimen used for umbilical cord blood and haploidentical bone marrow transplantation for aplastic anemia will result in outcomes similar to those of unrelated adult donor bone marrow transplantation.
Background
Survival after HLA-matched unrelated donor transplantations for aplastic anemia now exceeds 80% [39,40], leading to increased HCT referrals of patients failing immunosuppressive therapy. However, up to 40% of otherwise eligible patients, especially minorities, do not have HLA-matched adult donors. Historically, outcomes with alternative donors have been poor [41,42]. More recently, dose- and timing-optimized administration of antithymocyte globulin, along with a slight increase in total body irradiation (TBI) dose (from 2 to 4 Gy) has produced consistent engraftment of alternative donor cells in pilot studies [43]. This affords the possibility of offering HCT to more patients with aplastic anemia.
Trial design
The committee proposed a phase I/II study of HCT for aplastic anemia using haploidentical and cord blood donors, building upon the recently completed BMT CTN 0301 trial that optimized cyclophosphamide dosing for unrelated donor marrow transplantations. This new trial would first optimize antithymocyte globulin dose and timing and then optimize TBI dose, to minimize exposure. The primary endpoint would be 1-year graft-failure–free survival. The statistical design would be finalized after analysis of BMT CTN 0301 in late 2014, building on the latest available data.
Feasibility and logistics
Aplastic anemia is a rare disease and not all patients require transplantation. In 2012, there were 103 matched sibling donor, 98 unrelated donor, and 10 cord blood transplantations for aplastic anemia in the United States. This trial proposal targets the small fraction of the aplastic population without an HLA-matched adult donor. BMT CTN 0301 required more than 30 sites to enroll 94 patients over a period of 9 years. Thus, we anticipate that this trial would require a national and possibly international initiative and more than 5 years for accrual of a sufficient sample size; however, the BMT CTN is the only venue that will allow evaluation of HCT techniques in this disease.
Strategy 3: HCT for Sickle Cell Disease
Hypothesis
Allogeneic HCT will improve long-term survival of young adults with severe sickle cell disease (SCD).
Background
Supportive care allows most children to survive to adulthood, but young adults with severe SCD experience rapid disease progression and premature mortality. HLA-matched sibling donor HCT has curative potential but is applied sparingly. A Cochrane review identified the need for a randomized controlled clinical trial to assess its risks and benefits [44]. Although trials of allogeneic HCT for children with SCD exist, there is limited experience using HCT in adults. The CIBMTR database records only 42 transplantations in adults (≥21 years) for SCD in the period between 2008 and 2012 [45]. Limited donor availability, regimen-related toxicity, GVHD, and high graft rejection rates have been problematic but may be overcome by refinements in HCT approach [46].
Trial design
The committee proposed a phase III trial comparing HCT with non-HCT therapy for young adults with SCD, using a biologic assignment approach to treatment allocation, based on availability of an HLA-matched related or unrelated donor.
Feasibility and logistics
Technical issues, such as sibling and unrelated donor availability, the high rate of graft rejection, concern about GVHD, and the influence of patient comorbidities resulting from complications of SCD all affect the feasibility of this trial. The details of the transplantation regimen would need to wait for the outcome of several currently ongoing feasibility trials. Furthermore, trial enrollment may be affected by caution on the part of patients and their primary care physicians.
Summary of Discussion
Strategy 1, defining the role of AuHCT for MS, garnered significant support and was felt to be a high priority for the network in the near future. Concerns were raised about recruitment and the need to engage the neurology community. It was recognized that Strategy 2, improving the outcome of alternative donor transplantation for aplastic anemia, was important and only feasible through the BMT CTN. The importance of the unmet clinical need in SCD addressed by Strategy 3 was acknowledged. It was felt that a trial in SCD should be prioritized as soon as the outcomes of current feasibility trials become available.
COMMITTEE 5: PEDIATRIC INDICATIONS FOR HCT
Current State of the Science
The most common indication for HCT in children is acute lymphoblastic leukemia (ALL). Leukemia relapse remains the most common cause of failure after HCT for childhood ALL. Relapse is especially common in patients who have minimal residual disease (MRD) before HCT and who do not develop acute GVHD, a group that forms a sizable population available for clinical trials of novel interventions.
Allogeneic hematopoietic cells provide a source of enzyme for children with inborn errors of metabolism (IEM) and can ultimately prevent or ameliorate disease manifestations. However, neurological worsening can continue during the first post-transplantation year before underlying central nervous system (CNS) inflammation abates. Arresting disease progression in these patients could improve long-term neurologic outcomes.
Strategy 1: Post-HCT Intervention to Prevent Relapse in Patients with ALL
Hypothesis
Relapse after HCT for B cell ALL can be decreased by early administration of anti–B cell therapies that do not depend upon functional adaptive immunity, followed by maintenance therapy through the first year after HCT, when relapse risk is highest.
Background
Patients who do not develop acute GVHD by day +55 are at increased risk of relapse, especially if they have MRD before HCT. According to CIBMTR data, 2-year relapse rates are higher in children (31%) and adults (36%) without acute GVHD compared with children (19%) and adults (26%) with grades I to II acute GVHD. High pre-HCT MRD adds to this risk (67% versus 35%) [47]. CIBMTR data from 2008 to 2012 [45] and other studies [47] show that >90% of acute GVHD after HCT with myeloablative conditioning occurs by day +55. Thus, a population at high risk for relapse can be identified within 2 months of HCT. Because immune reconstitution is incomplete early after HCT, agents that do not require the adaptive immune system for efficacy are ideal for this setting. Moxetumomab and inotuzumab are 2 of several anti-CD22 or anti-CD19 conjugated immunotoxins with differing mechanisms that have shown impressive single-agent activity in refractory B cell ALL.
Trial Design
The committee proposed a 3-arm randomized phase II protocol comparing post-HCT therapy with each of the 2 conjugated immunotoxins with a placebo control. Eligibility would include children and adults undergoing non–T cell–depleted transplantation from any type of donor after myeloablative conditioning for B cell ALL who are alive without organ failure or acute GVHD at day +55. Patients would be stratified by pre-HCT MRD status and stem cell source. Therapy would continue for 9 months. According to CIBMTR data, 2-year DFS of children and adults not developing acute GVHD by day +55 is 59% and 47%, respectively (overall 53%). We hypothesize that the 2-year DFS would improve from 53% to 70%. To test 2 agents against a control with 80% power at the 1-sided α = .10 level for each comparison [48], 255 patients will be needed.
Feasibility and logistics
CIBMTR data show 285 patients per year undergoing HCT with myeloablative conditioning for non-Philadelphia+ BALL alive with no acute GVHD at day +55. We assume 71 per year (25%) would enroll, leading to an accrual time of 3.6 years. Modification of the study design to test agents sequentially with possible expansion to a phase III trial for a given agent if screening criteria are met could be considered, depending upon availability of agents and feasibility of comparative trials.
Strategy 2: Phase II Trial to Assess the Efficacy of Etanercept/Celecoxib in Preventing Post-HCT Neurological Progression in Children with IEM
Hypothesis
Children with IEM who receive CNS-active anti-inflammatory agents before and after HCT will experience reduced demyelination and better neurological outcomes.
Background
Myelination is essential for normal brain function and is a cornerstone of human neurodevelopment [49]. Disease stabilization after HCT in IEM patients is dependent upon engraftment of donor-derived microglia, which occurs slowly over the first post-HCT year. Disease progression/ demyelination occurring the first year after transplantation is common and thought to be related to persistent CNS inflammation caused by accumulation of toxic metabolites from the underlying disease process. Preclinical studies show that this inflammation is mediated by TNF-α and through the COX-2 pathway [50]. Coadministration of agents blocking these pathways improves myelination in animal models of these diseases.
Trial design
The committee proposed a phase III trial in patients with IEM randomizing patients to receive etanercept + celecoxib before and after allogeneic HCT versus not. The primary endpoint would be change in myelination from pre-HCT baseline to 6 months measured after HCT by magnetic resonance imaging–based quantitative susceptibility mapping [51]. Twenty-nine patients in each arm would provide 80% power with a 2-sided α = .05 to detect a .75 standard deviation difference.
Feasibility and logistics
CIBMTR data show that 47patients with these diagnoses undergo HCT annually. Assuming a participation rate of 40%, accrual could be completed in 3 years. One potential problem is the reluctance to randomize to the control arm, as the experimental agents are readily available outside the research setting.
Summary of Discussion
There was strong support for the post-HCT relapse prevention strategy in ALL because of compelling preliminary data and simplicity of design, although safety data for specific agents given as early as 2 months after HCT are lacking. The discussion group also encouraged the inclusion of adults in this study. There was less enthusiasm about the IEM proposal because of the possibility that inflammation may be needed after HCT to facilitate engraftment and, possibly, for establishment of donor-derived microglia in the CNS. It was felt that more preliminary data are required to justify this approach.
COMMITTEE 6: PEDIATRIC OUTCOMES
Current State of the Science
Most pediatric HCT recipients are surviving long-term but long-term toxicities are a problem, especially those linked to glucocorticoid therapy for GVHD, particularly chronic GVHD. Specific strategies are necessary to deal with GVHD in children, as opposed to allowing accrual of modest numbers of older children onto GVHD trials primarily focused on adults. Children may have different dosing requirements and response to therapies and brisker immune reconstitution than adults [52]. Infants and small children have increased body surface area to weight ratios relative to adults and, consequently, receive higher drug doses when dosed by body surface area, with potentially increased toxicity [53,54]. Key drug metabolizing enzymes develop throughout childhood, being less active at some ages than in adults, yet more active at other ages [55]. Toxicities, such as impaired growth and disruptive behavior have increased importance in children compared with adults. These important nuances are likely to be lost when children constitute a subpopulation of a large adult study, and they likely deter enrollment of children onto adult studies. Focusing on the most promising avenues likely to improve pediatric outcomes and endpoints is necessary.
Strategy 1: Daily versus Alternate Day Dosing of Steroids in Chronic GVHD
Hypothesis
Every-other day prednisone regimens lower the total cumulative dose of prednisone at 6 months and reduce toxicity.
Background
Despite being debated for 2 decades, the question of whether daily or alternate day steroid dosing is best for chronic GVHD remains unanswered. The committee conducted a survey that showed that 54% of respondents use alternate day and 46% use daily steroids, confirming lack of agreement. The case for alternate day dosing derives from old, poorly controlled literature comprising, primarily, case series in non-HCT populations. An answer to this question could not only unify practice but also inform the design of future clinical trials intent on testing novel steroid-sparing agents.
Trial design
The committee proposed a randomized phase II trial comparing daily to alternate day steroids for chronic GVHD, with specified criteria for proceeding to phase III. Both arms would follow specified taper schedules, unless over-ridden by patient tolerance. The primary endpoint will be a comparison of body mass index Z-scores between children in each arm at 6 months. Demonstration of a .5 reduction would require about 150 patients (75 per arm). Secondary end-points would evaluate steroid toxicities via calendar-driven measurements of bone health (development of avascular necrosis, or dual-energy x-ray absorptiometry (DEXA) scan Z-scores <−2.0), anthropometry (height velocity, total body fat by DEXA scan, arm-muscle area changes over time), number and type of behavioral interventions, myopathy (5-point manual muscle test and other simple physical therapy tests), number of medicines to control hypertension, hyperglycemia, and infection rates (invasive fungal, viral, bacterial).
Feasibility and logistics
Sample size estimates are based on the desire to demonstrate a .5 reduction in body mass index Z-score. There are approximately 300 children with chronic GVHD diagnosed annually. Enrollment of 20% would allow accrual of the necessary sample size in fewer than 2.5 years. A survey of tapers prescribed by 3 representative pediatric institutions suggests similar total prednisone exposures regardless of schedule, suggesting that a trial addressing schedule, without confounding by dose, is feasible.
Strategy 2: Bortezomib for the Prevention of Acute GVHD
Hypothesis
Three doses of bortezomib given on days 1, 4, and 7 after transplantation will reduce the incidence of acute GVHD by 50% in children receiving transplantation with myeloablative conditioning.
Background
A study in adults suggests that the incidence of acute GVHD can be reduced and immune reconstitution improved with use of bortezomib in the early post-HCT period [33].
Trial design
The committee proposed a randomized phase II trial enrolling children up to age 18 years old undergoing allogeneic umbilical cord, blood, or marrow transplantation after a myeloablative preparative regimen. Patients would receive either a standard calcineurin inhibitor (CNI) plus methotrexate or mycophenolate mofetil regimen or a similar regimen with the addition of bortezomib on days +1, +4, and +7. The primary endpoint would be incidence of acute GVHD at day 100. Given an expected grade 2 to 4 acute GVHD rate of 50%, the goal is to reduce grades 2 to 4 GVHD to 25%, which would require 74 patients per arm. Secondary end-points would include extensive chronic GVHD and time to recovery of normal lymphocyte counts.
Feasibility and logistics
CIBMTR data indicate that there are 300 eligible children annually. If 75 enroll per year, accrual could be completed in 2 years.
Summary of Discussion
The importance of developing methods to reduce late toxicities associated with prolonged steroid use in children is clearly recognized. The facts that steroids are widely used and that there are no studies defining the best approach make Strategy 1 of great interest and this proposal was met with high enthusiasm. There was less enthusiasm for Strategy 2, based, in part, on the view that the wide variability in stem cell sources (cord, related, unrelated), preparative regimens and disease types required to allow the study to be conducted in a timely fashion could obscure any possible benefit of the experimental intervention.
COMMITTEE 7: OPTIMAL DONOR AND GRAFT SOURCE
Current State of the Science
The lack of histocompatible graft sources is a major limitation to the treatment of hematological or immune disorders with allogeneic HCT.
Strategy 1: Overcoming the HLA Barrier with Post-transplantation Cyclophosphamide
Hypothesis
Post-transplantation cyclophosphamide (PTCY) prevents GVHD lethality of HLA-disparate, non–T cell–depleted related donor HCT in myeloablated hosts, and it produces outcomes similar to those of HLA-matched volunteer donor transplantations.
Background
PTCY controls alloreactivity safely and effectively across HLA disparity [56,57]. Although mostly utilized after non-myeloablative conditioning, the 1-year survival is ≥60% in younger patients after ablative conditioning [58]. An adult relative who shares 1 HLA haplotype is almost universally available for patients without an HLA-matched donor; availability of the latter type of donor is limited, particularly for patients from minority populations.
Trial design
The committee proposed a single-arm phase II study of HCT with conditioning including myeloablative doses of TBI or busulfan, haploidentical marrow or blood stem cell grafts, and PTCY followed by tacrolimus/mycophenolate mofetil maintenance. The population would include patients <60 years of age with adequate organ function, low comorbidity, and hematologic malignancies in CR or with minimal disease. Primary endpoint is 1-year survival. With a sample size of 62, the 2-sided 90% confidence interval of 1-year survival is 50% to 70%, and the power is 84% to rule out a survival of <43% with a 2-sided α = .10. An exploratory analysis will compare survival of trial patients with HLA-matched unrelated donor HCT recipients from the CIBMTR database.
Feasibility and logistics
Based on experience with BMT CTN 0603, which evaluated use of PTCY and haploidentical donors with RIC and accrued rapidly, and the wider use of myeloablative than RIC regimens, the study is expected to complete accrual within 18 months.
Strategy 2: Facilitate Engraftment of HLA-Disparate Cord Blood Transplantation
Hypotheses
Recipient conditioning, or ex vivo cord blood priming or expansion, or cotransplantation of third-party progenitors facilitate cord blood cell engraftment.
Background
Cord blood engraftment is delayed and graft failure is more frequent than it is after adult donor allografts, contributing to increased patient morbidity and mortality. Eight ongoing trials address this unmet need (see Table 3). The first 3 in the table are in more advanced stages of development.
Table 3.
Cord Blood Priming on Expansion Trials
| Strategy | Trial Phase, Number of Cord Blood Units | Expected Completion |
|---|---|---|
| Ex vivo expansion with mesenchymal cells plus cytokines [59] | Phase III, multicenter, randomized, double unit | >24 mo |
| Ex vivo priming with dimethyl-PGE2 [60] | Phase II, multicenter, randomized, double unit | >24 mo |
| Ex vivo expansion with notch ligand, freshly cultured or off-the-shelf products [61] | Phase II, multicenter, randomized, double unit | >24 mo |
| Recipient treatment with dipeptidylpeptidase (DPP)-4 inhibitor [62] | Phase II, multicenter, single | >24 mo |
| Ex vivo priming by fucosylation [63] | Phase II, multicenter, double unit | 6–12 mo |
| Ex vivo expansion with nicotinamide inhibitor, retains T cells [64] | Phase II, single-center, single unit | 6–12 mo |
| Cotransplantation of CD34+ cells from HLA haplo-mismatched donor, myeloablative regimen [65] | Phase II, single-center, double unit | 6–12 mo |
| Cotransplant of CD34+ cells from HLA haplo-mismatched donor, RIC [66] | Phase II, single-center, single unit | 6–12 mo |
Recommendation
Because of the relatively scarce population of adult patients receiving cord blood transplantation, the committee recommended that BMT CTN and other cord blood investigators collaborate to enroll eligible patients onto 1 or more of these potentially practice-changing open trials to facilitate timely completion. Suggested criteria for the most promising strategies are: (1) favorable single-center data, (2) likely to improve transplantation effectiveness and resource utilization, (3) exportable, (4) applicable to children and adults, and (5) effective independently of conditioning or immunosuppression regimens.
Strategy 3: Optimize HLA-DPB1 Compatibility for Nearly Every Patient with an Unrelated Donor
Hypothesis
The probability of identifying a permissive DPB1 mismatch (or DPB1 match) increases from 70% without DPB1 typing to 90% by testing up to 10 donors per patient.
Background
More than 80% of unrelated donors matched for HLA-A/B/ C/DRB1 are mismatched for DPB1 alleles sharing a T cell epitope (TCE) that are poorly stimulatory (permissive), whereas others are stimulatory (nonpermissive) [67]. Mismatching for nonpermissive DPB1 alleles is associated with a ~15% increase in mortality risk after transplantation [68,69]. Identifying a donor that is matched or has a permissive mismatch at DPB1 could improve survival by ~5%.
Trial design
The committee recommended a prospective single-arm trial in which DP testing would be done on multiple donors (for patients with multiple A, B, C, and DRB1-matched donors) to determine how many patients find a donor with a permissive-DPB1 mismatch and how many donors should be typed based on the patient DPB1 TCE. A sample of 460 patients would provide 99% power to test the primary hypothesis that the probability of a permissive mismatch increases from 70% to 90% with alpha of .05 and allow adequate subset analyses by TCE groups. For example, there will be 95% power with alpha of .02 to detect an increase in the probability of a permissive mismatch from 7.8% to 30% in the rarest TCE-subset. The protocol will also consider donor age, gender, and cytomegalovirus (CMV) serology and their influence on donor selection. Patient and donor DNA also could be used for studies of other gene complexes, such as the killer immunoglobulin-like receptors [70,71].
Feasibility and logistics
A retrospective study addressing similar questions would require substantially fewer resources.
Summary of Discussion
Based on available preliminary data, there was considerable enthusiasm for Strategy 1. Likewise, the committee generally agreed with the approach described in Strategy 2; there is a great need to improve the outcome of cord blood transplantation and several of the trials underway show promise. Given that these studies are already established and accruing, it is reasonable for the BMT CTN to encourage its members to participate in the most promising studies. Although Strategy 3 had merit, it was felt that similar information might be gained with fewer resources by doing a retrospective study.
COMMITTEE 8: GVHD
Current State of the Science
GVHD prophylaxis
An optimal GVHD prophylaxis regimen would effectively prevent both acute and chronic GVHD while allowing an effective graft-versus-tumor response and prompt immunologic reconstitution. BMT CTN 1203 and 1301 are designed to test new strategies previously developed in single institutions; accrual to these trials, projected to complete in 2015 and 2017, respectively, should have highest priority. Other strategies developed during the next 5 years could be similarly adopted for testing in BMT CTN trials.
Acute GVHD therapy
BMT CTN 0302 and 0802 (the latter closed for futility) failed to identify a strategy superior to single-agent predni-sone to ameliorate the inflammatory effects of acute GVHD while avoiding the mortality of severe immunosuppression. This remains an important goal.
Chronic GVHD therapy
BMT CTN 0801 is a phase II/III study based on the hypothesis that avoidance of CNIs in the treatment of chronic GVHD might facilitate tolerance and improve GVHD response rates compared with conventional CNI-containing immunosuppressive therapy. After interim analysis of data from the phase II portion of this trial, it was decided to discontinue accrual, although patients are being followed for the established phase III endpoints. While awaiting these data, additional agents may be tested in phase II studies.
Strategy 1: Validation of a Biomarker/Clinical Scoring System to Identify Patients at High and Low Risk of Response to Current Acute GVHD Therapy
Hypothesis
Patients can be segregated at diagnosis of acute GVHD into 2 or more groups with different likelihoods of response to therapy using currently available clinical and laboratory assessments.
Background
Therapy trials are limited by the inability to determine prognosis accurately at onset of acute GVHD. The original grading system correlated nonrelapse mortality (NRM) with maximal GVHD grade, which is affected by the response to treatment. Dichotomization into standard risk or high risk at the onset of GVHD is done implicitly in many centers and was formalized recently by the Minnesota group [72]. The Minnesota system identifies 15% of patients as high risk (with 41% versus 18% 6-month NRM) with similar findings in several independent cohorts from BMT CTN 0302, 0802, and 2 large centers (n = 1718). A newly described Ann Arbor grading system [73] uses clinical features and biomarkers in an algorithm that assigns patients into 3 risk groups containing 35% (low risk), 40% (intermediate risk), and 25% (high risk) of patients. The algorithm discriminated the 3 risk groups in 2 separate validation cohorts. The highest risk category was associated with 45% NRM at 6 months. The algorithm was validated in 2 datasets, 1 from 2 centers, and another including 300 patients from BMT CTN 0302 and 0802 who had plasma samples available.
Trial design
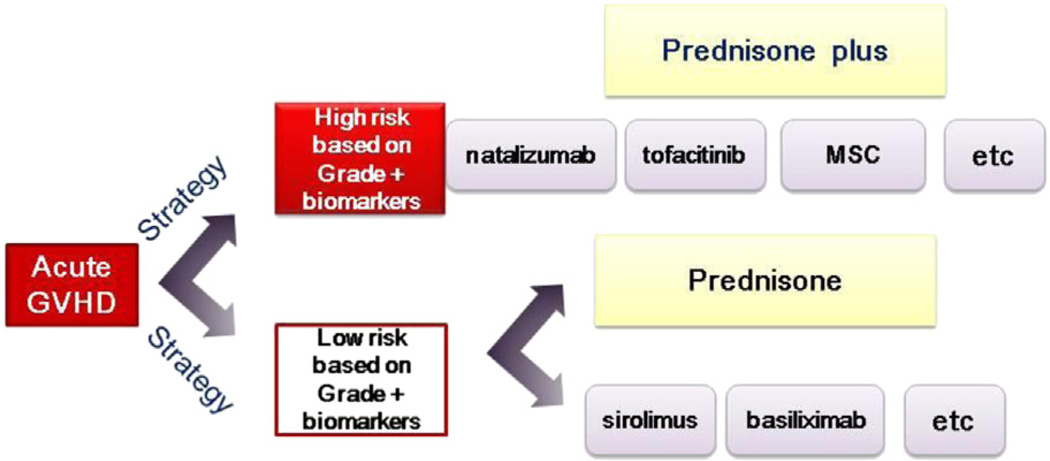
The committee proposed that a study be conducted in conjunction with BMT CTN 1202 (Prospective Multi-Center Cohort for the Evaluation of Biomarkers Predicting Risk of Complications and Mortality Following Allogeneic HCT) to validate a biomarker-based approach to risk stratification in protocols 1203 and 1301. Validation of the Ann Arbor grading system or any other prognostic factors would have important implications for future clinical trials testing new approaches for treatment of acute GVHD. Accurate identification of high-risk patients at the onset of GVHD would make it feasible to accrue patients to trials testing experimental agents in smaller phase II trials with a robust endpoint of 6-month NRM. Simultaneously, accurate identification of low-risk patients would make it feasible to test whether lower or no glucocorticoid doses (or other approaches) could control GVHD while minimizing steroid-related complications. The general strategy is outlined in Figure 1.
Figure 1.
The overall trial design depends on assignment to a high- or low-risk group based on GVHD grade and biomarkers. High-risk patients will be treated with prednisone, and new agents will be serially studied by addition to standard prednisone therapy. Low-risk patients will be randomized between a prednisone monotherapy or 1 of several single-agent nonsteroid alternatives.
Strategy 1A: Develop an Approach to Identify Patients at High Risk of Life-threatening Acute GVHD and Enroll Them in Serial Studies of Agents that Work in Synergy with Prednisone
Hypothesis
Addition of agents to prednisone will increase the effectiveness of therapy for newly diagnosed high-risk acute GVHD.
Trial design
Agents such as natalizumab, tofacitinib, abatacept, mesenchymal stem cells, extracorporeal photopheresis, and/ or others can be tested sequentially in the high-risk cohort. In a randomized phase II trial of 1 to 2 agents compared with control, assuming a day 28 CR rate of 15%, using 1-sided significance level of 15%, with 85% power to detect an increase in day 28 complete remission rate to 35%, we would need 49 patients per group.
Strategy 1B. Develop an Approach to Identify Patients at Low Risk of Life-threatening Acute GVHD and Enroll Them in a Study Designed to Limit Immunosuppression-related Toxicity [74]
Hypothesis
Patients with low-risk GVHD can be effectively treated with lower doses of immune suppression that minimize toxicity.
Trial design
The committee proposed a study to randomize primary therapy of acute GVHD to prednisone or sirolimus [75] basiliximab or another agent to determine whether a nonsteroid-containing regimen would provide equivalent control of GVHD with less corticosteroid-related toxicity. A randomized phase II trial of a new agent compared with control in low- or intermediate-risk patients, assuming a day 28 CR rate in the range of 40% to 65%, using 1-sided significance level of 15%, with 85% power to detect an increase in day 28 CR rate to 75%, would require 58 patients per group.
Feasibility and logistics
High-risk GVHD occurs in 15% to 25% of patients and low-risk GVHD occurs in 35% to 40% of patients; therefore, there is likely to be an adequate population to study. The design requires tight correlation with BMT CTN 1202 and real-time biomarker information. Critical to the success of this design is sufficient flexibility to allow serial studies that will facilitate rapid assessment of several drugs. Criteria to choose study drugs will include completion of phase I or II testing, a rational basis for targeting GVHD, known activity in an immunologic or inflammatory disorder, and drug availability.
Strategy 2: Evaluation of Novel Agents for Chronic GVHD
Background
There are candidate drugs studied outside of the BMT CTN that may be useful in the therapy of chronic GVHD. Interleukin-2 has phase I/II data that support a trial in early onset chronic GVHD [76]. Proteasome inhibitors have activity, but the drug, schedule, and timing need to be assessed [77]. Ibrutinib has activity that is theoretically interesting and a pilot study has been organized. Additional drugs can be evaluated once there are adequate preliminary data.
Summary of Discussion
Although there are considerable logistic concerns, the overall proposed strategies generated a high level of enthusiasm. Ways to diminish some of the barriers are discussed by Committee 13 (Clinical Trial Design).
COMMITTEE 9: CELL AND GENE THERAPY
State of the Science
Cell therapy has made rapid recent progress with immunotherapy being designated as the “advance of the year” by Science in 2014 [78]. In 2010, BMT CTN sponsored a meeting on CD19 chimeric antigen receptor (CAR) strategies [79]. Initial reports showing activity in lymphoma and chronic lymphocytic leukemia were published in 2010 and 2011 [80,81]. In the last year, several papers [82,83] showed even more encouraging response rates in ALL, resulting in the first 2 cell therapy proposals approved by BMT CTN for further development. These studies will evaluate T cells genetically modified to express CARs specific for CD19 in relapsed ALL after transplantation [83] or in patients with active disease to induce remission and enable transplantation. Multicenter cell and gene therapy studies have particular challenges as they require Investigational New Drug applications as well as production and shipping of cells, clinical grade vectors, and identification of sources for crucial ancillary reagents.
The committee reviewed a broad cadre of cell and gene therapy approaches for translation to future BMT CTN studies. There are several phase I studies evaluating regulatory T cells [84,85], but it was felt that more data were needed to identify the optimum cell type and clinical setting for a multicenter trial. Gene transfer to hematopoietic stem cells to treat immunodeficiency diseases is being evaluated through small ad hoc international consortia, which are the appropriate venues for such diseases. Gene therapy for |3-thalassemia or SCD, however, clearly falls within the purview of BMT CTN and several phase I trials testing different strategies and vectors will soon open. Although data from these studies are needed to determine the optimal approach for later phase testing, such results may be available within the next 2 to 3 years and BMT CTN should be prepared to definitively test these strategies. Similarly, results of the 2 currently planned CD19 CAR studies will be needed to guide the design of the next study of genetically modified T cells targeting CD19.
Strategy 1: Trial of Third-Party CMV-specific T Cells (Joint Proposal with Infection Committee) [86,87]
See Infection Committee.
Strategy 2: Bridging Trial of Haploidentical Donor Natural Killer Cells for AML Patients with Active Disease before Transplantation
Hypothesis
Lymphodepleting chemotherapy and infusion of related donor haploidentical natural killer (NK) cells will induce clinical remission in patients with refractory AML.
Background and significance
The University of Minnesota has treated more than 50 AML patients who failed standard therapy with the objective of achieving a CR as a bridge to transplantation. Data suggest that short-term success, defined as remission induction, is in the 25% to 50% range in small studies exploring different platforms [88]. One difficulty in interpreting these studies is the inability to separate antileukemia activity of the chemotherapy regimen from the NK cells. A study comparing chemotherapy ± NK cells is needed to definitively address the role of NK cells as a bridging therapy in AML with residual disease before transplantation. Additionally, we need to understand the optimal NK cell product [89,90].
Trial design
The committee proposed a randomized phase II study in patients with persistent refractory AML (up to 30% blasts on bone marrow within preceding 21 days) having received >2 cycles of standard induction chemotherapy. Arm 1 patients would receive lymphodepleting chemotherapy alone (cyclophosphamide 50 mg/kg × 2 and fludarabine 25 mg/ m2 × 3). Arm 2 would receive the same chemotherapy but with haploidentical freshly isolated and activated NK cells. Arm 3 would receive the same chemotherapy and ex vivo expanded NK cells. All arms would receive IL-2 (or IL-15) to promote in vivo expansion of endogenous or infused NK cells. The primary endpoint would be achievement of CR and an important secondary point would be the ability to proceed to allogeneic transplantation.
Feasibility and logistics
Assuming a baseline CR in this population of 20%, 38 patients per arm (total of 114) will result in 80% power with a 1-sided type I error of 5% to detect an improvement of 20% (20% to 40%) in the CR rate. There is a possibility of obtaining IL-15 for this trial, a cytokine believed to be superior for NK cell in vivo expansion.
Strategy 3: Phase III Randomized Trial of Autologous Epstein-Barr Virus–specific T Lymphocytes after AuHCT for Patients with Epstein–Barr encoding region–in situ hybridization (EBER-ISH)–Positive Hodgkin Lymphoma
Hypothesis
Infusion of autologous cytotoxic T lymphocytes enriched against Epstein-Barr virus (EBV) type II latency antigens after AuHCT for patients with EBER-ISH–positive Hodgkin lymphoma (HL) will result in an improved event-free survival compared with control patients.
Background and significance
AuHCT achieves a 2-year PFS rate of approximately 60% in patients with relapsed HL. Approximately 30% to 40% of patients with HL are EBER-ISH positive. Adoptive transfer of latent membrane protein (LMP)-specific T cells results in increased frequency of relevant EBV-antigen–specific T cells and memory T cell populations provide sustained antitumor responses [91].
Trial design
The committee proposed a multicooperative group randomized phase III study in patients with EBER-ISH positive HL. With 164 patients (82 per arm), considering a baseline 2-year PFS of 60% to 70%, the study has 80% or higher power to detect a 20% improvement in 2-year event-free survival at .05 significance level based on a 2-sided Z-test with binomial distribution.
Feasibility and logistics
According to CIBMTR, >800 patients per year in the United States undergo AuHCT for HL. If 25% are EBER-ISH positive, there would be 200 eligible patients per year making planned accrual of 80 to 100 feasible.
Summary of Discussion
The review of Strategy 1 is covered by Committee 11. Strategy 2 was viewed with enthusiasm because a multicenter study is the only way to compare 2 NK products that have been developed through an NCI-funded program project grant and the National Heart, Lung, and Blood Institute Production Assistance for Cellular Therapy program, but there will need to be process development to finalize the manufacturing and accessory cytokines before the study can be initiated. Although there was support for Strategy 3, which was developed through an NCI lymphoma specialized programs of research excellence, the sample size could not be calculated without knowing if the outcome after AuHCT differs for EBV-positive and EBV-negative HL patients [92]. This analysis is planned. Additionally, the analysis may be confounded if HL patients are receiving other maintenance therapies, such as brentuximab.
COMMITTEE 10: COMORBIDITY AND REGIMEN-RELATED TOXICITY
Current State of the Science
Efforts to decrease regimen-related toxicity (RRT) have focused on reducing regimen intensity rather than direct prevention or early treatments. Nevertheless, mortality from pulmonary, hepatic, gastrointestinal, and other RRT remains too frequent [93]. Although a significant fraction of younger patients with hematologic cancers are offered HCT, only a small minority of those 60 years or older receive HCT, despite the higher frequency of most hematologic cancers in this age group. Objective and reliable recipient health status measures are necessary to guide decision-making about transplantation eligibility and selection of the most appropriate transplantation strategy in older patients. Additionally, early detection and evaluation of organ dysfunction after HCT holds promise to improve outcome.
Strategy 1: Improving Prognostic Assessment for Patients 60 Years and Older Undergoing Allogeneic HCT
Hypothesis
Adding functional assessment and biomarkers to validated clinical indices will improve the ability to predict NRM and RRT in older patients.
Background and significance
Validated comorbidity indices (such as the HCT-specific comorbidity index, HCT-CI) substantially improved prediction of transplantation-related organ toxicities and NRM in adults undergoing allogeneic HCT [93–97]. Emerging data suggest the value of other measures to risk stratify patients including geriatric assessment (GA), which adds a functional and ability assessment, plasma biomarkers (particularly c-reactive protein, ferritin, and albumin), and genetic or epigenetic modifications [98–100]. We hypothesize that a composite health status risk score comprising GA, HCT-CI, and biomarkers could better predict NRM and RRT leading to improved interpretation of clinical studies, identification of populations for future investigations, better allocation of patients to different transplantation strategies, and improved HCT outcomes.
Trial design
The committee proposed a study designed to improve risk assessment for NRM. Patients ≥60 years undergoing allogeneic HCT will be evaluated before HCT with validated GA measures that capture physical, mental, social, emotional, and functional health; HCT-CI scores; plasma biomarkers (c-reactive protein, ferritin, and albumin); and also have cells banked for future whole genome and epigenetic screening. Functional and QOL evaluation will be performed every 6 months for 2 years. The study is designed for the following reasons: (1) to develop a composite model with a c-statistic estimate >.85 to predict NRM, and (2) to test the model’s prediction of secondary outcomes including overall and functional free survivals, QOL, and RRT. A sample of 700 patients will be used to develop the model to ensure adequate statistical power. Established thresholds for GA tools and cut-off values for the biomarkers will be used for modeling. Bootstrapping method will be used to estimate bias-corrected values of c-statistic for internal validation of the model.
Feasibility and logistics
This study is envisaged to be open to enrollment to most patients, whether or not they are enrolled on another BMT CTN trial. Some of the biomarker data are already collected routinely or in BMT CTN 1202. GA can be completed by patients on paper, electronically, or over the telephone, and functional tests take 5 minutes by a research assistant. Successful creation of the validated HCT-CI used similar methods and similar sample size.
Strategy 2: Novel Approaches for Diagnosis, Classification, and Biologic Assessment of Late-Onset Pulmonary Toxicity after HCT
Hypothesis
Early detection of pulmonary dysfunction after allogeneic HCT will improve outcomes.
Background and significance
Lung injury contributes significantly to morbidity and is the most common RRT cause of mortality after allogeneic HCT. Late-onset pulmonary complications (LOPC) are particularly problematic, including bronchiolitis obliterans syndrome and restrictive lung disease. Inconsistencies in terminology and diagnostic criteria contribute to a wide variation in early detection of these complications and a false impression of low incidence. Optimal monitoring and treatment for LOPC are not established and only 1 NIH symptom-based lung score (dyspnea) correlates with outcomes [101]. Once symptoms of LOPC occur, prognosis is poor [102–105]. New therapeutic strategies for bronchiolitis obliterans syndrome and restrictive lung disease hold promise for improved outcome, but early intervention is required to affect survival [106–109]. New imaging techniques and more frequent monitoring of pulmonary function utilizing readily available devices can detect lung changes earlier.
Trial design
The committee proposed an observational study determine whether an aggressive post-HCT monitoring program with a novel imaging biomarker will detect subclinical lung dysfunction early (3 to 6 months after HCT) and more frequently (20% to 30%) relative to historical controls. The incidence of late onset, noninfectious lung disease utilizing conventional methods is 10% to 15%, and earliest detection is usually after the development of symptoms (1 to 2 years after HCT), when prognosis is poor. Patients undergoing allogeneic HCT will undergo pulmonary function tests (PFTs) and plasma sampling before transplantation, at day 100, and every 3 months for 2 years. Hand-held spirometry will be used between conventional PFTs, with specific criteria for early PFTs [101,110]. A persistent 15% decline in forced vital capacity, forced expiratory volume in 1 second, or carbon monoxide diffusing capacity will initiate work-up including high-resolution computed tomography (HRCT), broncho-alveolar lavage, collection of broncho-alveolar lavage fluid, blood and stool samples for biomarker analysis, and next-generation sequencing for pathogen discovery. Digital HRCT files will be analyzed by a sensitive attenuation mapping technique [111]. Patients with noninfectious lung dysfunction will be available for state-of-the-art intervention.
Feasibility and logistics
A sample size of 300 subjects is expected to have sufficient power to determine whether early detection is possible. Broad eligibility criteria will ensure rapid accrual. The universal availability of PFTs and HRCT scans and the ease of biomarker collection suggest this study is feasible.
Strategy 3: Identifying Microbiological “Biomarkers” that Predict RRT
Hypothesis
Oligoclonality of the microbiome predicts poorer outcomes after HCT. Additionally, microbes that predict adverse outcomes or promote RRT can be identified allowing early intervention.
Background and significance
Our understanding of the underlying pathobiology of gastrointestinal and pulmonary complications after HCT remains incomplete [112,113]. Despite the susceptibility of the post-HCT population to infections, efforts to characterize microbial triggers are limited. Identification of some opportunistic pathogens, such as pneumocystis and CMV, has led to improved surveillance, prophylaxis, early treatment, and improved outcomes. Newer methods allow characterization of known and novel bacteria, viruses, and fungi in the microbiome [114]. A shotgun metagenomic sequencing approach, for example, resulted in the identification of a new colitis-associated pathogen [115].
Trial design
The committee proposed prospective collection of additional biospecimens (stool, sputum) from patients undergoing allogeneic transplantation and enrolled on BMT CTN 1202. Collected specimens will undergo central shotgun metagenomic sequencing. Shannon diversity index of microbial phyla will be calculated to quantify microbial diversity in each specimen. Spearman’s rank correlation coefficient will be calculated to determine the strength of the correlation between Shannon diversity index and outcomes. The genome sequences of novel organisms will be assembled and deposited to the National Center for Biotechnology Information. Unsupervised hierarchical clustering of cases/ controls will be performed to identify “microbiome” compositions or new organisms associated with RRT.
Feasibility and logistics
Feasibility of this trial is greatly enhanced by leveraging the evaluations and data collection schedule and forms in place for BMT CTN 1202. Specimens would be collected at the same time points as the blood samples collected on BMT CTN 1202 (weekly from day 0 through day 28, and on days 42, 56, and 91). A detailed kit and protocol for sample collection and shipment would be provided; samples would be stored in a central repository.
Summary of Discussion
Enthusiasm was high for the development of a composite health status risk score to improve the selection of older patients for HCT. The additional collection of biospecimens for future genetic and epigenetic analysis was recommended. There was general agreement for the need for earlier detection of pulmonary toxicity and the development of an observational trial of PFT and possibly novel imaging biomarkers. Trials of novel early therapeutic intervention, however, require further preliminary data before going forward, Finally, the potential for an improved understanding of microbiome alterations during HCT and the discovery of new pathogenic microbes by metagenomic sequencing techniques was acknowledged but felt not to be feasible as a standalone trial at this time.
COMMITTEE 11: INFECTION AND IMMUNE RECONSTITUTION COMMITTEE
Current State of the Science
Despite the introduction of multiple effective antimicrobial agents, more accurate diagnostics, and advances in anti-infective strategies, infection remains 1 of the most common causes of serious morbidity and death after HCT. Suboptimal or delayed immune reconstitution is the major risk factor for this.
Strategy 1: Randomized Phase II Trial of Adoptive Immunotherapy Using Banked Third-Party CMV-specific T Cells for Refractory CMV Infection [Developed Jointly with the Gene and Cell Therapy Committee]
Hypothesis
Adoptive immunotherapy using CMV-specific T cells can improve control of refractory CMV infection compared with antiviral drug therapy.
Background and significance
CMV mortality decreased with the introduction of effective antiviral drugs but substantial challenges remain [116]. CMV-specific T cell adoptive therapy has been shown in small studies to be safe and effective for prevention and treatment of CMV infection [86,117]. The utility of adoptive immunotherapy in refractory CMV infection has not been studied in a multicenter randomized trial.
Trial design
The committee proposed a randomized phase II study in allogeneic HCT recipients with refractory CMV infection (persistent DNAemia despite 2 weeks of anti-CMV drug therapy, and not receiving prednisone >1 mg/kg/day). Subjects would receive either banked CMV-specific T cells or placebo, to be given on days 1, 8, and 15 after study entry. Patients on both arms would continue to receive anti-CMV drug therapy. Approaches to antiviral drugs and tapering of immunosuppression would be standardized. The primary endpoint would be the incidence of CMV disease [118] in a time-to-event analysis. Secondary clinical, safety, clinical, virologic, immune, and survival endpoints would be assessed over 6 months. Block randomization and subset analysis will be conducted in those receiving ≤.5 and > .5 mg/kg/day of prednisone.
Feasibility and logistics
We estimate DNAemia to occur in 55% of CMV-seropositive patients. Twenty-nine percent of these patients will develop persistent DNAemia, and 22% of persistently DNAemic patients will develop disease [119]. We expect the response rate to CMV-specific cells to be 74% [86], thereby reducing CMV disease rates from 22% to 6%. We anticipate approximately 90 patients per arm will be needed to demonstrate this difference. Given that there about 4000 allotransplantations per year in the United States in CMV-positive patients, there should be about 650 eligible patients a year, making accrual in 2 years feasible.
Strategy 2: Randomized Phase II/III Trial of a Novel Parainfluenza Virus (PIV) Entry Inhibitor in HCT Recipients with PIV Upper Respiratory Tract Infection
Hypothesis
DAS181 (Ansun Biopharma, San Diego, CA) given for treatment of PIV upper respiratory tract infection (URTI) in HCT recipients will reduce progression to lower respiratory tract disease (LRTD).
Background and significance
PIVs are major respiratory tract pathogens, which can cause fatal viral LRTD and long-term airflow obstruction after HCT [120–123] and for which there are no effective therapy [124]. PIV URTI is a major risk factor for LRTD [120,121]. DAS181 is a novel sialidase viral receptor blocker on respiratory tract epithelial cells [125]. DAS181 is active against several respiratory viruses (including PIV and influenza) [126,127] and is currently in a phase II trial in HCT recipients with PIV LRTD.
Trial design
The committee proposed a phase III trial in which HCT recipients presenting with documented PIV URTI would be randomized 2:1 to either DAS181 or placebo for 7 days. The primary endpoint would be time to progression to LRTD. Secondary clinical and physiologic endpoints would also be assessed.
Feasibility and logistics
The rates of symptomatic PIV infections are 7% to 8% [122] in 2 core centers of the BMT CTN. If the rate is similar in all HCT centers, there should be more than 1000 eligible patients in the United States annually. We estimate the progression rate in the placebo group to be ~15%. With an estimated ~280 patients, the study would have 82% power to detect a reduction from 15% to 5%. Recruitment should be feasible in 2 to 3 years.
Strategy 3: Stepped Intervention Program to Reduce Infections in Allogeneic HCT Recipients
Hypothesis
A multifaceted stepped intervention using an evidence-based infection prevention guidelines checklist with feedback of post-HCT infection rates in allogeneic HCT recipients will reduce rates of CMV disease and preventable bacteremic and fungal infections.
Background and significance
Prevention strategies shown to reduce serious infections after HCT have been codified in guidelines [128]. Unfortunately, adherence to guidelines is variable [129] due to differences in systems and individuals [130]. Hospital safety studies show that barriers to guideline implementation can be overcome by a multifaceted approach [130–132], using an unambiguous checklist with interventions linked in time and space and reliance on systems, rather than on the actions of individual clinicians. Outcomes can be improved with use of technology to “flag” data, follow and report outcomes, and suggest actionable responses.
Trial design
The committee proposed a 3-year multicenter prospective, interventional, nonrandomized, stepped, quality-improvement cohort study designed to improve implementation of strategies to prevent infections in allogeneic HCT recipients. The primary intervention will be the use of pretransplantation and post-transplantation (weekly) goals checklists up to day 100 to improve implementation of evidence-based prevention strategies, tracking of severe infection data, and continuous feedback of guidelines adherence and infection rates.
Stepped interventions include the following: (1) development of BMT CTN infection definitions and prevention guidelines for the trial, (2) pilot infection tracking system (this will enable finalization of sample size estimates), and (3) implementation of prevention checklist and continuous feedback system. The primary endpoint will be the serious composite infection (SCI) rate (severe Gram-negative bacterial infections, CMV syndrome and/or disease, and invasive fungal infections) at day 100. Poisson regression modeling will be used to compare rates before, during, and up to 2 years after implementation of the primary intervention. Secondary endpoints will be compliance with guidelines, health care utilization measures, incidences of each serious infection, and transplantation-related mortality.
Feasibility and logistics
Historically, the estimated 3-month SCI rate is 30%. Through the application of a multifaceted stepped intervention (single arm), with a sample size of 246, a SCI rate of 20% (10% reduction from the historical rate) can be estimated to a desired precisionof5% with 95% confidence. The historical SCI rate measured after pilot data collection will be used to confirm sample size estimates, as these estimates are dependent on the actual baseline SCI rate measured after year 1.
Summary of Discussion
There was considerable enthusiasm for both Strategies 1 and 2, which were considered high priorities for the network in the near future. It was felt that Strategy 1 addressed a novel approach to a significant problem that would require the Network to be successfully tested. Concern was expressed about the estimate of the endpoints in that they were derived some time ago and may not reflect the event rates in a contemporaneous cohort of patients and in recipients of T cell–replete grafts. Moreover, quantitative PCR detection assays have replaced older, less sensitive antigen assays. The committee indicated that a current prospective study in allogeneic T cell–replete HCT recipients is underway and will be used to revise the estimates of the event rate. If this study indicates the event rate is too low, options include confining the study population to high-risk patients (patients receiving T cell–depleted or cord blood grafts and/or those receiving antithymocyte globulin or alemtuzumab) or discussing with the Food and Drug Administration an alternative or composite endpoint. For the respiratory syncytial virus antiviral trial in Strategy 2, there also was considerable enthusiasm. No major concerns were noted. Although there was enthusiasm for the stepped-intervention program in Strategy 3, concerns were raised regarding the current paucity of data to support key endpoint estimates and the need for a method to select centers with a heterogeneity of practices, as some centers may already have in place approaches that standardize adherence to guidelines practices. It was felt that there should be a pilot study to provide good estimates of how much of a reduction in SCI rates might be expected after adoption of the intervention.
COMMITTEE 12: LATE EFFECTS/QOL/ECONOMICS
Current State of the Science
The BMT CTN has conducted 1 trial of training in exercise and/or stress management to improve QOL. However, many BMT CTN trials have QOL or economic measures as secondary endpoints. After reviewing the pertinent literature, the committee focused on a primary prevention trial for bone loss. The committee wrote 3 white papers to codify methods to incorporate secondary endpoints of late effects, QOL, and economic outcomes into other BMT CTN clinical trials wishing to study these endpoints, available at https://web.emmes.com/study/bmt2/SOSS.html.
The committee considered multiple other research questions, but deferred them because of the reasons listed in Table 4.
Table 4.
Deferred Late Effects/Quality of Life Trials
| Domain | Rationale for Deferral |
|---|---|
| Preventive care | Smoking cessation – no preliminary data Vaccinations – could only be in a limited number of centers and may not need the Network Vitamin D supplementation – appropriate endpoints unclear |
| Survivor support | Survivorship care plan – ongoing funded multicenter study Internet-based survivorship support – ongoing funded multicenter study (INSPIRE) Survivorship support package – would require extensive development |
| QOL, economics Late effects |
Usually appropriate as secondary endpoints Cardiotoxicity – need very late follow-up, expensive study, rare outcome Sexual dysfunction – lack of preliminary data Iron overload – unclear clinical implications, premature for an intervention study Infertility – lack of a feasible intervention Avascular necrosis – lack of a feasible intervention |
Strategy 1: Randomized, Placebo-controlled Study to Evaluate the Efficacy of Zolendronic Acid for Prevention of Bone Loss after Allogeneic HCT
Hypothesis
Administration of zoledronic acid will prevent bone mineral density (BMD) loss during the first year after allogeneic HCT, as demonstrated in a single-center study [133].
Background and significance
Osteopenia is found in 20% to 30% of patients before transplantation, whereas 10% to 20% already have osteoporosis [134]. Studies show that significant bone loss occurs in the first 6 to 12 months after HCT [135], and remineralization can take years, if it occurs at all [136]. A single-center study has shown an 8% cumulative incidence risk of fracture at 3 years after HCT as well as decline in BMD with continued exposure to risk factors such as corticosteroids and CNIs [137]. Low BMD is a risk factor for fractures.
Trial design
The committee proposed a phase III randomized, placebo-controlled trial comparing a single 5 mg i.v. infusion of zoledronic acid to placebo. The randomization would be stratified to balance factors that may correlate with bone loss or response assessment (baseline BMD, steroid exposure, center). The primary endpoint is the change in BMD (g/cm2) as measured by DEXA scan. A sample size of 130 patients per group will give 90% power to detect a .5 standard deviation difference in the femoral BMD between enrollment and 1 year after transplantation, assuming we are unable to evaluate 30% at 1 year because of death or missing DEXA data. Both arms will receive calcium and vitamin D. Patients with baseline osteoporosis or prior bisphosphonate use will be excluded from the trial.
Feasibility and logistics
Zoledronic acid is approved for the prevention and treatment of bone loss at the proposed dose. There are an estimated 5000 potentially eligible patients in the United States annually, so recruitment should be brisk.
Strategy 2: Standardize Collection of Patient-Reported Outcomes, Late Effects and Economic Data in BMT CTN Therapeutic Trials
Background and recommendation
There is a growing interest in comparing the outcomes of different therapies beyond traditional endpoints of survival, NRM, and disease control. Many protocol teams would like to collect data on QOL, symptoms, late effects, or economic endpoints. They may be motivated by anticipated differences between treatment approaches, or because the design of their trial will support many secondary studies in the future. The clinical data and specimens collected for most BMT CTN clinical trials represent a robust resource upon which collection of QOL, late effects, and cost data for selected studies is likely to deliver high value. The BMT CTN should promote a core set of measurement tools for these endpoints and standardize approaches to collection of these data types to ensure useful and interpretable data. Not all trials will include these endpoints, but those that do should have a standardized approach.
Feasibility and logistics
The committee drafted 3 white papers to outline best practices regarding collection of these data types. These papers provide detailed recommendations for study design, implementation, and conduct, and suggested wording for protocols and consent forms. Members of the committee are available to help protocol teams integrate these secondary endpoints into their trials and to help teams monitor data collection while the trial is ongoing.
Summary of Discussion
There was high enthusiasm for Strategy 1. The primary issues raised concerned the most appropriate time for enrollment (before HCT versus day +100 versus when steroids are started), identifying appropriate high-risk patients for enrollment, the wisdom of including patients with normal BMD, the duration of follow-up, how the DEXA scans and zoledronic acid would be financed, and whether markers of bone turnover should be incorporated into the trial. Although these issues require resolution, there was high enthusiasm for this trial. Concerning Strategy 2, the primary suggestion was to coordinate with the European Society for Blood and Marrow Transplantation and other interested groups to make sure that international standardization is incorporated whenever possible.
COMMITTEE 13: CLINICAL TRIAL DESIGN
The SOSS clinical trial design committee identified several important issues to be considered when developing clinical trials in HCT.
Incorporation of Controls in the Design of Phase II Trials
A phase II study using a historical control rate makes interpretation of results difficult because unrecognized changes in patient characteristics over time can increase the probability of a false positive or false negative result [138,139]. Including a concurrent control group improves the design but requires substantially more patients for sufficient statistical power. Ideally, the control arm is randomized, although a nonrandomized convenience sample of contemporaneous patients could be used instead. A compromise involves using CIBMTR registry data with regression methods to partially adjust for differences between trial and registry patients. This approach is most appropriate when eligibility criteria can be easily reproduced and when outcomes and well-established risk factors are routinely collected by the registry. Differential patterns of assessment can be addressed by including additional prospectively enrolled CIBMTR patients, subject to additional participant consent. BMT CTN 1203 (A multicenter phase II trial randomizing novel approaches for GVHD prevention compared to contemporary controls) uses prospective enrollment of a registry control group receiving tacrolimus + methotrexate as GVHD prophylaxis. Another valuable source of control patients is BMT CTN 1202, where protocol-specified GVHD and toxicity assessments and biospecimens augment registry data. The Graft Sources SOSS committee proposed a haplo-identical HCT trial that could possibly use a registry control. Enrollment occurs just before HCT, facilitating identification of registry control patients, and the primary endpoint is a standard PFS outcome.
Patient Enrichment for Studying Infrequent Outcomes
A trial studying a new treatment for an infrequent but serious outcome risks exposing many patients to a treatment that may benefit few patients, because a large sample size is needed to identify a small treatment effect. Although eligibility could be restricted to patients with a higher likelihood of the outcome, drawbacks of such an “enrichment” strategy include slower accrual, less generalizability of the results, and the need for a randomized control group when it is difficult to identify higher-risk registry patients. The Pediatric Indications and Approaches SOSS committee proposed a relapse prevention trial in ALL that identifies patients at high risk of relapse based on pre-HCT MRD and absence of acute GVHD by day 55 after HCT, making detection of a risk reduction more feasible. However, the day 55 eligibility criterion makes it difficult to identify appropriate control patients from a registry, leading to the need for a randomized control arm.
Biomarkers in Eligibility Criteria as an Enrichment Strategy
Biomarkers may be used to identify high-risk patients for study inclusion, but the need for a pre-enrollment laboratory measurement adds logistical complexity. The biomarker’s predictive value can be established using standard metrics, such as Akaike Information Criterion, area under the receiver operating characteristics curve, and Brier score, or more controversial metrics, such as standardized-net benefit or net-reclassification index [140]. Interlaboratory measurement variability of the assay should be assessed as well as consistency of the biomarker’s ability to predict outcomes across subgroups of patients. The GVHD SOSS committee is proposing the use of a 3-biomarker panel to identify patients with high-risk acute GVHD and poor prognosis for survival. The benefit of this grading will be gauged by its ability to (1) identify a large enough group of high-risk patients to sufficiently power clinical studies, and (2) stratify GVHD severity among patients better than standard clinical assessment, such as the Consensus criteria.
Rapid Assessment of Agents in a Phase II Design
Assessment of multiple promising new agents is often slowed by administrative hurdles in multicenter trials including protocol development and approval. A “plug-in” protocol could be written with a primary and backup agent so that completion of accrual to the primary agent (either through study completion or early stopping) triggers a switch tothe backup agent without delay in implementation. Then a protocol amendment could add another backup agent, leaving a primary and secondary treatment to still be studied. Short-term endpoints, if reasonably likely to predict clinical benefit, can facilitate quick decisions of whether to switch to a phase III trial with that treatment or to switch to the next agent.
Novel Endpoints in HCT
Quantitative or ordinal endpoints typically require smaller sample sizes than PFS or time-to-event data, but analysis must appropriately handle patients who die or are otherwise not evaluable. Possible strategies include imputation (a procedure for inserting a value to stand in for missing data) and use of rank statistics for hypothesis testing, or an analysis conditional on survival. Imputation helps to preserve the intention-to-treat principle and may be less sensitive to bias due to differences in survival. However, it typically requires more patients than a conditional analysis because this approach shrinks the measurement distributions closer together because of the inclusion of patients who die. Careful consideration of study eligibility and timing of the intervention can reduce the impact of mortality on the outcome. The Late Effects SOSS committee proposed a study of zoledronic acid for prevention of bone loss, where the outcome is change in femoral neck BMD from 100 days to 1 year after transplantation. Restricting eligibility to early/ intermediate disease with good performance score can help minimize the impact of the competing risk of death.
Summary
Clinical trials in HCT are difficult because of the rarity of the diseases being studied and multiple competing risks in the post-transplantation setting. Strategies discussed above including comparisons to a registry control, patient enrichment and use of biomarkers, rapid assessment of new agents, and use of novel endpoints in HCT all have the potential to improve feasibility of HCT trials, although the advantages and disadvantages of each strategy must be carefully considered. Although the committee’s preference is for randomized controls, it recognizes that alternative designs must also be considered.
Prioritization
As noted earlier, after the committee presentations and open discussion, the SOSS Planning Group, committee chairs, and outside reviewers met, discussed the individual proposals, and formed a prioritization list. The 12 concepts listed in Table 5 received a high level of enthusiasm from the review group. They are listed in the order of presentation at the meeting. The highest priorities were reserved for those studies addressing impactful questions, with adequate preliminary data and no obvious impediments to their conduct. Many of the concepts not given high priority asked important questions and, if preliminary data are generated or certain barriers can be circumvented, might become equally compelling in the future.
Table 5.
2014 SOSS High Priority Trials
| Committee | Priority |
|---|---|
| Leukemia | Phase III study of postallogeneic transplantation maintenance using FLT3 inhibition versus placebo in patients with FLT3+ AML and azacytidine versus placebo in those with FLT3-AML |
| Lymphoma | Phase III study of postautologous transplantation maintenance using ibrutinib versus placebo in patients with relapsed or refractory DLBCL |
| Nonmalignant disease | Phase III study of autologous transplantation versus standard therapy for MS |
| Pediatric indications | Phase III study of post-transplantation maintenance using moxetumomab or inotuzumab versus placebo in pediatric and adult patients with B cell ALL |
| Pediatric outcomes | Phase II study of daily versus alternate day dosing of steroids for chronic GVHD |
| Optimal donor and graft source | Phase II study of haploidentical peripheral blood stem cells and PTCY after myeloablative conditioning |
| GVHD | In low-risk patients, randomized phase II studies of novel agents versus steroids, and in high-risk patients, randomized phase II studies of novel agents plus steroids versus steroids alone |
| Gene and cell therapy | Phase III study of haploidentical donor NK cells for AML |
| Comorbidity/RRT | Development of a more robust risk assessment method incorporating biomarkers and geriatric assessment tools |
| Infection/immune reconstitution | Phase III study of CMV-specific T cell adoptive therapy. |
| Infection/immune reconstitution | Phase II study of a novel PIV entry inhibitor in HCT recipients with upper respiratory tract infection |
| Late effects | Phase III randomized trial of zoledronic acid versus placebo for prevention of bone loss after allogeneic HCT |
ACKNOWLEDGMENTS
Support was provided by grant #U10HL069294 from the National Heart, Lung, and Blood Institute and the National Cancer Institute (Grant # U10HL069294) and by the Be The Match Foundation (Grant # U10HL069294).
Footnotes
Financial disclosure: The authors have nothing to disclose.
Conflict of interest statement: There are no conflicts of interest to report.
REFERENCES
- 1.Ferrara JL, Anasetti C, Stadtmauer E, et al. Blood and Marrow Transplant Clinical Trials Network State of the Science Symposium 2007. Biol Blood Marrow Transplant. 2007;13:1268–1285. doi: 10.1016/j.bbmt.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara JL. Blood and Marrow Transplant Clinical Trials Network: progress since the State of the Science Symposium 2007. Biol Blood Marrow Transplant. 2014;20:149–153. doi: 10.1016/j.bbmt.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Research Network. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368:2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pratcorona M, Brunet S, Nomdedeu J, et al. Favorable outcome of patients with acute myeloid leukemia harboring a low-allelic burden FLT3-ITD mutation and concomitant NPM1 mutation: relevance to post-remission therapy. Blood. 2013;121:2734–2738. doi: 10.1182/blood-2012-06-431122. [DOI] [PubMed] [Google Scholar]
- 5.Swords R, Freeman C, Giles F. Targeting the FMS-like tyrosine kinase 3 in acute myeloid leukemia (Review) Leukemia. 2012;26:2176–2185. doi: 10.1038/leu.2012.114. [DOI] [PubMed] [Google Scholar]
- 6.Griffiths E, Gore S. Epigenetic therapies in MDS and AML. In: Karpf AR, editor. Epigenetic Alterations in Oncogenesis. New York: Springer; 2013. pp. 253–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jabbour E, Giralt S, Kantarjian H, et al. Low-dose azacitidine after allogeneic stem cell transplantation for acute leukemia. Cancer. 2009;115:1899–1905. doi: 10.1002/cncr.24198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devine SM, Owzar K, Blum W, et al. A phase II study of allogeneic transplantation for older patients with AML in first complete remission using a reduced intensity conditioning regimen: results from CALGB 100103/BMT CTN 0502. Blood. 2012;120:230. doi: 10.1200/JCO.2015.62.7273. [abstract] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farag SS, Maharry K, Zhang MJ, et al. Comparison of reduced-intensity hematopoietic cell transplantation with chemotherapy in patients age 60–70 years with acute myelogenous leukemia in first remission. Biol Blood Marrow Transplant. 2011;17:1796–1803. doi: 10.1016/j.bbmt.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stiff PJ, Unger JM, Cook JR, et al. Autologous transplantation as consolidation for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 2013;369:1681–1690. doi: 10.1056/NEJMoa1301077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedberg JW. Double-hit diffuse large B-cell lymphoma. J Clin Oncol. 2012;30:3439–3443. doi: 10.1200/JCO.2012.43.5800. [DOI] [PubMed] [Google Scholar]
- 13.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 14.Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lym-phoma. N Engl J Med. 2002;346:1937–1947. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 15.Honigberg LA, Smith AM, Sirisawad M, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci U S A. 2010;107:13075–13080. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Advani RH, Buggy JJ, Sharman JP, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol. 2013;31:88–94. doi: 10.1200/JCO.2012.42.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson WH, Gerecitano JF, Goy A, et al. The Bruton’s tyrosine kinase (BTK) inhibitor, ibrutinib (PCI-32765), has preferential activity in the ABC subtype of relapsed/refractory de novo diffuse large B-cell lym-phoma (DLBCL): interim results of a multicenter, open-label, phase 2 study. Blood. 2012;120:686. [abstract] [Google Scholar]
- 18.Vose JM, Carter S, Burns LJ, et al. Phase III randomized study of rituximab/carmustine, etoposide, cytarabine, and melphalan (BEAM) compared with iodine-131 tositumomab/BEAM with autologous he-matopoietic cell transplantation for relapsed diffuse large B-cell lymphoma: Results from the BMT CTN 0401 trial. J Clin Oncol. 2013;31:1662–1668. doi: 10.1200/JCO.2012.45.9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gisselbrecht C, Schmitz N, Mounier N, et al. Rituximab maintenance therapy after autologous stem-cell transplantation in patients with relapsed CD20(+) diffuse large B-cell lymphoma: final analysis of the collaborative trial in relapsed aggressive lymphoma. J Clin Oncol. 2012;30:4462–4469. doi: 10.1200/JCO.2012.41.9416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson NA, Slack GW, Savage KJ, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30:3452–3459. doi: 10.1200/JCO.2011.41.0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green TM, Young KH, Visco C, et al. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30:3460–3467. doi: 10.1200/JCO.2011.41.4342. [DOI] [PubMed] [Google Scholar]
- 22.Gandhi M, Petrich AM, Cassaday RD, et al. Impact of induction regimen and consolidative stem cell transplantation in patients with double hit lymphoma (DHL): a large multicenter retrospective analysis. Blood. 2013;122:640. doi: 10.1182/blood-2014-05-578963. [abstract] [DOI] [PubMed] [Google Scholar]
- 23.Vose JM. Mantle cell lymphoma: 2013 update on diagnosis, risk-stratification, and clinical management (Review) Am J Hematol. 2013;88:1082–1088. doi: 10.1002/ajh.23615. [DOI] [PubMed] [Google Scholar]
- 24.Williams ME. Transplantation for mantle cell lymphoma: is it the right thing to do? Hematology. 2013;2013:568–574. doi: 10.1182/asheducation-2013.1.568. [DOI] [PubMed] [Google Scholar]
- 25.Rivera-Rodriguez N, Cabanillas F. Recent advances in the management of mantle cell lymphoma. Curr Opin Oncol. 2013;5:716–721. doi: 10.1097/CCO.0000000000000010. [DOI] [PubMed] [Google Scholar]
- 26.Kluin-Nelemans HC, Hoster E, Hermine O, et al. Treatment of older patients with mantle-cell lymphoma. N Engl J Med. 2012;367:520–531. doi: 10.1056/NEJMoa1200920. [DOI] [PubMed] [Google Scholar]
- 27.Dietrich S, Weidle J, Rieger M, et al. Rituximab maintenance therapy after autologous stem cell transplantation prolongs progression-free survival in patients with mantle cell lymphoma. Leukemia. 2014;28:708–709. doi: 10.1038/leu.2013.332. [DOI] [PubMed] [Google Scholar]
- 28.Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381:1203–1210. doi: 10.1016/S0140-6736(12)61763-2. [Erratum appears in Lancet.2013 Apr 6;381:1184] [DOI] [PubMed] [Google Scholar]
- 29.Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369:507–516. doi: 10.1056/NEJMoa1306220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fonseca R, Monge J. Myeloma: classification and risk assessment (Review) Semin Oncol. 2013;40:554–566. doi: 10.1053/j.seminoncol.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Puig N, Sarasquete ME, Balanzategui A, et al. Critical evaluation of ASO RQ-PCR for minimal residual disease evaluation in multiple myeloma. A comparative analysis with flow cytometry. Leukemia. 2014;28:391–397. doi: 10.1038/leu.2013.217. [DOI] [PubMed] [Google Scholar]
- 32.Lokhorst H, Einsele H, Vesole D, et al. International Myeloma Working Group consensus statement regarding the current status of allogeneic stem-cell transplantation for multiple myeloma. J Clin Oncol. 2010;28:4521–4530. doi: 10.1200/JCO.2010.29.7929. [DOI] [PubMed] [Google Scholar]
- 33.Koreth J, Stevenson KE, Kim HT, et al. Bortezomib-based graft-versus-host disease prophylaxis in HLA-mismatched unrelated donor transplantation. J Clin Oncol. 2012;30:3202–3208. doi: 10.1200/JCO.2012.42.0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar SK, Bensinger WI, Zimmerman TM, et al. Weekly dosing of the investigational oral proteasome inhibitor ixazomib in relapsed/refractory multiple myeloma: results from a phase 1 study. Blood. 2014;124:1047–1055. doi: 10.1182/blood-2014-01-548941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenblatt J, Avivi I, Vasir B, et al. Vaccination with dendritic cell/ tumor fusions following autologous stem cell transplant induces immunologic and clinical responses in multiple myeloma patients. Clin Cancer Res. 2013;19:3640–3648. doi: 10.1158/1078-0432.CCR-13-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pasquini MC, Wang Z. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR Summary Slides. 2013 Available at: http://www.cibmtr.org.
- 37.Pasquini MC, Griffith LM, Arnold DL, et al. Hematopoietic stem cell transplantation for multiple scerosis: collaboration of the CIBMTR and EBMT to facilitate international clinical studies. Biol Blood Marrow Transplant. 2010;16:1076–1083. doi: 10.1016/j.bbmt.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nash RA, Bowen JD, McSweeney PA, et al. Treatment of severe multiple sclerosis (MS) with high-dose immunosuppressive therapy (HDIT) and autologous stem cell transplantation (SCT): 2 year follow-up. Blood. 2002;100(Part 1):864a. #3408 [Abstract] [Google Scholar]
- 39.Bacigalupo A, Socie’ G, Lanino E, et al. Fludarabine, cyclophosphamide, antithymocyte globulin, with or without low dose total body irradiation, for alternative donor transplants, in acquired severe aplastic anemia: a retrospective study from the EBMT-SAA Working Party. Haematologica. 2010;95:976–982. doi: 10.3324/haematol.2009.018267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eapen M, Le Rademacher J, Antin JH, et al. Effect of stem cell source on outcomes after unrelated donor transplantation in severe aplastic anemia. Blood. 2011;118:2618–2621. doi: 10.1182/blood-2011-05-354001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshimi A, Kojima S, Taniguchi S, et al. Unrelated cord blood transplantation for severe aplastic anemia. Biol Blood Marrow Transplant. 2008;14:1057–1063. doi: 10.1016/j.bbmt.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 42.Peffault de Latour R, Purtill D, Ruggeri A, et al. Influence of nucleated cell dose on overall survival of unrelated cord blood transplantation for patients with severe acquired aplastic anemia: a study by eurocord and the aplastic anemia working party of the European group for blood and marrow transplantation. Biol Blood Marrow Transplant. 2011;17:78–85. doi: 10.1016/j.bbmt.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto H, Kato D, Uchida N, et al. Successful sustained engraft-ment after reduced-intensity umbilical cord blood transplantation for adult patients with severe aplastic anemia. Blood. 2011;117:3240–3242. doi: 10.1182/blood-2010-08-295832. [DOI] [PubMed] [Google Scholar]
- 44.Pulsipher MA, Skinner R, McDonald GB, et al. National Cancer Institute, National Heart, Lung and Blood Institute/Pediatric Blood and Marrow Transplantation Consortium First International Consensus Conference on late effects after pediatric hematopoietic cell transplantation: the need for pediatric-specific long-term follow-up guidelines. Biol Blood Marrow Transplant. 2012;18:334–347. doi: 10.1016/j.bbmt.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Center for International Blood and Marrow Transplant Research. CIBMTR: Available at: http://www.cibmtr.org.
- 46.Bolanos-Meade J, Fuchs EJ, Luznik L, et al. HLA-haploidentical bone marrow transplantation with posttransplant cyclophosphamide expands the donor pool for patients with sickle cell disease. Blood. 2012;120:4285–4291. doi: 10.1182/blood-2012-07-438408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pulsipher MA, Langholz B, Wall DA, et al. The addition of sirolimus to tacrolimus/methotrexate GVHD prophylaxis in children with ALL: a phase III COG/PBMTC trial. Blood. 2014;123:2017–2025. doi: 10.1182/blood-2013-10-534297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rubinstein LV, Korn EL, Freidlin B, et al. Design issues of randomized phase II trials and a proposal for phase II screening trials. J Clin Oncol. 2005;23:7199–7206. doi: 10.1200/JCO.2005.01.149. [DOI] [PubMed] [Google Scholar]
- 49.Deoni SC, Mercure E, Blasi A, et al. Mapping infant brain myelination with magnetic resonance imaging. J Neurosci. 2011;31:784–791. doi: 10.1523/JNEUROSCI.2106-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vinukonda G, Csiszar A, Hu F, et al. Neuroprotection in a rabbit model of intraventricular haemorrhage by cyclooxygenase-2, prostanoid receptor-1 or tumour necrosis factor-alpha inhibition. Brain. 2010;133:2264–2280. doi: 10.1093/brain/awq107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li W, Wu B, Batrachenko A, et al. Differential developmental trajectories of magnetic susceptibility in human brain gray and white matter over the lifespan. Hum Brain Mapp. 2014;35:2698–2713. doi: 10.1002/hbm.22360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Small TN, Papadopoulos EB, Boulad F, et al. Comparison of immune reconstitution after unrelated and related T-cell-depleted bone marrow transplantation: effect of patient age and donor leukocyte infusions. Blood. 1999;93:467–480. [PubMed] [Google Scholar]
- 53.Qaddoumi I, Bass JK, Wu J, et al. Carboplatin-associated ototoxicity in children with retinoblastoma. J Clin Oncol. 2012;30:1034–1041. doi: 10.1200/JCO.2011.36.9744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dreyer ZE, Dinndorf PA, Camitta B, et al. Analysis of the role of he-matopoietic stem-cell transplantation in infants with acute lympho-blastic leukemia in first remission and MLL gene rearrangements: a report from the Children’s Oncology Group. J Clin Oncol. 2011;29:214–222. doi: 10.1200/JCO.2009.26.8938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kearns GL, Abdel-Rahman SM, Alander SW, et al. Developmental pharmacology-drug disposition, action, and therapy in infants and children (Review) N Engl J Med. 2003;349:1157–1167. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 56.Brunstein CG, Fuchs EJ, Carter SL, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated umbilical cord blood grafts. Blood. 2011;118:282–288. doi: 10.1182/blood-2011-03-344853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luznik L, O’Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using non-myeloablative conditioning and high-dose, post-transplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raiola AM, Dominietto A, Ghiso A, et al. Unmanipulated hap-loidentical bone marrow transplantation and posttransplantation cyclophosphamide for hematologic malignancies after myeloablative conditioning. Biol Blood Marrow Transplant. 2013;19:117–122. doi: 10.1016/j.bbmt.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 59.de Lima M, McNiece I, Robinson SN, et al. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N Engl J Med. 2012;367:2305–2315. doi: 10.1056/NEJMoa1207285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cutler C, Multani P, Robbins D, et al. Prostaglandin-modulated umbilical cord blood hematopoietic stem cell transplantation. Blood. 2013;122:3074–3081. doi: 10.1182/blood-2013-05-503177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Delaney C, Heimfeld S, Brashem-Stein C, et al. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010;16:232–237. doi: 10.1038/nm.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Farag SS, Srivastava S, Messina-Graham S, et al. In vivo DPP-4 inhibition to enhance engraftment of single-unit cord blood transplants in adults with hematological malignancies. Stem Cells Dev. 2013;22:1007–1015. doi: 10.1089/scd.2012.0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Popat UR, Oran B, Hosing CM, et al. Ex vivo fucosylation of cord blood acclerates neutrophil and platelet engraftment. Blood. 2013;122:691. doi: 10.1182/blood-2015-01-607366. [Abstract] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Horwitz ME, Stiff PJ, Chao NJ, et al. NiCord® expanded haematopoietic progenitor cells are capable of outcompeting the unmanipulated cord blood unit following myeloablative dual umbilical cord blood transplantation. Bone Marrow Transplant. 2013:19. Supplement 188 [Abstract] [Google Scholar]
- 65.Barker JN, Ponce DM, Dahi PB, et al. Double-unit cord blood (CB) transplantation combined with haplo-identical CD34+ cell-selected PBSC results in 100% CB engraftment with enhanced myeloid recovery. Blood. 2013;122:298. [Abstract] [Google Scholar]
- 66.Liu H, Rich ES, Godley L, et al. Reduced-intensity conditioning with combined haploidentical and cord blood transplantation results in rapid engraftment, low GVHD, and durable remissions. Blood. 2011;118:6438–6445. doi: 10.1182/blood-2011-08-372508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crocchiolo R, Zino E, Vago L, et al. Nonpermissive HLA-DPB1 disparity is a significant independent risk factor for mortality after unrelated hematopoietic stem cell transplantation. Blood. 2009;114:1437–1444. doi: 10.1182/blood-2009-01-200378. [DOI] [PubMed] [Google Scholar]
- 68.Fleischhauer K, Shaw BE, Gooley T, et al. Effect of T-cell-epitope matching at HLA-DPB1 in recipients of unrelated-donor haemo-poietic-cell transplantation: a retrospective study. Lancet Oncol. 2012;13:366–374. doi: 10.1016/S1470-2045(12)70004-9. [Erratum appears in Lancet Oncol 2012;13:e134–5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pidala J, Lee SJ, Spellman SR, et al. HLA-mismatch is associated with worse outcomes after myeloablative conditioning and unrelated donor hematopoietic cell transplantation: a CIBMTR analysis: Abstract 5. Biol Blood Marrow Transplant. 2014:20. doi: 10.1016/j.bbmt.2015.05.028. Supplement:S25-S26 [Abstract] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cooley S, Weisdorf DJ, Guethlein LA, et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood. 2010;116:2411–2419. doi: 10.1182/blood-2010-05-283051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Venstrom JM, Pittari G, Gooley TA, et al. HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. N Engl J Med. 2012;367:805–816. doi: 10.1056/NEJMoa1200503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.MacMillan ML, Defor TE, Weisdorf DJ. What predicts high risk acute graft-versus-host disease (GVHD) at onset?: identification of those at highest risk by a novel acute GVHD risk score. Br J Haematol. 2012;157:732–741. doi: 10.1111/j.1365-2141.2012.09114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Levine JE, Harris AC, Taylor A, et al. A biomarker-based grading system at onset of GVHD predicts NRM better than the modified Glucksberg grading system. Blood. 2013;122:145. [Abstract] [Google Scholar]
- 74.Mielcarek M, Furlong T, Storer BE, et al. Efficacy and safety of lower-dose glucocorticoids for initial treatment of acute graft-versus-host disease: a randomized controlled trial. Blood. 2013;122:703. [Abstract] [Google Scholar]
- 75.Pidala J, Tomblyn M, Nishihori T, et al. Sirolimus demonstrates activity in the primary therapy of acute graft-versus-host disease without systemic glucocorticoids. Haematologica. 2011;96:1351–1356. doi: 10.3324/haematol.2011.041236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koreth J, Matsuoka K, Kim HT, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med. 2011;365:2055–2066. doi: 10.1056/NEJMoa1108188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Herrera AF, Kim HT, Bindra B, et al. A phase II study of proteasome-inhibition for initial therapy of chronic graft-versus-host disease. Blood. 2013;122:909. [Abstract] [Google Scholar]
- 78.Couzin-Frankel J. Breakthrough of the year 2013. Cancer immuno-therapy. Science. 2013;342:1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 79.Kohn DB, Dotti G, Brentjens R, et al. CARs on track in the clinic. Mol Ther. 2011;19:432–438. doi: 10.1038/mt.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kochenderfer JN, Wilson WH, Janik JE, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Porter DL, Levine BL, Kalos M, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brunstein CG, Miller JS, Cao Q, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117:1061–1070. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schneidawind D, Pierini A, Negrin RS. Regulatory T cells and natural killer T cells for modulation of GVHD following allogeneic hematopoietic cell transplantation (Review) Blood. 2013;122:3116–3121. doi: 10.1182/blood-2013-08-453126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Leen AM, Bollard CM, Mendizabal AM, et al. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood. 2013;121:5113–5123. doi: 10.1182/blood-2013-02-486324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Doubrovina E, Oflaz-Sozmen B, Prockop SE, et al. Adoptive immuno-therapy with unselected or EBV-specific T cells for biopsy-proven EBV+ lymphomas after allogeneic hematopoietic cell transplantation. Blood. 2012;119:2644–2656. doi: 10.1182/blood-2011-08-371971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 89.Denman CJ, Senyukov VV, Somanchi SS, et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS ONE. 2012;7:e30264. doi: 10.1371/journal.pone.0030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106:376–383. doi: 10.1182/blood-2004-12-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bollard CM, Gottschalk S, Torrano V, et al. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting epstein-barr virus latent membrane proteins. J Clin Oncol. 2014;32:798–808. doi: 10.1200/JCO.2013.51.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kanakry JA, Li H, Gellert LL, et al. Plasma Epstein-Barr virus DNA predicts outcome in advanced Hodgkin lymphoma: correlative analysis from a large North American cooperative group trial. Blood. 2013;121:3547–3553. doi: 10.1182/blood-2012-09-454694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sorror ML, Maris MB, Storer B, et al. Comparing morbidity and mortality of HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative and myeloablative conditioning: influence of pretransplant comorbidities. Blood. 2004;104:961–968. doi: 10.1182/blood-2004-02-0545. [DOI] [PubMed] [Google Scholar]
- 94.Diaconescu R, Flowers CR, Storer B, et al. Morbidity and mortality with nonmyeloablative compared to myeloablative conditioning before hematopoietic cell transplantation from HLA matched related donors. Blood. 2004;104:1550–1558. doi: 10.1182/blood-2004-03-0804. [DOI] [PubMed] [Google Scholar]
- 95.Artz AS, Pollyea DA, Kocherginsky M, et al. Performance status and comorbidity predict transplant-related mortality after allogeneic he-matopoietic cell transplantation. Biol Blood Marrow Transplant. 2006;12:954–964. doi: 10.1016/j.bbmt.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 96.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sorror ML, Sandmaier BM, Storer BE, et al. Long-term outcomes among older patients following nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation for advanced hematologic malignancies. JAMA. 2011;306:1874–1883. doi: 10.1001/jama.2011.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Muffly LS, Boulukos M, Swanson K, et al. Pilot study of comprehensive geriatric assessment (CGA) in allogeneic transplant: CGA captures a high prevalence of vulnerabilities in older transplant recipients. Biol Blood Marrow Transplant. 2013;19:429–434. doi: 10.1016/j.bbmt.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 99.Chien JW, Zhao LP, Storer B, et al. Improving hematopoietic cell transplant outcomes in a new era of genomic research. Biol Blood Marrow Transplant. 2009;15(Suppl. 1):42–45. doi: 10.1016/j.bbmt.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Artz AS, Wickrema A, Dinner S, et al. Pretreatment C-reactive protein is a predictor for outcomes after reduced-intensity allogeneic he-matopoietic cell transplantation. Biol Blood Marrow Transplant. 2008;14:1209–1216. doi: 10.1016/j.bbmt.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Palmer J, Williams K, Inamoto Y, et al. Pulmonary symptoms measured by the National Institutes of Health lung score predict overall survival, nonrelapse mortality, and patient-reported outcomes in chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2014;20:337–344. doi: 10.1016/j.bbmt.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yoshihara S, Yanik G, Cooke KR, et al. Bronchiolitis obliterans syndrome (BOS), bronchiolitis obliterans organizing pneumonia (BOOP), and other late-onset noninfectious pulmonary complications following allogeneic hematopoietic stem cell transplantation (Review) Biol Blood Marrow Transplant. 2007;13:749–759. doi: 10.1016/j.bbmt.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 103.Williams KM, Chien JW, Gladwin MT, et al. Bronchiolitis obliterans after allogeneic hematopoietic stem cell transplantation. JAMA. 2009;302:306–314. doi: 10.1001/jama.2009.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hildebrandt GC, Fazekas T, Lawitschka A, et al. Diagnosis and treatment of pulmonary chronic GVHD: report from the consensus conference on clinical practice in chronic GVHD (Review) Bone Marrow Transplant. 2011;46:1283–1295. doi: 10.1038/bmt.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Martin PJ, Chien JW. What we know and mostly do not know about bronchiolitis obliterans syndrome (Editorial) Bone Marrow Transplant. 2012;47:1–4. doi: 10.1038/bmt.2011.38. [DOI] [PubMed] [Google Scholar]
- 106.Yanik GA, Mineishi S, Levine JE, et al. Soluble tumor necrosis factor receptor: enbrel (etanercept) for subacute pulmonary dysfunction following allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18:1044–1054. doi: 10.1016/j.bbmt.2011.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Williams KM, Pavletic SZ, Lee SJ, et al. Interim analysis of a phase II trial of montelukast for the treatment of bronchiolitis obliterans syndrome after HSCT reveals immunobiology of disease. Biol Blood Marrow Transplant. 2013;19:S143. [Abstract] [Google Scholar]
- 108.Tizon R, Frey N, Heitjan DF, et al. High-dose corticosteroids with or without etanercept for the treatment of idiopathic pneumonia syndrome after allo-SCT. Bone Marrow Transplant. 2012;47:1332–1337. doi: 10.1038/bmt.2011.260. [DOI] [PubMed] [Google Scholar]
- 109.Yanik G, Ho VT, Horowitz M, et al. Randomized, double blind, placebo-controlled trial of a TNF inhibitor (ETANERCEPT) for the treatment of idiopathic pneumonia syndrome (IPS) after allogeneic stem cell transplant (SCT). A Blood and Marrow Transplant Clinical Trials Network (BMT CTN) study. Biol Blood Marrow Transplant. 2013;19:S169. doi: 10.1016/j.bbmt.2014.02.026. [Abstract] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chien JW, Martin PJ, Flowers ME, et al. Implications of early airflow decline after myeloablative allogeneic stem cell transplantation. Bone Marrow Transplant. 2004;33:759–764. doi: 10.1038/sj.bmt.1704422. [DOI] [PubMed] [Google Scholar]
- 111.Gálban CJ, Han MK, Boes JL, et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD pheno-types and disease progression. Nat Med. 2012;18:1711–1715. doi: 10.1038/nm.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Panoskaltsis-Mortari A, Griese M, Madtes DK, et al. An official American Thoracic Society research statement: noninfectious lung injury after hematopoietic stem cell transplantation: idiopathic pneumonia syndrome (Review) Am J Respir Crit Care Med. 2011;183:1262–1279. doi: 10.1164/rccm.2007-413ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tuncer HH, Rana N, Milani C, et al. Gastrointestinal and hepatic complications of hematopoietic stem cell transplantation (Review) World J Gastroenterol. 2012;18:1851–1860. doi: 10.3748/wjg.v18.i16.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jenq RR, Ubeda C, Taur Y, et al. Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J Exp Med. 2012;209:903–911. doi: 10.1084/jem.20112408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bhatt AS, Freeman SS, Herrera AF, et al. Sequence-based discovery of Bradyrhizobium enterica in cord colitis syndrome. N Engl J Med. 2013;369:517–528. doi: 10.1056/NEJMoa1211115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Boeckh M, Nichols WG, Papanicolaou G, et al. Cytomegalovirus in hematopoietic stem cell transplant recipients: current status, known challenges, and future strategies. Biol Blood Marrow Transplant. 2003;9:543–558. doi: 10.1016/s1083-8791(03)00287-8. [DOI] [PubMed] [Google Scholar]
- 117.Blyth E, Clancy L, Simms R, et al. Donor-derived CMV-specific T cells reduce the requirement for CMV-directed pharmacotherapy after allogeneic stem cell transplantation. Blood. 2013;121:3745–3758. doi: 10.1182/blood-2012-08-448977. [DOI] [PubMed] [Google Scholar]
- 118.Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients (Review) Clin Infect Dis. 2002;34:1094–1097. doi: 10.1086/339329. [DOI] [PubMed] [Google Scholar]
- 119.Almyroudis NG, Jakubowski A, Jaffe D, et al. Predictors for persistent cytomegalovirus reactivation after T-cell-depleted allogeneic he-matopoietic stem cell transplantation. Transpl Infect Dis. 2007;9:286–294. doi: 10.1111/j.1399-3062.2007.00235.x. [DOI] [PubMed] [Google Scholar]
- 120.Erard V, Chien JW, Kim HW, et al. Airflow decline after myeloablative allogeneic hematopoietic cell transplantation: the role of community respiratory viruses. J Infect Dis. 2006;193:1619–1625. doi: 10.1086/504268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Boeckh M. The challenge of respiratory virus infections in he-matopoietic cell transplant recipients. Br J Haematol. 2008;143:455–467. doi: 10.1111/j.1365-2141.2008.07295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Peck AJ, Englund JA, Kuypers J, et al. Respiratory virus infection among hematopoietic cell transplantation recipients: evidence for asymptomatic parainfluenza infection. Blood. 2007;110:1681–1688. doi: 10.1182/blood-2006-12-060343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sydnor ER, Greer A, Budd AP, et al. An outbreak of human parainfluenza virus 3 infection in an outpatient hematopoietic stem cell transplantation clinic. Am J Infect Control. 2012;40:601–605. doi: 10.1016/j.ajic.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 124.Falsey AR. Current management of parainfluenza pneumonitis in immunocompromised patients: a review. Infect Drug Resist. 2012;5:121–127. doi: 10.2147/IDR.S25874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Malakhov MP, Aschenbrenner LM, Smee DF, et al. Sialidase fusion protein as a novel broad-spectrum inhibitor of influenza virus infection. Antimicrob Agents Chemother. 2006;50:1470–1479. doi: 10.1128/AAC.50.4.1470-1479.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Moscona A, Porotto M, Palmer S, et al. A recombinant sialidase fusion protein effectively inhibits human parainfluenza viral infection in vitro and in vivo. J Infect Dis. 2010;202:234–241. doi: 10.1086/653621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Guzman-Suarez BB, Buckley MW, Gilmore ET, et al. Clinical potential of DAS181 for treatment of parainfluenza-3 infections in transplant recipients. Transpl Infect Dis. 2012;14:427–433. doi: 10.1111/j.1399-3062.2012.00718.x. [DOI] [PubMed] [Google Scholar]
- 128.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplant recipients: a global perspective. Bone Marrow Transplant. 2009;44:453–455. doi: 10.1038/bmt.2009.254. [DOI] [PubMed] [Google Scholar]
- 129.Pollack M, Heugel J, Xie H, et al. An international comparison of current strategies to prevent herpesvirus and fungal infections in hematopoietic cell transplant recipients. Biol Blood Marrow Transplant. 2011;17:664–673. doi: 10.1016/j.bbmt.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pronovost PJ. Enhancing physicians’ use of clinical guidelines. JAMA. 2013;310:2501–2502. doi: 10.1001/jama.2013.281334. [DOI] [PubMed] [Google Scholar]
- 131.Wachter RM, Pronovost P, Shekelle P. Strategies to improve patient safety: the evidence base matures. Ann Intern Med. 2013;158:350–352. doi: 10.7326/0003-4819-158-5-201303050-00010. [DOI] [PubMed] [Google Scholar]
- 132.Herzer KR, Pronovost PJ. Motivating physicians to improve quality: Light the intrinsic fire. Am J Med Qual. 2014;29:451–453. doi: 10.1177/1062860613510201. [DOI] [PubMed] [Google Scholar]
- 133.Hari P, Defor TE, Vesole DH, et al. Intermittent zoledronic acid prevents bone loss in adults after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2013;19:1361–1367. doi: 10.1016/j.bbmt.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 134.McClune BL, Polgreen LE, Burmeister LA, et al. Screening, prevention and management of osteoporosis and bone loss in adult and pediatric hematopoietic cell transplant recipients (Review) Bone Marrow Transplant. 2011;46:1–9. doi: 10.1038/bmt.2010.198. [DOI] [PubMed] [Google Scholar]
- 135.Savani BN, Donohue T, Kozanas E, et al. Increased risk of bone loss without fracture risk in long-term survivors after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:517–520. doi: 10.1016/j.bbmt.2007.01.085. [DOI] [PubMed] [Google Scholar]
- 136.Yao S, McCarthy PL, Dunford LM, et al. High prevalence of early-onset osteopenia/osteoporosis after allogeneic stem cell transplantation and improvement after bisphosphonate therapy. Bone Marrow Transplant. 2008;41:393–398. doi: 10.1038/sj.bmt.1705918. [DOI] [PubMed] [Google Scholar]
- 137.Petropoulou AD, Porcher R, Herr AL, et al. Prospective assessment of bone turnover and clinical bone diseases after allogeneic hematopoietic stem-cell transplantation. Transplantation. 2010;89:1354–1361. doi: 10.1097/TP.0b013e3181d84c8e. [DOI] [PubMed] [Google Scholar]
- 138.Cannistra SA. Phase II trials in Journal of Clinical Oncology. J Clin Oncol. 2009;27:3073–3076. doi: 10.1200/JCO.2009.23.1811. [DOI] [PubMed] [Google Scholar]
- 139.Hunsberger S, Zhao Y, Simon R. A comparison of phase II study strategies (Review) Clin Cancer Res. 2009;15:5950–5955. doi: 10.1158/1078-0432.CCR-08-3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pencina MJ, D’Agostino RB, Vasan RS. Statistical methods for assessment of added usefulness of new biomarkers (review) Clin Chem Lab Med. 2010;48:1703–1711. doi: 10.1515/CCLM.2010.340. [DOI] [PMC free article] [PubMed] [Google Scholar]