Abstract
Photon irradiation has been repeatedly suspected of increasing tumor cell motility and promoting locoregional recurrence of disease. This study was set up to analyse possible mechanisms underlying the potentially radiation-altered motility in medulloblastoma cells. Medulloblastoma cell lines D425 and Med8A were analyzed in migration and adhesion experiments with and without photon and carbon ion irradiation. Expression of integrins was determined by quantitative FACS analysis. Matrix metalloproteinase concentrations within cell culture supernatants were investigated by enzyme-linked immunosorbent assay (ELISA). Statistical analysis was performed using Student's t-test. Both photon and carbon ion irradiation significantly reduced chemotactic medulloblastoma cell transmigration through 8-μm pore size membranes, while simultaneously increasing adherence to fibronectin- and collagen I- and IV–coated surfaces. Correspondingly, both photon and carbon ion irradiation downregulate soluble MMP9 concentrations, while upregulating cell surface expression of proadhesive extracellular matrix protein-binding integrin α5. The observed phenotype of radiation-altered motility is more pronounced following carbon ion than photon irradiation. Both photon and (even more so) carbon ion irradiation are effective in inhibiting medulloblastoma cell migration through downregulation of matrix metalloproteinase 9 and upregulation of proadhesive cell surface integrin α5, which lead to increased cell adherence to extracellular matrix proteins.
Keywords: medulloblastoma, migration, adhesion, particle radiotherapy, integrin, matrix metalloproteinase
INTRODUCTION
Medulloblastoma is the most frequently diagnosed malignant brain tumor in children, with a peak incidence at the age of 5 years [1]. Despite being rare, it can also be found in adults, in whom pediatric treatment regimes appear feasible and effective, based upon similar presentation and course [2]. Pathologically, medulloblastomas comprise at least seven subtypes of infratentorial central nervous system (CNS) primitive neuroectodermal tumor, which originate from the cerebellum or the roof of the fourth ventricle [3].
Treatment usually involves neurosurgical resection to the most reachable extent, followed by concomitant chemoradiation and additional maintenance chemotherapy [1]. Due to the characteristic propensity of medulloblastoma to spread throughout the CNS along cerebrospinal fluid (CSF) pathways, radiotherapy is usually administered to the complete craniospinal axis [4].
The unique phenomenon of early, rapid and extensive CNS dissemination is based on the high capability of medulloblastoma cells to migrate along leptomeningeal surfaces [5–7]. To initiate tumor cell motility, medulloblastoma cells interact with typical components of the leptomeningeal extracellular matrix (ECM), such as fibronectin, collagen I and collagen IV [7, 8]. Migration is then promoted by exposure to gradients of serous chemoattractants, which can abundantly be found within CSF or blood plasma [9, 10]. However, in addition to intrinsic promoters of cell migration, treatment-related factors have been reported as increasing tumor cell motility. Photon irradiation (IR) has been repeatedly claimed to enhance cell migration in sarcoma [11], colorectal cancer [12], and glioma [13]. In medulloblastoma, preclinical studies have demonstrated increased tumor cell invasiveness through upregulation of urokinase plasminogen activator receptor (uPAR) signaling [6, 14]. In D283 and UW228 medulloblastoma cells, single photon doses of 6 Gy promoted uPAR-dependent Wnt/β-catenin-signaling and consequently increased the neurosphere-forming ability [14]. Moreover, single photon doses of 7 Gy induced expression of α3, α5 and β1 integrins, leading to enhanced wound healing migration in D283 and DAOY cells [6]. To date, little data has focused on dose-dependent alterations in medulloblastoma cell motility, and none has addressed the potential impact of carbon ion IR.
IR with carbon ions has recently been identified as exerting inhibitory influence on tumor cell migration. Correspondingly, expression of motility-promoting factors has been found to be suppressed even at sublethal doses [11, 15]. However, controversial and ambiguous findings have also reported distinct tumor cell lines within one tumor entity as not succumbing to the decelerating effect of carbon ion IR [16], but as possibly increasing their invasive potential instead [17].
As for medulloblastoma, clinicians have repeatedly postulated the use of particle radiotherapy due to its beneficial dose distribution [3] and increased biological effectiveness [18]. Previous works have mainly focused on proton particle IR. This manuscript addresses the role of carbon ion IR in motility changes in two medulloblastoma cell lines.
MATERIALS AND METHODS
Cell culture
D425 medulloblastoma cells were purchased from ATCC and kept at 37°C and 5% CO2 in improved MEM (Biochrom AG) supplemented with 1% Penicillin/Streptomycin and 10% fetal calf serum (FCS). Med8A medulloblastoma cells were a kind gift from Michael D. Taylor (Toronto, Ontario, Canada) and were kept at 37°C and 5% CO2 in DMEM (FG0415 Biochrom AG) supplemented with 1% Penicillin/Streptomycin and 10% FCS. At 24 h before adhesion and migration experiments, cells were serum starved in DMEM containing 1% Penicillin/Streptomycin and 0.5% FCS. Passaging of cells was performed every week.
Migration assays using membrane coated with extracellular matrix proteins
For migration assays, polycarbonate membranes with 8-μm pores, and for adhesion assays, 96-well cell culture dishes, were coated with 50 ng/cm2 fibronectin, 0.5 μg/cm2 collagen I and 0.5 μg/cm2 collagen IV, kept overnight at 4°C and washed in twice distillated and salt-free water prior to the experiments. Next, 5 × 103 cells were loaded into the upper chamber of a 48-well modified microchemotaxis chamber (Multiwell Chemotaxis Chamber, Neuro Probe). The lower well contained cell culture medium 10% FCS, as indicated. Lower and upper chambers were separated by an 8-µm pore size polycarbonate membrane that had been coated with fibronectin (50 ng/cm2), collagen I (0.5 μg/cm2) and collagen IV (0.5 μg/cm2) 24 h before the start of migration. Radiation treatments were performed 24 h before assessment of migration. Transmigrated cells were stained with DiffQuick® and counted with a Leica DC300F microscope. All assays were done in (at least) triplicates, and the wells were counted by an investigator blinded to the experimental set-up. Cell numbers are expressed as multiples of controls or as proportion of inputs.
Adhesion assay
At 24 h after IR, 5 × 102 cells were loaded into 96-well cell culture dishes coated with fibronectin (FN), collagen I and IV and allowed to adhere for 1 h before assessment of adhesion. Adherent cells were stained and counted with a Leica DC300F microscope by an investigator blinded to the experimental set-up. All assays were done in triplicates. Cell numbers are expressed as absolute numbers.
Enzyme-linked immunosorbent assay
For this assay, 1 × 105 cells were incubated for 24 h following IR. The cell culture supernatants were then harvested and stored at −18°C. The enzyme-linked immunosorbent assay (ELISA) protocol followed the R&D Systems® guidelines for MMP2/9 assays (human MMP-9 Immoassay DMP900 quantitative determination of active and pro MMP9, mouse monoclonal anti-MMP9) (human MMP-2 Immunoassay DMP2F0 quantitative determination of MMP2, polyclonal anti-MMP2). Analysis and evaluation were performed with the Infinite® F50/Robotic ELISA plate reader (absorbance at 450 nm, correction wavelength at 570 nm) and Magellan for F50 software (Tecan Group Ltd).
Fluorescence-activated cell sorting
At 24 h after IR, cells were fixed with 70% ethanol and stained with labeled anibodies directed against ανβ3 (FITC mouse anti-human-CD51/61, 555505, BD), α5 (PE mouse anti-human CD49e, 555617, BD), β1 (mouse anti-human-CD29, 556049, BD) and ανβ5 (PE mouse anti-human Integrin ανβ5 FAB2528P, R&D). Med8A and D425 cells were analyzed with a three-colour fluorescence-activated cell sorting (FACS) scan flow cytometer and CellQuestPro software (BD Biosciences).
Radiation treatment
Photon IR was performed using a biological X-ray irradiator with 320 kV and 12.50 mA (Precision X-Ray Inc., Model No.: XRAD 320, North Branford, USA). We applied single doses of 1, 2 and 10 Gy 24 h prior to migration experiments at a dose rate of 1.2 Gy/min. Carbon ion IR was applied with an extended Bragg peak (E = (128 ± 7) MeV/u, LET = (91.5 ± 1.5) keV/μm) at single carbon ion doses of 0.5, 1 and 3 Gy at the Heidelberg Ion Therapy Center. Carbon ion doses, assuming a relative biological effectiveness (RBE) of 3–4, were derived from comparable glioma studies [15], since no RBE calculations for medulloblastoma cells have been published thus far.
Statistics
All experiments were performed at least three times using triplicates in each individual experimental set-up. Data are displayed as means ± standard deviations. Comparisons between two groups were performed with Student's t-test. Statistical significance was noted for two-tailed P-values of ≤0.05.
RESULTS
Radiogenic alterations of medulloblastoma cell transmigration
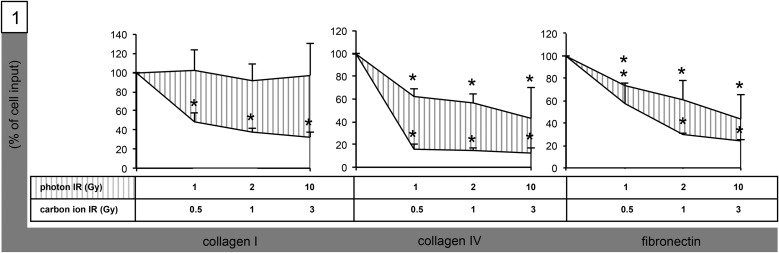
We investigated transmigration of medulloblastoma cell lines Med8A and D425 through polycarbonate membranes coated with ECM proteins FN, collagen I, and collagen IV following single doses of both photon (1, 2 and 10 Gy) and carbon ion (0.5, 1 and 3 Gy) IR. Medulloblastoma cells were motile and demonstrated robust serum-induced transmigration through collagen-I- (Med8A: n = 720 (14%); D425: n = 480 (9.6%)), collagen-IV- (Med8A: n = 1520 (30.4%); D425: 640 (12.8%)) and FN-coated membranes (Med8A: n = 1680 (33.6%); D425: n = 1260 (25.6%)). Following IR, we observed a significant reduction of migration in most experiments for both D425 and Med8A cells (Fig. 1). Impairment of migration was strongest for carbon ion–irradiated Med8A cells on collagen IV–coated surfaces (0.5 Gy: −84%, 1 Gy: −85.5%, 3 Gy: −87%). In general, carbon ion IR was more effective than photon IR in impairing medulloblastoma cell transmigration and caused significantly reduced transmigration through FN- and collagen I/IV–coated membranes in both cell lines, even at sublethal doses of 0.5 GyE. Photon IR, too, inhibited medulloblastoma transmigration in most set-ups; however, Med8A cell transmigration remained unaltered in experiments using collagen-I–coated membranes. Quantitative analyses of both photon- and carbon ion-altered transmigration are summarized in Table 1.
Fig. 1.
Radiogenic inhibition of Med8A medulloblastoma cell migration. Graphical analysis of transmigration through DiffQuik®-stained 8-µm pore size polycarbonate membranes coated with Fn, collagen I and IV following IR with single photon doses [31] of 1, 2 and 10 Gy, and single carbon ion doses (striped) of 0.5, 1 and 3 GyE. Statistical analysis performed using Student's t-test (asterisk indicates statistical significance P < 0.05).
Table 1.
Quantitative analysis of medulloblastoma cell transmigration
| Transmigration of medulloblastoma cells | |||
|---|---|---|---|
| Med8A (%) | D425 (%) | ||
| Fibronectin | control | 100 | 100 |
| 1 Gy photon IR | 74 ± 1.4 (*) | 103 ± 23.4 (ns) | |
| 2 Gy photon IR | 61 ± 17 (*) | 71 ± 9.3 (*) | |
| 10 Gy photon IR | 51 ± 4.2 (*) | 53 ± 2.9 (*) | |
| 0.5 GyE 12C IR | 57 ± 18.4 (*) | 79 ± 1.4 (ns) | |
| 1 GyE 12C IR | 30 ± 1.4 (*) | 66 ± 14 (ns) | |
| 3 GyE 12C IR | 25 ± 4.2 (*) | 51 ± 4.2 (*) | |
| Collagen I | control | 100 | 100 |
| 1 Gy photon IR | 102 ± 21.2 (ns) | 89 ± 16.5 (ns) | |
| 2 Gy photon IR | 91.5 ± 17.7 (ns) | 69.3 ± 9.1 (*) | |
| 10 Gy photon IR | 97 ± 23 (ns) | 55 ± 21.2 (*) | |
| 0.5 GyE 12C IR | 49 ± 9.2 (*) | 81 ± 10.6 (*) | |
| 1 GyE 12C IR | 38 ± 4.2 (*) | 59 ± 5.7 (*) | |
| 3 GyE 12C IR | 33 ± 4.9 (*) | 24 ± 25 (*) | |
| Collagen IV | control | 100 | 100 |
| 1 Gy photon IR | 62 ± 7.1 (*) | 109 ± 12.7 (ns) | |
| 2 Gy photon IR | 57 ± 7.1 (*) | 96 ± 44.5 (ns) | |
| 10 Gy photon IR | 43 ± 26.9 (*) | 74 ± 1.4 (*) | |
| 0.5 GyE 12C IR | 16 ± 4.2 (*) | 54 ± 7.1 (*) | |
| 1 GyE 12C IR | 15 ± 2.1 (*) | 49 ± 9.2 (*) | |
| 3 GyE 12C IR | 13 ± 4.2 (*) | 46 ± 4.2 (*) | |
Asterisk = statistical significance, P < 0.05, ns = absence of statistical significance, P > 0.05.
Matrix metalloproteinase release following carbon ion and photon IR
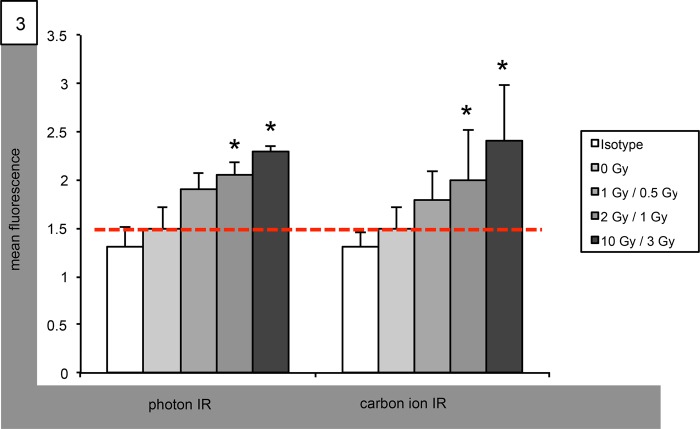
We determined secretion of matrix metalloproteinases (MMPs) into the cell supernatants following IR using ELISA. In Med8A cells, MMP9 secretion was significantly reduced after IR with carbon ions at doses as low as 1 Gy. Photon IR, too, decreased MMP9 concentration in Med8A supernatants; however, statistical significance was only reached for single doses of 10 Gy (Fig. 2). Supernatant concentrations of MMP9 in experiments using D425 cells remained unchanged by photon IR at all doses tested, whereas carbon ion IR supressed MMP9 secretion at single doses of both 1 and 3 Gy. In both Med8A and D425 cells, MMP2 concentration remained unaltered following both photon and carbon ion IR at all doses tested (data not shown).
Fig. 2.
Radiation-altered secretion of MMP9 in Med8A medulloblastoma cells. Graphical ELISA analysis of MMP9 secretion following IR with single photon doses (left group) of 1, 2 and 10 Gy, and with single carbon ion doses (right group) of 0.5, 1 and 3 GyE. Statistical analysis performed using Student's t-test (asterisk indicates statistical significance P < 0.05, red bar references basal unirradiated control).
Integrin expression following carbon ion and photon IR
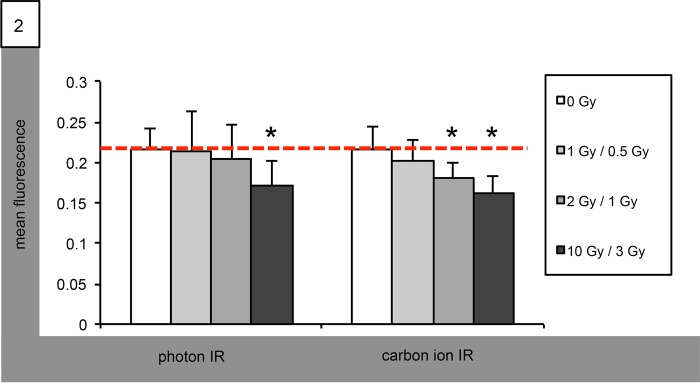
FACS analyses were performed in order to investigate IR-altered integrin expression. We did not observe any significant modification of ανβ3, ανβ5 or β1 integrin expression in Med8A and D425 cells following either photon or carbon ion IR. On the contrary, both cell lines stained significantly positive for α5 integrins, and following photon IR, Med8A cell surface expression of α5 was increased in a dose-dependent manner, yielding significant enhancements at both 2 and 10 Gy. In carbon ion–irradiated Med8A cells, expression of α5 was also continuously increased, with significant enhancements at both 1 and 3 Gy (Fig. 3). Expression of α5 following IR with 1 Gy photons or 0.5 Gy carbon ions was mildly increased, but did not reach statistical significance (Fig. 3). In D425 cells, photon IR increased α5 expression in a non-significant manner, while carbon ion IR significantly upregulated α5 expression at all doses tested (data not shown).
Fig. 3.
Radiation-altered expression of integrin α5 in Med8A medulloblastoma cells. Graphical FACS analysis of isotype-controlled integrin α5 surface expression following IR with single photon doses (left group) of 1, 2 and 10 Gy, and with single carbon ion doses (right group) of 0.5, 1 and 3 GyE. Statistical analysis performed using Student's t-test (asterisk indicates statistical significance P < 0.05, red bar references basal unirradiated control).
Radiogenic alterations of medulloblastoma adhesion
We next assessed radiogenic modifiability of medulloblastoma cell adhesion to FN- and collagen-I/IV–coated surfaces. We observed a significant dose-dependent increase in adhesion following carbon ion IR in Med8A cells (Fig. 4). Photon IR also caused increased adherence to all coated surfaces; however, the effect was less pronounced than in carbon ion–irradiated cells (Fig. 4). The same phenomenon of both photon- and carbon ion–increased adhesion to coated surfaces was observed in D425 cells (Table 2). Also, radiogenic adhesion was more evident in carbon ion–irradiated cells than in photon-based experiments.
Fig. 4.
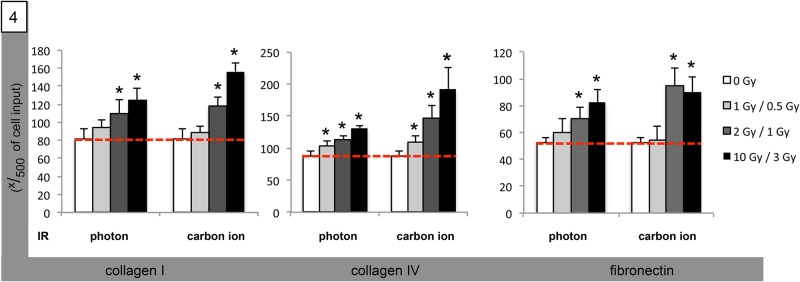
Radiogenic stimulation of Med8A medulloblastoma cell adhesion. Graphical analysis of cell adherence to polycarbonate surfaces coated with collagen I (left group), collagen IV (middle group), and FN (right group) following IR with single photon doses (left group) of 1, 2 and 10 Gy, and with single carbon ion doses (right group) of 0.5, 1 and 3 GyE. Statistical analysis performed using Student's t-test (asterisk indicates statistical significance P < 0.05, red bar references basal unirradiated control).
Table 2.
Quantitative analysis of medulloblastoma cell adhesion
| Adhesion of medulloblastoma cells |
(cell input 500 cells) | ||
|---|---|---|---|
| Med8A (n) | D425 (n) | ||
| Fibronectin | control | 52 ± 3.7 | 61 ± 11.3 |
| 1 Gy photon IR | 61 ± 6.3 (*) | 76 ± 7.1 (ns) | |
| 2 Gy photon IR | 71 ± 8.5 (*) | 109 ± 2.2 (*) | |
| 10 Gy photon IR | 82 ± 10.1 (*) | 118 ± 4.6 (*) | |
| 0.5 GyE 12C IR | 55 ± 9.6 (*) | 71 ± 0.5 (ns) | |
| 1 GyE 12C IR | 95 ± 12.7 (*) | 113 ± 8.3 (*) | |
| 3 GyE 12C IR | 90 ± 11.7 (*) | 150 ± 2.6 (*) | |
| Collagen I | control | 81 ± 12.0 | 30 ± 5.7 |
| 1 Gy photon IR | 95 ± 7.9 (ns) | 43 ± 11.7 (ns) | |
| 2 Gy photon IR | 110 ± 15.9 (*) | 54 ± 9.9 (*) | |
| 10 Gy photon IR | 125 ± 12.8 (*) | 74 ± 10.1 (*) | |
| 0.5 GyE 12C IR | 89 ± 7.4 (ns) | 46 ± 5.1 (*) | |
| 1 GyE 12C IR | 118 ± 9.4 (*) | 54 ± 8.0 (*) | |
| 3 GyE 12C IR | 157 ± 9.2 (*) | 92 ± 14.6 (*) | |
| Collagen IV | control | 88 ± 7.1 | 24 ± 5.1 |
| 1 Gy photon IR | 104 ± 6.4 (*) | 35 ± 5.4 (*) | |
| 2 Gy photon IR | 114 ± 5.8 (*) | 46 ± 6.6 (*) | |
| 10 Gy photon IR | 131 ± 5.1 (*) | 70 ± 10.2 (*) | |
| 0.5 GyE 12C IR | 110 ± 9.9 (*) | 36 ± 6.3 (*) | |
| 1 GyE 12C IR | 147 ± 19.8 (*) | 50 ± 9.6 (*) | |
| 3 GyE 12C IR | 192 ± 34.2 (*) | 105 ± 12.0 (*) | |
Asterisk = statistical significance, P < 0.05, ns = absence of statistical significance, P > 0.05.
DISCUSSION
In the present manuscript we investigated the modifiability of medulloblastoma cell motility through photon and carbon ion IR. In opposition to observations on various non-medulloblastoma tumor cells [11–13, 19], IR did not promote tumor cell migration but significantly impaired ECM-based transmigration, while simultaneously causing increased adherence to ECM-coated surfaces.
We have recently studied radiation-altered migration in malignant glioma cells and found migration to be significantly increased following sublethal photon IR through upregulation of FN-binding integrins [19]. This phenomenon has previously been observed for several other tumor cell lines, including sarcoma [11], colorectal [12] and pulmonary cancer cells [20]. Clinically, the finding of radiation-induced migration concerns radiation oncologists, because it imposes a theoretical risk of radiation-induced locoregional tumor infiltration and distant dissemination and, therefore, challenges current concepts of target volume delineation with limited safety margins for improved sparing of organs at risk.
Medulloblastomas are characterized by their tendency for early and rapid spread throughout the CNS [21]. Therefore, photon-induced migration would represent yet another stimulation of increased medulloblastoma cell motility.
In opposition to findings in other medulloblastoma cell lines, we detected migration to be decreased following both photon and carbon ion IR. Nalla et al. described DAOY medulloblastoma cell migration to be enhanced by 27% following single photon doses of 7 Gy [6]. This difference from our results may be explained by differences in experimental design. They investigated migration of cells towards one another in scratch assays, instead of analysis of transmigration away from the initial cell localization. Tabatabei et al. observed irradiated cells to secrete promigratory ligands [22] that may stimulate the reported migration in scratch assays. Therefore, the supposedly opposing notion of both promotion and inhibition of medulloblastoma cell migration through photon IR may be reconciled in terms of which migratory phenomena are being addressed: emigration from or immigration to previously irradiated tumor cell clusters.
Asuthkar et al. found single photon doses of 6 Gy to increase uPAR expression, suggestive of increased invasion. Therefore, they proposed targeting of uPAR in combination with photon IR as a promising option [14]. In contrast to these previously published works, we used two cell lines that are known to carry genetic amplifications of the myc oncogene. This aberration has been associated with early dissemination and poor outcome [23, 24], but it was also reported to sensitize medulloblastoma cells to IR [25]. These findings help to explain the opposing findings in different medulloblastoma cells, and support our results of IR-suppressed cell migration.
Inhibition of migration was strongest in FN- and collagen-IV–based experiments, which confirms previous works that have identified both FN and collagen IV to be key ECM proteins in radiation-induced motility alterations [19, 26, 27]. Carbon ion IR had significantly higher inhibitory effects on radiation than did photon IR. Again, this is in line with prior works that described particle IR as effectively impairing cell migration, even at sublethal doses, as were used in this project [11, 15].
Following IR, several tumor cell lines have been observed to upregulate their expression and secretion of MMPs [13, 20, 28]. This finding suggests MMPs might be involved in the phenomon of radiation-induced migration and infiltration, and MMP2 and MMP9 have been reported as characteristic in the context of photon-associated motility changes [13]. We determined concentrations of soluble MMP2 and MMP9 following both photon and carbon ion IR, and found significantly reduced MMP9 concentrations, even doses as low as 1 GyE. Following photon IR, MMP9 secretion was also suppressed, but with statistical significance only being reached for doses of 10 Gy. This finding may explain the difference in inhibitory effect on migration between photon and carbon ion IR. Measuring MMP2 after IR yielded no significant changes, suggesting an inferior role of MMP2 in the radiogenic changes in medulloblastoma cell motility. Ogata et al. also investigated IR-induced changes in MMP2 activity and found no influence of photon IR in sarcoma cells at various doses [11].
Since radiation-increased migration has repeatedly been correlated with increased integrin expression [6, 15, 19, 26, 29], we performed FACS analyses of various cell surface integrins. We did not detect any relevant radiogenic alteration in the expression of those integrins that have previously been identified as mediators of radiation-induced migration in other tumor entities (ανβ3, ανβ5 and β1). However, the expression of α5 was significantly enhanced in a dose-dependent manner after both photon and carbon ion IR. Our results are supported by Meineke et al. who found α5 to be significantly enhanced in a strictly time-dependent manner, with peak values being reached after 24 h following single photon doses of 5 Gy in colorectal cancer cells [26]. Expression of integrin α5 was iteratively confirmed to be a key integrin in mediating adherence to FN and collagen I/IV [30].
Besides its migratory impact, irradiation is also known to affect cell adherence to ECM proteins. Therefore, we finally performed adhesion experiments in order to determine radiogenic alterations that might establish a concept of radiation-decreased migration, while simultaneously increasing cell adherence. We found adhesion to FN, collagen I and IV to be significantly enhanced following both photon and carbon ion IR. A finding of increased cell adhesion to collagen I remains somewhat surprising because, in prior experiments, no corresponding decrease in cell chemotaxis was observed—as was detected for other matrix proteins; However, similar findings were reported by Tsutsumi et al., who found increased adhesion to collagen I to be connected to enhanced cell invasion in non–small cell lung cancer [20].
The observed increase in adhesion was strictly dose-dependent, reaching statistical significance after clinically relevant doses of 2 and 10 Gy, and 1 and 3 Gy, respectively. This phenotype corresponds with the radiogenic increase in ECM protein–binding α5 integrins, and is supported by several other works addressing radiogenic motility alterations: Nalla et al. reported enhancements of adhesion by 38% and 50% in collagen I-/FN-exposed medulloblastoma cells following single photon doses of 7 Gy [6 ]; Meineke et al. demonstrated photon-induced upregulation of integrin expression to significantly enhance adhesion capacity in colorectal cancer cells [26].
Whether these adherent tumor cells are less aggressive resident cells or cells predestined to infiltrate local structures remains controversial. We are aware that our present data cannot resolve this conflict. However, we want to state that, in D425 and Med8A medulloblastoma cells, neither photon nor carbon ion IR promote migration, and that radiogenic integrin-based adhesion does not go along with an increase in invasion-initiating MMPs.
In opposition to other CNS tumors, in medulloblastoma cells, both photon and carbon ion IR effectively prevent migration through impaired MMP9-secretion and upregulation of pro-adhesive FN- and collagen-binding integrin α5. Therefore, radiogenic stimulation of tumor cell infiltration and dissemination must not be feared when administering radiotherapy in medulloblastoma patients. Photon-associated findings have been consistently confirmed and are even more pronounced in carbon ion–irradiation experiments, suggesting particle irradiation to be a promising radiation modality in the treatment of medulloblastoma patients.
FUNDING
This work was supported by institutional funding. Funding to pay the Open Access publication charges for this article was provided by the Department of Radiation Oncology, University Hospital of Heidelberg, Heidelberg, Germany.
REFERENCES
- 1.Gerber NU, Mynarek M, von Hoff K, et al. Recent developments and current concepts in medulloblastoma. Cancer Treat Rev 2014;40:356–65. [DOI] [PubMed] [Google Scholar]
- 2.Friedrich C, von Bueren AO, von Hoff K, et al. Treatment of adult nonmetastatic medulloblastoma patients according to the paediatric HIT 2000 protocol: a prospective observational multicentre study. Eur J Cancer 2013;49:893–903. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett F, Kortmann R, Saran F. Medulloblastoma. Clin Oncol (R Coll Radiol) 2013;25:36–45. [DOI] [PubMed] [Google Scholar]
- 4.von Hoff K, Rutkowski S. Medulloblastoma. Curr Treat Options Neurol 2012;14:416–26. [DOI] [PubMed] [Google Scholar]
- 5.Fiorilli P, Partridge D, Staniszewska I, et al. Integrins mediate adhesion of medulloblastoma cells to tenascin and activate pathways associated with survival and proliferation. Lab Invest 2008;88:1143–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nalla AK, Asuthkar S, Bhoopathi P, et al. Suppression of uPAR retards radiation-induced invasion and migration mediated by integrin beta1/FAK signaling in medulloblastoma. PLOS ONE 2010;5:e13006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wikstrand CJ, Friedman HS, Bigner DD. Medulloblastoma cell–substrate interaction in vitro. Invasion Metastasis 1991;11:310–24. [PubMed] [Google Scholar]
- 8.Liang Y, Diehn M, Bollen AW, et al. Type I collagen is overexpressed in medulloblastoma as a component of tumor microenvironment. J Neurooncol 2008;86:133–41. [DOI] [PubMed] [Google Scholar]
- 9.Dudu V, Able RA, Jr, Rotari V, et al. Role of epidermal growth factor-triggered PI3 K/Akt signaling in the migration of medulloblastoma-derived cells. Cell Mol Bioeng 2012;5:502–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubin JB, Kung AL, Klein RS, et al. A small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc Natl Acad Sci U S A 2003;100:13513–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogata T, Teshima T, Kagawa K, et al. Particle irradiation suppresses metastatic potential of cancer cells. Cancer Res 2005;65:113–20. [PubMed] [Google Scholar]
- 12.Goetze K, Scholz M, Taucher-Scholz G, et al. The impact of conventional and heavy ion irradiation on tumor cell migration in vitro. Int J Radiat Biol 2007;83:889–96. [DOI] [PubMed] [Google Scholar]
- 13.Wild-Bode C, Weller M, Rimner A, et al. Sublethal irradiation promotes migration and invasiveness of glioma cells: implications for radiotherapy of human glioblastoma. Cancer Res 2001;61:2744–50. [PubMed] [Google Scholar]
- 14.Asuthkar S, Gondi CS, Nalla AK, et al. Urokinase-type plasminogen activator receptor (uPAR)-mediated regulation of WNT/beta-catenin signaling is enhanced in irradiated medulloblastoma cells. J Biol Chem 2012;287:20576–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rieken S, Habermehl D, Wuerth L, et al. Carbon ion irradiation inhibits glioma cell migration through downregulation of integrin expression. Int J Radiat Oncol Biol Phys 2012;83:394–9. [DOI] [PubMed] [Google Scholar]
- 16.Eke I, Storch K, Kastner I, et al. Three-dimensional invasion of human glioblastoma cells remains unchanged by X-ray and carbon ion irradiation in vitro. Int J Radiat Oncol Biol Phys 2012;84:e515–23. [DOI] [PubMed] [Google Scholar]
- 17.Fujita M, Otsuka Y, Imadome K, et al. Carbon-ion radiation enhances migration ability and invasiveness of the pancreatic cancer cell, PANC-1, in vitro. Cancer Sci 2012;103:677–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerelchuluun A, Hong Z, Sun L, et al. Induction of in situ DNA double-strand breaks and apoptosis by 200 MeV protons and 10 MV X-rays in human tumour cell lines. Int J Radiat Biol 2011;87:57–70. [DOI] [PubMed] [Google Scholar]
- 19.Rieken S, Habermehl D, Mohr A, et al. Targeting alphanubeta3 and alphanubeta5 inhibits photon-induced hypermigration of malignant glioma cells. Radiat Oncol 2011;6:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsutsumi K, Tsuda M, Yazawa N, et al. Increased motility and invasiveness in tumor cells that survive 10 Gy irradiation. Cell Struct Funct 2009;34:89–96. [DOI] [PubMed] [Google Scholar]
- 21.Engelhard HH, Corsten LA. Leptomeningeal metastasis of primary central nervous system (CNS) neoplasms. Cancer Treat Res 2005;125:71–85. [DOI] [PubMed] [Google Scholar]
- 22.Tabatabai G, Bahr O, Mohle R, et al. Lessons from the bone marrow: how malignant glioma cells attract adult haematopoietic progenitor cells. Brain 2005;128:2200–11. [DOI] [PubMed] [Google Scholar]
- 23.Pfister S, Remke M, Benner A, et al. Outcome prediction in pediatric medulloblastoma based on DNA copy-number aberrations of chromosomes 6q and 17q and the MYC and MYCN loci. J Clin Oncol 2009;27:1627–36. [DOI] [PubMed] [Google Scholar]
- 24.Ryan SL, Schwalbe EC, Cole M, et al. MYC family amplification and clinical risk-factors interact to predict an extremely poor prognosis in childhood medulloblastoma. Acta Neuropathol 2012;123:501–13. [DOI] [PubMed] [Google Scholar]
- 25.von Bueren AO, Oehler C, Shalaby T, et al. c-MYC expression sensitizes medulloblastoma cells to radio- and chemotherapy and has no impact on response in medulloblastoma patients. BMC Cancer 2011;11:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meineke V, Gilbertz KP, Schilperoort K, et al. Ionizing radiation modulates cell surface integrin expression and adhesion of COLO-320 cells to collagen and fibronectin in vitro. Strahlenther Onkol 2002;178:709–14. [DOI] [PubMed] [Google Scholar]
- 27.Stahler C, Roth J, Cordes N, et al. Impact of carbon ion irradiation on epidermal growth factor receptor signaling and glioma cell migration in comparison to conventional photon irradiation. Int J Radiat Biol 2013;89:454–61. [DOI] [PubMed] [Google Scholar]
- 28.Jadhav U, Mohanam S. Response of neuroblastoma cells to ionizing radiation: modulation of in vitro invasiveness and angiogenesis of human microvascular endothelial cells. Int J Oncol 2006;29:1525–31. [PMC free article] [PubMed] [Google Scholar]
- 29.Eke I, Dickreuter E, Cordes N. Enhanced radiosensitivity of head and neck squamous cell carcinoma cells by beta1 integrin inhibition. Radiother Oncol 2012;104:235–42. [DOI] [PubMed] [Google Scholar]
- 30.Maschler S, Wirl G, Spring H, et al. Tumor cell invasiveness correlates with changes in integrin expression and localization. Oncogene 2005;24:2032–41. [DOI] [PubMed] [Google Scholar]
- 31.White DP, Caswell PT, Norman JC. alpha v beta3 and alpha5beta1 integrin recycling pathways dictate downstream Rho kinase signaling to regulate persistent cell migration. J Cell Biol 2007;177:515–25. [DOI] [PMC free article] [PubMed] [Google Scholar]