Abstract
Breast cancer is the second leading cause of cancer deaths in women in the United States. Diethylstilbestrol (DES) is a synthetic estrogen that has been shown to cause cancer in animals and humans, altering cell viability as well as inducing DNA damage. Diallyl sulfide (DAS) is a garlic organosulfide that has been shown to inhibit both the initiation and promotion phases of cancer in vivo and in vitro, as well as reduce the risk of cancer in epidemiological studies. MCF-10A cells, regarded as a normal breast epithelial cell line, were treated with varying concentrations of DES, DAS or various dose combinations of DES and DAS concomitantly, and assessed for cell viability, DNA strand breaks, and lipid peroxidation. DES (10 μM) in combination with 1, 10, or 100 μM DAS resulted in a 31%, 34%, or 36% respective increase in cell viability compared to the DES treatment alone, after 24 h. At the same time point, 1, 10, and 100 μM DAS were all effective in significantly reducing DES (100 μM)-induced strand breaks to near that of the vehicle control. Additionally, 1 μM DAS was effective in significantly reducing DES (100 μM)-induced lipid peroxidation after 3 h. The results of this research suggest that DAS is effective in recovering cell viability, attenuating DNA strand breaks, and decreasing lipid peroxidation in MCF-10A cells.
Keywords: DNA damage, Diallyl sulfide, Diethystilbestrol, Breast epithelial cells chemoprevention
1. Introduction
Historically, researchers have referred to the cell growth promoting attributes of estrogen to explain cell transformation and tumorigenesis in in vitro and in vivo studies. Although, estrogen acts as a cell growth promoter in estrogen responsive tissue, many of the observed deleterious effects of estrogens in humans, animal models, and estrogen receptor-negative cell cultures cannot be solely attributed to the increases in cellular growth [1–4]. Oxidative metabolites of estrogen have been shown to react with DNA thereby leading to DNA damage [5,6]. These metabolites have been observed to covalently bind to DNA and form stable and depurinating DNA adducts. The resultant DNA damage can lead to genetic errors increasing the risk of cancer initiation in estrogen responsive and non-responsive tissues.
Diethylstilbestrol (DES) is a synthetic estrogen that mimics the metabolism of endogenous estrogens. DES metabolizes into reactive intermediates, including its semiquinone and quinone metabolites [7,8] The primary metabolite, catechol 3’-OH-DES is oxidized into DES-3’,4’-quinone [8,9], and attacks DNA [10] by forming dep-urinating 3’-OH-DES-6’-N3Ade and 3’-OH-DES-6’-N7Gua adducts. DES metabolism can also lead to the formation of peroxides, which have the potential to attack electrophilic sites in nucleic acids and lipids [8,11,12], which models the actions of metabolized endogenous estrogen [6,13,14].
Diallyl sulfide (DAS), a lipophilic organosulfide compound found in garlic (Allium sativum), has been found to have chemopreventive properties in in vivo and in vitro studies. Animal models have shown that DAS can induce expression of nuclear excision repair enzymes, and reduce the redox cycling of DES, leading to increased in vitro and in vivo viability in liver microsomes and rat models [15–17]. The chemopreventive effects of DAS have been attributed to its inhibitory effects on CYP2E1-mediated bioactivation of carcinogenic chemicals [18]. CYP2E1 preferentially catalyzes oxidation of the sulfur atom to form the sulfoxide and the sulfone (DASO and DASO2). This final metabolism of DASO2 leads to the autocatalytic destruction of CYP2E1, which is mainly responsible for the chemoprotective effects of DAS in vivo [19]. Most importantly, DAS has been shown to inhibit DES induced formation of DNA adducts (in vivo) in the breast tissue of ACI rats. [20]. The current study hypothesizes that DAS will prevent DES induced cell death via the inhibiting formation of lipid peroxides and DNA strand breaks in the MCF-10A breast epithelial cell model.
2. Experimental
2.1. Chemicals and reagents
Dulbecco's Modified Eagle's Medium/Nutrient (DMEM/F12) 1:1 mix basal media, horse serum, penicillin, and streptomycin were purchased from Invitrogen (Carlsbad, CA). Hydrocortisone, cholera enterotoxin, human insulin, dimethylsulfoxide (DMSO), Triton X-100, (PBS, NaOH, Trizma base, NaCl, NA2 Ethylenediaminetetraacetic acid (EDTA), diethylpyrocarbonate (DEPC) ethanol, DNA se/ RNAse free water, DES and DAS were all purchased from Sigma Aldrich (St. Louis, MO). Human epidermal growth factor was purchased from BD Pharmingen (San Jose, CA) and fully frosted microscope slides and cover slips were purchased from Fisher Scientific (Pittsburgh, PA).
2.2. Cell culture
MCF-10A human breast epithelial cells were a generous gift from the laboratory of Dr. Thomas Kocarek at Wayne State University (Detroit, MI). The cells were cultured in a humidified incubator at 37 °C under 5% CO2 atmospheric conditions. The cells were grown, maintained and treated in media containing phenol red free DMEM/F-12 1:1 mix supplemented with human insulin (10 μg/ml), epidermal growth factor (20 ng/ml), cholera toxin (50 μl), hydrocortisone (0.5 μg/ml), horse serum (5%), and penicillin/streptomycin (10,000 units/ml).
2.3. Cell treatments
MCF-10A human breast epithelial cells were treated for 3 and 24 h with DES (10 or 100 μM) and/or varying concentrations of DAS (1, 10 and 100 μM). DES and DAS were both dissolved in DMSO, establishing a 0.1% concentration of DMSO in each treatment. DMSO (0.1%) served as the vehicle control.
2.4. Cell harvesting
Treated cells grown in cell culture flasks were harvested using disassociation media containing RNase free water (450 ml), phosphate buffered saline (PBS) 10 × (50 ml), EDTA (0.5 mM) and placed in centrifuge tubes. The cells were then centrifuged at 250G for 5 min to pellet the cells. The disassociation media was decanted off the cells, maintaining the integrity of the pellet, and the cells were resuspended in 1 ml 1 × PBS.
2.5. Cell viability
Cellular viability was quantified by using the CellTiter 96® AQueousOne Solution Cell Proliferation assay (MTS Assay) (Promega, Madison, WI). Cells (5 × 104) were plated per well in flat bottom 96-well plates and treated with DES, DAS or DES/DAS as described above. At 24 h, a 20 μl aliquot of AqueousOne Solution was added to each well and the plate was incubated at 37 °C for 2–4 h to allow for color development. The plates were analyzed on a BioTek Ex800 microplate reader using KC Junior software at 480 nm (Biotek, Winooski, VT).
2.6. Comet assay
The Comet assay was used to detect DNA strand breaks in response to treatment with the carcinogen DES in the presence and/or absence of the chemopreventive agent DAS. This assay sensitively detects DNA damage at the level of individual cells in the form of DNA strand breaks, Cellular DNA undergoes electrophoresis, and as the positively charged DNA migrates to the negatively charged electrode, damaged DNA migrates creating a comet like pattern. The olive tail moment (OTM) is defined as the product of the tail length and the fraction of total DNA in the tail. The OTM integrates the measurement of the smallest detectable size of migrating DNA (reflected in the comet tail length) and the number of relaxed/broken pieces, which creates the intensity of DNA in the tail. MCF-10A cells were treated and harvested from T-25 cm2 cell culture flasks. A 100 μl aliquot of harvested cells suspended in 1 × PBS was mixed with 900 μl of 0.75% low melting point agarose and placed in a 37 °C water bath. A 100 μl aliquot of cell/agarose suspension was placed on three microscope slides pre-coated with 1% normal melting point agarose (NMPA), covered with a coverslip and solidified on ice. Following the removal of the coverslip, a top coat of NMPA was placed on each slide and solidified on ice. The slides were placed in ice-cold lysis buffer (pH = 10) containing 1% Triton X-100 and refrigerated (4 °C) for at least 1 h. After lysis, the slides were placed in a highly alkaline (pH > 13) electrophoresis buffer for 30 min to allow the DNA to unwind followed by electrophoresis for 30 min at 280 A/25 V. The slides were then deactivated by rinsing with a neutralizing buffer (Tris buffer, pH = 7) three times and fixed with 100% ice-cold ethanol for 5 min. In preparation for analysis, the slides were stained with 100 μl of propidium iodide (20 μg/ml) and evaluated under a fluorescent microscope. A total of 150 cell images were examined per treatment under 20× magnification using Kinetic Imaging Komet 5.5 software (Nottingham, UK). The mean olive tail moment (MOTM) was used as a parameter of DNA fragmentation.
2.7. Lipid peroxidation
The determination of the degree of free radicals present in a sample can be measured by the level of peroxides present. Lipid peroxidation is associated with several pathophysical cell and tissue abnormalities, including cancer. Lipid peroxidation is the oxidative degradation of lipids and is considered a basic mechanism of cellular damage induced by free radicals. Lipids were isolated using the Bligh and Dyer method [21]. The isolated lipids were analyzed with the Sigma PeroxiDetect kit. Lipid peroxides are measured with a methanolic reagent containing XO and butylated hydroxytoluene (BHT), an antioxidant that prevents the effects of excess peroxidation. The nmole of peroxide/ml is then calculated from a standard curve with 200 μM tert-BuOOH as a standard. The lipids from cells treated and harvested from T-175 cm2 cell culture flasks. The cellular lipids from 1 × 106 cells were analyzed for lipid peroxidation by using a PeroxiDetect kit (Sigma–Aldrich). A total of 100 μL of isolated lipids from each sample was placed in a clean microcentrifuge tube, followed by 1 ml of Working Color Reagent (4 mM butylated hydroxytoluene and 125 μM xylenol orange in 90% methanol, with:1:100 volume of Ferrous Ammonium Sulfate Reagent (25 mM ferrous ammonium sulfate in 2.5 M sulfuric acid)). The solution was mixed by vortexing and incubated at room temperature for 30–60 min to complete color formation. A Kayak HP 8453 UV–visible Spectroscopy System (Hewlett Packard, Waldbronn, Germany) was used determine the absorbance of each sample at 560 nm.
2.8. Statistical analysis
Results are expressed as means ± SEM from a minimum of three independent experiments. Data were analyzed utilizing one way analysis of variance (ANOVA) and Bonferroni's multiple comparison test to determine significant differences (p < 0.05) between the treatment groups. Dunnett's multiple comparison tests were used as a post hoc analysis to compare the treatments to the control.
3. Results
3.1. Cell viability (MTS assay)
The MTS assay was performed on MCF-10A cells treated to determine alterations in cell viability associated with treatment with DAS and DES for 24 h. Additionally, concomitant treatments containing both DES and DAS were evaluated to determine the effect of DAS on DES induced cell toxicity. Increasing concentrations of DES alone induced dose-dependent decreases in cell viability (Fig. 1A). Furthermore, it was demonstrated that DES at 100 μM caused a 57.3% reduction in cell viability relative to the vehicle control (DMSO). From this data, the LD50 of DES in MCF-10A cells was determined to be 10 μM. Cells treated with both 10 μM DES and 1, 10, or 100 μM DAS resulted in an attenuation of DES-induced cytotoxicity and increased cell viability by 38%, 53% and 76%, respectively (Fig. 1C). As concentrations of DES increased from 1 μM to 100 μM DNA damage increased (Fig. 2A).
Fig. 1.
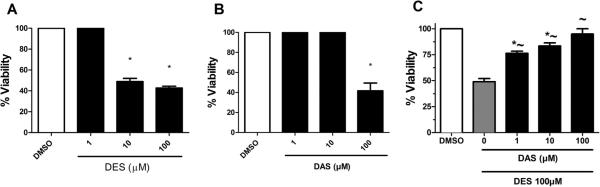
Cell viability of MCF-10A cells treated with 1,10, and 100 μM of (A) DES, (B) DAS, and (C) DES/DES for 24 h. DES concentrations of 10 μM and 100 μM statistically lowered MCF10-A cells viability by ~50%. Only the 100 μM DAS dose reduced cell viability, and all concentrations of DAS attenuated DES-induced reduction in viability of MCF-10A cells. MTS assay was performed as described in Section 2 (n = 3). The cells were normalized to the DMSO vehicle control as 100%. The results are expressed as the mean ± SEM, where * indicates a significance of p < 0.05 relative to the DMSO vehicle control and ~ indicates a significance of p < 0.05 relative to 100 μM DES. DAS is shown to inhibit DES induced cell toxicity, and ~ indicates a significance of p < 0.05 relative to 100 μM DES. DAS is shown to inhibit DES induced cell toxicity.
Fig. 2.
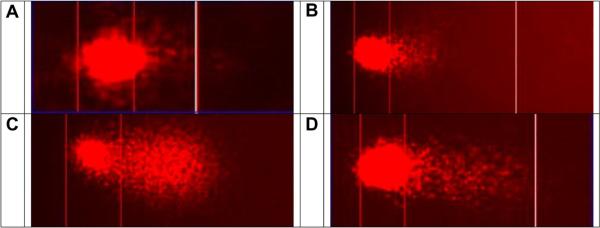
Images of the inhibition of DES induced DNA strand breaks by DAS in MCF-10A cells during Comet assay. (A) Cell treated with DMSO vehicle control; (B) A cell treated with 100 μM DAS; (C) A cell treated with 100 μM DES; (D) a cell treated with 100 μM DES and 100 μM DAS. In the concomitant treatment, DAS attenuates DES-induced DNA strand breaks.
3.2. Alkaline single cell gel electrophoresis (Comet assay)
A representative example of the treatment groups and DNA integrity is illustrated in the DNA strand break propidium iodide illuminated image (Fig. 2). MCF-10A cells were treated with varying concentrations of DES and DAS for 3 h with concomitant treatments of 100 μM DES and increasing doses of DAS for 3 and 24 h (Figs. 3 and 4). DES treatments were conducted to measure the initial DNA damage caused by this carcinogen. DES at concentrations of 1, 10, and 100 μM produced DNA strand breaks in a dose-dependent manner with the 100 μM dose producing a statistically significant increase in DNA damage relative to DMSO (Fig. 3A). From these results, it was determined that the highest concentration of DES would be the most effective in determining DAS attenuation of DES-induced strand breaks. DAS treatments produced strand breaks less than that of the vehicle control, indicating that the garlic organosulfide in this experiment was not a major contributor to DNA damage at 3 h (Fig. 3B). Concomitantly treating cells for 3 h resulted in a significant decrease in DES-induced strand breaks with 1 and 10 μM DAS, however, the 100 μM dose was not effective in attenuating the carcinogen induced strand breaks (Fig. 3C). However, by 24 h, all three concentrations of DAS were effective at attenuating DES-induced DNA strand breaks to levels similar of that of the vehicle and control cells only (Fig. 4).
Fig. 3.
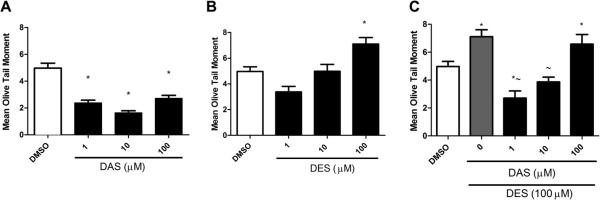
DNA damage of MCF-10A cells with 3 h treatments of 1,10, and 100 μM of (A) DAS, (B) DES, and (C) DES & DAS. DAS did not cause additional DNA single strand breaks, while 100 μM DES increased the mean olive tail moment by ~30%. In concomitant treatments of DAS and 100 μM DES, 1 μM DAS was most effective in attenuating DES induced single strand breaks as measured by mean olive tail moment. COMET assay was performed as described in Section 2. Results are expressed as mean ± SEM. Assays were carried out in triplicate. The asterisk (*) denotes significance p < 0.05 compared to vehicle control, DMSO. The tilda (~) denotes significance p < 0.05 compared to 100 μM DES.
Fig. 4.
DNA damage of MCF-10A cells with 24 h DAS/DES exposure. DAS at all treatment concentrations reduced 100 μM DES-induced DNA single strand breaks. COMET assay was performed as described in Section 2. Results are expressed as mean ± SEM. Assays were carried out in triplicate. The tilda (~) denotes significance p < 0.05 compared to 100 μM DES.
3.3. Lipid peroxidation
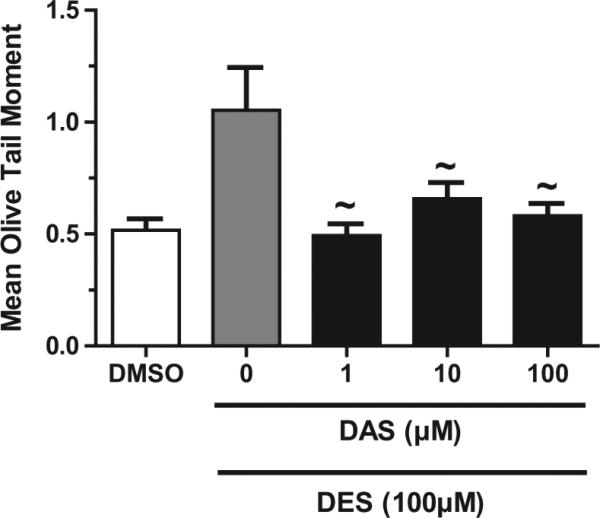
To determine whether DES in the presence or absence of DAS produces reactive oxygen species, cells treated for 3 h were harvested and lipid peroxides were quantified from isolated lipids as a measure of hydrogen peroxide formation. The 100 μM DES positive control produced 1.59 nmoles of peroxide/106 cells, a 279% increase relative to DMSO (Fig. 5). Lipid peroxides were reduced in cells co-treated with 100 μM DES and 1, 10, and 100 μM DAS by 51.2%, 16.5%, and 7.7%, respectively (Fig. 5).
Fig. 5.

Lipid damage in MCF-10A cells with 3 h DAS exposure. 1 μM DAS was most effective at attenuating 100 μM DES induced lipid peroxides. Results are expressed as mean ± SEM. Assays were carried out in triplicate. The asterisk (*) denotes significance p < 0.05 compared to vehicle control DMSO. The tilda (~) denotes significance p < 0.05 compared to 100 μM DES.
4. Discussion
This study presents new evidence that DAS attenuates a DES-induced reduction in MCF-10A cellular viability. The results of our cell viability assays with DES treatments at lower concentrations are consistent with the human embryonic cell viability experiment of Benachour et al. in which cell death was seen with DES concentrations of 20 μM or more [22]. These results are supported by previous studies using Flathead minnows exposed to a synthetic, non-steroidal, pharmaceutical estrogen (DES) for 16 days at an exposure concentration of 20 ng/L, which found that DES elicited a vitellogenic response [23]. This observation led us to conclude that DES effects may be due to its estrogen mimicking activity. The organosulfur compound DAS has previously been shown to reduce the effects of DES in vivo [15]. This study establishes that the same effects are seen in vitro. In Fig. 1B, cell viability was not affected by low concentrations of DAS compared to DMSO, while higher concentrations of DAS decreased cell viability after 24 h of treatment. In studies performed by Wu et al., DAS at low concentrations did not change J5 cell viability (100 μM DAS) after 24 h of exposure as compared to control [24]. However, our studies indicate that the viability of MCF-10A cells was increased by 10% when treated with concentrations as low as 1 μM DAS concomitantly with 10 μM DES for 24 h. When MCF-10A cells were treated with 100 μM DAS and 10 μM DES for 24 h, there was a 36% increase in cell viability.
This study demonstrates that DES produced DNA strand breaks in a dose dependent fashion in MCF-10A cells. Induction of these strand breaks is significant in that it illustrates how DES in normal cells can cause genetic damage that could possibly lead to cancer. Previously it has been shown that DES undergoes redox cycling producing DES quinone and oxygen radicals, which subsequently produce adducts [20]. These DES induced DNA strand breaks could be the results of the production of superoxide via DES redox cycling. This supposition is supported by our data which shows that concomitant treatment of 100 μM DES and DAS had statistically significant (p < 0.05) high mean olive tail moments which is indicative of DNA damage. In addition, the lower concentration of 1 μM DAS also prevented DES-induced DNA strand breaks at the 3 h and 24 h concomitant treatment groups. Higher concentrations of DES/DAS concomitant treatment at 3 h produced significantly more DNA strand breaks compared to the 24 h time period. These results may be attributed to the ability of the cells that have survived the high concomitant treatment for 24 h to undergo DNA repair, leading to fewer observed strand breaks. DAS decreased DNA stand breaks in a dose dependent fashion. This attribute has been detailed in other in vitro and in vivo experiments [20].
The formation of lipid peroxides results in the alteration of lipids leading to the loss of integrity of the membranes. This increases the resulting damage by reducing the integrity of the membranes and damaging DNA, which will lead to deleterious effects following continuous exposure. This study shows that high levels of lipid peroxides were formed in MCF-10A cells treated with 1 μM, 10 μM, or 100 μM DES. Lipid peroxide damage can be attenuated by antioxidants, such as vitamin E, structural separation or low oxygen tension [25,26]. This study focused on DAS as an antioxidant. Lipid peroxide levels were reduced when the MCF-10A cells were concomitantly exposed to DAS and DES. Lipid peroxides were reduced in the concomitant treatment of cells with 100 μM DES and 1, 10 or 100 μM DAS at 51.2%, 16.5%, and 7.7%, respectively.
Ultimately, moderate concentrations of DAS in vitro have beneficial effects as a chemopreventive agent due to its ability to inhibit DNA damage produced by estrogenic chemicals similar to DES. These results validate the effectiveness of DAS at decreasing DNA strand breaks as well as reducing the deleterious effects of lipid peroxides at all experimental concentrations. The lowest concentration of DAS used in these studies was the most effective in reducing the level of DNA damage. This result correlates with research seen in rat liver hepatocytes, although the level of protection from xenobiotics is higher in liver cells. This effect was documented with a decrease in viability with higher doses (5 mM) of DAS [27]. This study is a reinforcing statement describing the lipid peroxide reducing capabilities of DAS in MCF-10A exposed to DES. Since DES is an effective estrogen mimic, [28,29], inferences can also be made about the effectiveness of DAS to reduce endogenous estrogen related lipid peroxides. Further research is needed to investigate the usefulness of DAS as a therapeutic or chemopreventive agent.
Acknowledgements
This research was funded in part by NIH Programs RCMI #5G12-RR-003020 and ARCH #5S11ES011182-05.
References
- 1.Feigelson H, Henderson B. Estrogens and breast cancer. Carcinogenesis. 1996;17(11):2279–84. doi: 10.1093/carcin/17.11.2279. [DOI] [PubMed] [Google Scholar]
- 2.Henderson BE, Feigelson HS. Hormonal carcinogenesis. Carcinogenesis. 2000;21(3):427–33. doi: 10.1093/carcin/21.3.427. [DOI] [PubMed] [Google Scholar]
- 3.Henderson BE, Ross R, Bernstein L. Estrogens as a cause of human cancer: the Richard and Hinda Rosenthal Foundation award lecture. Cancer Res. 1988;48(2):246–53. [PubMed] [Google Scholar]
- 4.Nutter L, Ngo E, Abul-Hajj YJ. Characterization of DNA damage induced by 3,4-estrone-o-quinone in human cells. J Biol Chem. 1991;266(25):16380–6. [PubMed] [Google Scholar]
- 5.Cavalieri E, Rogan E, Chakravarti D, Helmut S, Lester P. The role of endogenous catechol quinones in the initiation of cancer and neurodegenerative diseases, in methods in enzymology. Academic Press; 2004. pp. 293–319. [DOI] [PubMed] [Google Scholar]
- 6.Cavalieri EL, Stack DE, Devanesan PD, Todorovic R, Dwivedy I, Higginbotham S, et al. Molecular origin of cancer: catechol estrogen-3,4-quinones as endogenous tumoric initiators. Proc Natl Acad Sci USA. 1997;94(20):10937–42. doi: 10.1073/pnas.94.20.10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roy D, Thomas R. Catalysis of the oxidation and reduction reactions of steroid and stilbene estrogens by nuclear enzymes. Arch Biochem Biophys. 1994;315(2):310–6. doi: 10.1006/abbi.1994.1505. [DOI] [PubMed] [Google Scholar]
- 8.Liehr J, Roy D. Free radical generation by redox-cycling of estrogens. Free Radic Biol Med. 1990;8:415–23. doi: 10.1016/0891-5849(90)90108-u. [DOI] [PubMed] [Google Scholar]
- 9.Roy D, Liehr JG. Catalysis of the oxidation of steroid and stilbene estrogens to estrogen quinone metabolites by the beta-naptaflavone-inducable cytrochrome p459Ia family. Arch Biochem Biophys. 1992;296:450–6. doi: 10.1016/0003-9861(92)90596-o. [DOI] [PubMed] [Google Scholar]
- 10.Liehr JG, DaGue BB, Ballatore AM, Henkin J. Diethylstilbestrol (DES) quinone: a reactive intermediate in DES metabolis. Biochem Pharmacol. 1983;32(24):3711–8. doi: 10.1016/0006-2952(83)90139-9. [DOI] [PubMed] [Google Scholar]
- 11.Liehr JG, Roy D. Free radical generation by redox cycling of estrogens. Free Radic Biol Med. 1990;8(4):415–23. doi: 10.1016/0891-5849(90)90108-u. [DOI] [PubMed] [Google Scholar]
- 12.Boyd NF, McGuire V. The possible role of lipid peroxidation in breast cancer risk. Free Radic Biol Med. 1991;10(3–4):185–90. doi: 10.1016/0891-5849(91)90074-d. [DOI] [PubMed] [Google Scholar]
- 13.Cavalieri E, Frenkel K, Liehr JG, Rogan E, Roy D. Chapter 4: Estrogens as Endogenous Genotoxic Agents–DNA Adducts and Mutations. J Natl Cancer Inst Monogr. 2000;2000(27):75–94. doi: 10.1093/oxfordjournals.jncimonographs.a024247. [DOI] [PubMed] [Google Scholar]
- 14.Cavalieri E, Chakravarti D, Guttenplan J, Hart E, Ingle J, Jankowiak R, et al. Catechol estrogen quinones as initiators of breast and other human cancers: implications for biomarkers of susceptibility and cancer prevention. Biochim Biophys Acta BBA Rev Cancer. 2006;1766(1):63–78. doi: 10.1016/j.bbcan.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Green M, Newell O, Aboyade-Cole A, Darling-Reed S, Thomas RD. Diallyl sulfide induces the expression of estrogen metabolizing genes in the presence and/or absence of diethylstilbestrol in the breast of female ACI rats. Toxicol Lett. 2007;168(1):7–12. doi: 10.1016/j.toxlet.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Thomas R, Roy D. Mitochondrial enzyme-catalyzed oxidation and reduction reactions of stilbene estrogen. Carcinogenesis. 1995;16(4):891–5. doi: 10.1093/carcin/16.4.891. [DOI] [PubMed] [Google Scholar]
- 17.Thomas R, Green M, Wilson C, Sadrud-Din S. Diallyl sulfide inhibits the oxidation and reduction reactions of stilbene estrogens catalyzed by microsomes, mitochondria and nuclei isolated from breast tissue of female ACI rats. Carcinogenesis. 2004;25(5):787–91. doi: 10.1093/carcin/bgg161. [DOI] [PubMed] [Google Scholar]
- 18.Tauberta D, Glöckner R, Müllerb D. The garlic ingredient diallyl sulfide inhibits cytochrome p450 2E1 dependent bioactivation of acrylamide to glycidamide. Toxicol Lett. 2006;164(1):1–5. doi: 10.1016/j.toxlet.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Jin L, Baillie T. Metabolism of the chemoprotective agent Diallyl sulfide to Glutathione conjugates in rats. Chem Res Toxicol. 1997;10(3):318–27. doi: 10.1021/tx9601768. [DOI] [PubMed] [Google Scholar]
- 20.Green M, Wilson C, Sakeenah S-D, Thomas R. Diallyl sulfide inhibits the oxidation and reaction and reduction reactions of stilbene estrogens catalyzed by microsomes, mitochondria and nuclei isolated from breast tissue of female ACI rats. Carcinogenesis. 2004;25(5):787–91. doi: 10.1093/carcin/bgg161. [DOI] [PubMed] [Google Scholar]
- 21.Benachour N, Moslemi S, Sipahutar H, Seralini G-E. Cytotoxic effects and aromatase inhibition by xenobiotic endocrine disrupters alone and in combination. Toxicol Appl Pharmacol. 2007;222(2):129–40. doi: 10.1016/j.taap.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 22.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–7. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 23.Folmar L, Hemmer R, Hemmer C, Bowman KK, Denslow ND. Comparative estrogenicity of estradiol, ethynyl estradiol and diethylstilbestrol in an in vivo, male sheepshead minnow (Cyprinodon variegatus), vitellogenin bioassay. Aquat Toxicol. 2000;49(1–2):77–88. doi: 10.1016/s0166-445x(99)00076-4. [DOI] [PubMed] [Google Scholar]
- 24.Wu C, Chung J, Tsai S, Yang J, Sheen L. Differential effects of allyl sulfides from garlic essential oil on cell cycle regulation in human liver tumor cells. Food Chem Toxicol. 2004;42(12):1937–47. doi: 10.1016/j.fct.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Kohen R, Nyska A. Invited review: oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol. 2002;30(6):620–50. doi: 10.1080/01926230290166724. [DOI] [PubMed] [Google Scholar]
- 26.Clark SF. The biochemistry of antioxidants revisited. Nutr Clin Pract. 2002;17(1):5–17. doi: 10.1177/011542650201700105. [DOI] [PubMed] [Google Scholar]
- 27.Sheen L, Lii C, Sheu S, Meng RHC, Tsai SJ. Effect of the active principle of garlic–Diallyl sulfide–On cell viability, detoxification capability and the antioxidation system of primary rat hepatocytes. Food Chem Toxicol. 1996;34(10):971–8. doi: 10.1016/s0278-6915(96)00066-x. [DOI] [PubMed] [Google Scholar]
- 28.Colburn T, vom Saal F, Soto AM. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect. 1993;101(378):4. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saeed M, Rogan E, Cavalieri E. Mechanism of tumor initiation by the human carcinogen diethylstilbestrol: the defining link to natural estrogens. AACR Meeting Abstr. 2005;2005(1):499. doi: 10.1002/ijc.24113. [DOI] [PMC free article] [PubMed] [Google Scholar]


