Abstract
The aim of this study was to evaluate whether the lesion regression rate (ΔLR) based on the Response Evaluation Criteria in Solid Tumors (RECIST) criteria could be used for the prediction of treatment outcome in head and neck squamous cell carcinoma (HNSCC) patients treated with chemoradiotherapy (CRT) compared with FDG PET-CT. A total of 33 patients underwent MRI and PET-CT at pretreatment and at 8 weeks after CRT. We assessed the treatment outcome by analyzing the following parameters: the RECIST criteria, ΔLR, the European Organization for Research and Treatment of Cancer (EORTC) criteria, and pretreatment SUVmax of the primary tumor and node. The correlation between the analysis of the parameters and the results of the long-term follow-up of the patients was determined. The RECIST did not significantly correlate with locoregional control (LRC) or survival. The ΔLR was significantly lower for the lesions with locoregional failure (LRF) than for those with LRC. A threshold ΔLR of 48% revealed a sensitivity of 72.7% and specificity of 77.3% for the prediction of LRF. Progression-free survival (PFS) of patients with ΔLR ≥ 48% was significantly better than that of patients with ΔLR < 48% (P = 0.001), but not overall survival. There was a significant correlation between LRC and the EORTC (P = 0.02). The patients who achieved a complete response by the EORTC criteria showed significantly better PFS and overall survival (P = 0.01 and 0.04, respectively). The ΔLR was inferior to FDG PET-CT with respect to the prediction of patient survival; however, it may be useful for selecting patients in need of more aggressive monitoring after CRT.
Keywords: head and neck cancer, chemoradiotherapy, treatment outcome, RECIST, PET-CT
INTRODUCTION
During the past two decades, organ preservation strategies using chemoradiotherapy (CRT) have become an important treatment approach for locally advanced head and neck squamous cell carcinoma (HNSCC). However, the disease control achieved with CRT remains heterogeneous, since the treatment responses and clinical outcomes may differ among patients whose clinical staging and treatment method are the same.
For patients with persistent disease following CRT, salvage surgery to the primary site and/or a neck dissection has curative potential. However, salvage surgery to the primary site and/or a neck dissection post-CRT are associated with significant morbidity [1, 2]. Therefore, the accurate estimation of the treatment outcome, including differentiation between responders and non-responders, will contribute to appropriate patient management and the avoidance of unnecessary surgical intervention in patients who have been successfully treated.
Evaluations of HNSCC patients' responses to CRT have routinely used both clinical examinations and anatomical imaging modalities such as CT and/or MRI. However, post-CRT changes make the interpretation of images difficult, since the anatomical images have distinct limitations in the accurate identification of viable tumors within residual masses, the identification of sub-centimeter residual tumors in normal-sized lymph nodes, and the characterization of secondary enlarged inflammatory lymph nodes [3]. However, in HNSCC, there have been several reports showing a significant association between changes in primary tumor size at the completion of chemotherapy and/or radiotherapy and treatment outcome [4–6]. In addition, the RECIST criteria, which rely solely on changes in the size of the primary tumor and lymph nodes based on CT or MRI, have become widely recognized. The RECIST has been the gold standard in evaluating tumor response in routine oncology practice [7, 8]. However, in our knowledge, there has been no study evaluating the usefulness of the lesion regression rate (ΔLR) based on RECIST criteria for the prediction of treatment outcome in HNSCC patients treated with CRT.
In many HNSCC studies, PET-CT has been identified as a valuable imaging biomarker to assess treatment response and long-term survival [9–13]. The most widely available PET tracer is 18fluorine fluorodeoxyglucose (FDG), a glucose analog. Regarding approaches for objectively assessing treatment responses using FDG-PET, the European Organization for Research and Treatment of Cancer (EORTC) criteria are based on the standard uptake value (SUV), which has become the standard for the assessment of metabolic tumor response and follow-up [14]. However, FDG uptake in active inflammatory tissue or reactive lymph nodes may occur, and these false-positive findings limit the interpretation of PET imaging post-chemotherapy and/or radiotherapy [15, 16]. Moreover, PET-CT is costly and administers a high dose of radiation.
The aim of this study was to evaluate whether the ΔLR and the treatment response categories based on RECIST criteria could be used for the prediction of treatment outcome in HNSCC patients treated with CRT compared with FDG PET-CT.
MATERIALS AND METHODS
Patient population
This prospective study was approved by the Committee on Clinical Study at our institution, and all patients signed informed consent forms before the MRI and PET-CT studies. The study population consisted of patients with histologically confirmed primary HNSCC who were treated with chemoradiotherapy between January 2008 and July 2011 at our institution. The inclusion criteria were: no previous treatment history (primary case); tumor volume and site appropriate to CRT with curative intent; no history of radiotherapy in the head and neck region; performance status of 0–1 (Eastern Cooperative Oncology Group scale); age ≤80 years. The exclusion criteria were active invasive malignancies in the 3 years leading up to protocol entry, distant metastases, and serious complications: active infectious disease, interstitial pneumonia, cardiac failure, renal failure, liver dysfunction. A total of 33 patients who met these criteria were enrolled. The patient characteristics are displayed in Table 1.
Table 1.
Patient characteristics
| Characteristics | No. of patients (n = 33) |
|---|---|
| Age | |
| Median | 67.6 |
| Range | 50–80 |
| Sex, male/female | 30/3 |
| Tumor location | |
| Oropharynx | 7 |
| Hypopharynx | 12 |
| Larynx | 11 |
| Oral cavity | 3 |
| T stage (UICC) | |
| T2 | 12 |
| T3 | 12 |
| T4 | 9 |
| N stage (UICC) | |
| N0 | 12 |
| N1 | 3 |
| N2 | 16 |
| N3 | 2 |
| Stage (UICC) | |
| II | 4 |
| III | 9 |
| IV | 20 |
UICC = Union Internationale Contre le Cancer.
Therapeutic regimen for chemoradiotherapy
All patients underwent concurrent CRT. External radiotherapy was administered in 2-Gy daily standard fractions using 4-MV X-rays. The use of CT-based 3D conformal radiotherapy was mandatory. The gross tumor volume and the bulky lymph nodes were treated with up to 60–70 Gy (median: 68.4 Gy). A prophylactic nodal area was irradiated with up to 40–50 Gy (median: 44.6 Gy). Patients received concurrent chemotherapy using S-1 and cisplatin; S-1 at the dose of 60 mg/m2 for 3 weeks followed by 1 week of rest plus weekly cisplatin at the dose of 30 mg/m2 for 3 weeks followed by 1 week of rest (n = 28) or cisplatin 100 mg/m2 at weeks 1 and 4 (n = 5). The chemotherapy was repeated every four weeks for two courses.
Imaging examination and analysis
Pretreatment MRI (MRIbaseline) and PET-CT (PETbaseline) were obtained within the 4 weeks prior to the start of treatment, and the second MRI (MRIfollow) and PET-CT (PETfollow) were obtained at 8 weeks after the completion of treatment in each patient.
MR imaging
MR imaging was performed using a 1.5-T system (Avant; Siemens Medical System, Erlangen, Germany) with a neck coil or a neurovascular coil. T2-weighted axial and coronal images were acquired using a fast spin-echo sequence (TR/TE = 4000/90 ms, 512 × 256 matrix), and T1-weighted axial and coronal images were acquired using a spin-echo sequence (TR/TE = 630/12 ms, 512 × 256 matrix). T1-weighted images were repeated after administration of 0.1 mmol/kg of body weight of gadopentetate dimeglumine (Magnevist; Schering, Berlin, Germany) in the axial and coronal planes. The field of view was 25 cm and the slice thickness was 6 mm.
The target lesions of the primary tumor and lymph nodes were defined as lesions that could be measured only unidimensionally, as defined by RECIST version 1.1 [8]. In this study, by RECIST guideline, the minimum size of measurable target lesions was 12 mm because of the slice thickness of 6 mm on MRI, but measurable target lymph node size was ≥15 mm. The sum of the unidimensional measurements (UMs) of the target lesions in each patient was calculated in MRIbaseline and MRIfollow. Then, the change in the sum of the UMs of the target lesions was calculated in terms of the ΔLR using the following formula:
On the basis of the RECIST criteria, ΔLR was used to classify the treatment response as: complete response (CR) = disappearance of all target lesions; partial response (PR) = reduction in ΔLR ≧30%; stable disease (SD) = reduction in ΔLR <30% to increase in ΔLR <20%; and progressive disease (PD) = increase in ΔLR ≧20% and absolute increase of ≧5 mm or one or more new lesions was observed.
FDG PET-CT
All patients fasted at least 6 h before the PET-CT examination. PET/CT (Biograph Sensation 16; Siemens Medical Systems, Erlangen, Germany) imaging was initiated 60 min after an intravenous injection of FDG (250–300 MBq). CT images were matched to the pixel size of the PET data in order to match the in-slice resolution of the PET emission images. Without changing the patient position, a whole-body PET emission scan was performed over the same area as was covered by CT with six bed positions. The protocol comprised an emission scan with 3 min/bed position. PET images were reconstructed using CT attenuation maps. PET-CT images were analyzed on a dedicated workstation.
All PET-CT images were evaluated by a semi-quantitative analysis of the primary tumor and lymph node lesions on PET using the parameter SUVmax, which was measured as the maximum value of SUV in each voxel within the volume of interest drawn on the lesions. The primary tumor and lymph node with the highest SUVmax were chosen as target lesions at PETbaseline and subsequently analyzed at PETfollow. SUVmax measurement from the target lesions were summed on each PET-CT scan, giving ΣSUVmax. The metabolic response was calculated as ΔΣSUVmax using the following formula:
On the basis of the EORTC criteria [14], ΔΣSUVmax was used to classify the treatment response as: CR = FDG uptake within the lesion was indistinguishable from that of the surrounding normal tissue; PR = reduction in the ΔΣSUVmax >25%; SD = reduction in the ΔΣSUVmax ≤ 25% up to an increase in the ΔΣSUVmax < 25%; PD = increase in the ΔΣSUVmax ≥ 25% or FDG uptake in one or more new lesions, or when a visible increase in the extent of FDG uptake was observed.
Patient follow-up
After the treatment, patients were followed up to evaluate the locoregional control (LRC) by clinical examination and a pan-endoscopy, followed by biopsy in cases of suspected residual disease. In addition, routine follow-up contrast-enhanced CT and MRI were performed every 6 months. Locoregional failure (LRF) was defined as a persistent or recurrent primary lesion or adenopathy during the follow-up period consisting of either histopathological proof or clinically suspected locoregional recurrence resulting in a clinical assessment and an increase in lesion size on serial CT or MRI examinations.
The progression-free survival (PFS) period was defined as the time between assignment and disease progression, death, or last known follow-up, and the overall survival (OS) period was defined as the time from registration until death from any cause. The median follow-up period for all patients was 32.0 months (range: 10–64 months); the median follow-up of the survivors (no evidence of disease) was 35.1 months (range: 24–64 months).
Statistical analysis
The treatment responses assessed by RECIST and EORTC criteria were correlated with LRC using the Pearson's chi-square test to determine statistical significance. We estimated the PFS and OS by the Kaplan–Meier method followed by the log-rank test to evaluate the differences in patient survival between the CR and non-CR groups assessed by RECIST and EORTC. The pretreatment SUVmax in the primary tumor (pre T-SUVmax), the pretreatment SUVmax in the metastatic node (pre N-SUVmax), and the ΔLR were compared for LRC and LRF using the Mann–Whitney U-test. In addition, the correlation between the ΔLR and the sum of UMs at MRIbaseline was evaluated in order to ascertain the whether the sum of UMs might be the confounding factor of the treatment outcome.
We performed a receiver operating characteristic (ROC) analysis with the area under the curve (AUC) to investigate the discriminatory capability of variables as indicators distinguishing LRF from LRC. For the calculation of the sensitivity and specificity of the significant predictive value of variables for LRF, the optimal threshold was determined by giving equal weighting to sensitivity and specificity on the ROC curve.
To determine the usefulness of variables for the prediction of prognosis after CRT, we compared the PFS and OS for the two groups divided by the optimal threshold value, using the Kaplan–Meier method followed by the log-rank test.
Statistical calculations were performed using statistical analysis software (SPSS, version 15.0; SPPS Inc., Chicago, IL), and P-values <0.05 were considered significant.
RESULTS
Treatment outcomes
During the follow-up period, complete LRC was achieved in 22 of the 33 patients (66.7%), and the remaining 11 patients showed LRF. Six of the 33 patients (18.2%) developed an isolated local recurrence. Three of the 33 patients (9.1%) developed a regional recurrence without primary tumor recurrence. Two of the 33 patients (6.1%) developed a simultaneous locoregional tumor recurrence. Another two patients showed lung metastasis. Patients with regional recurrence were treated with neck dissection with adjuvant chemotherapy. For the patients who were inoperable, only chemotherapy was performed. Eight of the 33 patients (24.2%) died during the follow-up period because of the extent of the local recurrent tumor.
Treatment response assessment by EORTC and RECIST
In the RECIST, 8 patients were assessed as achieving a CR, and 25 patients were assessed as achieving a PR after treatment. In contrast, in the EORTC, 20 patients were assessed as achieving a CR, 7 patients as achieving a PR, and 6 patients as showing SD after treatment. There was poor agreement between the response evaluations using the RECIST and EORTC (weighted kappa: 0.02), which were identical in only 33.3% of the patients.
In our evaluation of the correlations among LRC and response assessment by RECIST and EORTC (Table 2), a significant correlation was found between LRC and response assessment by EORTC (P = 0.02). In addition, for the prediction of LRF, CR by EORTC had a sensitivity of 61.5%, specificity of 85%, positive predictive value (PPV) of 72.7%, and negative predictive value (NPV) of 77.3%, respectively. In contrast, there was no significant correlation between LRC and response assessment by RECIST. The corresponding PFS and OS curves in the patients with and without CR by EORTC are shown in Figs 1and 2. The patients with CR by EORTC had a significantly better PFS compared with those without CR by EORTC (P = 0.01). Moreover, the patients with CR by EORTC had a significantly better OS compared with those without CR by EORTC (P = 0.04). However, there was no significant difference in PFS and OS between the patients with and without CR by RECIST.
Table 2.
Assessment of treatment response and correlation with locoregional control
| Assessment of treatment response | Number of patients | Number of patients with LRC (rate %) | P-value |
|---|---|---|---|
| RECIST | |||
| CR | 8 | 6 (75.0) | 0.89 |
| Non-CR | 25 | 16 (64.0) | |
| EORCT | |||
| CR | 20 | 17 (85.0) | 0.02 |
| Non-CR | 13 | 5 (38.5) |
CR = complete response, Non-CR = non-complete response, LRC = locoregional control.
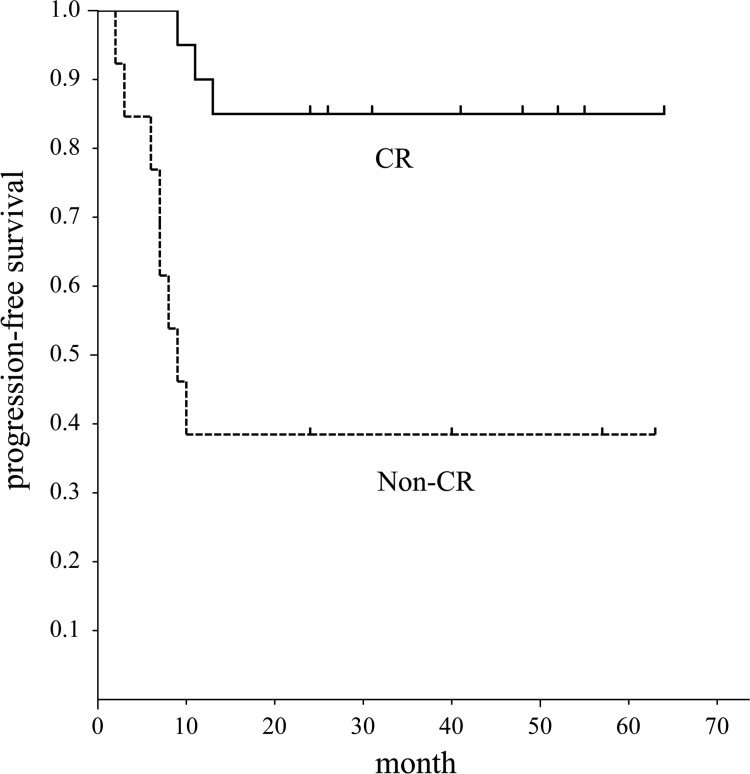
Fig. 1.
Progression-free survival of patients with head and neck squamous cell carcinoma assessed by EORTC criteria. Graph shows progression-free survival period of patients with CR was significantly better than that of patients without CR (P = 0.01).
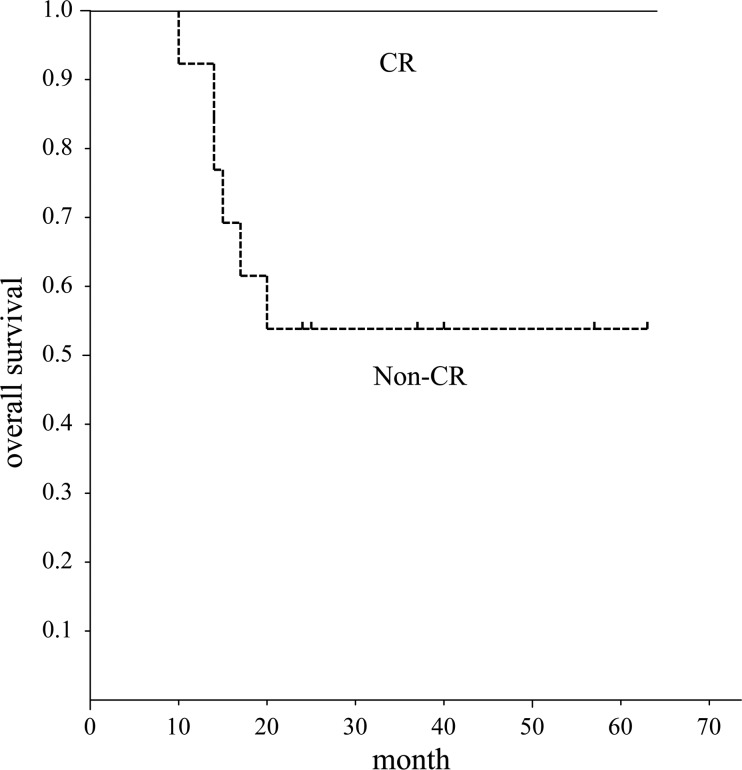
Fig. 2.
Overall survival of patients with head and neck squamous cell carcinoma assessed by EORTC criteria. Graph shows overall survival period of patients with CR was significantly better than that of patients without CR (P = 0.04).
Associations between variables and locoregional control
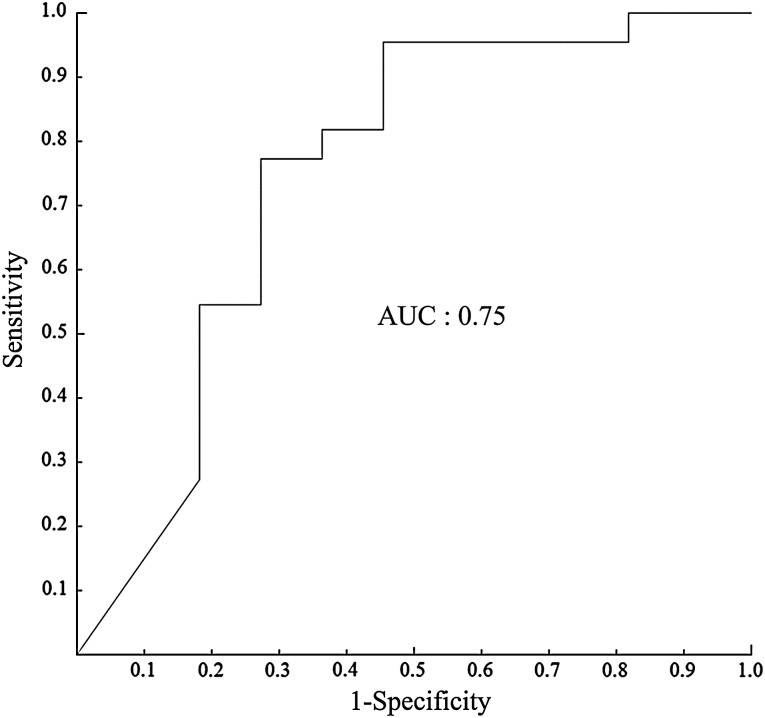
The ΔLR were significantly lower for the lesions with LRF than those with LRC, but there was no significant difference between the lesions with LRC and those with LRF in pre T-SUVmax, or pre N-SUVmax (Table 3). There was no significant correlation between the ΔLR and the sum of UMs at MRIbaseline (Fig. 3). The result of the ROC analysis for the discriminate capability of ΔLR as indicator for distinguishing LRF from LRC showed that the AUC value of ΔLR was 0.75 (Fig. 4). The feasible threshold value for distinguishing LRF from LRC of ΔLR was 48%. When this threshold value was adopted, sensitivity, specificity, PPV, and NPV of ΔLR was 72.7%, 77.3%, 61.4% and 85.0%, respectively.
Table 3.
Comparison of variables between locoregional control and failure
| LRC | LRF | P value | |
|---|---|---|---|
| Pre T-SUVmax | 11.58 ± 4.47 | 13.59 ± 3.64 | NS |
| Pre N-SUVmax | 6.75 ± 2.85 | 8.62 ± 3.38 | NS |
| ΔLR | 0.60 ± 0.26 | 0.39 ± 0.10 | 0.02 |
Data are expressed as mean ± S.D. NS = P-value > 0.05, LRC = locoregional control, LRF = locoregioinal failure, Pre T-SUVmax = pretreatment SUVmax in primary tumor, Pre N-SUVmax = pretreatment SUVmax in metastatic node, ΔLR = lesion regression rate.
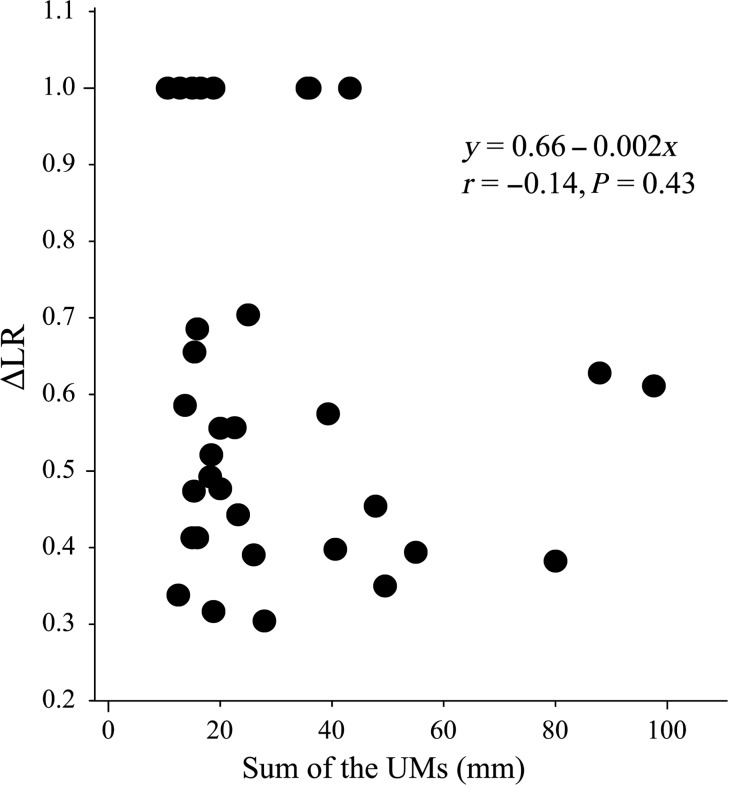
Fig. 3.
The correlation between the lesion regression rate (ΔLR) and sum of the unidimensional measurements (UMs) of target lesions at MRI baseline. There was no significant correlation between them.
Fig. 4.
ROC curve using the lesion regression rate (ΔLR) as indicator for distinguishing locoregional failure from locoregional control. Area under the curve is 0.75, and the best cut-off value is 0.48.
Progression-free survival and overall survival
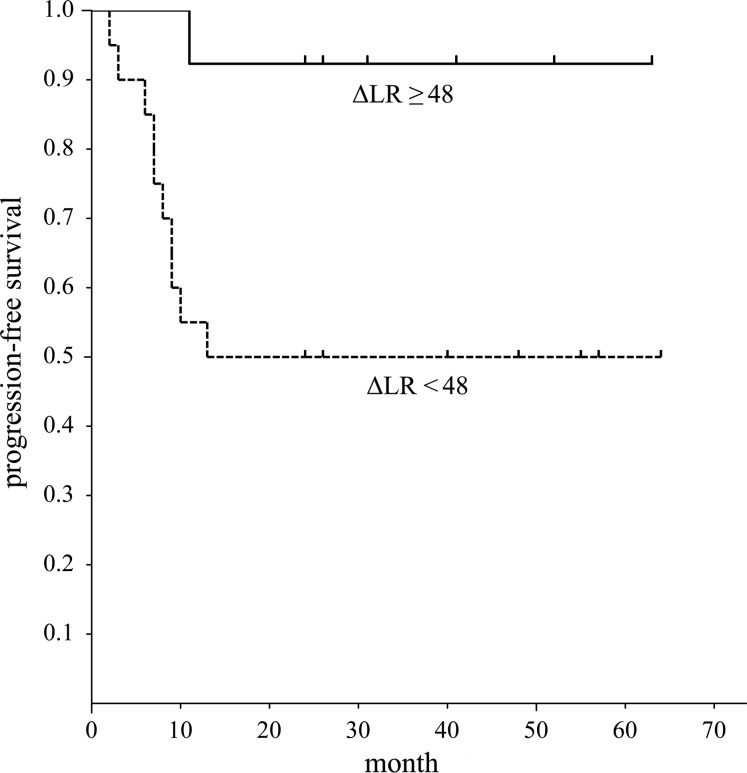
The difference in PFS between the two groups divided by the threshold values of ΔLR (Fig. 5) was significant (P = 0.001). But there was no significant difference in OS between the two groups divided by the threshold value of ΔLR.
Fig. 5.
Progression-free survival of patients with head and neck squamous cell carcinoma assessed by the lesion regression rate (ΔLR). Graph shows the progression-free survival period of patients with ΔLR ≥48(%) was significantly better than that of patients with ΔLR <48(%) (P = 0.001).
DISCUSSION
In this study, we evaluated the correlation between RECIST and LRC, and we found no statistically significant correlation between them. In addition, there was no significant difference in PFS and OS between the patients with and without CR by RECIST. On the other hand, with respect to EORTC, the concordance with treatment response evaluations using the EORTC and RECIST was found to be in poor agreement in this study. Furthermore, we observed that among patients with locally advanced HNSCC, the EORTC at 8 weeks after the completion of CRT was significantly correlated with LRC, and the patients who achieved a CR as assessed by EORTC showed significantly better prognoses in PFS and OS compared with the non-CR patients by EORTC. Our results are comparable with those of several previous reports. Xie et al. [9] reported that their patients with nasopharyngeal carcinoma who did not show any abnormal FDG uptake at 1–5 months after treatment had significantly higher OS and disease-free survival (DFS) rates compared with the patients with persistent abnormal FDG uptake. Passero et al. [10] reported that HNSCC patients with disappearance of FDG uptake attributable to malignancy on PET-CT at 8 weeks after treatment showed significantly better PFS than those with persistent abnormal FDG uptake. Therefore, a negative FDG uptake on PET-CT after CRT may be a powerful predictor of outcome in patients with HNSCC, and PET-CT may be indicated for response evaluation in this setting to improve the accuracy of post-treatment assessment by tumor size changes.
However, several previous studies showed a significant association between tumor regression at the completion of radiotherapy/chemotherapy and the probability of local control. Jaulerry et al. [4] reported that the probability of local relapse was significantly associated with the tumor regression rate at 5 weeks after the start of radiotherapy in HNSCC patients. Clavel et al. [5] reported that a regression of the tumor diameter of ≥80% and a residual largest diameter of 15 mm of nodes at 6 to 8 weeks after CRT in patients with node-positive HNSCC showed negative pathologic values of 100% and 86%, respectively. Bhatia et al. [6] reported that tumor volume reduction during and after CRT in HNSCC patients was significantly associated with local failure, and 6 weeks after CRT, volume reduction (<35%) achieved 100% PPV for local failure.
In the present study, we assessed whether the ΔLR based on RECIST criteria can be used to obtain prognostic information. We found that the ΔLR was significantly lower for the patients with LRF compared with those with LRC. In addition, the PFS of the two groups divided by the threshold value of 48% of ΔLR for distinguishing LRF from LRC showed a significant difference, and PFS of patients with ΔLR ≥48% was significantly better than that with ΔLR <48%. However, the difference in OS between the two groups divided by the ΔLR threshold as 48% was not significant. The ΔLR was defined as the change in the sum of the unidimensional measurements of all target lesions. Therefore, it was improbable that the ΔLR might be associated with various cellular characteristics such as cell viability and proliferative activity. For this reason, it was thought that the ΔLR did not show significant association with OS. However, it was suggested that the ΔLR may be a valid clinical factor to prognose LRC and PFS after CRT in patients with HNSCC. In addition, the ΔLR using MRI may be of advantage with respect to the cost and radiation exposure in comparison with PET-CT.
Many investigators have demonstrated a significant impact of primary tumor volume on treatment outcome in HNSCC patients after radiotherapy or CRT [17, 18]. Tumor volume is generally calculated by a summation-of-area technique, and the contouring of tumor boundaries depends on a hand-drawn region of interest on CT or MRI. Therefore, tumor volume may be overestimated owing to the inadvertent inclusion of peri-tumoral inflammation and/or edema. For this reason, we did not evaluate volumetric analysis. On the other hand, the unidimensional index recommended in RECIST may be an easily measurable parameter that can estimate the therapeutic response of both primary tumor and metastatic nodes.
Early prediction of potential treatment failures during or shortly after CRT is an important goal, as salvage surgery is the only subsequent curative option and should be performed early to prevent the residual tumor from becoming surgically unresectable. Several previous studies pointed out that FDG PET and PET-CT after CRT have a high NPV and specificity for excluding residual locoregional disease [19, 20]. In this study, sensitivity, specificity, PPV and NPV of CR by EORTC for the prediction of LRF were 61.5%, 85%, 72.7% and 77.3%, respectively. On the other hand, those of optimal threshold of the ΔLR for the prediction of LRF were 72.7%, 77.3%, 61.4% and 85%, respectively. Therefore, although the ΔLR was inferior to CR by EORTC in specificity and PPV for the prediction of LRF, the ΔLR demonstrated a high NPV when predicting LRF after CRT and seemed to be superior to CR by EORTC in NPV. Therefore, it was thought that ΔLR does not have inferiority to FDG PET-CT in its value for predicting LRF of HNSCC after CRT. In addition, the ΔLR might be useful in clinical practice for identifying a subset of patients that is likely to have LRF on the basis of the 8 weeks scan; for these patients, more aggressive monitoring is justified.
Allal et al. [11, 12] reported that the pretreatment SUVmax was correlated with LRC and DFS, and that the pretreatment SUVmax was an independent prognostic factor in HNSCC patients. In addition, in a meta-analysis on the predictive value of pretreatment SUVmax measurements, Xie et al. [13] found that in comparison with patients with a high SUVmax, patients with a low SUVmax had a reduced risk of progression, death and recurrence by 77, 76 and 73%, respectively. These results indicated that high primary tumor SUVmax can serve as a prognostic marker in patients with HNSCC, with higher values correlating with poorer outcomes. However, in the present study, the pretreatment SUVmax values of the primary tumor and nodes did not show a significant association with LRC. Similarly, several previous studies reported that pretreatment SUVmax of primary and/or adenopathy did not correlate with tumor recurrence [21, 22]. Therefore, a single measurement of pretreatment SUVmax may not be sufficient to predict the degree of treatment-induced metabolic damage. It was thought that the change in the SUVmax before and after CRT might be important for predicting the LRC or patient's survival.
Recently, there have been a few studies evaluating the prognostic value of early FDG PET-CT in patients with HNSCC treated with CRT. Castaldi et al. [23] evaluated the prognostic value of early (after 2 weeks of treatment) and late (8–12 weeks after treatment) PET-CT in patients with HNSCC treated with CRT, and they found that the late FDG uptake reduction in the primary tumor and metastatic nodes was significantly correlated with both relapse-free survival and disease-specific survival. However, the predictive role of the early metabolic response was not confirmed. In contrast, Hentschel et al. [24] reported that the decrease in SUVmax from pretreatment to Weeks 1 and 2 of CRT might be a potential prognostic marker for HNSCC patients. It was thus thought that the predictive value of the early change in the FDG uptake of interim PET-CT may be contradictory in HNSCC patients treated with CRT.
There are limitations to our study. First, the patient population was relatively small and heterogeneous, including tumors from various head and neck sites. Therefore, further studies with a large number of patients without potential selection bias are needed. Second, biological differences in squamous cell carcinomas due to differences in the patients' smoking and alcohol use, as well as due to molecular markers such as epidermal growth factor receptor expression and human papillomavirus infection, have been suggested as prognostic factors. In particular, human papillomavirus-positive oropharyngeal carcinoma has emerged as a new entity with an excellent overall survival rate [25], but the patients in the present study were not tested for human papillomavirus infection.
In conclusion, the results of this study demonstrated that the ΔLR based on RECIST after 8 weeks of CRT in patients with HNSCC may be a valid clinical factor for providing prognostic information and may be useful for selecting patients in need of more aggressive monitoring after CRT. However, the ΔLR seemed to be inferior to FDG PET-CT with respect to the prediction of a patient's survival.
FUNDING
This work was supported by the Japan Society for the Promotion of Science KAKENHI (23591801).
REFERENCES
- 1.Ferlito A, Corry J, Silver CE, et al. Planned neck dissection for patients with complete response to chemoradiotherapy: a concept approaching obsolescence. Head Neck 2010;32:253–61. [DOI] [PubMed] [Google Scholar]
- 2.Esteller E, Vega MC, Lopez M, et al. Salvage surgery after locoregional failure in head and neck carcinoma patients treated with chemoradiotherapy. Eur Arch Otorhinolaryngol 2011;268:295–301. [DOI] [PubMed] [Google Scholar]
- 3.McCabe KJ, Rubinstein D. Advances in head and neck imaging. Otolaryngol Clin North Am 2005;38:307–19. [DOI] [PubMed] [Google Scholar]
- 4.Jaulerry C, Dubray B, Brunin F, et al. Prognostic value of tumor regression during radiotherapy for head and neck cancer: a prospective study. Int J Radiat Oncol Biol Phys 1995;33:271–9. [DOI] [PubMed] [Google Scholar]
- 5.Clavel S, Charron MP, Belair M, et al. The role of computed tomography in the management of the neck after chemoradiotherapy in patients with head and neck cancer. Int J Radiat Oncol Biol Phys 2012;82:567–73. [DOI] [PubMed] [Google Scholar]
- 6.Bhatia KSS, King AD, Yu KH, et al. Does primary tumor volumetry performed early in the course of definitive concomitant chemoradiotherapy for head and neck squamous cell carcinoma improve prediction of primary site outcome? Br J Radiol 2010;83:964–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Therasse P, Eisenhauer EA, Verweij J. RECIST revisited: a review of validation studies on tumour assessment. Eur J Cancer 2006;42:1031–9. [DOI] [PubMed] [Google Scholar]
- 8.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumor: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 9.Xie P, Yue JB, Fu Z, et al. Prognostic value of 18F-FDG PET/CT before and after radiotherapy for locally advanced nasopharyngeal carcinoma. Ann Oncol 2010;21:1078–82. [DOI] [PubMed] [Google Scholar]
- 10.Passero VA, Branstetter BF, Shuai Y, et al. Response assessment by combined PET-CT scan versus CT scan alone using RECIST in patients with locally advanced head and neck cancer treated with chemoradiotherapy. Ann Oncol 2010;21:2278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allal AS, Dulguerov P, Allaoua M, et al. Standardized uptake value of 2-[18F] fluoro-2-deoxy-D-glucose in predicting outcome in head and neck carcinoma treated by radiotherapy with or without chemotherapy. J Clin Oncol 2002;20:1398–404. [DOI] [PubMed] [Google Scholar]
- 12.Allal AS, Slosman DO, Kebdani T, et al. Prediction of outcome in head-and-neck cancer patients using the standardized uptake value of 2-[18F]fluoro-2-deoxy-D-glucose. Int J Radiat Oncol Biol Phys 2004;59:1295–300. [DOI] [PubMed] [Google Scholar]
- 13.Xie P, Li M, Zhao H, et al. 18F-FDG PET or PET-CT to evaluate prognosis for head and neck cancer: a meta-analysis. J Cancer Res Clin Oncol 2011;137:1085–93. [DOI] [PubMed] [Google Scholar]
- 14.Young H, Baum R, Cremerius U, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer 1999;35:1773–82. [DOI] [PubMed] [Google Scholar]
- 15.Chen AY, Vilaseca I, Hudgins PA, et al. PET-CT vs contrast-enhanced CT: what is the role for each after chemoradiation for advanced oropharyngeal cancer? Head Neck 2006;28:487–95. [DOI] [PubMed] [Google Scholar]
- 16.Malone JP, Gerberi MAT, Vasireddy S, et al. Early prediction of response to chemoradiotherapy for head and neck cancer. Arch Otolaryngol Head Neck Surg 2009;135:1119–25. [DOI] [PubMed] [Google Scholar]
- 17.Knegjens JL, Hauptmann M, Pameijier FA, et al. Tumor volume as prognostic factor in chemoradiation for advanced head and neck cancer. Head Neck 2011;33:375–82. [DOI] [PubMed] [Google Scholar]
- 18.Rutkowski T. The role of tumor volume in radiotherapy of patients with head and neck cancer. Radiat Oncol 2014;9:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCabe KJ, Rubinstein D. Advances in head and neck imaging. Otolaryngol Clin North Am 2005;38:307–19. [DOI] [PubMed] [Google Scholar]
- 20.Ong SC, Schoder H, Lee NY, et al. Clinical utility of 18F-FDG PET/CT in assessing the neck after concurrent chemoradiotherapy for locoregional advanced head and neck cancer. J Nucl Med 2008;49:532–40. [DOI] [PubMed] [Google Scholar]
- 21.Greven KM, Williams DW, III, McGuirt WF, et al. Serial positron emission tomography scans following radiation therapy with head and neck cancer. Head Neck 2001;23:942–6. [DOI] [PubMed] [Google Scholar]
- 22.Vemon MR, Maheshwari M, Schultz CJ, et al. Clinical outcomes of patients receiving integrated PET/CT-guided radiotherapy for head and neck carcinoma. Int J Radiat Oncol Biol Phys 2008;70:678–84. [DOI] [PubMed] [Google Scholar]
- 23.Castaldi P, Rufini V, Bussu F, et al. Can “early” and “late” 18F-FDG PET-CT be used as prognostic factors for the clinical outcome of patients with locally advanced head and neck cancer treated with radio-chemotherapy? Radiother Oncol 2012;103:63–8. [DOI] [PubMed] [Google Scholar]
- 24.Hentschel M, Appold S, Schreiber A, et al. Early FDG PET at 10 or 20 Gy under chemoradiotherapy is prognostic for locoregional control and overall survival in patients with head and neck cancer. Eur Nucl Mol Imaging 2011;38:1203–11. [DOI] [PubMed] [Google Scholar]
- 25.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus: positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 2008;100:261–9. [DOI] [PubMed] [Google Scholar]