Abstract
The Wnt signaling pathway is essential for cell fate decisions during embryonic development as well as for homeostasis after birth. Dapper antagonist of catenin-1 (Dact1) plays an important role during embryogenesis by regulating Wnt signaling pathways. Consequently, targeted disruption of the Dact1 gene in mice leads to perinatal lethality due to severe developmental defects involving the central nervous system, genitourinary system and distal digestive tract. However, the expression and potential function of Dact1 in other tissues during development and postnatal life have not been well studied. Here, we have generated reporter mice in which LacZ expression is driven by the Dact1 gene promoter and characterized Dact1-LacZ expression in embryos and adult tissues. Our data show that while Dact1-LacZ is expressed in multiple mesoderm- and neuroectoderm-derived tissues during development, high expression of Dact1-LacZ is restricted to a small subset of adult tissues, including the brain, eye, heart, and some reproductive organs. These results will serve as a basis for future investigation of Dact1 function in Wnt-mediated organogenesis and tissue homeostasis.
Keywords: Dact1, LacZ reporter, Wnt signal, Embryogenesis, Homeostasis
Wnt signaling plays important roles in cell fate decisions during embryonic development and in tissue homeostasis after birth (Moon et al., 2004; Logan and Nusse, 2004). Wnt signaling is initiated by binding of extracellular Wnt ligands to Frizzled (Fz) transmembrane receptor complexes, leading to the recruitment and activation of Dishevelled (Dvl) proteins, central to both canonical and noncanonical Wnt signaling pathways. Activation of the canonical Wnt pathway results in the stabilization of cytoplasmic/nuclear β-catenin, leading to changes in T-cell factor (TCF)/lymphocyte enhancer factor (Lef)-mediated gene transcription (Huang and He, 2008; Angers and Moon, 2009). In contrast, noncanonical Wnt signaling pathways, such as the planar cell polarity (PCP) pathway, regulate cytoskeleton remodeling and cell polarity during development, producing an elongation and narrowing of the tissue along the anterior-posterior axis (Kibar et al., 2001; Curtin et al., 2003; Montcouquiol et al., 2003; Wang et al., 2005; Zallen, 2007).
Dapper antagonist of catenin-1 (Dact1) was initially identified by its ability to interact with Dvl, and was shown to control embryogenesis by modulating Wnt signaling pathways by antagonizing Dvl functions in Xenopus (Cheyette et al., 2002). However, subsequent studies show that Dact1 can also cooperate with Dvl proteins to promote Wnt signaling (Gloy et al., 2002; Waxman et al., 2004; Park et al., 2006; Suriben et al., 2009; Yang and Cheyette, 2013; Arguello et al., 2013; Yang et al., 2013). Regulation of these apparently opposing functions occurs through differential phosphorylation, suggesting that Dact1 acts as a molecular switch in Wnt signaling (Teran et al., 2009).
Dact1 is required for notochord and head structure development in Xenopus embryos (Cheyette et al., 2002). In mice, loss of Dact1 leads to severe abnormalities at birth, including neural tube defects due to post-translational alterations of Vangle2, a transmembrane component of the Wnt/PCP pathway, at the primitive streak (Suriben et al., 2009; Wen et al., 2010). Recently, missense heterozygous mutations in the DACT1 gene have been identified in patients with neural tube defects (Shi et al., 2012). These studies suggest that Dact1 function is evolutionarily conserved and that a major function of Dact1 is to regulate the PCP pathway during neural tube development.
Previous studies have shown that Dact1 is expressed in the central nervous system during embryogenesis in mice (Fisher et al., 2006; Hunter et al., 2006). However, information regarding Dact1 expression in other tissues during development and in postnatal organs other than the brain is limited (Fisher et al., 2006; Kettunen et al., 2010). As Wnt signaling is essential for organogenesis in multiple embryonic tissues as well as for adult tissue homeostasis (Moon et al., 2004; Logan and Nusse, 2004; Wang and Wynshaw-Boris, 2004), a more thorough investigation of Dact1 expression will provide insight into the potential role of Dact1 in these processes.
To address this issue, we have generated Dact1-LacZ reporter mice and characterized Dact1 expression in developing embryos and adult tissues. Our data show that while Dact1-LacZ is expressed at high levels in multiple mesoderm- and neuroectoderm-derived tissues during embryonic development, its expression is restricted to a small number of postnatal tissues, including the brain, eye, heart, testis and female reproductive tract.
1. Results
1.1. Generation of Dact1-LacZ reporter mice
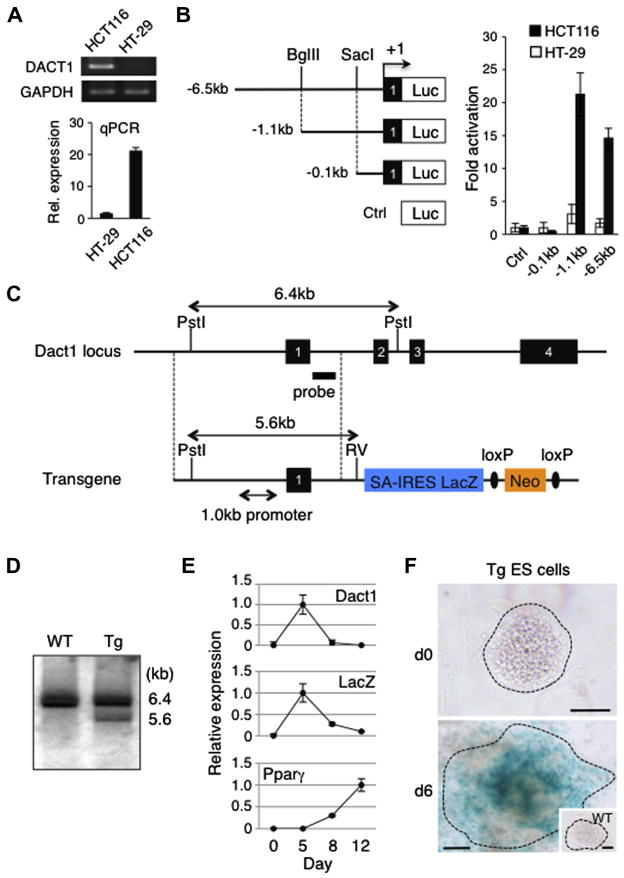
To first identify the Dact1 gene promoter, we generated luciferase constructs harboring upstream sequences of the mouse Dact1 gene with various 5′ deletions. Each construct was transfected into HCT116 colorectal carcinoma cells, which express a high level of endogenous Dact1 compared to HT-29 colorectal adenocarcinoma cells (Fig. 1A), followed by luciferase reporter assays. Our data show that the luciferase activity driven by a 1.1 kb fragment upstream of the transcription initiation site was as high as that of the 6.5 kb full-length promoter in HCT116 cells while both promoter constructs showed significantly reduced activity in HT-29 cells (Fig. 1B). A further deletion revealed that a reporter construct harboring a 0.1 kb fragment exhibited basal reporter activity in HCT116 cells (Fig. 1B). These data indicate that a 1.0 kb promoter region spanning from the position −1.1 to −0.1 kb, relative to the transcription initiation site, contains regulatory elements sufficient for driving Dact1 gene expression.
Fig. 1.
Characterization of the Dact1 gene promoter and Tg ES cells used for the generation of Dact1LacZ reporter mice. (A) Evaluation of the DACT1 gene expression in two colorectal cancer cell lines. Differential expression of the DACT1 gene in HCT116 and HT-29 cells was determined by semi-quantitative RT-PCR, followed by gel electrophoresis and staining with ethidium bromide (top) and real-time PCR (qPCR), expressed as the mean ± s.d. (bottom). In both RT-PCR and qPCR, expression of GAPDH served as an internal control. (B) Reporter assays with Dact1 gene promoter constructs. A series of Firefly luciferase reporter constructs were co-transfected with a Renilla luciferase control plasmid into HCT116 and HT-29 cells. Forty-eight hours after transfection, luciferase activities were measured and Firefly luciferase activity was normalized to that of Renilla luciferase. Data shown are the mean ± s.d. from three independent experiments. Luc, a promoter-less luciferase cassette; +1, transcription initiation site within exon 1 of the Dact1 gene; Ctrl, control. (C) Endogenous Dact1 allele (top) and a transgenic construct (bottom) used for the generation of Dact1LacZ Tg mice. The transgene contains a 6.0 kb fragment homologous to the endogenous Dact1 locus (indicated by dotted lines) including the 1.0 kb Dact1 promoter region, followed by a reading frame-independent LacZ gene cassette composed of an En2 splice acceptor (SA), internal ribosomal entry site (IRES) and β-galactosidase cDNA with SV40 poly A (LacZ), and a neomycin resistant gene cassette (Neo) flanked by loxP sites (ovals). Dact1LacZ-neo Tg mice were crossed with Actβ-Cre Tg mice to delete the Neo cassette, generating Dact1LacZ Tg mice. Solid boxes with numbers, Dact1 exons 1–4; RV, EcoRV. (D) Southern blot analysis. Genomic DNAs from WT and Tg ES cells were digested with Pst I and EcoRV and hybridized with a DNA probe shown in (C). (E) Real-time PCR analysis of Dact1, Dact1-LacZ and Pparγ expression in Tg ES cells upon differentiation into the adipogenic lineage. Expression of each gene was normalized to Rps18 transcript levels. The highest expression was set at 1.0 and data shown are the mean ± s.d. from three independent experiments. (F) Dact1-LacZ activities as determined by X-gal staining of Tg ES cells before and after differentiation into the adipogenic lineage. WT ES cells were used as a negative control (inset). Data shown are representative of three independent experiments with similar results. Bars = 100 μm.
We next obtained embryonic stem (ES) cells harboring a Dact1-LacZ reporter transgene from the European Mouse Mutant Cell Repository (EuMMCR). The transgene is composed of a 6.0 kb fragment homologous to the wild type (WT) Dact1 locus including the 1.0 kb Dact1 promoter region identified above and a reading frame-independent LacZ gene cassette (SA-IRES-LacZ), thereby allowing for LacZ expression under the transcriptional control of the Dact1 regulatory elements (Fig. 1C). Integration of the intact promoter into genomic DNA was confirmed by Southern blot using transgenic (Tg) ES cell DNA (Fig. 1D). Notably, our data show that the ratios of endogenous Dact1 alleles and the transgene were approximately 2:1, suggesting that a single copy of the transgene was integrated in these Tg ES cells.
To determine if Dact1-LacZ transcription recapitulates endogenous Dact1 gene expression, we induced Tg ES cells into the adipogenic lineage and performed real-time PCR. Dact1 is highly expressed at the preadipocyte stage and its expression declines during terminal differentiation into mature adipocytes (Lagathu et al., 2009). Our data show that expression of Dact1-LacZ in Tg ES cells was significantly upregulated at an early stage of preadipocyte differentiation and decreased to basal levels as expression of peroxisome proliferator-activated receptor γ (Pparγ), a marker for mature adipocytes (Tontonoz et al., 1994), increased (Fig. 1E). We also confirmed that Tg ES cells differentiating into the adipogenic lineage expressed LacZ by whole mount staining with X-gal (Fig. 1F). As differentiating WT ES cells did not show detectable signals with X-gal staining, the observed LacZ activity was not due to endogenous β-galactosidase activity. These data indicate that expression of Dact1-LacZ can be used to monitor endogenous Dact1 gene expression in these Tg ES cells.
Finally, we microinjected Dact1-LacZ Tg ES cells into WT blastocysts and generated Dact1-LacZ reporter mice by germline transmission (Dact1LacZ-neo Tg mice). Heterozygous Dact1LacZ-neo Tg mice were crossed with Actβ-Cre Tg mice to remove the neomycin cassette, resulting in the generation of Dact1LacZ Tg mice. Expression of LacZ in Dact1LacZ-neo Tg and Dact1LacZ Tg mice was indistinguishable throughout life, suggesting that the presence of the neomycin cassette does not interfere with the spatiotemporal expression of LacZ from the Dact1-LacZ reporter construct (data not shown). In this study, we used heterozygous Dact1LacZ Tg mice maintained in a mixed C57BL/6; 129/Sv; FVB/N background (see Section 3.2) by intercrossing heterozygous Dact1LacZ Tg mice with WT littermates in the absence of the Actβ-Cre transgene.
1.2. Expression of Dact1-LacZ in developing mouse embryos
We first investigated Dact1-LacZ expression in developing mouse embryos at E12.5, when endogenous Dact1 expression reaches its peak as determined by Northern blot using whole embryo RNAs (Fisher et al., 2006). Whole mount X-gal staining of Dact1LacZ Tg mouse embryos revealed strong signals in the cranial ganglion including the inner ears, the dorsal root ganglion and the heart, where it was expressed in the vessel wall and myocardium in the atrioventricular junction (Fig. 2A, B and E–I). Dact1-LacZ was also expressed in the intrinsic muscle of the tongue (Fig. 2J) and mesenchyme of the wrists/ankles with a stronger signal in the anterior than posterior region (Fig. 2C and K). These results are consistent with previous studies by in situ hybridization (Fisher et al., 2006; Hunter et al., 2006) and suggest that LacZ expression in Dact1LacZ Tg mice recapitulates Dact1 expression in vivo.
Fig. 2.
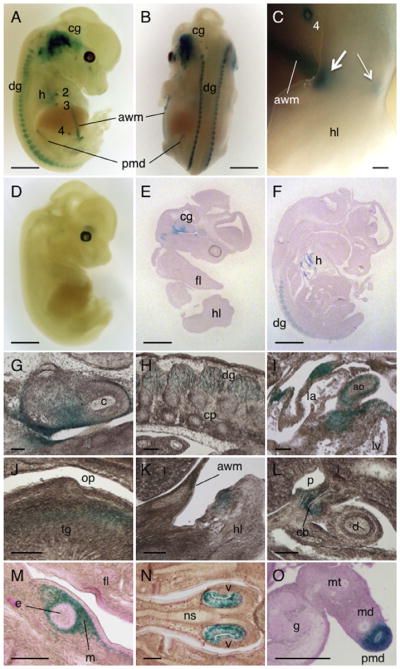
Expression of Dact1-LacZ in embryos. (A–D) E12.5 embryos stained for LacZ activity and photographed as whole mount preparations. (A and B) Dact1LacZ Tg mouse embryos showing strong LacZ signals in the cranial ganglion, dorsal root ganglion, heart, nipples, paramesonephric duct, and abdominal wall mesenchyme. (C) An enlarged view of the hindlimb showing LacZ signals in the ankle with a stronger signal in anterior (thick arrow) than posterior region (thin arrow). (D) WT embryo serving as a negative control. (E and F) Sagittal sections of X-gal-stained E12.5 Dact1LacZ Tg mouse embryos showing strong LacZ signals in the cranial ganglion, heart and dorsal root ganglion. (G–O) Enlarged views of embryonic tissues prepared from E12.5 (G–L) and E14.5 (M–O) Dact1LacZ Tg mouse embryos stained for LacZ activity using X-gal, followed by sectioning. Dact1-LacZ expression was detected in the inner ear (G) and the dorsal root ganglion (H). In the heart, Dact1-LacZ was expressed in the vessel wall and myocardium in the atrioventricular junction (I). Dact1-LacZ was also detected in the intrinsic muscle of the tongue (J) and mesenchyme surrounding some developing structures such as the limb (K), common bile duct (L) and nipple (M). The vomeronasal organ (N) and paramesonephric duct (O) also expressed Dact1-LacZ. cg, cranial ganglion; dg, dorsal root ganglion; h, heart; 2–4, mammary placodes 2–4; awm, abdominal wall mesenchyme; pmd, paramesonephric duct; hl, hindlimb; fl, forelimb; c, cochlea; cp, cartilage primordium; ao, aorta; la, left atrium; lv, left ventricle; op, oropharynx; tg, tongue; l, liver; cb, common bile duct; p, portal vein; d, duodenum; e, epithelial component of mammary placode; m, mesenchymal component of mammary placode; v, vomeronasal organ; ns, nasal septum; md, mesonephric duct; mt, mesonephric tubule; g, gonad (ovary). Scale bars = 1 mm (A, B and D–F), 0.1 mm (C) and 100 μm (G–O).
In addition to the expected results observed above, we found that Dact1-LacZ was also expressed in mesenchyme surrounding the common bile duct (Fig. 2L) and the nipples (Fig. 2A, C and M) as well as in abdominal wall (Fig. 2A, B and K). We also found that Dact1-LacZ was expressed in the vomeronasal organ (Jacobson’s organ), an auxiliary olfactory sense organ (Fig. 2N). Notably, our data show that the paramesonephric duct (Müllerian duct), which eventually forms the reproductive tract in the female but is lost in the male, expressed high levels of Dact1-LacZ (Fig. 2A, B and O). In contrast, the mesonephric duct (Wolffian duct) that develops into reproductive organs in the male but is largely obliterated in the female was devoid of Dact1-LacZ expression (Fig. 2O). Together, these results demonstrate that Dact1 is expressed in multiple mesoderm- and neuroectoderm-derived tissues during embryonic development in mice. Our data also suggest that Dact1 may play an important role in the specification of the female reproductive tract at the sex determination stage.
1.3. Expression of Dact1-LacZ in adult mouse brain
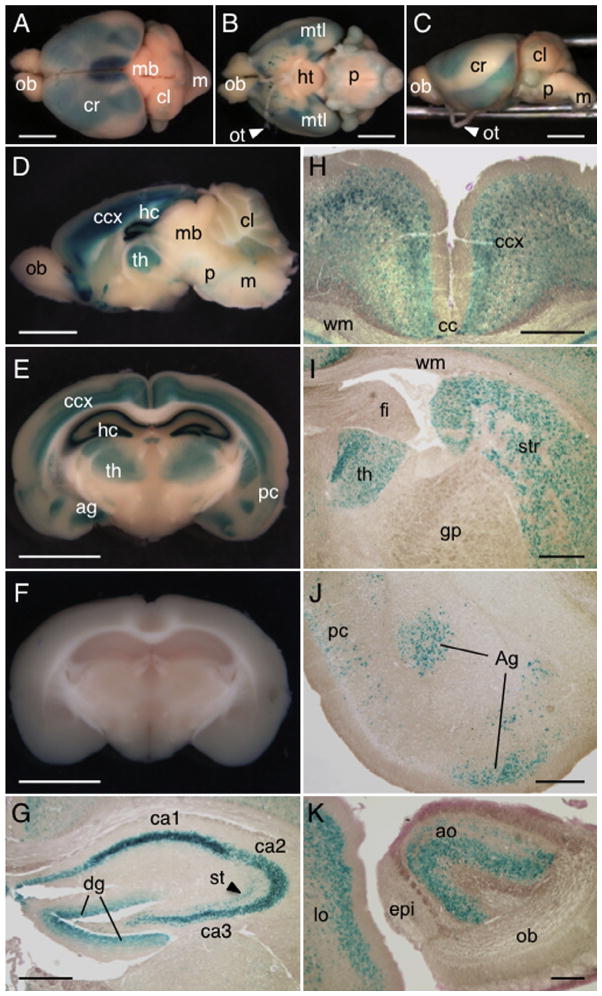
As previous analyses had revealed Dact1 gene expression in adult brain (Fisher et al., 2006), we next assessed Dact1-LacZ expression in the brain. Our data show that Dact1-LacZ was highly expressed in the cerebrum, with weaker signals in the cerebellum, pons and medulla; the olfactory bulb and midbrain were LacZ-negative (Fig. 3A–D).
Fig. 3.
Expression of Dact1-LacZ in adult brain. (A–C) The brains from 8 week-old Dact1LacZ Tg mice were stained for LacZ activity using X-gal and photographed as whole mount preparations. Shown are dorsal (A), ventral (B) and lateral (C) views of the brain. (D–F) The brains from Dact1LacZ Tg mice (D and E) and WT littermates (F) were sliced sagittally along the midline (D) or coronally in the central region of the cerebrum (E and F) and stained for LacZ activity using X-gal. The strongest LacZ signals were detected in the hippocampus, followed by cerebral cortex, ventral forebrain, thalamus and the medial temporal lobes. Weak signals were also noted in the cerebellum, pons and medulla. (G) Within the hippocampus, Dact1-LacZ was detected in the pyramidal neurons in the cornu ammonis regions, granular cells in the dentate gyrus and some interneurons in the stratum radiatum. (H–J) Expression of Dact1-LacZ in other regions of the cerebrum including the cerebral cortex (H), thalamus and striatum (I), amygdaloid nucleus and piriform cortex (J). (K) Expression of Dact1-LacZ in anterior olfactory nucleus and lateral orbital cortex in the ventral forebrain. We analyzed the brains from postnatal day 7 (P7) to 36-week old mice and found similar results. ob, olfactory bulb; cr, cerebrum; mb, midbrain; cl, cerebellum; m, medulla; ht, hypothalamus; p, pons; mtl, medial temporal lobe; ot, optic tract; ccx, cerebral cortex; hc, hippocampus; th, thalamus; ag, amygdaloid nucleus; pc, piriform cortex; ca1–3, cornu ammonis areas 1–3; dg, dentate gyrus; st, stratum radiatum; cc, central canal; wm, white matter; fi, fimbria of hippocampus; gp, external globus pallidus; str, striatum; ao, anterior olfactory nucleus; lo, lateral orbital cortex; epi, accessory olfactory bulb. Scale bars = 3 mm (A–F) and 500 μm (G–K).
To determine neuronal cell types expressing Dact1-LacZ in the cerebrum in more detail, we prepared 3 mm coronal sections of the central region of the cerebrum and stained them for LacZ activity using X-gal (Fig. 3E and F), followed by histological analysis (Fig. 3G–J). Consistent with previous studies by in situ hybridization (Fisher et al., 2006), a high level of Dact1-LacZ expression was detected in the hippocampus, where it was expressed in the pyramidal neurons in the cornu ammonis regions (Fig. 3G). Other neuronal cell types such as granular cells in the dentate gyrus and some interneurons in the stratum radiatum also expressed Dact1-LacZ (Fig. 3G). In the cerebral cortex, Dact1-LacZ was expressed in all layers except for the outermost layer (Fig. 3H). Moving more medially, Dact1-LacZ was found broadly in the thalamus and the striatum (Fig. 3I). We also found that Dact1-LacZ was expressed at high levels in the piriform cortex and amygdaloid nucleus (Fig. 3J) within the medial temporal lobes of the forebrain. We also performed additional staining and sectioning of the ventral forebrain where Dact1-LacZ activity was high (Fig. 3D) and found that Dact1-LacZ was expressed in the anterior olfactory nucleus and lateral orbital cortex (Fig. 3K).
1.4. Expression of Dact1-LacZ in other adult tissues in mice
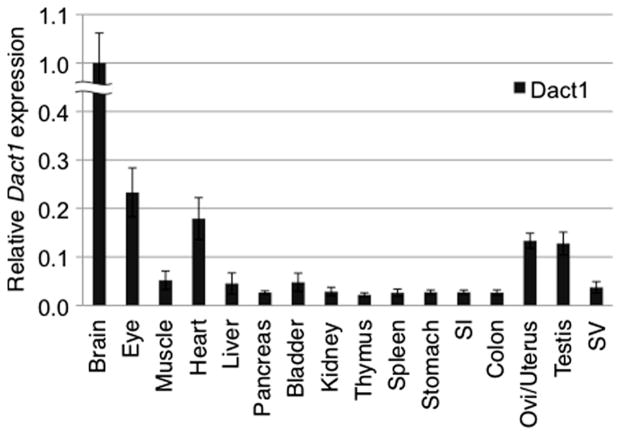
Next, we investigated Dact1-LacZ expression in other adult tissues by whole mount X-gal staining, taking advantage of a rapid processing of X-gal staining in large specimens. We stained more than twenty different postnatal tissues from Dact1LacZ Tg mice ranging from 0 to 35 weeks of age and found that Dact1-LacZ activity was detected in a relatively small number of postnatal tissues, including the eye, heart, oviduct, uterus and testis as described below. These observations were consistent with data from real-time PCR analysis showing that endogenous Dact1 was expressed in these tissues at significantly higher levels than in other tissues examined (Fig. 4).
Fig. 4.
Expression of Dact1 in adult mouse tissues. Real-time PCR was performed using cDNA prepared from 4-week old WT adult mouse tissues. Dact1 was highly expressed in the brain, followed by the eye, heart, oviduct/uterus and testis. The expression level of Dact1 was normalized to the expression of a housekeeping gene Rps18. Data shown are representative of two independent experiments with similar results and expressed as the mean ± s.d. SI, small intestine; Ovi, oviduct; SV, seminal vesicle.
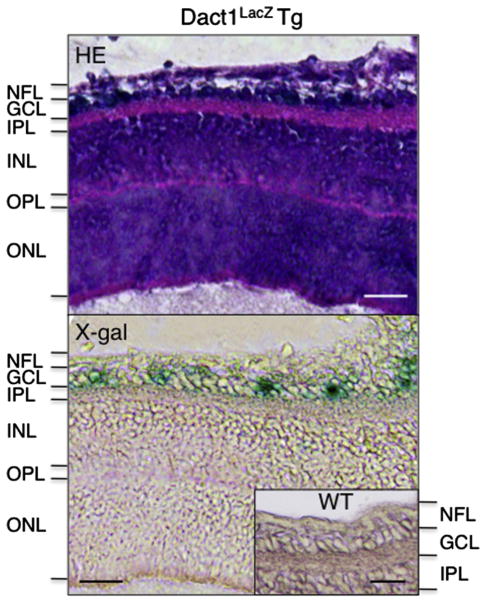
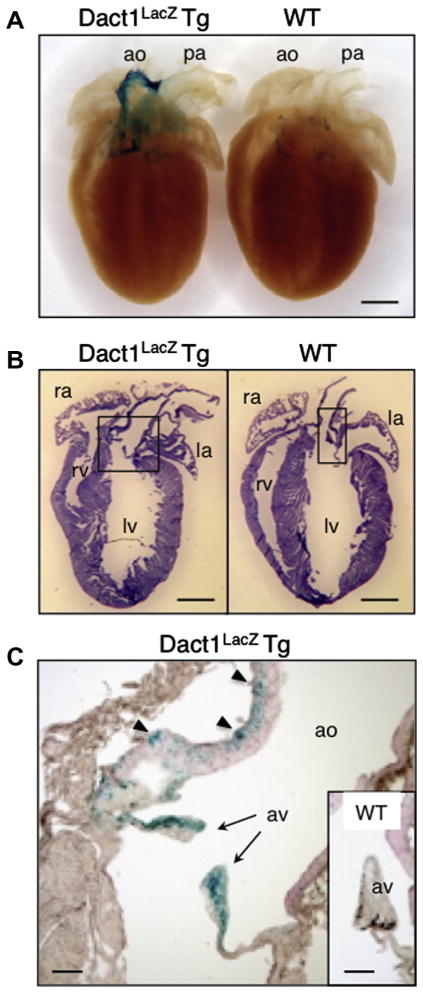
In the eyes, Dact1-LacZ was expressed in the retinal ganglion cells, while other cell types such as bipolar cells, rods and cones were LacZ-negative (Fig. 5). In the heart, Dact1-LacZ was expressed at high levels in the conotruncal region (Fig. 6A) including the connective tissue of the aortic valve and the muscle between elastic fibers in the aorta, while the pulmonary artery was LacZ-negative (Fig. 6A–C).
Fig. 5.
Expression of Dact1-LacZ in adult eye. The eyes from 8 week-old Dact1LacZ Tg mice were stained for LacZ activity and cut into serial sections, followed by staining with hematoxylin and eosin (HE, top) or eosin alone (bottom). Inset in the lower panel indicates the retina from a WT littermate serving as a negative control. We analyzed the eyes from P1 to 15-week old mice and found similar results. NFL, optic nerve fiber layer; GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer. Scale bars = 25 μm.
Fig. 6.
Expression of Dact1-LacZ in adult heart. (A and B) The hearts from 8 week-old Dact1LacZ Tg mice and WT littermates were stained for LacZ activity and photographed as whole mount preparations (A) or cut into sections, followed by hematoxylin and eosin (HE) staining (B). (C) An enlarged view of the conotruncal region in the Dact1LacZ Tg mouse heart showing LacZ staining in the connective tissue of the aortic valve (arrows) and the muscle between elastic fibers in aorta (arrow heads). Inset in (C) shows the aortic valve in a WT littermate mouse serving as a negative control. We analyzed the hearts from P0 to 35-week old mice and found similar results. ao, aorta; pa, pulmonary artery; ra, right atrium; la, left atrium; rv, right ventricle; lv, left ventricle; av, aortic valve. Scale bars = 1 mm (A and B) and 200 μm (C).
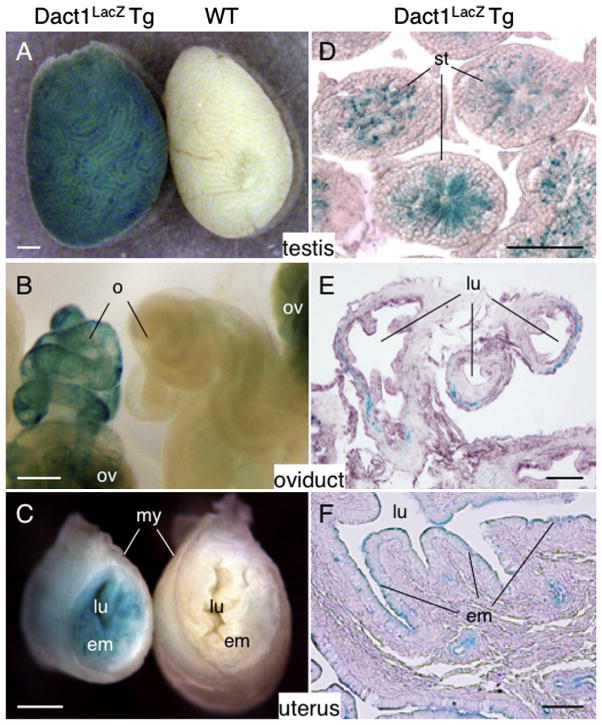
Among male and female reproductive organs, Dact1-LacZ was expressed in the testis, oviduct and uterus (Fig. 7). In the testis, Dact1-LacZ was expressed in the late spermatids (Fig. 7A and D). Consistent with Dact1-LacZ expression in late spermatids, Dact1-LacZ activity was first detected in the testis at around 3 weeks of age and persisted thereafter. In the ovary, non-specific LacZ signals were detected in some stromal cells, but oocytes themselves were LacZ-negative irrespective of their developmental stage (Fig. 7B and data not shown).
Fig. 7.
Expression of Dact1-LacZ in adult reproductive organs. (A–F) The testis, oviduct and uterus from 8 week-old Dact1LacZ Tg mice and WT littermates were stained for LacZ activity and photographed as whole mount preparations (A–C) or cut into sections, followed by counterstaining with eosin (D–F). Dact1-LacZ was expressed in the late spermatids (D), the connective tissue in the outer wall of the oviduct (E) and the uterine endometrium (F). Note that the ovary shows non-specific LacZ signals (B). We analyzed the tissues from P1 to 35-week old mice and found similar results except for the testes that were negative for LacZ activity before 3 weeks of age. st, seminiferous tubule; o, oviduct; ov, ovary; lu, lumen; em, endometrium; my, myometrial layer. Scale bars = 0.5 mm (A–C), 50 μm (D) and 100 μm (E and F).
In the oviduct, Dact1-LacZ was expressed in a mosaic pattern in the outer wall (Fig. 7B and E) while it was expressed uniformly in the endometrium, the inner mucous membrane of the uterus (Fig. 7C and F). Unlike the testis, Dact1-LacZ was detected as early as the neonatal stage in both oviduct and the uterus. Some male reproductive organs (i.e., epididymis, vas deferens and coagulating gland) show high endogenous β-galactosidase activities and we were not able to determine specific LacZ expression in these tissues (data not shown).
2. Discussion
We have shown in this study that while Dact1-LacZ was expressed in multiple mesoderm- and neuroectoderm-derived tissues during development, high expression of Dact1-LacZ was restricted to a small subset of cell types in adult tissues. As Wnt signaling pathways play a critical role in the development, morphogenesis and homeostasis of many of these tissues, our results suggest that Dact1 may also play an important functional role in these processes, as discussed below.
2.1. Neuronal subtypes
Synapse formation and axon guidance are two distinct, but closely related, developmental processes that are essential for establishing neuronal circuitry. Previous studies in multiple model organisms suggest that these two processes may share common regulatory machinery (Chen and Cheng, 2009). Although recent studies indicate that Dact1 regulates synapse formation in hippocampal neurons and cortical interneurons (Okerlund et al., 2010; Arguello et al., 2013), its potential role in axon guidance remains unknown. Our data showing that Dact1-LacZ is highly expressed in some neuronal types including dorsal root ganglion (Fig. 2A, B, F and H), retinal ganglion cells (Fig. 5), olfactory sensory neurons (Fig. 2N) and anterior olfactory nucleus (Fig. 3K), in combination with the known role of Wnt signaling in regulating axon guidance in these neuronal types (Lu et al., 2004; Sato et al., 2006; Schmitt et al., 2006; Rodriguez-Gil and Greer, 2008), suggest that Dact1 may also play a role in axon guidance in some neuronal cells. Thus, further investigation of Dact1 function may provide insight into how axon guidance and synapse formation are coordinated at the molecular level.
2.2. The heart
Our data show that Dact1-LacZ is expressed at high levels in the conotruncal region of the heart during embryogenesis and the cardiac outflow tract in the adult (Figs. 2I and 6). Notably, approximately 30% of all congenital heart diseases are due to defects in the conotruncal region (Chien, 2000; Kioussi et al., 2002). Normal development of the heart outflow tract involves extensive remodeling of the conotruncal region through which the single vessel is separated into the aorta and pulmonary artery (Hutson and Kirby, 2003). Defects in the development of the conotruncal region lead to phenotypes such as double outlet right ventricle (DORV), where both the pulmonary artery and the aorta connect to the right ventricle, transposition of the great arteries (TGA) and persistent truncus arteriosis (PTA), where the outflow tract fails to divide into an aorta and pulmonary artery. Notably, homozygous mutants for Dact1-interactors such as Dvl2, Dvl3 and Van Gogh-like 2 (Vangl2) exhibit cardiac outflow tract abnormalities (Henderson et al., 2001; Hamblet et al., 2002; Etheridge et al., 2008). Similar outflow tract defects including TGA and PTA have also been observed in mice lacking Wnt11 and Wnt5a ligands (Schleiffarth et al., 2007; Zhou et al., 2007). Such findings support further investigation of a potential role for Dact1 in Wnt-mediated development and pathogenesis of the conotruncal region of the heart and the cardiac outflow tract.
2.3. Female reproductive tract
We showed that Dact1-LacZ is expressed at a high level in the Müllerian duct in developing embryos (Fig. 2O) and that its expression persists in the oviducts and the uterus of the adult (Fig. 7B, C, E and F). Wnt signaling plays important roles in the development of the Müllerian duct, including the initiation, outgrowth, patterning and differentiation into vagina, cervix, uterus and oviducts (van der Horst et al., 2012). Notably, targeted deletion of the Dact1 gene in mice leads to a lack of vagina, resulting in hydrometrocolpos by impaired uterine outflow (Suriben et al., 2009; Wen et al., 2010). Similar developmental abnormalities in the female reproductive tract have been reported in several Wnt ligand mutant mice. For instance, Wnt5a deficient mice lack the posterior Müllerian-derived structures (cervix and vagina) while anterior derived structures (oviducts and uterine horns) appear normal (Mericskay et al., 2004). By contrast, the oviducts in Wnt7a nullizygous mice are either absent or remained uncoiled, resembling uterine morphology, while the uterus itself showed marked resemblance to the vaginal morphology (Miller and Sassoon, 1998; Parr and McMahon, 1998).
In adults, Wnt signaling controls the monthly balance between proliferation and differentiation of the endometrium and deregulated Wnt signaling has been reported in endometrial cancers (Fukuchi et al., 1998; Jeong et al., 2009; van der Zee et al., 2013). Thus, Wnt signaling is critical not only for the development of the female reproductive tract but also for the maintenance of endometrial homeostasis in adult. It would be interesting to investigate whether Dact1 cooperates with different Wnt signals to regulate the development and specification of the Müllerian duct and whether deregulated Dact1 activity contributes to endometrial carcinogenesis.
2.4. Conclusions
The results in this study will serve as a basis for future investigation of biological functions of Dact1 in Wnt-mediated organogenesis during development and tissue homeostasis in adult.
3. Experimental procedures
3.1. Dact1 promoter reporter assay
A 6.5 kb Dact1 promoter region was amplified by PCR using mouse BAC clone (RP23-288F14, Children’s Hospital Oakland Research Institute) as a template. The 5′ and 3′ primer sequences used were 5′-GTA TGG TAC CGA CTC TAG CTG CAT ACA CTG C-3′ containing a Kpn I linker and 5′-GTA TGG ATC CGC GCT GCG TCC GGC TTC ATG A-3′ containing a BamHI linker, respectively. PCR-amplified fragments were digested with Kpn I and BamHI and subcloned into the pGL3-basic vector (Promega), followed by direct sequencing to verify correct amplification. Reporter constructs harboring 1.1 kb and 0.1 kb promoter regions were generated by digesting the full-length promoter construct with Bgl II and SacI, respectively, followed by self-ligation.
HCT116 and HT-29 cells (American Type Culture Collection, ATCC) were grown in DMEM medium containing 10% fetal calf serum (Atlanta Biologicals), 10 U/ml penicillin and 100 μg/ml streptomycin (Invitrogen). Cells were seeded at a density of 1 × 105 cells/well in 12-well plates. Subsequently, 1 μg of each reporter construct was transfected into cells per well along with 0.3 μg of the Renilla Luciferase vector pRL-TK (Promega) by SuperFect reagent (Qiagen). Forty-eight hours after the transfection, cells were lysed and luciferase activities were measured using Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer’s instructions. Firefly luciferase activities were normalized to Renilla luciferase control activities.
3.2. Generation of Dact1LacZ reporter mice
Transgenic (Tg) ES cells harboring a Dact1LacZ-neo transgene (Dact1tm2a(EUCOMM)Hmgu) were obtained from the European Mouse Mutant Cell Repository (EuMMCR; http://www.eucomm.org). The parental ES cells used for transgenesis (JM8.N4) were originally derived from C57BL/6N mice. After verifying by Southern blot, Tg ES cells were microinjected into 129/Sv blastocysts to obtain chimeric mice. Male chimeras were mated to female 129/Sv mice to achieve germline transmission and F2 generations of Dact1LacZ-neo Tg mice were crossed with Actβ-Cre Tg mice in a FVB/N background (Jackson Labs) to remove the neomycin cassette, resulting in the generation of Dact1LacZ Tg mice. The Dact1LacZ transgene was identified by PCR using tail DNA with a primer pair 5′-GGT CGC TAC CAT TAC CAG-3′ and 5′-AAA CCA CTC AAG TAA ATA GTA AGT ATA AG-3′ (466 bp), while the Cre transgene was detected by a primer pair 5′-TCC AAT TTA CTG ACC GTA CAC CAA-3′ and 5′-CCT GAT CCT GGC AAT TTC GGC TA-3′ (540 bp). In this study, heterozygous Dact1LacZ Tg mice were maintained by intercrossing with WT littermates. For staged embryos, the day of the vaginal plug was designated E0.5. All experimental procedures were performed in accordance with a protocol approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Pennsylvania.
3.3. Southern blot analysis
Genomic DNA was extracted from WT and Tg ES cells and 20 μg DNA was digested with Pst I and EcoRV. As a probe, 1.1 kb EcoRI fragment downstream of exon 1 was radio-labeled with [α-32P] dCTP (GE Healthcare) by random priming using Rediprime II DNA Labeling System (GE Healthcare).
3.4. Differentiation of ES cells into the adipogenic lineage
WT and Tg ES cells were differentiated into the adipogenic lineage as described previously (Dani et al., 1997) with some modifications. Briefly, embryoid bodies (EBs) were formed and maintained in bacteriological plates for 2 days in DMEM medium supplemented with 10% fetal bovine serum (FBS) (Atlanta Biologicals), 1 × MEM non-essential amino acids, 2 mM L-glutamine (Life Technologies), 10 U/ml penicillin, 100 μg/ml streptomycin and 100 μM 2-mercaptoethanol. Cultures were then primed by 1 μM all-trans retinoic acid (Sigma–Aldrich) for 3 days, followed by cultivation in gelatin-coated ϕ35 mm dishes for additional 1–7 days in DMEM media supplemented with 10% FBS, 0.5 mg/ml insulin, 2 nM triiodothyronine (T3) (Sigma–Aldrich), 5 μg/ml troglitazone (Cayman Chemicals), 10 U/ml penicillin and 100 μg/ml streptomycin. LacZ enzymatic activity was determined in culture by X-gal staining as described in Section 3.5 while expression of genes was analyzed by real-time PCR (see Section 3.6 for detail).
3.5. LacZ whole mount enzyme histochemistry
Embryos and adult tissues were dissected from F3 to F6 generations of Dact1LacZ Tg mice. Embryos older than E12.5 and adult tissues were cut open or sliced into thin sections to ensure good penetration of fixation and staining solutions. Embryos and tissue specimens and differentiating ES cells in cultures were fixed in 4% paraformaldehyde at 4 °C for 1 h and 10 min, respectively, washed 3 times for 15 min each in rinse buffer (0.2 M sodium phosphate [pH7.3], 2 mM magnesium chloride, 0.02% NP-40 and 0.01% sodium deoxycholate), and incubated at 30 °C until an appropriate staining intensity was obtained in staining buffer containing 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide and 1 mg/ml 5-bromo-4-chloro-3-indolyl-β-D-pyranoside (X-gal) in rinse buffer. After staining, samples were washed in phosphate buffered saline (PBS) and post-fixed in 4% paraformaldehyde. Embryos and tissue samples were dehydrated in a graded ethanol series and xylene, embedded in paraffin, sectioned at 8–20 μm, and then counterstained with 1% eosin for histological analysis.
3.6. RNA isolation and gene expression analysis
Total RNA was isolated using Trizol reagent (Invitrogen) according to the manufacturer’s instructions. One microgram total RNA was reverse transcribed using the ProtoScript M-MuLV First Strand cDNA Synthesis Kit (New England Biolabs). Real-time PCR was performed using SYBR Green PCR Master Mix (Applied Biosystems) in an ABI 7900 HT machine according to the manufacturer’s instructions. Relative expression of each gene to the house keeping genes GAPDH and Rps18 was determined by the ΔΔCt method.
Primer sequences used were as follows: human DACT1 (forward: 5′-TTG AAC TGT TTG AGG CGA AGA G-3′ and reverse: 5′-ACT GAA CAC CGA GTT AGA GGA AT-3′), mouse Dact1 (forward: 5′-AAG AGA TGC CGG TTT GTT GAA-3′ and reverse: 5′-GCC CGA AGC TCC ATC ACT C-3′), Dact1-LacZ (forward: 5′-GAA GAG AAC ATC TTG CTG-3′ and reverse: 5′-TCA GCC TTG AGC CTC TGG AGC-3′), mouse Pparγ (forward: 5′-TGA AAG AAG CGG TGA ACC ACT G-3′ and reverse: 5′-TGG CAT CTC TGT GTC AAC CAT G-3′), human GAPDH (forward: 5′-AGC CAC ATC GCT CAG ACA C-3′ and reverse: 5′-GCC CAA TAC GAC CAA ATC C-3′) and mouse Rps18 (forward: 5′-CAT GCA GAA CCC ACG ACA GTA-3′ and reverse: 5′-CCT CAC GCA GCT TGT TGT CTA-3′).
Relative expression of DACT1 in human cell lines was also determined by semi-quantitative RT-PCR. The cDNAs were amplified by 28 cycles of PCR for GAPDH (66 bp) and 30 cycles for DACT1 (201 bp), with the primer pairs described above. Each PCR cycle consists of 95 °C for 20 s, 58 °C for 20 s and 72 °C for 20 s. Amplified products were analyzed on 2% agarose gels.
Acknowledgments
The authors thank Dr. Leslie King for critical reading of the manuscript. This study was supported in part by Grants from the Skin Disease Research Center (5-P30-AR-057217) and Pennsylvania Department of Health (4100042754) to M.S.
References
- Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10:468–477. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- Arguello A, Yang X, Vogt D, Stanco A, Rubenstein JL, Cheyette BN. Dapper antagonist of catenin-1 cooperates with Dishevelled-1 during postsynaptic development in mouse forebrain GABAergic interneurons. PLoS One. 2013;8:e67679. doi: 10.1371/journal.pone.0067679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SY, Cheng HJ. Functions of axon guidance molecules in synapse formation. Curr Opin Neurobiol. 2009;19:471–478. doi: 10.1016/j.conb.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheyette BN, Waxman JS, Miller JR, Takemaru K, Sheldahl LC, Khlebtsova N, Fox EP, Earnest T, Moon RT. Dapper, a Dishevelled-associated antagonist of beta-catenin and JNK signaling, is required for notochord formation. Dev Cell. 2002;2:449–461. doi: 10.1016/s1534-5807(02)00140-5. [DOI] [PubMed] [Google Scholar]
- Chien KR. Myocyte survival pathways and cardiomyopathy: implications for trastuzumab cardiotoxicity. Semin Oncol. 2000;27:9–14. [PubMed] [Google Scholar]
- Curtin JA, Quint E, Tsipouri V, Arkell RM, Cattanach B, Copp AJ, Henderson DJ, Spurr N, Stanier P, Fisher EM, Nolan PM, Steel KP, Brown SD, Gray IC, Murdoch JN. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr Biol. 2003;13:1129–1133. doi: 10.1016/s0960-9822(03)00374-9. [DOI] [PubMed] [Google Scholar]
- Dani C, Smith AG, Dessolin S, Leroy P, Staccini L, Villageois P, Darimont C, Ailhaud G. Differentiation of embryonic stem cells into adipocytes in vitro. J Cell Sci. 1997;110:1279–1285. doi: 10.1242/jcs.110.11.1279. [DOI] [PubMed] [Google Scholar]
- Etheridge SL, Ray S, Li S, Hamblet NS, Lijam N, Tsang M, Greer J, Kardos N, Wang J, Sussman DJ, Chen P, Wynshaw-Boris A. Murine dishevelled 3 functions in redundant pathways with dishevelled 1 and 2 in normal cardiac outflow tract, cochlea, and neural tube development. PLoS Genet. 2008;4:e1000259. doi: 10.1371/journal.pgen.1000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher DA, Kivimäe S, Hoshino J, Suriben R, Martin PM, Baxter N, Cheyette BN. Three Dact gene family members are expressed during embryonic development and in the adult brains of mice. Dev Dyn. 2006;235:2620–2630. doi: 10.1002/dvdy.20917. [DOI] [PubMed] [Google Scholar]
- Fukuchi T, Sakamoto M, Tsuda H, Maruyama K, Nozawa S, Hirohashi S. Beta-catenin mutation in carcinoma of the uterine endometrium. Cancer Res. 1998;58:3526–3528. [PubMed] [Google Scholar]
- Gloy J, Hikasa H, Sokol SY. Frodo interacts with Dishevelled to transduce Wnt signals. Nat Cell Biol. 2002;4:351–357. doi: 10.1038/ncb784. [DOI] [PubMed] [Google Scholar]
- Hamblet NS, Lijam N, Ruiz-Lozano P, Wang J, Yang Y, Luo Z, Mei L, Chien KR, Sussman DJ, Wynshaw-Boria A. Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation and neural tube closure. Development. 2002;129:5827–5838. doi: 10.1242/dev.00164. [DOI] [PubMed] [Google Scholar]
- Henderson DJ, Conway SJ, Greene ND, Gerrelli D, Murdoch JN, Anderson RH, Copp AJ. Cardiovascular defects associated with abnormalities in midline development in the Loop-tail mouse mutant. Circ Res. 2001;89:6–12. doi: 10.1161/hh1301.092497. [DOI] [PubMed] [Google Scholar]
- Huang H, He X. Wnt/beta-catenin signaling: new (and old) players and new insights. Curr Opin Cell Biol. 2008;20:119–125. doi: 10.1016/j.ceb.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter NL, Hikasa H, Dymecki SM, Sokol SY. Vertebrate homologues of Frodo are dynamically expressed during embryonic development in tissues undergoing extensive morphogenetic movements. Dev Dyn. 2006;235:279–284. doi: 10.1002/dvdy.20609. [DOI] [PubMed] [Google Scholar]
- Hutson MR, Kirby ML. Neural crest and cardiovascular development: a 20-year perspective. Birth Defects Res C Embryo Today. 2003;69:2–13. doi: 10.1002/bdrc.10002. [DOI] [PubMed] [Google Scholar]
- Jeong JW, Lee HS, Franco HL, Broaddus RR, Taketo MM, Tsai SY, Lydon JP, DeMayo FJ. Beta-catenin mediates glandular formation and dysregulation of beta-catenin induces hyperplasia formation in the murine uterus. Oncogene. 2009;28:31–40. doi: 10.1038/onc.2008.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettunen P, Kivimäe S, Keshari P, Klein OD, Cheyette BN, Luukko K. Dact1-3 mRNAs exhibit distinct expression domains during tooth development. Gene Expr Patterns. 2010;10:140–143. doi: 10.1016/j.gep.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibar Z, Vogan KJ, Groulx N, Justice MJ, Underhill DA, Gros P. Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nat Genet. 2001;28:251–255. doi: 10.1038/90081. [DOI] [PubMed] [Google Scholar]
- Kioussi C, Briata P, Baek SH, Rose DW, Hamblet NS, Herman T, Ohgi KA, Lin C, Gleiberman A, Wang J, Brault V, Ruiz-Lozano P, Nguyen HD, Kemler R, Glass CK, Wynshaw-Boris A, Rosenfeld MG. Identification of a Wnt/Dvl/beta-Catenin→Pitx2 pathway mediating cell-type-specific proliferation during development. Cell. 2002;111:673–685. doi: 10.1016/s0092-8674(02)01084-x. [DOI] [PubMed] [Google Scholar]
- Lagathu C, Christodoulides C, Virtue S, Cawthorn WP, Franzin C, Kimber WA, Nora ED, Campbell M, Medina-Gomez G, Cheyette BN, Vidal-Puig AJ, Sethi JK. Dact1, a nutritionally regulated preadipocyte gene, controls adipogenesis by coordinating the Wnt/beta-catenin signaling network. Diabetes. 2009;58:609–619. doi: 10.2337/db08-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Lu W, Yamamoto V, Ortega B, Baltimore D. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell. 2004;119:97–108. doi: 10.1016/j.cell.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Mericskay M, Kitajewski J, Sassoon D. Wnt5a is required for proper epithelial–mesenchymal interactions in the uterus. Development. 2004;131:2061–2072. doi: 10.1242/dev.01090. [DOI] [PubMed] [Google Scholar]
- Miller C, Sassoon DA. Wnt-7a maintains appropriate uterine patterning during the development of the mouse female reproductive tract. Development. 1998;125:3201–3211. doi: 10.1242/dev.125.16.3201. [DOI] [PubMed] [Google Scholar]
- Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, Kelley MW. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature. 2003;423:173–177. doi: 10.1038/nature01618. [DOI] [PubMed] [Google Scholar]
- Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and β-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- Okerlund ND, Kivimäe S, Tong CK, Peng IF, Ullian EM, Cheyette BN. Dact1 is a postsynaptic protein required for dendrite, spine, and excitatory synapse development in the mouse forebrain. J Neurosci. 2010;30:4362–4368. doi: 10.1523/JNEUROSCI.0354-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JI, Ji H, Jun S, Gu D, Hikasa H, Li L, Sokol SY, McCrea PD. Frodo links Dishevelled to the p120-catenin/Kaiso pathway: distinct catenin subfamilies promote Wnt signals. Dev Cell. 2006;11:683–695. doi: 10.1016/j.devcel.2006.09.022. [DOI] [PubMed] [Google Scholar]
- Parr BA, McMahon AP. Sexually dimorphic development of the mammalian reproductive tract requires Wnt-7a. Nature. 1998;395:707–710. doi: 10.1038/27221. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Gil DJ, Greer CA. Wnt/Frizzled family members mediate olfactory sensory neuron axon extension. J Comp Neurol. 2008;511:301–317. doi: 10.1002/cne.21834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Umetsu D, Murakami S, Yasugi T, Tabata T. DWnt4 regulates the dorsoventral specificity of retinal projections in the Drosophila melanogaster visual system. Nat Neurosci. 2006;9:67–75. doi: 10.1038/nn1604. [DOI] [PubMed] [Google Scholar]
- Schleiffarth JR, Person AD, Martinsen BJ, Sukovich DJ, Neumann A, Baker CV, Lohr JL, Cornfield DN, Ekker SC, Petryk A. Wnt5a is required for cardiac outflow tract septation in mice. Pediatr Res. 2007;61:386–391. doi: 10.1203/pdr.0b013e3180323810. [DOI] [PubMed] [Google Scholar]
- Schmitt AM, Shi J, Wolf AM, Lu CC, King LA, Zou Y. Wnt-Ryk signalling mediates medial-lateral retinotectal topographic mapping. Nature. 2006;439:31–37. doi: 10.1038/nature04334. [DOI] [PubMed] [Google Scholar]
- Shi Y, Ding Y, Lei YP, Yang XY, Xie GM, Wen J, Cai CQ, Li H, Chen Y, Zhang T, Wu BL, Jin L, Chen YG, Wang HY. Identification of novel rare mutations of DACT1 in human neural tube defects. Hum Mutat. 2012;33:1450–1455. doi: 10.1002/humu.22121. [DOI] [PubMed] [Google Scholar]
- Suriben R, Kivimäe S, Fisher DA, Moon RT, Cheyette BN. Posterior malformations in Dact1 mutant mice arise through misregulated Vangl2 at the primitive streak. Nat Genet. 2009;41:977–985. doi: 10.1038/ng.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teran E, Branscomb AD, Seeling JM. Dpr Acts as a molecular switch, inhibiting Wnt signaling when unphosphorylated, but promoting Wnt signaling when phosphorylated by casein kinase Idelta/epsilon. PLoS One. 2009;4:e5522. doi: 10.1371/journal.pone.0005522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. MPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994;8:1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- van der Horst PH, Wang Y, van der Zee M, Burger CW, Blok LJ. Interaction between sex hormones and WNT/β-catenin signal transduction in endometrial physiology and disease. Mol Cell Endocrinol. 2012;358:176–184. doi: 10.1016/j.mce.2011.06.010. [DOI] [PubMed] [Google Scholar]
- van der Zee M, Jia Y, Wang Y, Heijmans-Antonissen C, Ewing PC, Franken P, DeMayo FJ, Lydon JP, Burger CW, Fodde R, Blok LJ. Alterations in Wnt-β-catenin and Pten signalling play distinct roles in endometrial cancer initiation and progression. J Pathol. 2013;230:48–58. doi: 10.1002/path.4160. [DOI] [PubMed] [Google Scholar]
- Wang J, Mark S, Zhang X, Qian D, Yoo SJ, Radde-Gallwitz K, Zhang Y, Lin X, Collazo A, Wynshaw-Boris A, Chen P. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nat Genet. 2005;37:980–985. doi: 10.1038/ng1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wynshaw-Boris A. The canonical Wnt pathway in early mammalian embryogenesis and stem cell maintenance/differentiation. Curr Opin Genet Dev. 2004;14:533–539. doi: 10.1016/j.gde.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Waxman JS, Hocking AM, Stoick CL, Moon RT. Zebrafish Dapper1 and Dapper2 play distinct roles in Wnt-mediated developmental processes. Development. 2004;131:5909–59021. doi: 10.1242/dev.01520. [DOI] [PubMed] [Google Scholar]
- Wen J, Chiang YJ, Gao C, Xue H, Xu J, Ning Y, Hodes RJ, Gao X, Chen YG. Loss of Dact1 disrupts planar cell polarity signaling by altering dishevelled activity and leads to posterior malformation in mice. J Biol Chem. 2010;285:11023–11030. doi: 10.1074/jbc.M109.085381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Fisher DA, Cheyette BN. SEC14 and spectrin domains 1 (Sestd1), Dishevelled 2 (Dvl2) and Dapper antagonist of catenin-1 (Dact1) co-regulate the Wnt/planar cell polarity (PCP) pathway during mammalian development. Commun Integr Biol. 2013;6:e26834. doi: 10.4161/cib.26834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Cheyette BN. SEC14 and spectrin domains 1 (Sestd1) and Dapper antagonist of catenin 1 (Dact1) scaffold proteins cooperatively regulate the Van Gogh-like 2 (Vangl2) four-pass transmembrane protein and planar cell polarity (PCP) pathway during embryonic development in mice. J Biol Chem. 2013;288:20111–20120. doi: 10.1074/jbc.M113.465427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallen JA. Planar polarity and tissue morphogenesis. Cell. 2007;129:1051–1063. doi: 10.1016/j.cell.2007.05.050. [DOI] [PubMed] [Google Scholar]
- Zhou W, Lin L, Majumdar A, Li X, Zhang X, Liu W, Etheridge L, Shi Y, Martin J, Van de Ven W, Kaartinen V, Wynshaw-Boris A, McMahon AP, Rosenfeld MG, Evans SM. Modulation of morphogenesis by noncanonical Wnt signaling requires ATF/CREB family mediated transcriptional activation of TGFbeta2. Nat Genet. 2007;39:1225–1234. doi: 10.1038/ng2112. [DOI] [PMC free article] [PubMed] [Google Scholar]