Abstract
Cardiopulmonary late toxicity is of concern in concurrent chemoradiotherapy (CCRT) for esophageal cancer. The aim of this study was to examine the benefit of proton beam therapy (PBT) using clinical data and adaptive dose–volume histogram (DVH) analysis. The subjects were 44 patients with esophageal cancer who underwent definitive CCRT using X-rays (n = 19) or protons (n = 25). Experimental recalculation using protons was performed for the patient actually treated with X-rays, and vice versa. Target coverage and dose constraints of normal tissues were conserved. Lung V5–V20, mean lung dose (MLD), and heart V30–V50 were compared for risk organ doses between experimental plans and actual treatment plans. Potential toxicity was estimated using protons in patients actually treated with X-rays, and vice versa. Pulmonary events of Grade ≥2 occurred in 8/44 cases (18%), and cardiac events were seen in 11 cases (25%). Risk organ doses in patients with events of Grade ≥2 were significantly higher than for those with events of Grade ≤1. Risk organ doses were lower in proton plans compared with X-ray plans. All patients suffering toxicity who were treated with X-rays (n = 13) had reduced predicted doses in lung and heart using protons, while doses in all patients treated with protons (n = 24) with toxicity of Grade ≤1 had worsened predicted toxicity with X-rays. Analysis of normal tissue complication probability showed a potential reduction in toxicity by using proton beams. Irradiation dose, volume and adverse effects on the heart and lung can be reduced using protons. Thus, PBT is a promising treatment modality for the management of esophageal cancer.
Keywords: esophageal cancer, concurrent chemoradiotherapy, proton beam therapy, DVH analysis, deformation adaptation
INTRODUCTION
Surgery is the standard treatment for esophageal cancer, but concurrent chemoradiotherapy (CCRT) has benefits with regard to prognosis, mortality and quality of life after treatment [1–3]. Outcomes of CCRT are promising, but late adverse events in the heart and lung are of concern, with reported risks of adverse effects of Grade ≥3 of 6–46% [2–6]. Reduction of irradiation doses to the organ at risk (OAR) is a simple and robust method to reduce the rate of adverse events. In this context, proton beam therapy (PBT) may provide therapeutic advantages over conformal X-ray therapy for patients with esophageal cancer [7, 8]. These advantages are based on the fundamental physical dose distribution of particle-ion beams [9]. Compared with 4D intensity-modulated radiation therapy (IMRT), PBT reduces lung V5, V10, V20 and the mean lung dose (MLD) in dosimetric studies [8]. Thus, previous reports have shown theoretical therapeutic advantages of PBT [7, 8], but a correlation between the dose advantage and clinical outcome has not been established.
A further problem of previous studies using dose–volume histogram (DVH) analysis is that anatomical changes and planning re-evaluation during fractionated radiation are not taken in count. Simple summation of DVH data between different computed tomography (CT) scans does not reflect the true DVH, particularly when beam directions are changed. An adaptive DVH analysis that reflects close to the true dose–volume relationship is needed by utilization of CT–CT deformation techniques. In this study, by cross-referencing treatment plans for each patient using adaptive DVH analysis with the actual incidence of adverse effects, we compared the possible adverse effects of X-rays with those in PBT. Then, using normal tissue complication probability (NTCP) calculations, we examined the potential therapeutic advantages of PBT over X-rays.
MATERIALS AND METHODS
Patients
Consecutive patients who underwent definitive CCRT utilizing X-rays (n = 19) or protons (n = 25) between 2009 and 2011 were enrolled in the study. Patient characteristics are shown in Table 1. The staging evaluation of each patient was done with upper GI series, CT scans, esophagogastroduodenoscopy (EGD), and positron emission tomography (PET)/CT scans. Endoscopic ultrasound was also used if the depth of invasion was unclear. The X-ray group included more advanced stage cases than the proton group. The median dose of radiation was 60 Gy in both groups. Most tumor sites were in the thoracic esophagus, and all tumors were squamous cell carcinoma.
Table 1.
Characteristics of the enrolled patients
| X-ray group | Proton group | |
|---|---|---|
| Number of patients | 19 | 25 |
| Alive/dead | 13/6 | 20/5 |
| Median follow-up period | 20 months (± 4.7 months 95% CI) | 24 months (± 5.1 months 95% CI) |
| Irradiated dose | 60 Gy | 60–70 GyE (Median 60 Gy) |
| Site Cervical | 7 | 3 |
| Thoracic | 12 | 22 |
| Abdominal | 0 | 0 |
| Stage (UICC 7th) | ||
| 0 | 0 | 1 |
| IA | 4 | 7 |
| IB | 0 | 3 |
| IIA | 0 | 1 |
| IIB | 1 | 4 |
| IIIA | 4 | 3 |
| IIIB | 4 | 1 |
| IIIC | 6 | 5 |
Immediately after the start of radiotherapy, all patients received the first cycle of chemotherapy. This consisted of an intravenous infusion of cisplatin (70 mg/m2 body surface area) over 3 h followed by fluorouracil (2800 mg/m2) over 96 h. Therefore, concurrent chemotherapy was administered during Days 1 to 5 of radiotherapy. Additional cycles of chemotherapy were scheduled at 3-week intervals. Thus, patients received two cycles of chemotherapy during fractionated radiotherapy. Patients in the X-ray group were treated with only X-rays and chemotherapy, and those in the proton group were treated with only protons and the same chemotherapy regimen. Thus, no patient received a combination of X-rays and proton beams.
Radiotherapy systems
The X-ray therapy system consisted of a linear accelerator (Clinac iX, Varian Medical Systems, Palo Alto, CA, USA) equipped with a 5–10 mm multileaf collimator (MLC), a rotational treatment couch, and a treatment-planning system (Xio ver. 4.8, Elekta, Stockholm, Sweden). The PBT system consisted of a 250-MeV synchrotron equipped with an isocentric rotational gantry, a 15 × 15 cm passive scattering port with a 5-mm MLC, a rotational treatment couch, a treatment-planning system (Hitachi 3D Treatment Planning System ver. 2.0, Tokyo, Japan), a treatment-planning CT scanner, and an X-ray simulator without any system modification.
Principles of treatment planning
The gross tumor volume (GTV) was defined as all diseased tissue seen on CT images and other diagnostic imaging. To confirm the tumor location on CT, two to three metal markers were placed in the normal esophageal wall at the tumor edges during endoscopy prior to the initial treatment planning. The initial clinical target volume (CTV1) included all areas of potential disease spread, such as the esophageal wall and mediastinal lymph nodes. For cancer of the thoracic esophagus (n = 34), the whole thoracic esophagus was included in CTV1 in most patients. The second CTV (CTV2) included the GTV with 20- to 25-mm margins in the cranial and caudal directions of the tumor and 10-mm margins in other directions. The lung contour was defined as the thoracic cavity excluding the bilateral main bronchus. The heart contour was defined as described by Feng et al. [10]. In brief, superiorly the heart starts just inferior to the left pulmonary artery. For simplification, a round structure including the great vessels was contoured. Inferiorly, the heart blends with the diaphragm. The superior vena cava was included for simplification and consistency.
A total dose of 40 gray (Gy) or gray equivalent (GyE), with relative biological effectiveness (RBE) set to 1.1 for the proton beam, was given to CTV1 using conventional fractionation with a fractional dose of 2 Gy. An additional dose of 20 Gy or GyE in 10 fractions was given to CTV2. The planning target volume (PTV) was made by adding adequate margins to the CTVs. The total dose was 60 Gy in most patients; however, five patients in the proton group received an additional boost for GTV with a short margin, since an endoscopic examination at a total dose of 50 Gy suggested persistent tumor tissue. If necessary, treatment plans were revised repeatedly to adapt to tumor volume changes and patient constitutional changes. Replanning was performed using new planning CT scans.
For X-ray planning, a two-field anteroposterior and posteroanterior (AP/PA) beam arrangement was used for CTV1 up to 40 Gy, and then a two-field right anterior oblique (RAO) and left posterior oblique (LPO) arrangement was used for the boost to CTV2. Field-in-field techniques and wedge compensators were sometimes used to maintain dose distribution uniformity. For PBT planning, a two-field AP/PA beam arrangement was used throughout treatment in most thoracic cases. When spinal cord doses became an issue, even in PBT, an LPO arrangement was used instead of a PA arrangement. RAO and left anterior oblique arrangements were used in cervical cases. In cases where the PTV exceeded the maximum field size (14 cm) in our system, two fields were ‘patched’ using the D-SLIT technique [11]. All plans were revised and approved by multiple radiation oncology specialists with experience in particle therapy and X-ray therapy.
Experimental planning and cross referencing
Experimental PBT plans were made for patients who received X-ray therapy, using the actual planning CT. Alternatively, X-ray plans were made using the planning CT for patients who received PBT. Original CTVs and PTVs used in the actual treatment were maintained in the experimental plans. To evaluate cumulative dose and volume from different planning CTs accurately, all planning CTs and doses linked to CT images were merged using deformation techniques [12–14]. Data conversion and translation between systems, CT–CT deformation, and dose volume studies were performed using MIM Maestro ver. 6 (Cleveland, OH, USA). The concept of dose delivery to the target and normal tissue was exactly the same in X-ray and proton planning. Coverage of PTVs was provided by > 95% of prescribed doses, and the maximum spinal dose was restricted up to 44 Gy. After summation of each X-ray and proton treatment plan, dosimetric factors such as percentage volume of whole lung, heart receiving more than a certain dose (Vx), and the mean lung dose (MLD) were calculated using DVH analysis. The experimental plans were compared with the actual treatment plans. Typical dose distributions and the total DVH for X-rays and protons are shown in Fig. 1.
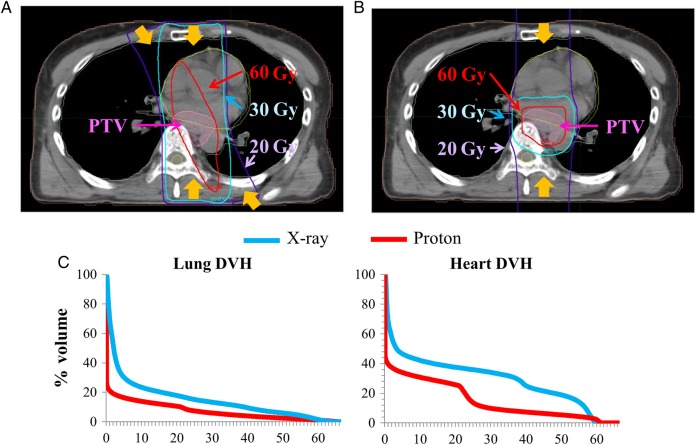
Fig. 1.
Typical dose distributions and dose–volume histograms in treatment of esophageal cancer in (A) X-ray 3D-CRT and (B) PBT. In 3D-CRT, 20 Gy is delivered widely to the lung, 30 Gy is delivered to most of the heart, and 60 Gy is also delivered widely to the heart. (C) Typical dose–volume histograms of the lung and heart. PBT results in lower irradiation doses in both OARs.
Normal tissue complication probability calculation
NTCP was calculated using the Lyman–Kutcher–Burman (LKB) model following Emami et al. and Burman et al. [15–17]:
where TD is the tolerance dose and v is the fraction of organ irradiated. The model has four parameters: Vref, TD50, n and m. TD50 is the dose to the whole organ that gives a complication probability of 50%. Volume dependence is determined by n; the slope of the complication probability vs dose curve is determined by m; and Vref is the reference volume for TD50.
Follow-up
Follow-up included physical examinations, blood tests and imaging studies. Squamous cell carcinoma antigen was measured as a serum tumor marker at 1 month after completion of radiotherapy and at 2- to 3-month intervals thereafter. EGD and CT scans were performed 1 to 2 months after radiotherapy for assessment of the initial tumor response. Similar scans were performed every 3 months for the first year and every 6 months thereafter for evaluation of tumor recurrence at lymph nodes and distant organs. Treatment-related morbidities were evaluated by physical examination and imaging. Events were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Effects (CTCAE), ver. 4.
Statistical analysis
Statistical analysis was performed by Student t-test or paired t-test using R Project software (http://www.r-project.org/). Receiver operating characteristic (ROC) curves and specificity/sensitivity calculations were also performed using this software.
RESULTS
Adverse effects
Cardiopulmonary late adverse effects of Grade ≥2 in all 44 patients are shown in Table 2. Pulmonary events of this grade occurred in 8 cases (18.2%), and cardiac events such as pericardial effusion occurred in 11 cases (25.0%). All pulmonary events of Grade ≥2 were in the X-ray group. Cardiac events of Grade ≥2 occurred in 10 cases (52.6%) in the X-ray group and in 1 case (4.0%) in the proton group. Six cases in the X-ray group had both pulmonary and cardiac events of Grade ≥2.
Table 2.
Clinical outcomes for adverse effects
| All data | ≤G1 | G2 | G3 | G4 | G5 |
| Pharmacological pneumonitis | 43 (97.7%) | 0 | 0 | 0 | 1 (2.3%) |
| Lung infection | 43 (97.7%) | 0 | 0 | 0 | 1 (2.3%) |
| Radiation pneumonitis | 40 (90.9%) | 3 (9.1%) | 1 (2.3%) | 0 | 0 |
| Pulmonary effusion | 42 (95.5%) | 1 (2.3%) | 1 (2.3%) | 0 | 0 |
| Pericardial effusion | 33 (75.0%) | 11 (25.0%) | 0 | 0 | 0 |
| X-ray group | ≤G1 | G2 | G3 | G4 | G5 |
| Pharmacological pneumonitis | 18 (94.7%) | 0 | 0 | 0 | 1 (5.3%) |
| Lung infection | 18 (94.7%) | 0 | 0 | 0 | 1 (5.3%) |
| Radiation pneumonitis | 15 (78.9%) | 3 (15.8%) | 1 (5.3%) | 0 | 0 |
| Pulmonary effusion | 17 (89.5%) | 1 (5.3%) | 1 (5.3%) | 0 | 0 |
| Pericardial effusion | 9 (47.4%) | 10 (52.6%) | 0 | 0 | 0 |
| Proton group | ≤G1 | G2 | G3 | G4 | G5 |
| Pharmacological pneumonitis | 25 (100%) | 0 | 0 | 0 | 0 |
| Lung infection | 25 (100%) | 0 | 0 | 0 | 0 |
| Radiation pneumonitis | 25 (100%) | 0 | 0 | 0 | 0 |
| Pulmonary effusion | 25 (100%) | 0 | 0 | 0 | 0 |
| Pericardial effusion | 24 (96.0%) | 1 (4.0%) | 0 | 0 | 0 |
Correlation of DVH parameters and adverse effects
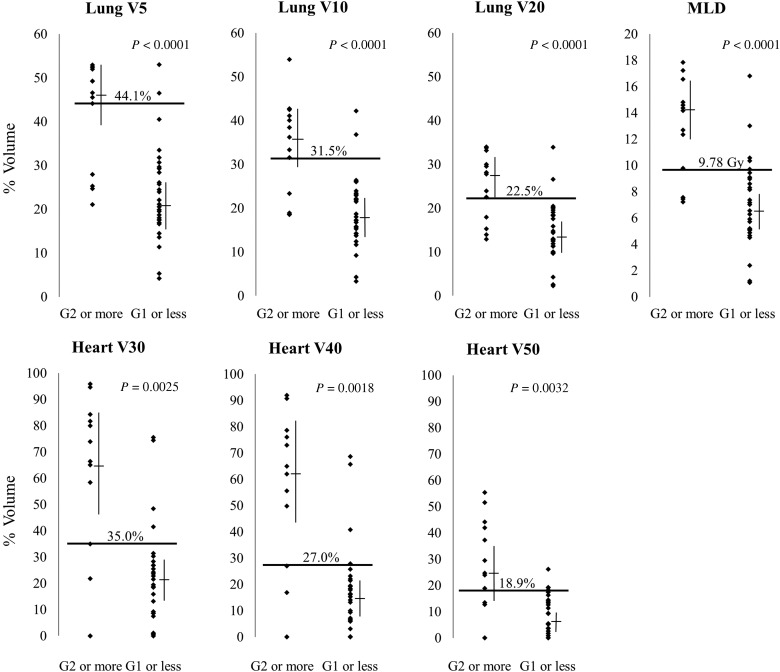
DVH parameters such as Vx and MLD for patients with and without adverse events of Grade ≥2 are shown in Fig. 2. The irradiated OAR volume in patients with events of Grade ≥2 was significantly higher than in those with events of Grade ≤1. The data were used to calculate ROC curves, which gave cut-off values of V5 44.1% (specificity 93.1%, sensitivity 69.2%), V10 31.5% (93.1%, 69.2%), V20 22.5% (93.1%, 69.2%) and MLD 9.78 Gy (86.2%, 76.9%) for the lung; and V30 35.0% (86.2%, 76.9%), V40 27% (86.2%, 76.9%) and V50 18.9% (89.7%, 69.2%) for the heart.
Fig. 2.
Parameters in patients with and without adverse events of Grade ≥2. All parameters were significantly higher in patients with late adverse events of Grade ≥2. Bars show the median and 95% CI. Horizontal bars show cut-off lines calculated from ROC curves.
DVH parameters in the organ at risk based on cross-referencing of treatment plans
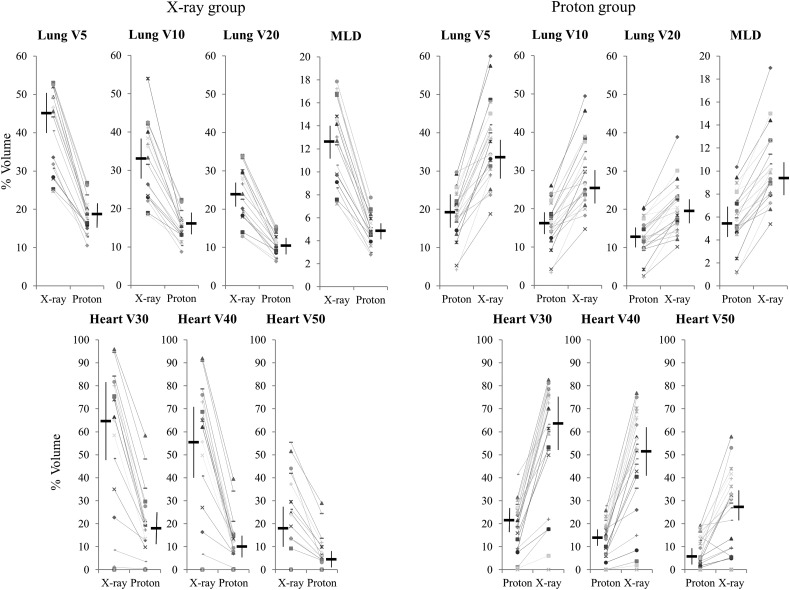
Dose–volume data in actual and experimental treatment plans are shown in Table 3. Lung V5–V20, MLD, and heart V30–V50 were all lower in the PBT plans (P < 0.001 by paired t-test). The X-ray group included more cases with advanced disease, but experimental dose–volume data calculated for protons were mostly the same as actual data in the proton group. Similarly, experimental dose–volume data calculated for X-rays in the proton group were mostly the same as actual data in the X-ray group. Differences in DVH parameters in actual treatment and experimental plans in each group are shown in Fig. 3. All patients in the X-ray group had reduced irradiated lung and heart volumes using protons (P < 0.001), while irradiation exposure worsened using X-rays for all patients in the proton group (P < 0.001).
Table 3.
DVH parameters in both actual treatment and experimental planning
| X-ray group | |||||
| Actual X-ray plan (95% CI) | Experimental proton plan (95% CI) | ||||
| Lung V5 | 45.57 | (40.64–50.50) | 18.89 | (16.90–20.88) | P = 1.48*10−10 |
| Lung V10 | 33.36 | (28.68–38.04) | 15.51 | (13.87–17.15) | P = 2.15*10−8 |
| Lung V20 | 23.98 | (20.56–27.40) | 10.61 | (9.36–11.86) | P = 8.35*10−9 |
| MLD | 12.69 | (11.03–14.35) | 4.72 | (4.12–5.32) | P = 3.84*10−10 |
| Heart V30 | 65.03 | (48.58–81.48) | 18.84 | (11.26–26.42) | P = 9.10*10−4 |
| Heart V40 | 55.65 | (40.13–71.17) | 9.54 | (4.42–14.66) | P = 1.41*10−4 |
| Heart V50 | 18.89 | (10.33–27.45) | 5.41 | (1.65–9.17) | P = 4.70*10−3 |
| Proton group | |||||
| Actual proton plan (95% CI) |
Experimental X-ray plan (95% CI) | ||||
| Lung V5 | 19.56 | (22.29–16.83) | 34.30 | (29.89–38.71) | P = 1.75*10−8 |
| Lung V10 | 16.77 | (14.33–19.21) | 26.77 | (23.12–30.42) | P = 8.01*10−7 |
| Lung V20 | 12.54 | (10.44–14.64) | 19.58 | (16.83–22.33) | P = 5.90*10−5 |
| MLD | 5.73 | (4.73–6.73) | 9.32 | (8.03–10.61) | P = 6.39*10−6 |
| Heart V30 | 21.51 | (16.94–26.08) | 63.29 | (52.43–74.15) | P = 8.22*10−8 |
| Heart V40 | 15.29 | (11.74–18.84) | 51.78 | (41.45–62.11) | P = 7.53*10−7 |
| Heart V50 | 5.51 | (2.63–8.39) | 28.96 | (21.54–36.38) | P = 2.62*10−4 |
Fig. 3.
Differences in parameters in the lung and heart in actual treatment plans and experimental plans. All dose parameters were significantly lower in PBT (P < 0.001 by paired t-test). Horizontal bars show median values and vertical bars show the 95% CI.
Potential change in dose distribution
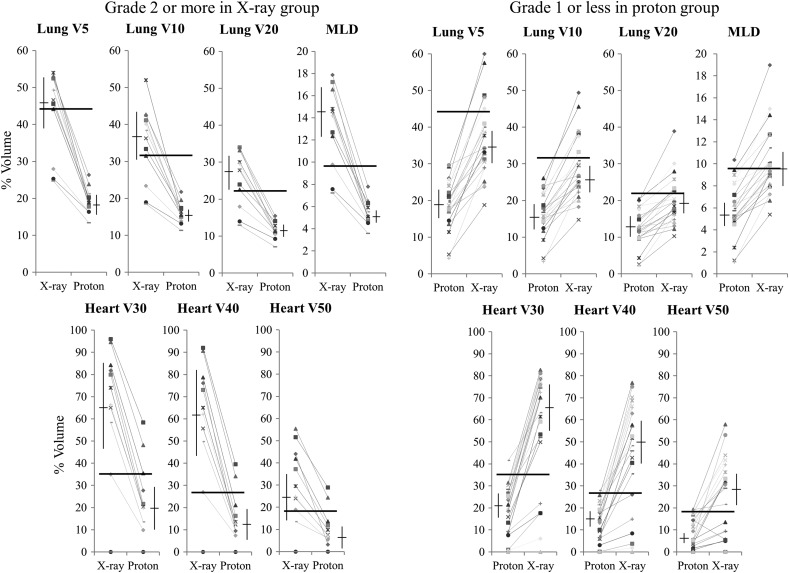
DVH parameters for proton treatment in patients in the X-ray group with adverse events of Grade ≥2 are shown in Fig. 4. There were 13 cardiopulmonary late adverse events of Grade ≥2 in this group, and dose parameters decreased in all these cases in the experimental proton plans. In the proton group, 24 cases had cardiopulmonary adverse events of Grade ≤1, and the mean values of all parameters increased in all these patients in the X-ray plans.
Fig. 4.
Potential changes in parameters in patients with adverse effects of Grade ≥2 in the X-ray group and in patients with adverse effects of Grade ≤1 in the proton group. Horizontal bars show cut-off lines calculated from ROC curves.
Calculated normal tissue complication probability
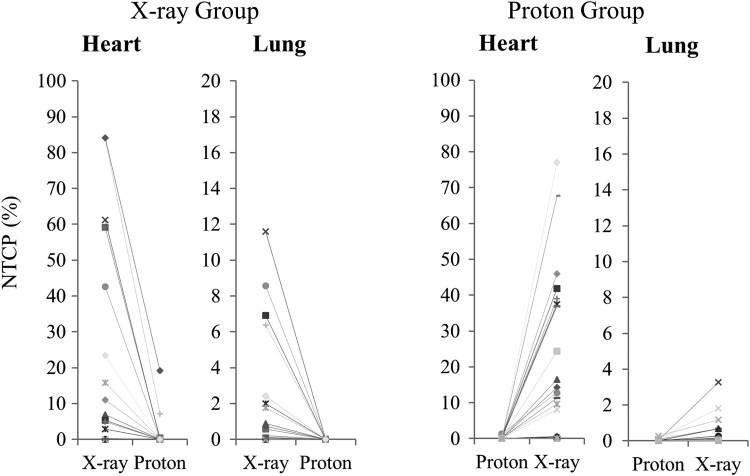
Changes in NTCP for the lung and heart in each patient are shown in Fig. 5. NTCP decreased in proton plans in the X-ray group and increased in X-ray plans in the proton group. No case had an increased NTCP in proton plans or a decreased NTCP in X-ray plans.
Fig. 5.
Normal tissue complication probability (NTCP) changes between plans in the X-ray and proton groups.
DISCUSSION
3D conformal radiation therapy (3D-CRT) using X-rays is a widely used technique worldwide. However, X-ray treatment of esophageal cancer results in large parts of the heart and lung being irradiated at low to medium doses [18, 19] and this causes late adverse events in these organs [2–6]. Ishikura et al. reported a rate of 11.5% for late cardiopulmonary toxicity of Grade ≥3, and the RTOG 85-01 and Intergroup 0123 studies found rates of 28% and 37–46%, respectively, for any severe late toxicity of Grade ≥3.
IMRT can be used to focus high dose areas to the target, which leads to lower heart doses [20] and fewer cardiac late adverse events. The clinical superiority of IMRT over 3D-CRT has been suggested using propensity scores [21]. However, the basic physical characteristics of X-rays of multiple field directions leads to wider low-dose areas, and IMRT results in irradiation of larger areas at low doses than 3D-CRT; this is especially critical in organs with low tolerance doses, such as the lung [22]. The risk of pneumonitis may also be higher with IMRT in thoracic irradiation [23, 24]. Additionally, IMRT is considered unsuitable for treatment of cancer with respiratory movement because it is difficult to apply respiratory gating during IMRT due to the complicated beam arrangement.
PBT provides distinct therapeutic advantages over 3D-CRT for esophageal cancer based on DVH studies [7, 8] because it is possible to reduce OAR doses without affecting the PTV coverage of the prescribed dose. Compared with 4D IMRT, PBT can reduce V5, V10, V20 and MLD from 49.5% to 13.9%, 32.5% to 12%, 15.6% to 9.8%, and 9.65 Gy to 4.55 Gy, respectively [8]. However, previous DVH studies have not examined the correlation with treatment outcomes in esophageal cancer. The current study provides a link between the dose distribution superiority of PBT with actual clinical results by cross-referencing individual patient plans.
In this study, morbidity rates in the heart and lung in the proton group were lower than those in the X-ray group, consistent with the lower OAR doses and volumes in the proton group. However, there were more advanced cases in the X-ray group than in the proton group, and this may have affected the morbidity rate. Previous Phase II studies of definitive radiation therapy of 60 Gy/30 fr concurrently combined with CDDP and 5-FU for advanced esophageal cancer (JCOG 9906) revealed that late toxicities compromised Grade 3 or severe pericardial (13%) and pleural effusions (9%) and radiation pneumonitis (4%) [25].
Furthermore, results of a randomized Phase II study for Stage II–IVA esophageal cancer was recently reported by Nishimura [26], and 91 patients were treated with radiation therapy of 60 Gy/30fr concurrently combined with CDDP plus 5FU. In the study, Grade 3 or severe late toxicities of pericardial and pleural effusions were observed in 9% and 7%, respectively, but the corresponding rates of a Phase II study (JCOG9708) performing CCRT using a similar treatment protocol for Stage I esophageal cancer were 0% and 3%, respectively [27]. Therefore, there seems to be no doubt that the size of the irradiation fields affects the development of late cardiopulmonary toxicities.
In our study however 11 of 14 patients with Stage IIIA–C esophageal cancer in the X-ray group experienced Grade 2 or severe cardiopulmonary events, whereas none of 9 patients with Stage IIIA–C in the proton group did. Although some lung dose parameters of the X-ray plans in the proton group were lower than in the X-ray group, no significant difference in OAR doses between the two groups was observed.
Furthermore, experimental dose–volume data calculated for protons in the X-ray group were mostly the same as actual data in the proton group. Similarly, experimental dose–volume data calculated for X-rays in the proton group were mostly the same as actual data in the X-ray group (Fig. 3). In addition, we also found a clear correlation between OAR doses and late adverse events in the present study. Therefore, we think that differences in incidences of the cardiopulmonary events between X-ray and proton therapy in the study may be mainly caused by the difference in dose distribution of the different types of radiation beams.
NTCP is a useful method for estimating adverse effects in radiotherapy. In this study, we calculated NTCP using the model described in Burman et al. [17] using data from Emami et al. [16]. However, the rates of observed adverse events in chemoradiotherapy were higher than those expected from the calculated NTCP. The data in Enami et al. [16] were obtained from studies using radiotherapy alone, and this may explain why we observed more adverse events than expected from the calculated NTCP. Definitive radiotherapy often includes concurrent chemotherapy, and thus parameters reflecting this approach are in demand.
Clinically, new planning CTs are obtained when tumors shrink during radiotherapy. This makes it difficult to evaluate the cumulative doses accurately for two plans based on different CT series. Simple accumulation of DVH data does not reflect the true DVH, particularly when beam directions are changed. To evaluate the cumulative DVH as accurately as possible, and to reflect actual time-dependent changes of the patient and tumor, we used a CT–CT deformation technique reported in adaptive RT and 4D CT studies [12–14]. Therefore, more accurate DVHs of OARs were obtained in this study compared with previous studies that did not utilize this deformation technique.
This study does not directly show superiority of PBT over X-rays because it is a non-randomized retrospective study. However, by cross-referencing proton and X-ray plans, we believe that bias has been reduced to a minimal level. Thus, the superiority of PBT for esophageal cancer with regard to irradiation doses to the heart and lung and the occurrence of cardiopulmonary adverse effects is apparent from the results of the study. The accumulation of further cases is required to validate this result, in part because our NTCP data underestimate the risk of adverse effects due to the small sample size and the use of chemoradiotherapy data.
FUNDING
This work was supported by the Ministry of Education, Science, Sports and Culture of Japan [Scientific Research (B) (24390286), Challenging Exploratory Research (24659556), Young Scientists (B) (25861064) and Scientific Research (C) (24591832)]. Funding to pay the Open Access publication charges for this article was provided by Grants-in-Aids for scientific research from the Ministry of Education, Culture, Sports, Science, and Technology (24390286, 24591832).
ACKNOWLEDGEMENTS
Study results were presented at the 53rd Annual Conference of the Particle Therapy Co-Operative Group (PTCOG53), Shanghai, China, 8–14 June 2014, and at the 17th ECCO/38th ESMO/32nd ESTRO European Cancer Congress, Amsterdam, Netherlands, 27 September–1 October 2013.
REFERENCES
- 1.al-Sarraf M, Martz K, Herskovic A, et al. Progress report of combined chemoradiotherapy versus radiotherapy alone in patients with esophageal cancer: an intergroup study. J Clin Oncol 1997;15:277–84. [DOI] [PubMed] [Google Scholar]
- 2.Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA 1999;281:1623–7. [DOI] [PubMed] [Google Scholar]
- 3.Herskovic A, Martz K, al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med 1992;326:1593–8. [DOI] [PubMed] [Google Scholar]
- 4.Morota M, Gomi K, Kozuka T, et al. Late toxicity after definitive concurrent chemoradiotherapy for thoracic esophageal carcinoma. Int J Radiat Oncol Biol Phys 2009;75:122–8. [DOI] [PubMed] [Google Scholar]
- 5.Ishikura S, Nihei K, Ohtsu A, et al. Long-term toxicity after definitive chemoradiotherapy for squamous cell carcinoma of the thoracic esophagus. J Clin Oncol 2003;21:2697–702. [DOI] [PubMed] [Google Scholar]
- 6.Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol 2002;20:1167–74. [DOI] [PubMed] [Google Scholar]
- 7.Lin SH, Komaki R, Liao Z, et al. Proton beam therapy and concurrent chemotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys 2012;83:e345–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X, Zhao KL, Guerrero TM, et al. Four-dimensional computed tomography-based treatment planning for intensity-modulated radiation therapy and proton therapy for distal esophageal cancer. Int J Radiat Oncol Biol Phys 2008;72:278–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pedroni E, Bacher R, Blattmann H, et al. The 200-MeV proton therapy project at the Paul Scherrer Institute: conceptual design and practical realization. Med Phys 1995;22: 37–53. [DOI] [PubMed] [Google Scholar]
- 10.Feng M, Moran JM, Koelling T, et al. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys 2011;79:10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okonogi N, Hashimoto T, Ishida M, et al. Designed-seamless irradiation technique for extended whole mediastinal proton-beam irradiation for esophageal cancer. Radiat Oncol 2012;7:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaus MR, Brock KK, Pekar V, et al. Assessment of a model-based deformable image registration approach for radiation therapy planning. Int J Radiat Oncol Biol Phys 2007;68:572–80. [DOI] [PubMed] [Google Scholar]
- 13.Lerma FA, Liu B, Wang Z, et al. Role of image-guided patient repositioning and online planning in localized prostate cancer IMRT. Radiother Oncol 2009;93:18–24. [DOI] [PubMed] [Google Scholar]
- 14.Nagano A, Minohara S, Kato S, et al. Adaptive radiotherapy based on the daily regression of a tumor in carbon-ion beam irradiation. Phys Med Biol 2012;57:8343–56. [DOI] [PubMed] [Google Scholar]
- 15.Burman C, Kutcher GJ, Emami B, et al. Fitting of normal tissue tolerance data to an analytic function. Int J Radiat Oncol Biol Phys 1991;21:123–35. [DOI] [PubMed] [Google Scholar]
- 16.Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991;21:109–22. [DOI] [PubMed] [Google Scholar]
- 17.Brown AP, Urie MM, Barest G, et al. Three-dimensional photon treatment planning for Hodgkin's disease. Int J Radiat Oncol Biol Phys 1991;21:205–15. [DOI] [PubMed] [Google Scholar]
- 18.Wang SL, Liao Z, Vaporciyan AA, et al. Investigation of clinical and dosimetric factors associated with postoperative pulmonary complications in esophageal cancer patients treated with concurrent chemoradiotherapy followed by surgery. Int J Radiat Oncol Biol Phys 2006;64:692–9. [DOI] [PubMed] [Google Scholar]
- 19.Gayed IW, Liu HH, Yusuf SW, et al. The prevalence of myocardial ischemia after concurrent chemoradiation therapy as detected by gated myocardial perfusion imaging in patients with esophageal cancer. J Nucl Med 2006;47:1756–62. [PubMed] [Google Scholar]
- 20.Kole TP, Aghayere O, Kwah J, et al. Comparison of heart and coronary artery doses associated with intensity-modulated radiotherapy versus three-dimensional conformal radiotherapy for distal esophageal cancer. Int J Radiat Oncol Biol Phys 2012;83:1580–6. [DOI] [PubMed] [Google Scholar]
- 21.Lin SH, Wang L, Myles B, et al. Propensity score-based comparison of long-term outcomes with 3-dimensional conformal radiotherapy vs intensity-modulated radiotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys 2012;84:1078–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nutting CM, Bedford JL, Cosgrove VP, et al. A comparison of conformal and intensity-modulated techniques for oesophageal radiotherapy. Radiother Oncol 2001;61:157–63. [DOI] [PubMed] [Google Scholar]
- 23.Vogelius IS, Westerly DC, Cannon GM, et al. Intensity-modulated radiotherapy might increase pneumonitis risk relative to three-dimensional conformal radiotherapy in patients receiving combined chemotherapy and radiotherapy: a modeling study of dose dumping. Int J Radiat Oncol Biol Phys 2011;80:893–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar G, Rawat S, Puri A, et al. Analysis of dose–volume parameters predicting radiation pneumonitis in patients with esophageal cancer treated with 3D-conformal radiation therapy or IMRT. Jpn J Radiol 2012;30:18–24. [DOI] [PubMed] [Google Scholar]
- 25.Kato K, Muro K, Minashi K, et al. Phase II study of chemoradiotherapy with 5-fluorouracil and cisplatin for Stage II–III esophageal squamous cell carcinoma: JCOG trial (JCOG 9906). Int J Radiat Oncol Biol Phys 2011;81:684–90. [DOI] [PubMed] [Google Scholar]
- 26.Nishimura Y, Hiraoka M, Koike R, et al. Long-term follow-up of a randomized Phase II study of cisplatin/5-FU concurrent chemoradiotherapy for esophageal cancer (KROSG0101/JROSG021). Jpn J Clin Oncol 2012;42:807–12. [DOI] [PubMed] [Google Scholar]
- 27.Kato H, Sato A, Fukuda H, et al. A phase II trial of chemoradiotherapy for stage I esophageal squamous cell carcinoma: Japan Clinical Oncology Group Study (JCOG9708). Jpn J Clin Oncol 2009;39:638–43. [DOI] [PubMed] [Google Scholar]