Abstract
INTRODUCTION
Genetics is behind our circadian machinery. CLOCK (Circadian Locomotor Output Cycles Kaput) 3111T/C single-nucleotide polymorphism (SNP) has been previously related to obesity and weight loss. However, phenotypic association and functionality of CLOCK 3111 locus is still unknown. The aim of this study was to determine, in free-living conditions, if the presence of CLOCK 3111C in overweight women could be related to (a) circadian disorders, and (b) changes in sleep quality, to improve understanding of the previously demonstrated associations with obesity and reduced weight loss of the C carriers.
METHODS
Wrist temperature, actimetry and position (TAP) and TAP variables were measured as markers of circadian functionality during 8 consecutive days. A rest–activity and food diary was also completed, whereas sleep quality was determined by domiciliary polysomnography. We recruited 85 women who were overweight with body mass index (BMI) of 28.59 ± 4.30 kgm−2 and age 43 ± 12 years. From this sample, we found that 43 women were carrying the minor allele (C) for CLOCK 3111T/C SNP and 42 women were TT carriers (major allele carriers). Both groups of patients were matched for number, age, obesity parameters and energy intake.
RESULTS
Compared with TT subjects, who showed more robust circadian rhythm profiles, patients with the C allele displayed significant circadian abnormalities: lower amplitude and greater fragmentation of the rhythm, a less stable circadian pattern and a significantly weakened circadian function, as assessed by the circadian function index (CFI). C subjects were also less active, started their activities later in the morning and were sleepier during the day, showing a delayed acrophase that characterizes ‘evening-type’ subjects.
CONCLUSION
C genetic variants in CLOCK 3111T/C display a less robust circadian rhythm than TT and a delayed acrophase that characterizes ‘evening-type’ subjects. We support the notion that identifying CLOCK genotypes in patients may assist the therapist in characterization of the roots of the metabolic problem.
Keywords: CLOCK, single-nucleotide polymorphism, 3111TC, circadian
INTRODUCTION
Our circadian timekeeping machinery is driven by a complex genetic network of checks and balances. The great inter-individual differences observed in chronotype, responses to sleep curtailment and association with obesity point to an underlying genetic component, and some limited data suggest that genetic variation at loci involved in circadian regulation may underlie these large phenotypic differences.
In this regard, genetic variation at the CLOCK (Circadian Locomotor Output Cycles Kaput), a key driver of the circadian rhythm, has been related to psychological alterations and eveningness,1–5 and one particular CLOCK single-nucleotide polymorphism (SNP) within the 3′-untranslated terminal region (3111T/C) has been associated with obesity.6 Moreover, this SNP has been associated with weight loss. Thus, carriers of the C allele displayed greater difficulty in losing weight than non-carriers.7 It has been proposed that the relationship between this SNP and weight loss could be mediated by sleep reduction and eveningness,8 as well as sleep quality;9 however, these findings are controversial partly owing to the fact that they are mostly performed with sleep quality questionnaires and not with polysomnography, considered the reference technique for this purpose. In addition, we might also postulate that CLOCK 3111T/C minor allele carriers (C) could be suffering from circadian misalignments, such as delayed or advanced sleep phase syndrome, free running syndrome or irregular circadian rhythms, that could also affect obesity and weight loss.
To evaluate and diagnose circadian disorders, actimetry has been considered the method of choice though, as with any other measurement, it is subject to masking and artifacts.10–11 Recently, the rhythm of skin peripheral temperature has been proposed as a marker in obesity.12 A more in-depth method, called the temperature, actimetry and position (TAP) algorithm, has been also proposed by Ortiz-Tudela et al.13 This measurement is based on integrating, after normalization, the following variables: skin temperature, motor activity and body position. The first of these variables, skin temperature, is mostly under endogenous control, while motor activity is modified voluntarily, but it has also an endogenous component. Lastly, of the three variables used for the integration, body position is the most closely dependent on voluntary control. These rhythmic variables considered to be circadian markers have artifacts and masking factors, as used separately. However, the combination of these measurements in one algorithm, TAP, diminishes the presence of masking factors, and converts this measurement in a suitable tool to evaluate the status of the human circadian system under normal living conditions.13
The aim of this study was to define, in free-living conditions, whether the presence of this particular genetic variant CLOCK 3111C in overweight or moderate obese women could be related to (a) circadian disorders using temperature, actimetry position and TAP variables as a marker of circadian disruption, and (b) changes in sleep quality as assessed by domiciliary polysomnography. Our primary objectives are to improve understanding of the previously demonstrated associations with obesity and reduced weight loss observed in CLOCK 3111C carriers.
METHODS
Study population
We recruited 85 women who were overweight with body mass index (BMI) of 28.6 ± 4.3 kgm−2 and age 43 ± 12 years. Subjects were randomly selected from a previous study in which we investigated the association between CLOCK genes and weight loss.7,8,14 From this sample, 43 women were carrying the CLOCK 3111C allele (CC and TC) and 42 women were TT carriers. C carriers were pooled for analyses based on previous studies showing that C/C had characteristics similar to that of T/C subjects.15 Both genotype-based groups were matched for age, obesity parameters, energy intake (Table 1) and menopausal status (27% were postmenopausal).
Table 1.
General characteristics of minor (TC and CC) and major (TT) allele carriers of CLOCK 3111T/C in women
| Obesity parameters | CLOCK 3111T>C | P-value | |||
|---|---|---|---|---|---|
| Minor allele carriers (TC + CC) n = 43 | Major allele carriers (TT) n = 42 | ||||
| Mean | s.d. | Mean | s.d. | ||
| Age (years) | 41.9 | 12.8 | 44.3 | 11.8 | 0.385 |
| Height (m) | 1.64 | 0.06 | 1.63 | 0.06 | 0.471 |
| Weight (kg) | 75.64 | 9.74 | 77.34 | 14.07 | 0.518 |
| BMI (kg m−2) | 28.1 | 3.6 | 29.1 | 4.9 | 0.297 |
| Waist (cm) | 89.2 | 11.3 | 92.9 | 11.3 | 0.135 |
| Hip (cm) | 108.5 | 9.5 | 110.3 | 10.6 | 0.419 |
| Body fat (%) | 35.3 | 4.3 | 36.1 | 5.8 | 0.488 |
| Glucose (mg dl−1) | 84 | 17 | 88 | 14 | 0.341 |
| Insulin (µUI ml−1) | 9.1 | 10.8 | 7.0 | 7.0 | 0.301 |
| Total triglicerydes (mg dl−1) | 105 | 49 | 96 | 53 | 0.442 |
| Total cholesterol (mg dl−1) | 193 | 25 | 186 | 28 | 0.302 |
| HDL cholesterol (mg dl−1) | 58.5 | 13.8 | 53.0 | 14.6 | 0.085 |
| LDL cholesterol (mg dl−1) | 113 | 22 | 115 | 26 | 0.783 |
| Leptin (ng ml−1) | 21.3 | 10.2 | 21.6 | 14.5 | 0.918 |
| adiponectin (ng ml−1) | 37.4 | 13.2 | 43.4 | 19.4 | 0.196 |
| IL-6 (pg ml−1) | 25.7 | 5.2 | 25.6 | 6.3 | 0.924 |
| Total energy intake (kcal) | 2078 | 771 | 1818 | 578 | 0.486 |
Abbreviations: BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein; IL, interleukin; s.d., standard deviation.
All subjects attended outpatient obesity clinics in the city of Murcia, located in southeastern Spain. Patients receiving thermogenic, lipogenic, sleep drugs or melatonin, or those diagnosed with insomnia, cognitive disorders, diabetes mellitus, chronic renal failure, hepatic diseases or cancer were excluded from the study. All procedures were in accordance with good clinical practice and patient data were codified to guarantee anonymity. The written informed consent was obtained before subjects were included in the study and was performed in accordance with the Helsinki Declaration of Human Studies and approved by the Ethical Committee of the University of Murcia (Murcia, Spain).
Obesity-related parameters
Body weight was determined in barefooted subjects wearing light clothes using a digital scale accurate to the nearest 0.1 kg. Height was measured using a Harpenden digital stadiometer (rank, 0.7–2.05). Height and weight measurements were always obtained at the same time of day. BMI was calculated as weight (kg)/height (m2)
Total body fat was measured by bioelectrical impedance, using TANITA TBF-300 (Tanita Corporation of America, Arlington Heights, IL, USA) equipment. Subjects were requested not to drink liquids during the 2 h before the measurement.
DNA isolation and CLOCK genotyping
DNA was isolated from blood samples using standard procedures (Qiagen, Valencia, CA, USA). We performed genotyping of the CLOCK 3111T/C SNP using a TaqMan assay with allele-specific probes on the ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA, USA) according to the standardized laboratory protocols.16
Dietary intake and biochemical analyses
Initial energy and nutrient intake was determined by means of a 24 h dietary recall, whereas biochemical markers were measured in fasting blood samples at the beginning of the treatment as described previously.17
Assessment of the circadian system status
Chronotype test: Horne and Ostberg questionnaire
Subjects completed the Horne and Ostberg18 questionnaire to assess the morningness–eveningness. This questionnaire establishes five behavioral categories: definitively morning types (score = 70–86), moderately morning types (score = 59–69), neither types (score = 42–58), moderately evening types (score = 31–41) and definitively evening types (score = 16–30). For the purpose of this study, we reduced the categories from five to three: morning type (score = 59–70), neither type (score = 42–58) and evening type (score = 41–16).19
Wrist temperature rhythm
Wrist temperature (WT) rhythm was assessed continuously for 8 days using a temperature sensor (Thermochron iButton DS1921H; Dallas, Maxim, Dallas, TX, USA) with a sensitivity of 0.125 °C and programmed to sample every 10 min. It was attached to a double-sided cotton sport wrist band, and the sensor surface was placed over the inside of the wrist on the radial artery of the non-dominant hand, as described previously by Sarabia et al.12 The information stored in the iButton was transferred through an adapter (DS1402D-DR8; Dallas, Maxim) to a personal computer using iButton Viewer v. 3.22 (Dallas Semiconductor MAXIM software provided by the manufacturer).
Data were recorded during the months of November to May, with environmental temperatures ranging between 16.1 °C and 21.3 °C (data obtained from the Center for Statistics of Murcia), to minimize the influence of extreme environmental temperatures on WT.
Body position and rest–activity rhythm
The body position and rest–activity rhythm was assessed over the same 8 days using a HOBO Pendant G Acceleration Data Logger UA-004-64 (Onset Computer, Bourne, MA, USA) placed on the non-dominant arm by means of a sports band, with its X axis parallel to the humerus bone. The sensor was programmed to record data every 30 s.
The information stored in the actimeter was transferred through an optical USB Base Station (MAN-BASE-U-4, HOBO; Onset Computer) to a personal computer using the software provided by the manufacturer (HOBOware 2.2). From the information provided by the actimeter, we defined two variables: motor activity (A) and body position (P). First, A was calculated as degrees of change in X, Y and Z axis position with respect to the previous sampling time as described by Ortiz-Tudela et al.13 Second, P was calculated as the angle between X axis of the actimeter and the horizontal plane, being its value 0° when the arm is in a horizontal position and 90° when it is vertically aligned.13
TAP
To obtain the integrated TAP variable, we first normalized the three variables (T, A and P) by calculating the 95th and 5th percentiles for each variable and volunteer.13
Motor activity and body position show their higher levels during daytime, when the subject is active. WT profile, on the contrary, shows its higher levels during the night, when the subject is resting. Thereafter, to calculate TAP, we reversed the T profile. In a third step, we calculated the mean of three normalized variables. Thus, 0 corresponded to complete rest and sleep, and 1 to periods of high arousal and movement.
Sleep characteristics
Over the 8 days of the experiment, all subjects were instructed to complete a rest–activity and food diary designed by the Chronobiology Laboratory at the University of Murcia (Murcia, Spain),13 and from these diaries we obtained the habitual sleep and food intake time recording.
Sleep quality was assessed with domiciliary polisomnography (SOMNOscreen EEG 10–20; SOMNOmedics GmbH, Randersacker, Germany). The conditions and the phases of the human sleep were defined according to the typical pattern observed by means of the electroencephalogram, the electrooculogram (a measurement of the ocular movements) and the surface electromiogram. With this test, a variety of corporal functions during the sleep was registered, such as the electrical activity of the brain, the ocular movement, the muscular activity, the heart rate, the respiratory effort, the entry of the air and oxygen concentration in the blood. The examination was carried out during the night in the patients’ house, to be able to study the normal patterns of dream. The specialist placed the electrodes in the chin, the hairy leather and in the external edge of the eyelids. The signs from the electrodes were registered while patient was with the closed eyes and during the sleep. Different indicators of the sleep quality of the patients were obtained, such as sleep efficiency (%), sleep latency (h), latency rapid eye movement (h), arousals (%), obstructive apneas (events per h), desaturations (events per hour >3%) and mean heart rate (b.p.m.), among others.
Statistical methods and variables obtained
To characterize WT, activity and position rhythms, we calculated the following parameters using parametric and non-parametric methods.
-
Cosinor’s analysis
Mesor: Mean value of the rhythm fitted to a cosine function.
Amplitude: Difference between the maximum (or minimum) value of the cosine function and mesor.
Acrophase: Timing of the maximum value of the cosine function.
Rayleigh test: A measure of the rhythm’s phase stability during successive days. This test provides an r vector with its origin at the center of a circumference of radius one. The r vector length (between 0 and 1) is proportional to the degree of phase homogeneity during the period analyzed.
-
Fourier analysis
First harmonic’s power (P1): Spectral power of the 24 h rhythm.
Twelfth harmonic’s accumulated power (PACUM12), which indicates the accumulated spectral power of the first twelfth harmonics (from 24 to 2 h periods).
-
Non-parametric analysis20
Interdaily stability: The similarity of the 24 h pattern over days. It varied between 0 for Gaussian noise and 1 for perfect stability, where the pattern repeated itself exactly day after day.
Intradaily variability (IV), which characterizes the rhythm fragmentation. Its values oscillated between 0, when the wave was perfectly sinusoidal, and 2, when the wave was as Gaussian noise.
Average of the 5 consecutive hours of maximum values (M5) of WT and its timing (TM5). In the case of A and P, the average 10 consecutive hours of maximum values (M10) and its timing (TM10) were calculated.
Hourly average during the 10 consecutive hours of minimum values (L10) of WT and its timing (TL10). For A and P variables, hourly average during the 5 consecutive hours of minimum values (L5) of WT and its timing (TL5) were obtained.
Relative amplitude, it was calculated by the difference between M5 and L10 divided by the sum of M5 and L10.
CFI: A numerical index that determines the circadian robustness and is based on three circadian parameters: interdaily stability, IV and relative amplitude.13
All these rhythmic parameters were obtained by using an integrated package for temporal series analysis ‘Circadianware’ (Chronobiology Laboratory, University of Murcia, 2010). To assess statistical differences between C and TT women in the waveforms, a repeated-measures analysis of variance was performed for the group of women (global analysis of variance, P for genetic variant influence), the kinetics of the response (P for time) and the interaction of both factors (genetic variant × time). When statistical differences were found by the repeated-measures analysis of variance, a multiple-comparison test, adjusted by the least significant difference, was applied to identify differences between the two groups of women for each timepoint of extraction.
RESULTS
Table 1 represents some relevant characteristics of the studied population. The two groups did not significantly differ in age, obesity-related characteristics or energy intake.
However, C carriers tended to be more evening type than CT + TT carriers as assessed by the Horne and Ostberg questionnaire (mean ± s.d.) (49.53 ± 8.31; 52.95 ± 9.01) for minor and major carriers, respectively (P = 0.072).
To show the functional relevance of 3111TC in chronodisruption, we assessed its influence on skin temperature, actimetry, position and TAP parameters (Table 2). Patients with the C allele displayed significant abnormalities in their circadian rhythmicity. These were particularly relevant among the WT parameters, with significantly lower amplitude (total and relative), lower first harmonic power (P1), higher temperature values during the active period (L10) and lower CFI, which indicated a diminished circadian robustness among minor allele women (Table 2).
Table 2.
Differences in wrist temperature, actimetry, position and TAP rhythmicity variables depending on the CLOCK 3111T/C genetic variant
| CLOCK 3111T>C | P-value | ||||
|---|---|---|---|---|---|
| Minor allele carriers (TC + CC) | Major allele carriers | ||||
| Mean | s.d. | Mean | s.d. | ||
| Wrist temperature | |||||
| Amplitude | 0.97 | 0.42 | 1.10 | 0.60 | 0.017 |
| P1 | 1.09 | 0.94 | 1.55 | 1.85 | 0.011 |
| L10 | 32.81 | 0.70 | 32.52 | 0.93 | 0.032 |
| Relative amplitude | 0.02 | 0.01 | 0.03 | 0.02 | 0.012 |
| CFI | 0.41 | 0.05 | 0.43 | 0.05 | 0.047 |
| Motor activity | |||||
| Rayleigh | 0.63 | 0.24 | 0.71 | 0.20 | 0.078 |
| Body position | |||||
| Acrophase (h) | 15:49 | 01:03 | 15:11 | 02:01 | 0.075 |
| Rayleigh | 0.65 | 0.20 | 0.73 | 0.20 | 0.049 |
| TAP | |||||
| IV | 0.29 | 0.08 | 0.27 | 0.07 | 0.034 |
| TL5 (h) | 04:22 | 00:53 | 03:59 | 00:48 | 0.037 |
Abbreviations: BMI, body mass index; CFI, circadian function index; h, hours; IV, intradaily variability; L10, hourly average during the 10 consecutive hours of minimum values; P1, first harmonic’s power; TAP, wrist Temperature, Actimetry, Position; s.d., standard deviation. Data adjusted for age, BMI, waist, pathologies and medication.
Actimetry and position parameters confirmed circadian misalignments in C carriers, with a delayed acrophase in the position, which suggested a tendency to the eveningness and a less stable rhythm (Rayleig test) (Table 2).
C women also showed a higher IV in TAP, which implies a greater fragmentation of the rhythm in C women as compared with their TT counterparts and a phase delay in the time of day when subjects were at rest and TAP presented the lowest values (TL5) (Table 2). This time can be considered as a valuable phase reference for the circadian system, and coincides with the maximum value of wrist skin temperature and the center of the sleep period.
Interestingly, Pearson’s correlations analyses showed an association between decreased amplitude, eveningness (delayed acrophase) (r = −0.26; P = 0.017) and an increased fragmentation of rhythms (IV) (r = −0.46, P < 0.0001), all of these features that characterize cronodisruption in evening-type individuals.
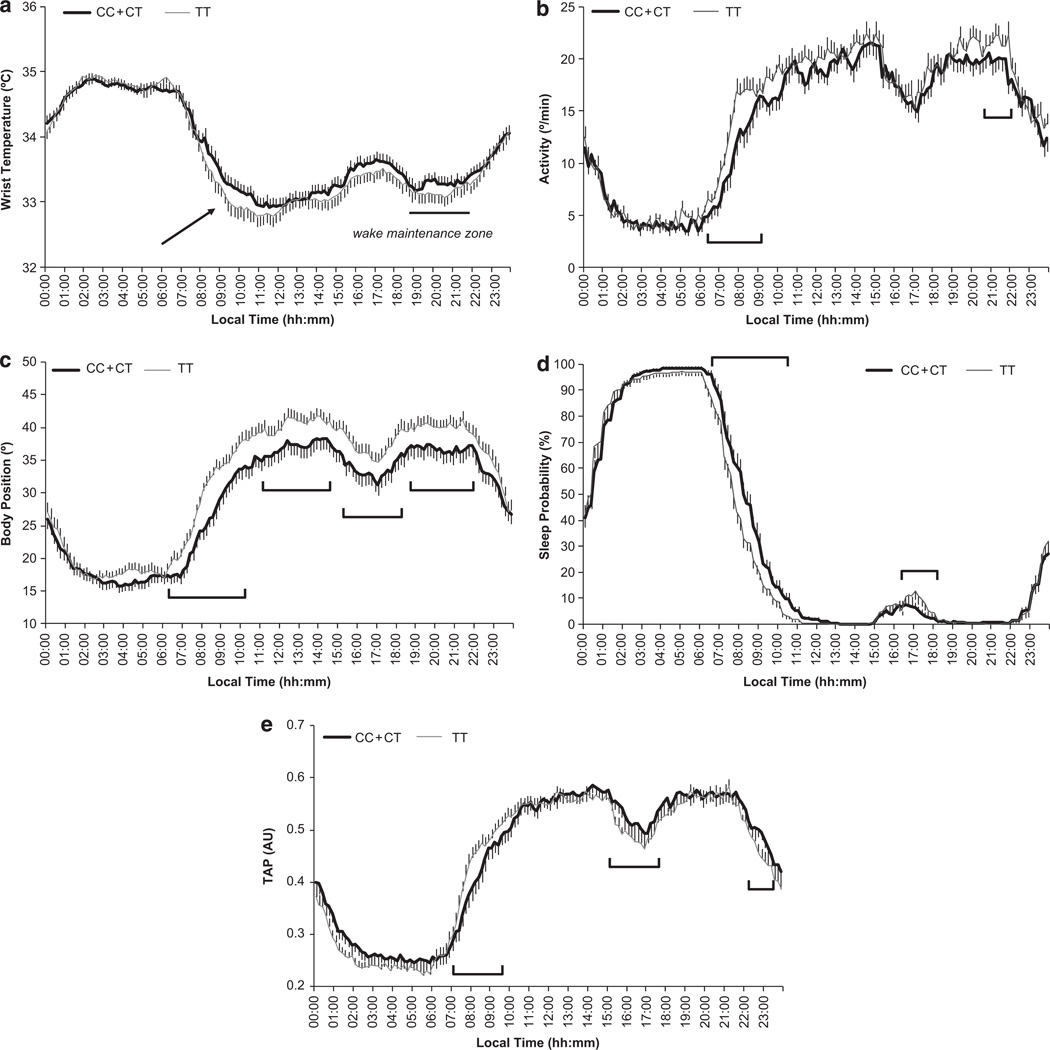
Daily mean waveforms in both C and TT women are represented in Figure 1a–e for WT, motor activity, body position, sleep probability and TAP. As expected, both groups exhibited similar daily WT patterns, characterized by an increase before the time of lights-off at bedtime, a nocturnal steady state with high temperatures and a pronounced drop after arising in the morning. There was a secondary peak around afternoon, a period associated with naps, and a dip between 2000 and 2200 h, a period already known as the ‘wake maintenance zone’.
Figure 1.
Daily mean waveform of wrist temperature (a), actimetry (b), position (c), sleep probability (d) and TAP (e) recorded over a 8-day period in C and TT overweight women. Red arrow points to the segment that represents the drop after arising in the morning, which is significantly different between both groups of women (P < 0.05). Moreover, those sections of the graph with significant differences (P < 0.05) between C and TT women are highlighted in all figures.
A roughly inverse pattern was observed for motor activity and body position (Figure 1b and c), which displayed higher values during the day and lower values at night, when the subject was resting. Figure 1d represents the sleep probability waveform, while the integrated TAP variable is shown in Figure 1e. Each rest period, whether diurnal or nocturnal, coincided with a series of very low TAP values, and implied the coexistence of low activity, horizontal position and high temperature. The TAP mean waveform was characterized by a broad dip during sleep time, and a consistent, but transient TAP dip around 1600–1700 h, following the subjects’ usual lunch time and during their normal nap period. Maximum TAP values were observed between 1200–1400 h and 2000–2200 h. Again, TAP values started to decrease in advance of bedtime.
When we analyzed potential differences in the mean waveforms of C and TT subjects, we observed that in the WT curve, C women showed a slower decrease after the morning arising, whereas in TT women this decrease tended to be sharper (P < 0.05) (Figure 1a). In contrast, during the ‘wake maintenance zone’ C women had a lower dip, characterized by higher mean temperatures (Figure 1a). In general, C women displayed higher temperature values than TT along the day, which is related to diurnal skin vasodilatation and propensity to sleepiness.
Significant differences were also obtained in activity, position and sleep probability waveforms depending on the genetic variant. Data consistently showed that TT homozygotes were more active during the day. Moreover, C women started to be active later in the morning, showing a delayed phase as compared with TT. These data were confirmed with position, sleep probability and TAP waveforms and with records from the rest– activity diary showing that C women used to get up later than TT, and as a consequence they tended to sleep longer (Table 3). When analysis of repeated measurements was performed, differences were statistically significant in the several sections of the curves as represented in Figure 1 (P < 0.05). However, no significant differences were found between C and TT women in the sleep quality as assessed by polysomnography or in the food intake timing (Table 3).
Table 3.
Results from polysomnography and rest–activity and food diary
| Polysomnography | CLOCK 3111T>C | P-value | |||
|---|---|---|---|---|---|
| Minor allele carriers (TC + CC) | Major allele carriers (TT) | ||||
| Mean | s.d. | Mean | s.d. | ||
| Total time (h) | 0602 | 0117 | 0557 | 0108 | 0.732 |
| Sleep efficiency (%) | 78.83 | 13.00 | 76.87 | 10.34 | 0.447 |
| Sleep latency (h) | 0027 | 0030 | 0027 | 0021 | 0.962 |
| Latency REM (h) | 0136 | 0101 | 0154 | 0102 | 0.179 |
| Arousals (%) | 31.05 | 16.31 | 27.88 | 17.51 | 0.393 |
| Deep sleep – total sleep (%) | 21.24 | 8.99 | 20.41 | 9.42 | 0.679 |
| Deep sleep – no REM (%) | 28.15 | 12.39 | 26.85 | 11.93 | 0.625 |
| Deep sleep – time record (%) | 18.97 | 9.37 | 17.96 | 9.03 | 0.619 |
| Time recorded (h) | 0727 | 0030 | 0727 | 0030 | 0.989 |
| Obstructive apneas (events per h) | 1.76 | 3.69 | 2.78 | 8.26 | 0.469 |
| Mixed apneas (events per h) | 0.29 | 1.02 | 0.81 | 4.17 | 0.431 |
| Central apneas (events per h) | 1.15 | 2.39 | 1.29 | 4.09 | 0.850 |
| Hypopneas (events per h) | 23.65 | 42.89 | 28.38 | 39.25 | 0.599 |
| Apnea index | 4.81 | 8.14 | 6.92 | 9.36 | 0.275 |
| SaO2 average (%) | 95.21 | 1.32 | 92.12 | 14.64 | 0.176 |
| SaO2 minimum (%) | 88.79 | 5.54 | 85.12 | 14.74 | 0.135 |
| SaO2 <90% | 1.39 | 3.63 | 2.23 | 5.05 | 0.814 |
| Desaturations (events per h >3%) | 5.12 | 8.35 | 6.47 | 9.71 | 0.497 |
| Rest–activity diary | |||||
| Sleep duration (h) | 0752 | 0045 | 0726 | 0045 | 0.016 |
| Initial time (h) | 0024 | 0057 | 0018 | 0043 | 0.884 |
| Final time (h) | 0821 | 0056 | 0745 | 0037 | 0.009 |
| Naps characteristics | |||||
| Women who take naps (%) | 52 | 48 | 0.457 | ||
| Naps duration (h) | 1.06 | 0.67 | 1.01 | 0.46 | 0.740 |
| Initial time (h) | 1558 | 0116 | 1608 | 0036 | 0.935 |
| Final time (h) | 1702 | 0130 | 1709 | 0042 | 0.947 |
| Food records | |||||
| Breakfast time (h) | 0924 | 0100 | 0848 | 0138 | 0.13 |
| Lunch time (h) | 1447 | 0030 | 1450 | 0033 | 0.67 |
| Dinner time (h) | 2127 | 0049 | 2126 | 0051 | 0.58 |
Abbreviations: h, hour; REM, rapid eye movement; SO2, oxygen saturation.
DISCUSSION
The aim of this study was to gain deeper understanding regarding the previously reported association of the CLOCK 3111T/C SNP with obesity and weight loss.
This study represents the first systematic evaluation of circadian rhythms of the CLOCK 3111T/C SNP in overweight women under habitual living conditions as determined by TAP and the integrated TAP variable. Compared with TT subjects, who showed healthier and more robust circadian rhythm profiles, patients with the C allele displayed significant circadian abnormalities: lower amplitude and greater fragmentation of the rhythm, a less stable circadian pattern and a significantly weakened circadian function, as assessed by the CFI index. C subjects were also less active than TT, started their activities later in the morning and were sleepier during the day, showing a delayed acrophase, which characterizes ‘evening-type’ subjects.
From all these circadian variables, the reduced amplitude of the rhythm is considered to be one of the most prominent circadian rhythm changes in aging.21 This parameter tends to be high in subjects with a healthy circadian system and who have a stable daily routine, whereas low amplitudes have been described in the elderly21 and also in illnesses such as Alzheimer20–21 or obesity.22 In this study, the C patients showed lower amplitudes in the mean waveform and in the resultant temperature parameter as compared with TT, which suggests a more aged circadian system. In addition, the Rayleigh index was lower in C women than in TT, signifying a less stable rhythm in the actimetry and position analyses.
IV in TAP was also related to C genetic variation among the women studied. This suggests a greater fragmentation of the rest–activity rhythm in C women as compared with their TT counterparts. This measure of the fragmentation is dependent on endogenous circadian disturbances. It shows a moderate correlation with functional, social and emotional well-being. For example, fragmentation increases in association with dementia, cognitive decline, and so on.23 Increased IV has been also related with aging,21 probably due to the increased daytime napping and nocturnal awakenings in the elderly, and also with obesity,22 where IV was also related to a decrease in the amplitude of the melatonin daily rhythmicity,22 a biological sign of chronodisruption.24
The minimum value of temperature during 10 consecutive hours was also significantly higher in C individuals than in TT. Higher temperatures have been related to diurnal skin vasodilatation, parasympathetic activation and sleepiness.25 Although the differences in mean temperature values between C and TT subjects may seem negligible (0.3 °C), this small difference appears to be sufficient to disrupt circadian rhythmicity. In fact, induction of a slight increase (0.4 °C) in skin temperature suppresses nocturnal wakefulness and shifts sleep to deeper stages.26
From all the circadian variables analyzed, CFI is a very suitable index because it integrates three circadian parameters, each one providing complementary information about the circadian system: IV, amplitude and interdaily stability.23 CFI allows us to classify the circadian system status of a population and has been shown to be accurate when trying to define the subjects’ circadian status.13 In general, the CFI of temperature was low in the women from the current sample for both C and TT subjects, and ranged between 0.57 (best) and 0.33 (worst) (data not shown), which indicated a lower circadian robustness in both groups of women as compared with healthy subjects in whom CFI of WT rhythm ranged from 0.73 to 0.43.13 These differences between the study subjects and the reference values may be due to the moderate obesity of the women participating in our study. In addition, CFI was lower in C women than in their TT counterparts.
Of note, position acrophase showed a phase delay in minor C allele carriers as compared with TT carriers. Moreover, the timing of the 5 consecutive hours of lowest values (TL5) in TAP was also seen later among C women. This phase delay was also evident when we analyzed the different mean waveforms of the women studied. Indeed, C subjects tended to start their activity later in the morning and to be sleepier during the morning than TT, whereas TT were more active as consistently reflected in the chronograms obtained from the temperature, activity, position and TAP.
Previous research suggests that in healthy subjects, carriers of the 3111C allele showed a significantly higher evening preference on questionnaires with a 10 to 44 min delay in preferred timing for activity or sleep episodes.27 Similar results were found by Benedetti et al.4 in depressed subjects by actimetry, although it was only registered during one day and in laboratory conditions, which may not reflect their habitual behavior. In that particular study, TT homozygotes tended to decrease, whereas carriers of the C allele tended to increase activity during the evening before lights off. A situation that was not present in this study. However, there have been non-replications of their finding,28–31 underscoring the need of further research in relation to this SNP. Data from this study confirm the evening preferences of C minor allele carriers as demonstrated by (a) Horne Ostberg questionnaire, (b) the delayed phase in activity, position and TAP waveforms in C women and (c) their tendency to get up later in the morning (rest–activity diary). Moreover, our results show significant associations between decreased amplitude, eveningness (delayed acrophase) and increased fragmentation of rhythms, all of these features that characterize cronodisruption in evening-type individuals.
Nevertheless, our results indicated that C women circadian impairment was not a consequence of changes in food intake timing or a less quality sleep, as deduced from the food records and domiciliary polysomnography data.
Particularly relevant were the data derived from TAP because it was mentioned previously, it integrates temperature, activity and position and reduces the presence of artifacts; thus, it is considered a suitable tool to evaluate the status of the human circadian system. TAP indicate that C women had several circadian rhythmicity alterations that could explain the predisposition of the minor C allele carriers of CLOCK 3111T/C to suffer from obesity32 and even cancer,33 as well as the lower success in weight loss.8,9,32
In summary, C genetic variants in CLOCK 3111T/C display a less robust circadian rhythm than TT and a delayed acrophase, which characterizes ‘evening-type’ subjects. We support the notion that identifying CLOCK genotypes in patients may assist therapist in characterization of the roots of the metabolic problem.
ACKNOWLEDGEMENTS
This study was supported by grants from Tomás Pascual and Pilar Gómez-Cuétara Foundations, Spanish Government of Science and Innovation (BFU2011-24720), Séneca Foundation from the Government of Murcia (15123/PI/10), National Heart, Lung and Blood Institute Grant HL-54776, National Institute of Diabetes and Digestive and Kidney Diseases Grant DK075030 and by contracts 53-K06-5-10 and 58-1950-9-001 from the US Department of Agriculture Research.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Serretti A, Benedetti F, Mandelli L, Lorenzi C, Pirovano A, Colombo C, et al. Genetic dissection of psychopathological symptoms insomnia in mood disorders and CLOCK gene polymorphism. Am J Med Genet B. 2003;121B:35–38. doi: 10.1002/ajmg.b.20053. [DOI] [PubMed] [Google Scholar]
- 2.Takao T, Tachikawa H, Kawanishi Y, Mizukami K, Asada T. CLOCK gene T3111C polymorphism is associated with Japanese schizophrenics: a preliminary study. Eur Neuropsychopharmacol. 2007;17:273–276. doi: 10.1016/j.euroneuro.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Benedetti F, Dallaspezia S, Fulgosi MC, Lorenzi C, Serretti A, Barbini B, et al. Actimetric evidence that CLOCK 3111T/C SNP influences sleep and activity patterns in patients affected by bipolar depression. Am J Med Genet B. 2007;144B:631–635. doi: 10.1002/ajmg.b.30475. [DOI] [PubMed] [Google Scholar]
- 4.Benedetti F, Radaelli D, Bernasconi A, Dallaspezia S, Falini A, Scotti G, et al. Clock genes beyond the clock: CLOCK genotype biases neural correlates of moral valence decision in depressed patients. Genes Brain Behav. 2008;7:20–25. doi: 10.1111/j.1601-183X.2007.00312.x. [DOI] [PubMed] [Google Scholar]
- 5.Kishi T, Kitajima T, Ikeda M, Yamanouchi Y, Kinoshita Y, Kawashima K, et al. Association study of clock gene (CLOCK) and schizophrenia and mood disorders in the Japanese population. Eur Arch Psychiatry Clin Neurosci. 2009;259:293–297. doi: 10.1007/s00406-009-0869-4. [DOI] [PubMed] [Google Scholar]
- 6.Sookoian S, Gemma C, Gianotti TF, Burgueño A, Castaño G, Pirola CJ. Genetic variants of Clock transcription factor are associated with individual susceptibility to obesity. Am J Clin Nutr. 2008;87:1606–1615. doi: 10.1093/ajcn/87.6.1606. [DOI] [PubMed] [Google Scholar]
- 7.Garaulet M, Corbalán MD, Madrid JA, Morales E, Baraza JC, Lee YC, et al. CLOCK gene is implicated in weight reduction in obese patients participating in a dietary programme based on the Mediterranean diet. Int J Obes (Lond) 2010;34:516–523. doi: 10.1038/ijo.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garaulet M, Sánchez-Moreno C, Smith CE, Lee YC, Nicolás F, Ordovás JM. Ghrelin, sleep reduction and evening preference: relationships to CLOCK 3111T/C SNP and weight loss. PLoS One. 2011;6:e17435. doi: 10.1371/journal.pone.0017435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barclay NL, Eley TC, Mill J, Wong CC, Zavos HM, Archer SN. Sleep quality and diurnal preference in a sample of young adults: associations with 5HTTLPR, PER3, and CLOCK 3111. Am J Med Genet B. 2011;156B:681–690. doi: 10.1002/ajmg.b.31210. [DOI] [PubMed] [Google Scholar]
- 10.Sadeh A, Acebo C. The role of actigraphy in sleep medicine. Sleep Med. 2002;6:113–124. doi: 10.1053/smrv.2001.0182. [DOI] [PubMed] [Google Scholar]
- 11.Acebo C, Le Bourgeois MK. Actigraphy. Respir Care Clin N Am. 2006;12:23–30. doi: 10.1016/j.rcc.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Sarabia JA, Rol MA, Mendiola P, Madrid JA. Circadian rhythm of wrist temperature in normal-living subjects—a candidate of new index of the circadian system. Physiol Behav. 2008;95:570–580. doi: 10.1016/j.physbeh.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Ortiz-Tudela E, Martinez-Nicolas A, Campos M, Rol MÁ, Madrid JA. New integrated variable based on thermometry, actimetry and body position (TAP) to evaluate circadian system status in humans. PLoS Comput Biol. 2010;6:e1000996. doi: 10.1371/journal.pcbi.1000996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garaulet M, Corbalán-Tutau MD, Madrid JA, Baraza JC, Parnell LD, Lee YC, et al. PERIOD2 variants are associated with abdominal obesity, psycho-behavioral factors, and attrition in the dietary treatment of obesity. J Am Diet Assoc. 2010;110:917–921. doi: 10.1016/j.jada.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benedetti F, Radaelli D, Bernasconi A, Dallaspezia S, Falini A, Scotti G, et al. Clock genes beyond the clock: CLOCK genotype biases neural correlates of moral valence decision in depressed patients. Genes Brain Behav. 2008;7:20–25. doi: 10.1111/j.1601-183X.2007.00312.x. [DOI] [PubMed] [Google Scholar]
- 16.Livak KJ. Allelic discrimination using fluorogenic probes and the 5′ nuclease assay. Genet Anal. 1999;14:143–149. doi: 10.1016/s1050-3862(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 17.Garaulet M, Lee YC, Shen J, Parnell LD, Arnett DK, Tsai MY, et al. Genetic variants in human CLOCK associate with total energy intake and cytokine sleep factors in overweight subjects (GOLDN population) Eur J Hum Genet. 2010;18:364–369. doi: 10.1038/ejhg.2009.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness–eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 19.Taillard J, Philip P, Chastang JF, Bioulac B. Validation of Horne and Ostberg morningness–eveningness questionnaire in a middle-aged population of Frenchworkers. J Biol Rhythms. 2004;19:76–86. doi: 10.1177/0748730403259849. [DOI] [PubMed] [Google Scholar]
- 20.Van Someren EJW, Mirmiran M, Swaab DF. Non-pharmacological treatment of sleep and wake disturbances in aging and Alzheimer’s disease: chronobiological perspectives. Behav Brain Res. 1993;57:235–253. doi: 10.1016/0166-4328(93)90140-l. [DOI] [PubMed] [Google Scholar]
- 21.Huang YL, Liu RY, Wang QS, Van Someren EJ, Xu H, Zhou JN. Age-associated difference in circadian sleep–wake and rest–activity rhythms. Physiol Behav. 2002;76:597–603. doi: 10.1016/s0031-9384(02)00733-3. [DOI] [PubMed] [Google Scholar]
- 22.Corbalán-Tutau MD, Madrid JA, Ordovás JM, Smith CE, Nicolás F, Garaulet M. Differences in daily rhythms of wrist temperature between obese and normalweight women: associations with metabolic syndrome features. Chronobiol Int. 2011;28:425–433. doi: 10.3109/07420528.2011.574766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carbalho-Bos S, Riemersma-van der Lek RF, Waterhouse J, Reilly T, Van Someren EJ. Strong association of the rest–activity rhythm with well-being in demented elderly women. Am J Geriatr Psychiatry. 2007;15:92–100. doi: 10.1097/01.JGP.0000236584.03432.dc. [DOI] [PubMed] [Google Scholar]
- 24.Corbalán-Tutau D, Madrid JA, Nicolás F, Garaulet M. Daily profile in two circadian markers ‘melatonin and cortisol’ and associations with metabolic syndrome components. Physiol Behav. 2012 doi: 10.1016/j.physbeh.2012.06.005. e-pub ahead of print 15 June 2012. [DOI] [PubMed] [Google Scholar]
- 25.Kistler A, Mariauzouls C, Von Berlepsch K. Fingertip temperature as an indicator for sympathetic responses. Int J Psychophysiol. 1998;29:35–41. doi: 10.1016/s0167-8760(97)00087-1. [DOI] [PubMed] [Google Scholar]
- 26.Raymann RJ, Swaab DF, Van Someren EJW. Skin temperature and sleep-onset latency: changes with age and insomnia. Physio. Behav. 2007;90:257–266. doi: 10.1016/j.physbeh.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Katzenberg D, Young T, Finn L, Lin L, King DP, Takahashi JS, et al. A CLOCK polymorphism associated with human diurnal preference. Sleep. 1998;21:569–576. doi: 10.1093/sleep/21.6.569. [DOI] [PubMed] [Google Scholar]
- 28.Robilliard DL, Archer SN, Arendt J, Lockley SW, Hack LM, English J, et al. The 3111 Clock gene polymorphism is not associated with sleep and circadian rhythmicity in phenotypically characterized human subjects. J Sleep Res. 2002;11:305–312. doi: 10.1046/j.1365-2869.2002.00320.x. [DOI] [PubMed] [Google Scholar]
- 29.Johansson C, Willeit M, Smedh C, Ekholm J, Paunio T, Kieseppä T, et al. Circadian clock-related polymorphisms in seasonal affective disorder and their relevance to diurnal preference. Neuropsychopharmacology. 2003;28:734–739. doi: 10.1038/sj.npp.1300121. [DOI] [PubMed] [Google Scholar]
- 30.Voinescu B, Thome J, Orasan R. The rs1801260 CLOCK polymorphism, links to depression, insomnia and diurnal preference—preliminary findings from a Romanian sample. Hum Vet Med. 2009;1:67–673. [Google Scholar]
- 31.Barclay NL, Eley TC, Mill J, Wong CC, Zavos HM, Archer SN, et al. Sleep quality and diurnal preference in a sample of young adults: associations with 5HTTLPR, PER3, and CLOCK 3111. Am J Med Genet B. 2011;156B:681–690. doi: 10.1002/ajmg.b.31210. [DOI] [PubMed] [Google Scholar]
- 32.Garaulet M, Lee YC, Shen J, Parnell LD, Arnett DK, Tsai MY, et al. CLOCK genetic variation and metabolic syndrome risk: modulation by monounsaturated fatty acids. Am J Clin Nutr. 2009;90:1466–1475. doi: 10.3945/ajcn.2009.27536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou F, He X, Liu H, Zhu Y, Jin T, Chen C, et al. Functional polymorphisms of circadian positive feedback regulation genes and clinical outcome of Chinese patients with resected colorectal cancer. Cancer. 2012;118:937–946. doi: 10.1002/cncr.26348. [DOI] [PubMed] [Google Scholar]