Abstract
With the widespread use of radio-frequency devices, it is increasingly important to understand the biological effects of the associated electromagnetic fields. Thus, we investigated the effects of radio-frequency electromagnetic fields (RF-EMF) on T cell responses during development due to the lack of science-based evidence for RF-EMF effects on developmental immune systems. Sprague Dawley (SD) rats were exposed to 2.14-GHz wideband code division multiple-access (W-CDMA) RF signals at a whole-body specific absorption rate (SAR) of 0.2 W/kg. Exposures were performed for a total of 9 weeks spanning in utero development, lactation and the juvenile period. Rats were continuously exposed to RF-EMF for 20 h/day, 7 days/week. Comparisons of control and exposed rats using flow cytometry revealed no changes in the numbers of CD4/CD8 T cells, activated T cells or regulatory T cells among peripheral blood cells, splenocytes and thymocytes. Expression levels of 16 genes that regulate the immunological Th1/Th2 paradigm were analyzed using real-time PCR in the spleen and thymus tissues of control and RF-EMF–exposed rats. Although only the Il5 gene was significantly regulated in spleen tissues, Il4, Il5 and Il23a genes were significantly upregulated in thymus tissues following exposure to RF-EMF. However, ELISAs showed no changes in serum IL-4 protein concentrations. These data indicate no adverse effects of long-term RF-EMF exposure on immune-like T cell populations, T cell activation, or Th1/Th2 balance in developing rats, although significant transcriptional effects were observed.
Keywords: RF-EMF exposure in vivo, developmental stage, T cell subset analyses, Th1/Th2 balance
INTRODUCTION
The use of wireless communication technology has increased significantly in recent years, raising public concerns about the health risks of radio-frequency electromagnetic fields (RF-EMF) from mobile phone systems, and prompting studies of the biological effects of RF-EMF exposure. However, Vijayalaxmi and Guenter suggest that DNA strand breaks or chromosomal aberrations in mammalian somatic cells exposed to RF-EMF are artifacts of various exposures and conditions, and reflect inaccurate assessments [1]. Thus, reproducible and accurate assessments are required to determine the effects of RF-EMF exposure. In the present study, we prepared an apparatus that uniformly emits high-power RF-EMF and calculated whole-body specific absorption rates (SARs) using dosimetric estimates in animals.
Although potentially harmful effects of RF-EMF exposure have been investigated in several animal models, rodent and human cell lines, and human peripheral blood lymphocytes [1–3], significant adverse effects have not been observed in many studies [1, 4–10]. Moreover, the biological effects of RF-EMF exposure have only been assessed in adult animals, and few studies have assessed immunological effects following in utero and postnatal exposure, despite recurrent concerns about possible adverse effects in children, and in embryos and fetuses during pregnancy [11]. The WHO indicated a high priority for in vivo studies ‘investigating effects from exposure of immature animals to RF fields on the development of hematopoietic and immune systems using functional, morphological, and molecular endpoints’ in the 2010 WHO Research Agenda, http://whqlibdoc.who.int/publications/2010/9789241599948_eng.pdf?ua=1. As for primer3, last accessed date is on March 20, 2015. Former studies have reported varying results regarding natural killer cell activity [12, 13], and immune functions such as phagocytosis and lymphocyte proliferation are frequently controversial or not confirmed in replicated experiments [9, 10, 14]. Humoral responses of functional mature B cells and antibody production in mice suggest a lack of effect of low-level RF-EMF exposure [10, 14, 15]. However, most of these immunological studies were performed in cells or adult rodent models. In contrast, while few studies eliminate the effects of RF-EMF exposure on T-cell function in vivo, various immune effects of RE-EMF exposure have been observed in lymphocyte subpopulations [9, 15–17]. Because T cell activation and differentiation play critical roles in immune system development during mammalian embryonic and postnatal stages, evaluation of the effects of RF-EMF exposure on T cell functions is warranted.
The Th1/Th2 paradigm provides a framework for understanding T cell biology and the interplay of innate and adaptive immunity. While Th1 cells regulate cell-mediated immunity, Th2 cells regulate humoral immunity [18, 19], and dysfunctions of the Th1/Th2 balance contribute to autoimmune [20], chronic inflammatory [21], and allergic diseases [22]. Several lines of evidence indicate that Th1, Th17 [23], and regulatory T cell (Treg) responses [24] control the induction and progression of autoimmune diseases. Therefore, the effects of continuous low-level RF-EMF exposure on T cell functions may reflect changes in T cell subsets in the Th1/Th2 paradigm during mammalian development. In particular, gene expression analyses of the immunotoxic effects of RF-EMF on Th1/Th2 immunity indicate that external factors, such as chemical products, play important roles [25], and make it possible to provide detailed quantitative data to supplement qualitative analyses like flow cytometry. Until now, transcriptional analyses have not satisfactorily investigated the immunological effects of RF-EMF in vivo.
In the present study, the immunological effects of RF-EMF were examined in rats using the analyses of T cell differentiation and activation and Th1/Th2 balance throughout neonatal, postnatal and juvenile phases. We especially focused on the transcriptional change associated with regulation of Th1/Th2 balance in rats following RF-EMF exposure compared with control rats. Freely moving rats were exposed to 2.14 GHz W-CDMA RF signals for 20 h/day for 9 weeks. To prevent thermal effects, whole-body average SARs were set at 0.2 W/kg, which is 2.5 times the International Commission on Non-Ionizing Radiation Protection (ICNIRP) guideline for the general public (0.08 W/kg) [26, 27]. Subsequently, immunotoxic effects of RF-EMF on T cell function were assessed at the molecular level using flow cytometry and real time PCR.
MATERIALS AND METHODS
Animals
All animal procedures were approved by the committee for animal experiments at the National Institute of Public Health. Sprague-Dawley (SD) rats were purchased from Japan SLC (Shizuoka, Japan), and 10-week-old female proestrus rats were screened using an Impedance Checker (Muromachi Kikai Co. Ltd, Tokyo, Japan) and were then individually mated with 10-week-old male rats. On the day after mating, individuals were selected, and the presence of sperm was confirmed using vaginal smears and microscopic tests. Pregnant females were individually bred and randomly assigned to either RF-EMF–exposed (n = 12) or sham-exposed (control) groups (n = 12). Rats were housed in acrylic cages in an animal room with a 12-h light/12-h dark cycle. Temperature and relative humidity were maintained at 23 ± 1°C and 50 ± 5%, respectively.
RF-EMF exposure
RF-EMF exposures were performed as described in previous reports [28, 29]. The apparatus comprised two boxes, one for sham exposure (control) and the other for RF-EMF exposure. The insides of the boxes were separated by four acrylic cages arranged in a 90° fan shape. The apparatus uniformly emitted high-power RF-EMF, and computer simulations were used to calculate SAR during the gestational period in one dam, during the lactation period in one dam and eight pups, and in four randomly selected male rats (from the eight pups) during the post-weaning period. A 2.14-GHz W-CDMA signal was emitted for 20 h per day (between 11 a.m. and 12 p.m.). SAR was set to 0.2 W/kg, which is 2.5 times higher than the ICNIRP guideline for the general public, per one dam before birth and per one pup after birth. Cages, bedding material, water and feed were changed during the remaining 4 h.
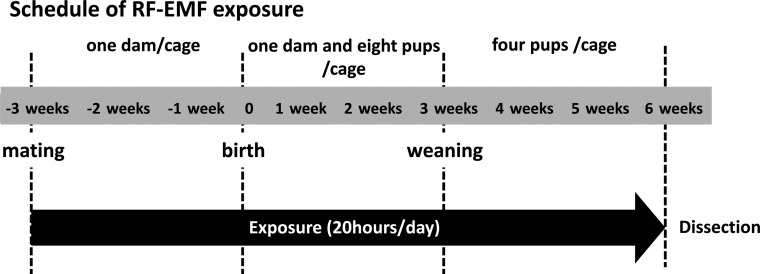
RF-EMF exposure was performed for 63 consecutive days (9 weeks) through embryonic, suckling and weaning stages (Fig. 1). To obtain more accurate SAR estimates from numerical calculations, each acrylic cage contained one dam from successful mating to birth, one dam and eight pups per cage from birth to weaning, and four pups per cage after weaning. Finally, a total of 48 RF-EMF–exposed rats and 48 sham-exposed rats were obtained. Subsequently, 12 rats from different dams were randomly selected from each group, and the remaining animals were used in other experiments. EMF exposure was performed in a specific-pathogen-free animal room with a shielded structure under the conditions described above. Rats were provided food and water ad libitum.
Fig. 1.
Schedule of RF-EMF exposure. Sprague–Dawley (SD) rats were exposed to RF-EMF for a total of 9 weeks. In brief, 10-week-old female proestrus rats were screened using an Impedance Checker after mating with 10-week-old male rats. Accurate calculation of whole-body SARs allowed the exposure of RF-EMF under the following conditions: one dam per cage from mating to birth, one dam and eight pups per cage from birth to weaning, and four pups after weaning.
Sample collection
Rats were dissected under anesthesia at the end of all exposures. Blood samples were collected, and splenocytes and thymocytes were extracted and analyzed using flow cytometry. Blood specimens were mixed with one-fifth volumes of 50-mM ethylenediaminetetraacetic acid (EDTA) solution, and 75-µl blood–EDTA mixtures were hemolyzed using 10-fold volumes of VersaLyse Lysing Solution (Beckman Coulter Inc., Brea, CA, USA), followed by incubation at room temperature for 10 min. Cells were washed twice in phosphate-buffered saline (PBS) and collected by centrifugation. Splenocytes and thymocytes were prepared in Hank's Balanced Salt Solution (HBSS; Thermo Fisher Scientific Inc., Waltham, MA, USA) by mincing spleen or thymus tissues between frosted edges of glass slides (Matsunami Glass Ind. Ltd, Osaka, Japan). Cells were filtered using nylon mesh sheets and washed with HBSS. Both cell types were collected by centrifugation and hemolyzed using VersaLyse Lysing Solution. After resuspension in 100 µl of PBS, cells were stained with labeled antibodies and were analyzed using flow cytometry (see below).
To obtain RNA samples, spleen and thymus tissues were removed from control and exposed rats, later soaked in RNA (Qiagen, Valencia, CA, USA), kept at 4°C for 2–5 days, and then at −80°C until RNA extraction.
Flow cytometry
T cell populations were detected using flow cytometry with the following antibodies: 0.25 μg fluorescein isothiocyanate conjugated anti-rat CD4 antibody (anti-CD4-FITC), 0.2 μg phycoerythrin anti-rat CD8 antibody (anti-CD8-PE), 0.2 μg phycoerythrin-cyanine-7 conjugated anti-rat CD3 antibody (anti-CD3-Cy7), 0.25 μg fluorescein isothiocyanate conjugated anti-rat CD86 antibodies (anti-CD86-FITC), 0.2 μg phycoerythrin conjugated anti-rat CD80 antibody (anti-CD80-PE), 0.2 μg phycoerythrin conjugated anti-rat CD28 antibody (anti-CD28-PE), 0.2 μg peridinin chlorophyll protein complex coupled with fluorochromes from eBioscience (tandem dyes) and conjugated anti-rat CD25 antibody (anti-CD25-PerCP-eFloure®710). All antibodies were purchased from eBioscience Inc. (San Diego, CA, USA) and added to blood, splenocyte and thymocyte (1−10 × 106 cells/test) samples. After incubation at 4°C for 30 min, cells were washed with PBS and collected by centrifugation. Cells were then analyzed using a Cytomics FC500 flow cytometer (Beckman Coulter Inc.). FITC, PE and PerCP-eFloure®710 or PE-Cy7 staining were detected in FL1, FL2 and FL4 channels, respectively. Analyses of CD4/CD8 lineage cells, CD4+CD25+ cells, CD4+CD28+ cells gated from lymphocytes, and CD80+ or CD86+ cells gated from white blood cells were performed in peripheral blood cells (PBCs), splenocytes and thymocytes. All data were analyzed using CXP software (Beckman Coulter Inc.).
Gene expression analysis
Total RNA was extracted from spleen and thymus tissues of 11 control (sham-exposed) rats and 11 RF-EMF–exposed rats using RNeasy Mini Kits (Qiagen, Hilden, Germany). RNA from one exposed rat was excluded from analyses due to poor preservation. RNA was reverse-transcribed using High Capacity cDNA Reverse Transcription Kits (Thermo Fisher Scientific Inc.), and cDNAs were used as template DNA for RT-PCR. Quantitative PCR was performed using a Fast SYBR Green Master Mix (Thermo Fisher Scientific Inc.) and 18 primer sets (Table 1), including 16 for genes involved in Th1/Th2 balance and 2 for the housekeeping genes Gapdh and Actb. All primers were designed using Primer3 (http://frodo.wi.mit.edu/primer3/input.htm) within the amplified fragment length of 150 base pairs and purchased from Exigen Inc., Tokyo, Japan. PCR reactions were performed for 45 cycles of 95°C for 10 s and 58°C for 30 s using a Stratagene Mx3000P QPCR system (Agilent Technologies Inc., Santa Clara, CA, USA). All samples were assayed in triplicates. Expression levels of target genes were normalized to the geometric mean of Gapdh and Actb expression, and relative mRNA levels were determined for each gene using the comparative threshold cycle (CT) method. Values of ΔCT were calculated for each sample by subtracting the mean CT of the two housekeeping genes from that of each gene. Baseline ΔCT was assigned to one control rat. Subsequently, ΔΔCT values were calculated by subtracting the baseline ΔCT from those of experimental samples, and were transformed into absolute values using the equation 2−ΔΔCT.
Table 1.
Primers for 16 genes that are associated with the Th1/Th2 paradigm
| Gene | Forward sequence (5′→3′) | Reverse sequence (5′→3′) |
|---|---|---|
| Il4 | TGATGTACCTCCGTGCTTGA | GTGAGTTCAGACCGCTGACA |
| Stat6 | GCATAGAGGCCTTTCAGCAC | TAGCTCCGAAGACAGCGTTT |
| Gata3 | GCCTGCGGACTCTACCATAA | CACAGGAAGTCCCTGCTCTC |
| Il5 | AAGAGAAGTGTGGCGAAGAG | CAGACTTCCATTGCCCACTC |
| Il10 | GAATTCCCTGGGAGAGAAGC | GCTCCACTGCCTTGCTTTTA |
| Ifng | GCCCTCTCTGGCTGTTACTG | CCAAGAGGAGGCTCTTTCCT |
| Il12a | GACAAAACCAGCACACTGGA | CTACCAAGGCACAGGGTCAT |
| Il12b | GCACAGAAACACGGACTTGA | GACAGAGATGCTCGTCCACA |
| Stat4 | ATCTTCCAATGGGAGCCTCT | GCCCTCGTTTCCTTTACTCC |
| Stat1 | GGAAGCGAAGACAGCAGAGT | GCTCTCTGCAACAATGGTGA |
| Tbx21 | CCGTGACTGCCTACCAGAAT | GTTGACAGTTGGGTCCAGGT |
| Tnf | TGCCTCAGCCTCTTCTCATT | GAGCCCATTTGGGAACTTCT |
| Tgfb1 | TGAGTGGCTGTCTTTTGACG | ACTGAAGCGAAAGCCCTGTA |
| Il6 | CCGGAGAGGAGACTTCACAG | CAGAATTGCCATTGCACAAC |
| Il23a | TCCAGTGTGGTGATGGTTGT | TAGAGAAGGCTCCCCTGTGA |
| Il17a | ACAAGCTCATCCCGTACCAG | AGTACCGCTGCCTTCACTGT |
| Gapdh | ATGACTCTACCCACGGCAAG | TACTCAGCACCAGCATCACC |
| Actb | GCTCTCTTCCAGCCTTCCTT | CGGATGTCAACGTCACACTT |
Enzyme-linked immunosorbent assay (ELISA)
ELISAs were performed with serum from control (n = 8) and RF-EMF–exposed animals (n = 8) using a Rat IL-4 ELISA Kit (RayBiotech Inc., Norcross, GA, USA) according to the manufacturer's protocols. The other four control and RF-EMF–exposed animals could not be detected because these animals were used in other experiments (data not shown).
Statistics
Differences in percentage distributions of CD4+CD8−, CD4−CD8+, CD4+CD8+, CD4−CD8−, CD80+, CD86+, CD4+CD28+ and CD4+CD25+ cells in control (n = 12) and RF-EMF–exposed animals (n = 12) were identified using Student's t test.
Relative mRNA expression levels of the 16 target genes were compared between control (n = 11) and RF-EMF–exposed (n = 11) animals using unpaired Student's t tests (assuming equal variances) or Welch's t test (assuming unequal variances). Meanwhile, the concentration of IL-4 was compared in sera between the control (n = 8) and RF-EMF–exposed (n = 8) animals using Student's t test. Differences were considered significant when P < 0.05 and P < 0.01. Statistical analyses were performed using Excel Statistics 2008 (Social Survey Research Information Inc., Tokyo, Japan).
RESULTS
T cell subpopulation frequencies in flow cytometry
At first, no significant differences in body weight, general hematological assessment or general blood chemistry analyses were observed between the control and RF-EMF–exposed animals (data not shown). These data indicate that RF-EMF exposure under the conditions described did not affect growth or blood cell production in developmental stages.
T cell subpopulation frequencies in flow cytometry were expressed as percentages (Table 2). CD4+CD8−, CD4−CD8+, CD4+CD8+ and CD4−CD8− cells among PBCs, splenocytes and thymocytes were analyzed, and populations of helper T lymphocytes and cytotoxic T lymphocytes were determined. No significant differences in proportions of cell populations were observed among PBCs (P = 0.91, 0.91, 0.71 and 0.58, respectively), splenocytes (P = 0.13, 0.06, 0.62 and 0.41, respectively) or thymocytes (P = 0.38, 0.56, 0.60 and 0.46, respectively). Moreover, no differences in the presence of CD4/CD8 T cell lineages were observed among PBCs, splenocytes or thymocytes from control and RF-EMF–exposed animals.
Table 2.
T cell subpopulation frequencies in flow cytometry
| Blood |
Spleen |
Thymus |
|||||
|---|---|---|---|---|---|---|---|
| Av. (%) | ±S.D. | Av. (%) | ±S.D. | Av. (%) | ±S.D. | ||
| CD4+CD8− | Sham | 66.74 | 3.20 | 40.45 | 3.19 | 55.79 | 4.40 |
| Expose | 66.59 | 2.85 | 42.74 | 3.69 | 54.19 | 3.90 | |
| CD4−CD8+ | Sham | 30.45 | 3.41 | 46.74 | 4.72 | 13.81 | 1.94 |
| Expose | 30.31 | 2.45 | 42.83 | 4.32 | 14.32 | 2.06 | |
| CD4+CD8+ | Sham | 1.85 | 0.43 | 2.16 | 0.23 | 28.70 | 4.64 |
| Expose | 1.90 | 0.23 | 2.11 | 0.24 | 29.72 | 4.26 | |
| CD4−CD8− | Sham | 0.97 | 0.33 | 10.68 | 4.75 | 1.84 | 0.34 |
| Expose | 1.21 | 1.37 | 12.36 | 4.59 | 1.97 | 0.48 | |
| CD4+CD25+ | Sham | 2.03 | 0.35 | 2.25 | 0.35 | 23.11 | 5.30 |
| Expose | 2.01 | 0.45 | 2.28 | 0.27 | 20.38 | 9.08 | |
| CD4+CD28+ | Sham | 39.07 | 3.98 | 21.36 | 3.22 | 90.29 | 0.78 |
| Expose | 40.55 | 6.84 | 21.27 | 2.26 | 90.70 | 1.22 | |
| CD80+ | Sham | 15.50 | 5.12 | 9.83 | 2.54 | 0.23 | 0.09 |
| Expose | 11.42 | 5.02 | 7.85 | 2.13 | 0.29 | 0.08 | |
| CD86+ | Sham | 32.21 | 6.43 | 14.86 | 1.91 | 0.14 | 0.04 |
| Expose | 29.98 | 7.01 | 14.00 | 2.40 | 0.17 | 0.07 | |
No differences in the frequencies of CD4+CD25+ cells were observed in peripheral blood, spleen or thymus, indicating no changes in activated regulatory T cells in these organs (PBCs, P = 0.93; splenocytes, P = 0.84; thymocytes, P = 0.99). Moreover, frequencies of CD80+ and CD86+ cells gated from white-blood-cell regions, and CD4+CD28+ cells gated from lymphocyte regions, which are co-stimulator cells associated with T cell activation, did not differ among PBCs (CD80+, P = 0.15; CD86+, P = 0.55; CD4+CD28+, P = 0.63), splenocytes (CD80+, P = 0.14; CD86+, P = 0.47; CD4+CD28+, P = 0.95), or thymocytes (CD80+, P = 0.21; CD86+, P = 0.24; CD4+CD28+, P = 0.14) in the control vs RF-EMF–exposed animals. These data showed that T cell and regulatory T cell activation was not promoted following exposure to RF-EMF under the present conditions.
Analyses of gene expression associated with the Th1/Th2 paradigm
Expression levels of the 16 genes associated with Th1/Th2 balance were analyzed using real-time PCR in spleen and thymus tissues from the control and RF-EMF–exposed animals (Table 3). Although Il5 gene expression was significantly upregulated in the spleen tissues following exposure to RF-EMF, no other significant differences in gene expression were observed. In particular, no significant changes were observed in the spleen tissues for the expression of Ifng or Il4, which are hallmarks of Th1/Th2-balance, or in the expression of the Th1 transcription factors Stat4, Stat1 or Tbx21, or of the Th2 transcription factors Stat6 and Gata3. Thus the Th1/Th2-balance revealed normal functionality. Moreover, no changes in the expression of the Treg-cell–associated cytokine genes (Tgfb1, Ifng and Il10) or the Th17-cell–associated genes (Tgfb1, Il6 and Il17) were observed in both tissues. In contrast, although Il4, Il5 and Il23a genes were significantly upregulated in the thymus tissues from exposed animals, no significant change in the expression of these genes was observed in the other organs. These data indicate changes in thymus gene expression following exposure to RF-EMF. However, no changes in Tgfb1, Ifng, Il17a, Il10 or Il6 gene expression were observed in the spleen, indicating that Treg or Th17 cell activation did not occur following exposure to RF-EMF.
Table 3.
Relative mRNA expression levels in RF-EMF-exposed and sham-exposed rats
| Gene | Spleen | Thymus |
|---|---|---|
| Il4 | ↑ (1.82*) | |
| Stat6 | ||
| Gata3 | ||
| Il5 | ↑ (1.53**) | ↑ (1.65*) |
| Il10 | ||
| Ifng | ||
| Il12a | ||
| Il12b | ||
| Stat4 | ||
| Stat1 | ||
| Tbx21 | ||
| Tnf | ||
| Tgfb1 | ||
| Il6 | ||
| Il23a | ↑ (1.72*) | |
| Il17a |
Significant changes in gene expression in RF-EMF-exposed rats are shown by arrows, and the relative ratios are shown in parentheses; *P < 0.05, and **P < 0.01.
Comparison of IL-4 concentrations in sera from control and RF-EMF–exposed animals
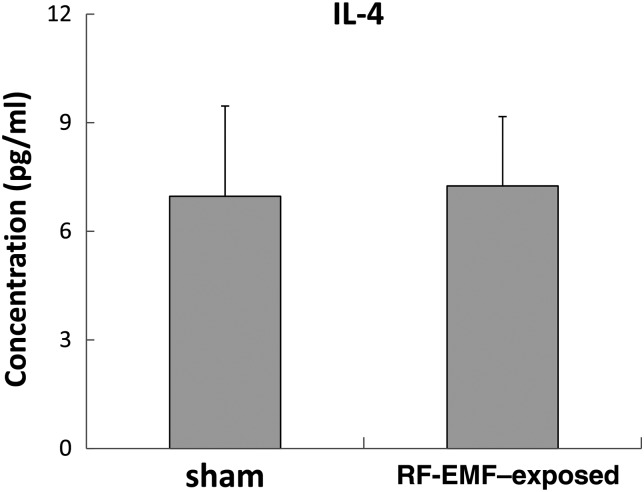
Concentrations of IL-4 were determined in sera from eight control and eight exposed rats using ELISAs for rat IL-4. Although the Il4 gene was upregulated in thymus tissues, no changes in IL-4 protein were observed in sera from RF-EMF–exposed rats (P = 0.74) (Fig. 2). The data suggest that exposure to RF-EMF is unlikely to elicit allergic responses.
Fig. 2.
Serum concentrations of IL-4 in control and RF-EMF–exposed rats. Cytokine concentrations were analyzed using ELISA. Values are presented as the mean ± standard deviation of eight mice per group; P > 0.05 for comparisons of IL-4 between control (sham-exposure).
DISCUSSION
In this study, we assessed the effects of exposure to 2.1-GHz RE-EMF W-CDMA signals on T cell kinetics and regulation, and on Th1/Th2 balance through developmental stages in rats. Although some transcripts that are associated with the Th1/Th2 paradigm were upregulated in the thymus and spleen after RF-EMF exposure, no changes in the proteins of T cell kinetics and regulation were observed. Our study provided evidence that chronic RF-EMF exposure without fever did not affect T cell function in the developmental stage.
The present study was performed using an RF-EMF exposure apparatus that allowed the uniform exposure of rats to high power RF-EMF during gestation and development [28]. Previously, Takahashi et al. evaluated relative organ weights and performed two behavioral tests using the same apparatus [29]; they adjusted exposure periods according to SAR and animal growth. In the present study, exposure conditions were set to SAR = 0.2 W/kg, and rats were exposed to RF-EMF for 20 h per day for 63 consecutive days (9 weeks), covering implantation, post-implantation, embryonic, neonatal, postnatal and lactation periods. To eliminate thermal effects, SARs were lower than those in previous studies by Tuschl et al. [4], Gatta et al. [9], Prisco et al. [15] and Jin et al. [16], in which high-powered RF-EMF exposure generated heat in rat bodies. In previous studies of the effects of RF-EMF on T lymphocytes, the cells or adult animals were more briefly (2 h/day) exposed to high SARs (2–4 W/kg), precluding assessment of the effects of chronic exposure. Thus, in the present study, we exposed rats to continuous but comparatively low SARs of RF-EMF.
In subsequent analyses of subpopulations of CD4+ T cells and CD8+ T cells (which are generally involved as helper T cells and cytotoxic T cells, respectively) the percentages of both CD4+ and CD8+ T cells in peripheral blood, spleen and thymus tissues were not significantly affected by RF-EMF exposure. These data indicate no effects on immune-related CD4+ and CD8+ T cell subsets. Specifically, the distribution of CD4+ and CD8+ T cell lineages, which play predominant roles in T cell differentiation and thymus cell proliferation, were unaffected under the RF-EMF exposure conditions described here.
To examine the effects of RF-EMF exposure on T cell activation, interactions between the co-stimulatory receptor CD28 and its ligands CD80 and CD86 were analyzed using flow cytometry. In these experiments, the percentages of CD4+CD28+ T cells, and CD80+ and CD86+ lymphocytes did not differ significantly between control and exposed rats. These data indicate that RF-EMF exposure does not induce T cell activation or proliferation, and prevents interactions between CD28 and CD80/CD86 during rat development [30, 31]. In contrast, percentages of CD4+CD25+ immune regulatory T cells in PBCs, splenocytes and thymocytes were unaffected by RF-EMF exposure during development. Because regulatory T cells maintain tolerance to self-antigens [32] and abrogate autoimmune disease [33], these data suggest that RF-EMF has no effect on the immune system. Moreover, flow cytometry of CD4+CD25+ cells, and the expression levels of Tgfb, Ifng and Il17a genes (which are associated with regulation of regulatory T cells or of the Treg/Th17 balance [34–36]) revealed no effects of RF-EMF exposure in spleen and thymus tissues. These data suggest that RF-EMF does not significantly affect regulatory T cell activation.
In further experiments, the effects of RF-EMF exposure on the Th1/Th2 balance were assessed by determining the expression of related genes in spleen and thymus tissues using real-time PCR. With the exception of Il5 in spleen tissues, and Il4, Il5 and Il23a in thymus tissues, no significant effects were observed. Dysfunction of the Th1/Th2 paradigm has been associated with autoimmune disease [20], chronic inflammation [21], and allergic disease [22]. Moreover, several lines of evidence suggest that the induction and progression of autoimmune diseases follows both Th1 responses and Th17 and Treg responses [23, 24]. Thus, expression analyses of genes associated with Th17 and Treg cell production were performed following exposure to RF-EMF, with a focus on the major helper T cell subsets Th1, Th2, Th17 and Treg. Previous studies show that helper T cells are induced by cytokines and transcription factors and secrete other (or the same) cytokines [20–22, 24, 37, 38]. For example, Th1 cells are induced by IL-4, Stat6 and Gata3, whereas Th2 cells are induced by IFN-γ, IL-12a, IL-12b, Stat4, Stat1 and Tbx21, Treg are induced by Tgf-β and IFN-γ, and Th17 cells are induced by Tgf-β and IL-6. Subsequently, Th1 cells secrete IFN-γ and/or TNF, Th2 cells secrete IL-4, IL-5 and/or IL-10, Th17 cells secrete IL-17, and Treg cells secrete Tgf-β1 and/or IL-10. In the present experiments, Il5 expression was increased in spleen and thymus tissues, potentially playing a role in the stimulation of B cell growth and eosinophil activation. However, comparisons of percentages of eosinophils between control and exposed rats showed no significant differences (data not shown). The IL-4 cytokine has been associated with Th2 cells and is produced by activated CD4+ T cells, CD8+ T cells, mast cells, basophil cells and natural killer T cells. Thus, the upregulation of Il4 may reflect high percentages of T lymphocytes among lymphocytes of the thymus. Koyanagi et al. reported that murine neonatal thymic CD4+ T cells were immunologically immature, but that they highly expressed IL-4 protein in the final stage of maturation [39]. Therefore, the upregulation of Il4 transcription may reflect the effects of continuous long-term RF-EMF exposure on T cell differentiation during gestation and development. However, IL-4 was not upregulated in the present ELISA experiments, warranting an assessment of gene expression associated with T cell differentiation during the lactation period. IL-23 plays important roles in Th17 cell proliferation, and its function is dependent on IL-6 and Tgf-β [24]. However, no changes in Il6 or Tgfb gene expression were observed (Table 3), indicating that the upregulation of Il23 after RF-EMF exposure was unrelated to Th17 cell proliferation. Dudacov et al. reported that the administration of IL-22 enhanced thymic recovery after injury following the induction of Il23 by γ-irradiation [40]. However, in the present study, no damage to the thymus was observed following exposure to RF-EMF, and Il22 transcription was very low (not detected). Thus, we suggest that immunological imbalance in Th1/Th2/Treg/Th17 was not induced by the three upregulated genes because the expression of other regulatory genes, which associate with each signaling pathway to regulate the Th1, Th2, Treg and Th17 cells, was not upregulated that of other genes regulating the pathway. These findings suggest that the gene induction by RF-EMF exposure observed here likely has little effect on Th1/Th2 balance during development.
To confirm whether the upregulation of Il4 genes in thymus tissues reflected changes in the Th1/Th2 balance, we compared serum concentrations of IL-4 in control and RF-EMF–exposed rats using ELISA. IL-4 concentrations were unaffected, indicating the absence of adverse effects of RF-EMF exposure on allergic responses, despite the present transcriptional observations. Therefore, no changes in CD4/CD8 lineages, T cell or regulatory T cell activation, or Th1/Th2 balance were observed in flow cytometry or ELISA experiments. In addition, the relative ratio of the three genes (Il4, Il5 and Il23a) was smaller than 2.0 (Table 3), so the expression changes in messenger RNA may not have been reflected in upregulation of the protein. However, we should observe the gene expression changes even though RF-EMF exposure was not damage. Further investigation is required to validate the biological effect of RF-EMF on transcriptional and/or translational changes in living systems.
In summary, the present flow-cytometry and real-time PCR analyses were designed to assess T cell activation, differentiation and Th1/Th2 balance after chronic RF-EMF exposure to 2.14-GHz W-CDMA signals at 0.2 W/kg. With the exception of transcriptional responses in the thymus (Il4, Il5 and Il23a) and spleen (Il5) tissues, the ensuing data showed no differences between exposed and control rats during development. Specifically, no changes in populations of helper T cells, cytotoxic CD4/CD8 T cells, T cell–activating CD28–CD80/86 interactions, activated CD4+CD25+ regulatory T cell populations, or Tgfb, Ifng or Il17a gene expression were observed. Similarly, RF-EMF exposure had no effect on serum IL-4 concentrations, indicating no effect on Th1/Th2 balance. Taken together, RF-EMF exposure under the conditions described presents no safety issues with respect to immune functions during development.
FUNDING
Funding to pay the Open Access publication charges for this article was provided by a Grant from the Ministry of Internal Affairs and Communications, Japan. This work was also supported by a Grant from the Ministry of Internal Affairs and Communications, Japan.
ACKNOWLEDGEMENTS
The authors would like to thank Enago (www.enago.jp) for editorial assistance, Dr Kanako Wake for technical help provided during the assembly of the exposure apparatus, Mrs Masako Ohsawa and Mrs Kasumi Yamanaka for their assistance of laboratory animal care and experiments, and Professor Junji Miyakoshi for his expert advice.
REFERENCES
- 1.Vijayalaxmi Obe G. Controversial cytogenetic observations in mammalian somatic cells exposed to radiofrequency radiation. Radiat Res 2004;162:481–96. [DOI] [PubMed] [Google Scholar]
- 2.Kheifets L, Shimkhada R. Childhood leukemia and EMF: review of the epidemiologic evidence. Bioelectromagnetics 2005;Suppl 7:S51–9. [DOI] [PubMed] [Google Scholar]
- 3.Stankiewicz W, Dabrowski MP, Kubacki R, et al. Immunotropic influence of 900 MHz microwave GSM signal on human blood immune cells activated in vitro. Electromagn Biol Med 2006;25:45–51. [DOI] [PubMed] [Google Scholar]
- 4.Tuschl H, Novak W, Molla-Djafari H. In vitro effects of GSM modulated radiofrequency fields on human immune cells. Bioelectromagnetics 2006;27:188–96. [DOI] [PubMed] [Google Scholar]
- 5.Black DR, Heynick LN. Radiofrequency (RF) effects on blood cells, cardiac, endocrine, and immunological functions. Bioelectromagnetics 2003;Suppl 6:S187–95. [DOI] [PubMed] [Google Scholar]
- 6.Meltz ML. Radiofrequency exposure and mammalian cell toxicity, genotoxicity, and transformation. Bioelectromagnetics 2003;Suppl 6:S196–213. [DOI] [PubMed] [Google Scholar]
- 7.Jauchem JR. Effects of low-level radio-frequency (3kHz to 300 GHz) energy on human cardiovascular, reproductive, immune, and other systems: a review of the recent literature. Int J Hyg Environ Health 2008;211:1–29. [DOI] [PubMed] [Google Scholar]
- 8.Scarfi MR, Fresegna AM, Villani P, et al. Exposure to radiofrequency radiation (900 MHz, GSM signal) does not affect micronucleus frequency and cell proliferation in human peripheral blood lymphocytes: an interlaboratory study. Radiat Res 2006;165:655–63. [DOI] [PubMed] [Google Scholar]
- 9.Gatta L, Pinto R, Ubaldi V, et al. Effects of in vivo exposure to GSM-modulated 900 MHz radiation on mouse peripheral lymphocytes. Radiat Res 2003;160:600–5. [DOI] [PubMed] [Google Scholar]
- 10.Nasta F, Prisco MG, Pinto R, et al. Effects of GSM-modulated radiofrequency electromagnetic fields on B-cell peripheral differentiation and antibody production. Radiat Res 2006;165:664–70. [DOI] [PubMed] [Google Scholar]
- 11.Otto M, von Mühlendahl KE. Electromagnetic fields (EMF): do they play a role in children's environmental health (CEH)? Int J Hyg Environ Health 2007;210:635–44. [DOI] [PubMed] [Google Scholar]
- 12.Smialowicz RJ, Rogers RR, Garner RJ, et al. Microwaves (2,450 MHz) suppress murine natural killer cell activity. Bioelectromagnetics 1983;4:371–81. [DOI] [PubMed] [Google Scholar]
- 13.Yang HK, Cain CA, Lockwood J, et al. Effects of microwave exposure on the hamster immune system. I. Natural killer cell activity. Bioelectromagnetics 1983;4:123–39. [DOI] [PubMed] [Google Scholar]
- 14.Sambucci M, Laudisi F, Nasta F, et al. Prenatal exposure to non-ionizing radiation: effects of WiFi signals on pregnancy outcome, peripheral B-cell compartment and antibody production. Radiat Res 2010;174:732–40. [DOI] [PubMed] [Google Scholar]
- 15.Prisco MG, Nasta F, Rosado MM, et al. Effects of GSM-modulated radiofrequency electromagnetic fields on mouse bone marrow cells. Radiat Res 2008;170:803–10. [DOI] [PubMed] [Google Scholar]
- 16.Jin YB, Pyun BJ, Jin H, et al. Effects of simultaneous combined exposure to CDMA and WCDMA electromagnetic field on immune functions in rats. Int J Radiat Biol 2012;88:814–21. [DOI] [PubMed] [Google Scholar]
- 17.Chagnaud JL, Veyret B. In vivo exposure of rats to GSM-modulated microwaves: flow cytometry analysis of lymphocyte subpopulations and of mitogen stimulation. Int J Radiat Biol 1999;75:111–3. [DOI] [PubMed] [Google Scholar]
- 18.Glimcher LH, Murphy KM. Lineage commitment in the immune system: the T helper lymphocyte grows up. Genes Dev 2000;14:1693–711. [PubMed] [Google Scholar]
- 19.Murphy KM, Ouyang W, Farrar JD, et al. Signaling and transcription in T helper development. Annu Rev Immunol 2000;18:451–94. [DOI] [PubMed] [Google Scholar]
- 20.Kuchroo VK, Das MP, Brown JA, et al. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell 1995;80:707–18. [DOI] [PubMed] [Google Scholar]
- 21.Dieleman LA, Palmen MJ, Akol H, et al. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol 1998;114:385–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maggi E. The TH1/TH2 paradigm in allergy. Immunotechnology 1998;3:233–44. [DOI] [PubMed] [Google Scholar]
- 23.Bettelli E, Korn T, Oukka M, et al. Induction and effector functions of T(H)17 cells. Nature 2008;453:1051–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Afzali B, Lombardi G, Lechler RI, et al. The role of T helper 17 (Th17) and regulatory T cells (Treg) in human organ transplantation and autoimmune disease. Clin Exp Immunol 2007;148:32–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamoto S, Win-Shwe TT, Yoshida Y, et al. Suppression of Th1- and Th2-type immune responses in infant mouse spleen after prenatal and postnatal exposure to low-level toluene and peptidoglycan. Inhal Toxicol 2009;21:793–802. [DOI] [PubMed] [Google Scholar]
- 26.International Commission on Non-Ionizing Radiation Protection. ICNIRP statement on the “Guidelines for limiting exposure to time-varying electric, magnetic, and electromagnetic fields (up to 300 GHz).” Health Phys 2009;97:257–8. [DOI] [PubMed] [Google Scholar]
- 27.Guidelines for limiting exposure to time-varying electric, magnetic, and electromagnetic fields (up to 300 GHz). International Commission on Non-Ionizing Radiation Protection. Health Phys 1998;74:494–522. [PubMed] [Google Scholar]
- 28.Wang J, Wake K, Kawai H, et al. Statistical determination of whole-body average SARs in a 2 GHz whole-body exposure system for unrestrained pregnant and newborn rats. Phys Med Biol 2012;57:143–54. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi S, Imai N, Nabae K, et al. Lack of adverse effects of whole-body exposure to a mobile telecommunication electromagnetic field on the rat fetus. Radiat Res 2010;173:362–72. [DOI] [PubMed] [Google Scholar]
- 30.Michel F, Attal-Bonnefoy G, Mangino G, et al. CD28 as a molecular amplifier extending TCR ligation and signaling capabilities. Immunity 2001;15:935–45. [DOI] [PubMed] [Google Scholar]
- 31.Carreno BM, Collins M. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu Rev Immunol 2002;20:29–53. [DOI] [PubMed] [Google Scholar]
- 32.Gershon RK, Kondo K. Infectious immunological tolerance. Immunology 1971;21:903–14. [PMC free article] [PubMed] [Google Scholar]
- 33.Sakaguchi S, Sakaguchi N, Asano M, et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 1995;155:1151–64. [PubMed] [Google Scholar]
- 34.Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25-naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med 2003;198:1875–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol 2010;40:1830–5. [DOI] [PubMed] [Google Scholar]
- 36.Veldhoen M, Hocking RJ, Atkins CJ, et al. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells . Immunity 2006;24:179–89. [DOI] [PubMed] [Google Scholar]
- 37.Chalubinski M, Broncel M. Influence of statins on effector and regulatory immune mechanisms and their potential clinical relevance in treating autoimmune disorders . Med Sci Monit 2010;16:RA245–51. [PubMed] [Google Scholar]
- 38.Afkarian M, Sedy JR, Yang J, et al. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat Immunol 2002;3:549–57. [DOI] [PubMed] [Google Scholar]
- 39.Koyanagi M, Imanishi K, Arimura Y, et al. Immunologic immaturity, but high IL-4 productivity, of murine neonatal thymic CD4 single-positive T cells in the last stage of maturation. Int Immunol 2004;16:315–26. [DOI] [PubMed] [Google Scholar]
- 40.Dudakov JA, Hanash AM, Jenq RR, et al. Interleukin-22 drives endogenous thymic regeneration in mice. Science 2012;336:91–5. [DOI] [PMC free article] [PubMed] [Google Scholar]