Abstract
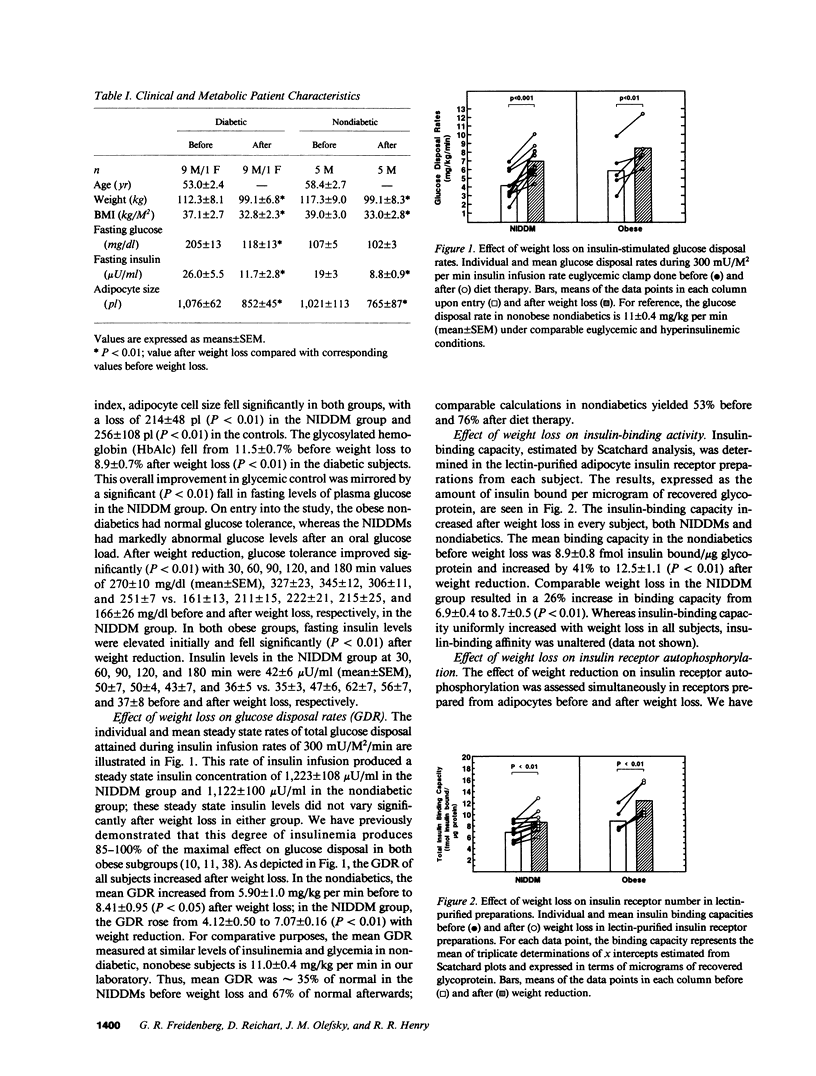
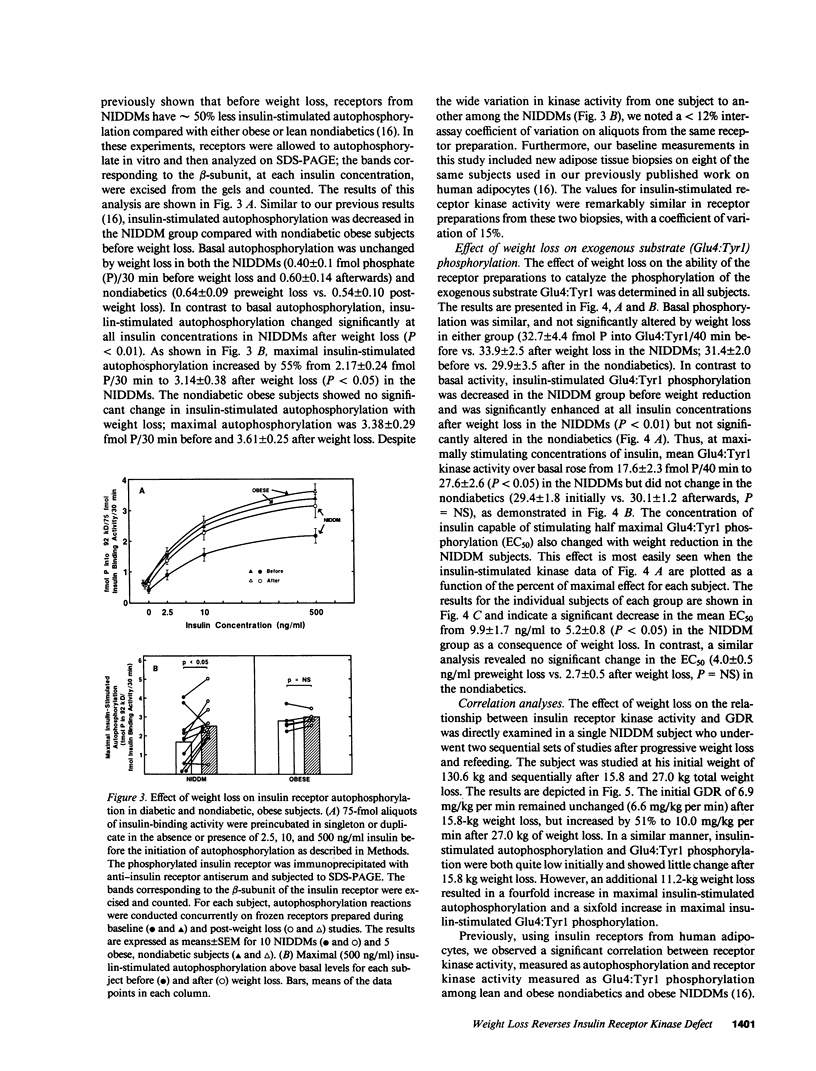
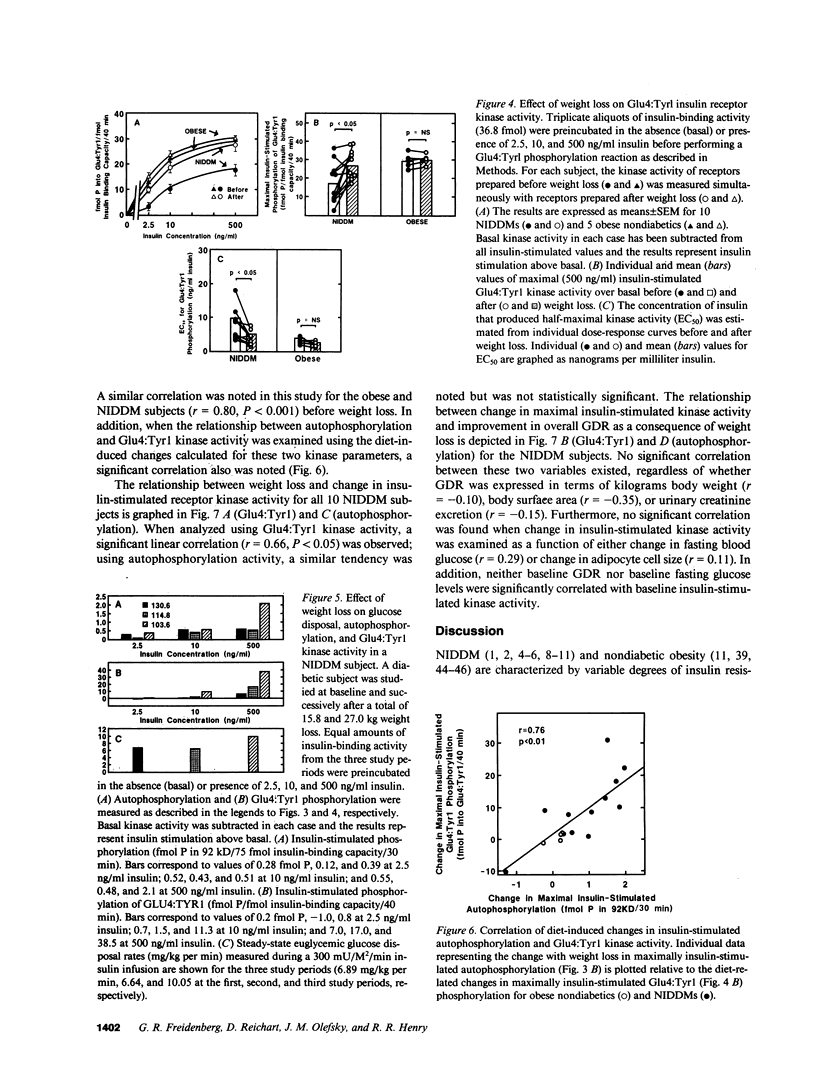
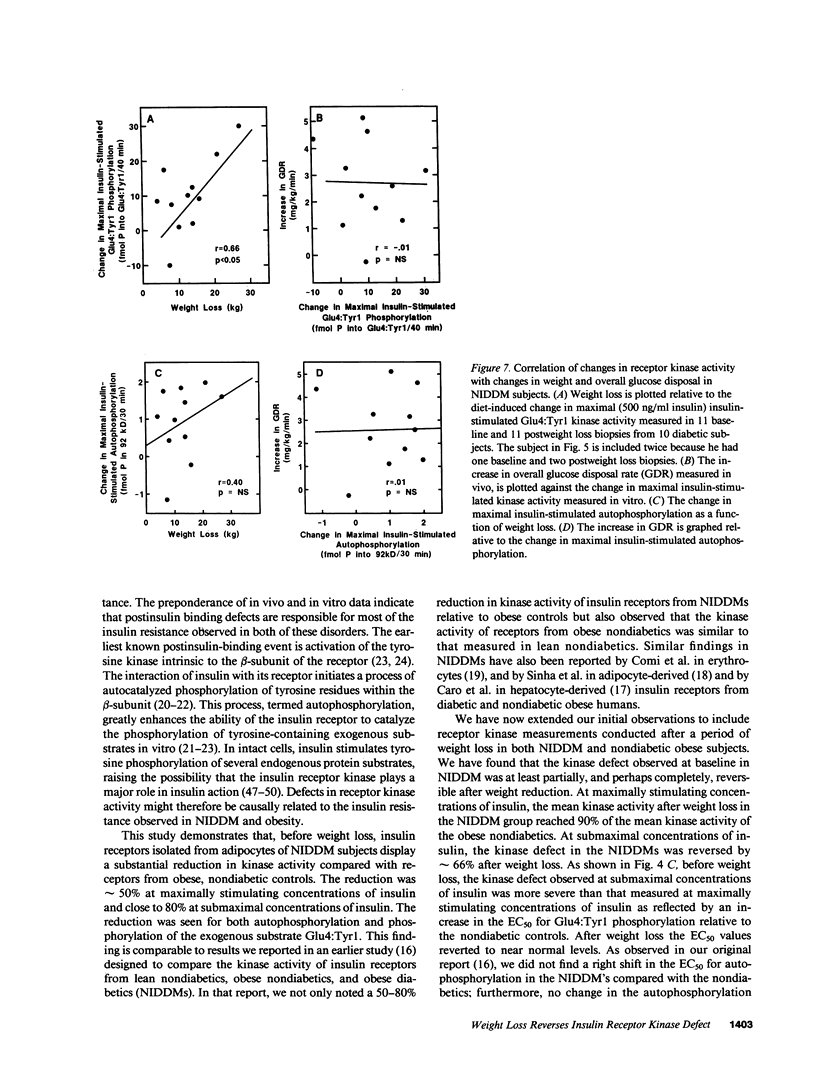
Insulin-stimulated kinase activity of adipocyte-derived insulin receptors is reduced in subjects with non-insulin-dependent diabetes mellitus (NIDDM) but normal in obese nondiabetics. To assess the reversibility of the kinase defect in NIDDM, insulin receptor kinase activity was measured before and after weight loss in 10 NIDDM and 5 obese nondiabetic subjects. Peripheral insulin action was also assessed in vivo by glucose disposal rates (GDR) measured during a hyperinsulinemic (300 mU/M2 per min) euglycemic clamp. In the NIDDMs, insulin receptor kinase activity was reduced by 50-80% and rose to approximately 65-90% (P less than 0.01) of normal after 13.2 +/- 2.0 kg (P less than 0.01) weight loss; comparable weight loss (18.2 +/- 1.5 kg, P less than 0.01) in the nondiabetics resulted in no significant change in insulin receptor kinase activity. Relative to GDR measured in lean nondiabetics, GDR in the NIDDMs was 35% of normal initially and 67% (P less than 0.01) of normal after diet therapy; weight loss in the nondiabetics resulted in an increase in GDR from 53 to 76% of normal (P less than 0.05). These results indicate that the insulin receptor kinase defect that is present in NIDDM is largely reversible after weight reduction. In contrast, the improvement in GDR, in the absence of any change in insulin receptor kinase activity in the nondiabetics, suggests that the main cause of insulin resistance in obesity lies distal to the kinase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arner P., Pollare T., Lithell H., Livingston J. N. Defective insulin receptor tyrosine kinase in human skeletal muscle in obesity and type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1987 Jun;30(6):437–440. doi: 10.1007/BF00292549. [DOI] [PubMed] [Google Scholar]
- Beck-Nielsen H., Pedersen O., Sørensen N. S. Effects of dietary changes on cellular insulin binding and in vivo insulin sensitivity. Metabolism. 1980 May;29(5):482–487. doi: 10.1016/0026-0495(80)90174-2. [DOI] [PubMed] [Google Scholar]
- Bernier M., Laird D. M., Lane M. D. Insulin-activated tyrosine phosphorylation of a 15-kilodalton protein in intact 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1844–1848. doi: 10.1073/pnas.84.7.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollag G. E., Roth R. A., Beaudoin J., Mochly-Rosen D., Koshland D. E., Jr Protein kinase C directly phosphorylates the insulin receptor in vitro and reduces its protein-tyrosine kinase activity. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5822–5824. doi: 10.1073/pnas.83.16.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burant C. F., Treutelaar M. K., Buse M. G. Diabetes-induced functional and structural changes in insulin receptors from rat skeletal muscle. J Clin Invest. 1986 Jan;77(1):260–270. doi: 10.1172/JCI112285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro J. F., Ittoop O., Pories W. J., Meelheim D., Flickinger E. G., Thomas F., Jenquin M., Silverman J. F., Khazanie P. G., Sinha M. K. Studies on the mechanism of insulin resistance in the liver from humans with noninsulin-dependent diabetes. Insulin action and binding in isolated hepatocytes, insulin receptor structure, and kinase activity. J Clin Invest. 1986 Jul;78(1):249–258. doi: 10.1172/JCI112558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro J. F., Sinha M. K., Raju S. M., Ittoop O., Pories W. J., Flickinger E. G., Meelheim D., Dohm G. L. Insulin receptor kinase in human skeletal muscle from obese subjects with and without noninsulin dependent diabetes. J Clin Invest. 1987 May;79(5):1330–1337. doi: 10.1172/JCI112958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou C. K., Dull T. J., Russell D. S., Gherzi R., Lebwohl D., Ullrich A., Rosen O. M. Human insulin receptors mutated at the ATP-binding site lack protein tyrosine kinase activity and fail to mediate postreceptor effects of insulin. J Biol Chem. 1987 Feb 5;262(4):1842–1847. [PubMed] [Google Scholar]
- Ciaraldi T. P., Kolterman O. G., Scarlett J. A., Kao M., Olefsky J. M. Role of glucose transport in the postreceptor defect of non-insulin-dependent diabetes mellitus. Diabetes. 1982 Nov;31(11):1016–1022. doi: 10.2337/diacare.31.11.1016. [DOI] [PubMed] [Google Scholar]
- Colca J. R., DeWald D. B., Pearson J. D., Palazuk B. J., Laurino J. P., McDonald J. M. Insulin stimulates the phosphorylation of calmodulin in intact adipocytes. J Biol Chem. 1987 Aug 25;262(24):11399–11402. [PubMed] [Google Scholar]
- Comi R. J., Grunberger G., Gorden P. Relationship of insulin binding and insulin-stimulated tyrosine kinase activity is altered in type II diabetes. J Clin Invest. 1987 Feb;79(2):453–462. doi: 10.1172/JCI112833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A., Ferrannini E., Koivisto V. New concepts in the pathogenesis and treatment of noninsulin-dependent diabetes mellitus. Am J Med. 1983 Jan 17;74(1A):52–81. doi: 10.1016/0002-9343(83)90654-x. [DOI] [PubMed] [Google Scholar]
- DeFronzo R., Deibert D., Hendler R., Felig P., Soman V. Insulin sensitivity and insulin binding to monocytes in maturity-onset diabetes. J Clin Invest. 1979 May;63(5):939–946. doi: 10.1172/JCI109394. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Desbuquois B., Aurbach G. D. Use of polyethylene glycol to separate free and antibody-bound peptide hormones in radioimmunoassays. J Clin Endocrinol Metab. 1971 Nov;33(5):732–738. doi: 10.1210/jcem-33-5-732. [DOI] [PubMed] [Google Scholar]
- Ebina Y., Araki E., Taira M., Shimada F., Mori M., Craik C. S., Siddle K., Pierce S. B., Roth R. A., Rutter W. J. Replacement of lysine residue 1030 in the putative ATP-binding region of the insulin receptor abolishes insulin- and antibody-stimulated glucose uptake and receptor kinase activity. Proc Natl Acad Sci U S A. 1987 Feb;84(3):704–708. doi: 10.1073/pnas.84.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Freidenberg G. R., Henry R. R., Klein H. H., Reichart D. R., Olefsky J. M. Decreased kinase activity of insulin receptors from adipocytes of non-insulin-dependent diabetic subjects. J Clin Invest. 1987 Jan;79(1):240–250. doi: 10.1172/JCI112789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freidenberg G. R., Klein H. H., Cordera R., Olefsky J. M. Insulin receptor kinase activity in rat liver. Regulation by fasting and high carbohydrate feeding. J Biol Chem. 1985 Oct 15;260(23):12444–12453. [PubMed] [Google Scholar]
- Garvey W. T., Huecksteadt T. P., Matthaei S., Olefsky J. M. Role of glucose transporters in the cellular insulin resistance of type II non-insulin-dependent diabetes mellitus. J Clin Invest. 1988 May;81(5):1528–1536. doi: 10.1172/JCI113485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorescu F., Flier J. S., Kahn C. R. Defect in insulin receptor phosphorylation in erythrocytes and fibroblasts associated with severe insulin resistance. J Biol Chem. 1984 Dec 25;259(24):15003–15006. [PubMed] [Google Scholar]
- Grunberger G., Comi R. J., Taylor S. I., Gorden P. Tyrosine kinase activity of the insulin receptor of patients with type A extreme insulin resistance: studies with circulating mononuclear cells and cultured lymphocytes. J Clin Endocrinol Metab. 1984 Dec;59(6):1152–1158. doi: 10.1210/jcem-59-6-1152. [DOI] [PubMed] [Google Scholar]
- Grunberger G., Zick Y., Gorden P. Defect in phosphorylation of insulin receptors in cells from an insulin-resistant patient with normal insulin binding. Science. 1984 Mar 2;223(4639):932–934. doi: 10.1126/science.6141638. [DOI] [PubMed] [Google Scholar]
- Harrison L. C., King-Roach A. P. Insulin sensitivity of adipose tissue in vitro and the response to exogenous insulin in obese human subjects. Metabolism. 1976 Oct;25(10):1095–1101. doi: 10.1016/0026-0495(76)90017-2. [DOI] [PubMed] [Google Scholar]
- Henry R. R., Wallace P., Olefsky J. M. Effects of weight loss on mechanisms of hyperglycemia in obese non-insulin-dependent diabetes mellitus. Diabetes. 1986 Sep;35(9):990–998. doi: 10.2337/diab.35.9.990. [DOI] [PubMed] [Google Scholar]
- Hughes T. A., Gwynne J. T., Switzer B. R., Herbst C., White G. Effects of caloric restriction and weight loss on glycemic control, insulin release and resistance, and atherosclerotic risk in obese patients with type II diabetes mellitus. Am J Med. 1984 Jul;77(1):7–17. doi: 10.1016/0002-9343(84)90429-7. [DOI] [PubMed] [Google Scholar]
- Häring H. U., White M. F., Machicao F., Ermel B., Schleicher E., Obermaier B. Insulin rapidly stimulates phosphorylation of a 46-kDa membrane protein on tyrosine residues as well as phosphorylation of several soluble proteins in intact fat cells. Proc Natl Acad Sci U S A. 1987 Jan;84(1):113–117. doi: 10.1073/pnas.84.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez J., Zuniga-Guajardo S., Zinman B., Angel A. Effects of weight loss in massive obesity on insulin and C-peptide dynamics: sequential changes in insulin production, clearance, and sensitivity. J Clin Endocrinol Metab. 1987 Apr;64(4):661–668. doi: 10.1210/jcem-64-4-661. [DOI] [PubMed] [Google Scholar]
- Jochen A. L., Berhanu P., Olefsky J. M. Insulin internalization and degradation in adipocytes from normal and type II diabetic subjects. J Clin Endocrinol Metab. 1986 Feb;62(2):268–274. doi: 10.1210/jcem-62-2-268. [DOI] [PubMed] [Google Scholar]
- Kadowaki T., Kasuga M., Akanuma Y., Ezaki O., Takaku F. Decreased autophosphorylation of the insulin receptor-kinase in streptozotocin-diabetic rats. J Biol Chem. 1984 Nov 25;259(22):14208–14216. [PubMed] [Google Scholar]
- Kashiwagi A., Verso M. A., Andrews J., Vasquez B., Reaven G., Foley J. E. In vitro insulin resistance of human adipocytes isolated from subjects with noninsulin-dependent diabetes mellitus. J Clin Invest. 1983 Oct;72(4):1246–1254. doi: 10.1172/JCI111080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga M., Zick Y., Blithe D. L., Crettaz M., Kahn C. R. Insulin stimulates tyrosine phosphorylation of the insulin receptor in a cell-free system. Nature. 1982 Aug 12;298(5875):667–669. doi: 10.1038/298667a0. [DOI] [PubMed] [Google Scholar]
- Klein H. H., Freidenberg G. R., Kladde M., Olefsky J. M. Insulin activation of insulin receptor tyrosine kinase in intact rat adipocytes. An in vitro system to measure histone kinase activity of insulin receptors activated in vivo. J Biol Chem. 1986 Apr 5;261(10):4691–4697. [PubMed] [Google Scholar]
- Kolterman O. G., Gray R. S., Griffin J., Burstein P., Insel J., Scarlett J. A., Olefsky J. M. Receptor and postreceptor defects contribute to the insulin resistance in noninsulin-dependent diabetes mellitus. J Clin Invest. 1981 Oct;68(4):957–969. doi: 10.1172/JCI110350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolterman O. G., Insel J., Saekow M., Olefsky J. M. Mechanisms of insulin resistance in human obesity: evidence for receptor and postreceptor defects. J Clin Invest. 1980 Jun;65(6):1272–1284. doi: 10.1172/JCI109790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Marchand-Brustel Y., Grémeaux T., Ballotti R., Van Obberghen E. Insulin receptor tyrosine kinase is defective in skeletal muscle of insulin-resistant obese mice. Nature. 1985 Jun 20;315(6021):676–679. doi: 10.1038/315676a0. [DOI] [PubMed] [Google Scholar]
- Lockwood D. H., Amatruda J. M. Cellular alterations responsible for insulin resistance in obesity and type II diabetes mellitus. Am J Med. 1983 Nov 30;75(5B):23–31. doi: 10.1016/0002-9343(83)90250-4. [DOI] [PubMed] [Google Scholar]
- Mandarino L. J., Campbell P. J., Gottesman I. S., Gerich J. E. Abnormal coupling of insulin receptor binding in noninsulin-dependent diabetes. Am J Physiol. 1984 Nov;247(5 Pt 1):E688–E692. doi: 10.1152/ajpendo.1984.247.5.E688. [DOI] [PubMed] [Google Scholar]
- McClain D. A., Maegawa H., Lee J., Dull T. J., Ulrich A., Olefsky J. M. A mutant insulin receptor with defective tyrosine kinase displays no biologic activity and does not undergo endocytosis. J Biol Chem. 1987 Oct 25;262(30):14663–14671. [PubMed] [Google Scholar]
- Olefsky J. M., Ciaraldi T. P., Kolterman O. G. Mechanisms of insulin resistance in non-insulin-dependent (type II) diabetes. Am J Med. 1985 Sep 20;79(3B):12–22. doi: 10.1016/s0002-9343(85)80003-6. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M. LIlly lecture 1980. Insulin resistance and insulin action. An in vitro and in vivo perspective. Diabetes. 1981 Feb;30(2):148–162. doi: 10.2337/diab.30.2.148. [DOI] [PubMed] [Google Scholar]
- Pang D. T., Sharma B. R., Shafer J. A., White M. F., Kahn C. R. Predominance of tyrosine phosphorylation of insulin receptors during the initial response of intact cells to insulin. J Biol Chem. 1985 Jun 10;260(11):7131–7136. [PubMed] [Google Scholar]
- Pfeifer M. A., Halter J. B., Porte D., Jr Insulin secretion in diabetes mellitus. Am J Med. 1981 Mar;70(3):579–588. doi: 10.1016/0002-9343(81)90579-9. [DOI] [PubMed] [Google Scholar]
- RABINOWITZ D., ZIERLER K. L. Forearm metabolism in obesity and its response to intra-arterial insulin. Characterization of insulin resistance and evidence for adaptive hyperinsulinism. J Clin Invest. 1962 Dec;41:2173–2181. doi: 10.1172/JCI104676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza R. A., Mandarino L. J., Gerich J. E. Mechanism and significance of insulin resistance in non-insulin-dependent diabetes mellitus. Diabetes. 1981 Dec;30(12):990–995. doi: 10.2337/diab.30.12.990. [DOI] [PubMed] [Google Scholar]
- Rosen O. M., Herrera R., Olowe Y., Petruzzelli L. M., Cobb M. H. Phosphorylation activates the insulin receptor tyrosine protein kinase. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3237–3240. doi: 10.1073/pnas.80.11.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha M. K., Pories W. J., Flickinger E. G., Meelheim D., Caro J. F. Insulin-receptor kinase activity of adipose tissue from morbidly obese humans with and without NIDDM. Diabetes. 1987 May;36(5):620–625. doi: 10.2337/diab.36.5.620. [DOI] [PubMed] [Google Scholar]
- Takayama S., Kahn C. R., Kubo K., Foley J. E. Alterations in insulin receptor autophosphorylation in insulin resistance: correlation with altered sensitivity to glucose transport and antilipolysis to insulin. J Clin Endocrinol Metab. 1988 May;66(5):992–999. doi: 10.1210/jcem-66-5-992. [DOI] [PubMed] [Google Scholar]
- Yu K. T., Czech M. P. Tyrosine phosphorylation of the insulin receptor beta subunit activates the receptor-associated tyrosine kinase activity. J Biol Chem. 1984 Apr 25;259(8):5277–5286. [PubMed] [Google Scholar]



