Abstract
Stress is a common occurrence in everyday life and repeated or traumatic stress can be a precipitating factor for illnesses of the central nervous system, as well as peripheral organ systems. For example, severe or long-term psychological stress can not only induce depression, a leading illness worldwide, but can also cause psychosomatic diseases such as asthma and rheumatoid arthritis. Related key questions include how psychological stress influences both brain and peripheral systems, and what detection mechanisms underlie these effects? A clue is provided by the discovery of the pathways underlying the responses to host “danger” substances that cause systemic diseases, but can also contribute to depression. The inflammasome is a protein complex that can detect diverse danger signals and produce the accompanying immune-inflammatory reactions. Interestingly, the inflammasome can detect not only pathogen-associated molecules, but also cell damage-associated molecules such as ATP. Here, we propose a new inflammasome hypothesis of depression and related comorbid systemic illnesses. According to this hypothesis, the inflammasome is a central mediator by which psychological and physical stressors can contribute to the development of depression, and as well as a bridge to systemic diseases. This hypothesis includes an explanation for how psychological stress can influence systemic diseases, and conversely how systemic diseases can lead to psychiatric illnesses. The evidence suggests that the inflammasome may be a new target for the development of treatments for depression, as well as psychosomatic and somatopsycho diseases.
Keywords: Inflammatory cytokine, Interleukin-1, Antidepressant, NLRP3 inflammasome, Toll like receptor
1. Introduction
Major depressive disorder (MDD) is characterized by depressed mood, low self-esteem, anhedonia, and disrupted sleeping, eating, and cognition, and has now been recognized to have an overall impact on global illness that is projected to be second only to ischemic heart disease in social and economical burden by 2020 (Greden, 2001; Murray and Lopez, 1997). In the United States, about 14 million people, or about 10% of the population, suffer from depression at any point in time (Kessler et al., 2003). Because approximately one third of MDD patients do not respond to traditional pharmacological medications such as monoamine reuptake inhibitors, there is a major unmet need for the development of novel, more efficacious therapeutic agents.
Although it is clear that genetic factors are involved [heritability estimates from twin studies are reported to be 37%–38% (Kendler et al., 2006; Sullivan et al., 2000)], environmental factors such as stress are strongly implicated in the pathology of depression (Kessler, 1997). Levels of stress have increased with growing social and economic demands in recent decades, resulting in a rapid rise in the prevalence of depression (Kessler et al., 2003). Here, we discuss evidence that psychological, as well as physical stressors can activate immune and inflammation processes and lead to increased cytokine levels, contributing to structural and functional alterations of neurons and the development of MDD.
A role for inflammation and related cytokines in MDD was first suggested in the 1980’s and since then evidence has accumulated in support of this hypothesis (Ader and Cohen, 1993; Maes, 1995; Maier et al., 1994; Tecoma and Huey, 1985). There have been several excellent reviews of this work, but the current paper examines a novel aspect of the immune-inflammation process in the response to stress and depression: that interkeukin-1β (IL-1β) and its regulator, “the Nod-like receptor (NLR) family, pyrin domain-containing 3 (NLRP3) inflammasome”, are a bridge between psychological stress and depression. Related to this hypothesis is the possibility that the inflammasome provides a bidirectional pathway between depression and comorbid systemic illnesses, suggesting novel strategies for treating depression.
2. Comorbidity of mood disorders and systemic/peripheral diseases
Psychosocial stress and systemic disease can both affect the onset of depression. For example, the comorbidity of depression in patients with diabetes, cancer, or cardiac disease is 17%–29%, much higher than that of the general population (10.3%) (% comorbidity of depression with specific systemic illnesses is shown in Table 1) (Evans et al., 2005). Moreover, chronic inflammation is implicated in the pathology of these diseases, and the possible mechanisms by which the NLRP3 inflammasome may serve as a key mediator is being elucidated (Table 1). There is also evidence from clinical procedures that inflammation can cause depression. For example, interferon (IFN) a cytokine that strongly activates the immune system (e.g., natural killer cells and macrophages), is used as a treatment for certain types of cancer and viral infections and causes a high prevalence of dose-dependent depressive symptoms (~50%) (Raison et al., 2006). Immune reactivity and inflammation has thus been implicated as a common factor underlying depressive effects of IFN.
Table 1.
Systemic/Peripheral diseases comorbid with depression and the role of IL-1β and the NLRP3 inflammasome.
| Physical diseases associated with NLRP3 inflammasome | Stimulator | Pathology/Implication of NLRP3 on physical disease | Prevelence of Depression | References |
|---|---|---|---|---|
| Metabolic Disorders | ||||
| Type II Diabetes Mellitus | Hyperglycemia Fatty acids |
β cell death, Insulin resistance | 26% |
Zhou et al. (2010),Mason et al. (2012) Evans et al.(2005) |
| Obesity | Fatty acids | Decrease insulin sensitivity | 20%–30% | Wen et al. (2011), Vandanmagsar et al. (2011), Evans et al.(2005) |
| Cardiovascular disease | Cholesterol crystals | Inflammation / polymorphism in the NLRP3 locus and concordance with fibrinogen gene variants | 17%–27% | Duewell et al. (2010), Mason et al. (2012), Evans et al.(2005) |
| Autoimmuno Diseases | ||||
| Rheumatoid Arthritis | ? | Chronic inflammation of the synovium / Increased expression of NLRP3 | 13%–20% | Kastbom et al. (2008), Mason et al. (2012), Sheehy et al. (2006) |
| Systemic lupus erythematosus (SLE) | U1-snRNP | Inflammatory response causes cell death and organ failure | 22.5%–42% | Bachen et al. (2009), Shin et al. (2012), Nery et al. (2007) |
| Alzheimer’s disease | Amyloid β | Nlrp3−/ − microglia demonstrated a significant reduction in the proinflammatory mediators when treated with amyloid-β | 30%–50% | Mitroulis et al. (2010), Mason et al. (2012), Evans et al. (2005), Halle et al. (2008) |
| Multiple Sclerosis (MS) | ? | Nlrp3 found to play a role in the progression of disease in an experimental autoimmune encephalomyelitis model | 25%–50% | Gris et al. (2010), Mason et al. (2012), Fischer et al. (2012) |
| Infection / Environmental Disease | ||||
| HIV | HIV | Activation of immune system against the virus | 5%–30% | Pontillo et al. (2012), Evans et al. (2005) |
| Asthma | Allergen? | A substaital reduction in inflammation and leukocyte recruitment to the lung in Nlrp3−/ − and IL-1R−/ − mice compared to WT mice | 18% | Besnard et al. (2011), Mason et al. (2012) |
| Chronic obstructive pulmonary disorders | Inflammation (uric acid ?) | Chronic bronchitis and emphysema | 10%–42% |
Eisner et al. (2005) Gasse et al. (2009), Mason et al. (2012) |
| Healthy Subject | ||||
| General population | 10.3% (12-month) 7.1% (Lifetime) |
Caferella et al. (2012) Kessler et al. (1994), Evans et al. (2005) |
||
Listed are the systemic diseases associated with NLRP3, as well as the different types of danger substances that lead to IL-1β release (or activate caspase-1). The prevalence of depression in populations with these different diseases is much higher (up to 30% or even higher) than that of the general population, which is 10.3%.
(Bachen et al., 2009; Besnard et al., 2011; Cafarella et al., 2012; Duewell et al., 2010; Eisner et al., 2005; Evans et al., 2005; Fischer et al., 2012; Gasse et al., 2009; Gris et al., 2010; Halle et al., 2008; Kastbom et al., 2008; Kessler et al., 1994; Mason et al., 2012; Mitroulis et al., 2010; Nery et al., 2007; Pontillo et al., 2012; Sheehy et al., 2006; Shin et al., 2012; Vandanmagsar et al., 2011; Wen et al., 2011; Zhou et al., 2010).
Further support for this hypothesis is provided by studies demonstrating that serum levels of pro-inflammatory cytokines such as IL-1β, IL-6 and TNFα are elevated in depressed patients (Dowlati et al., 2010; Howren et al., 2009). It is well known that these cytokines can induce somatic symptoms, referred to as sickness behavior, including fatigue and loss of appetite (Schiepers et al., 2005), which overlap with common symptoms of major depression. Further, cytokines can affect mood, causing dysphoria and anxiety (Schiepers et al., 2005). Here, we explore the possibility that repeated psychological stress can induce a chronic immune reaction and elevation of cytokines and discuss how this could contribute to depression and comorbid immune/inflammation illnesses.
3. Innate immune response mechanisms
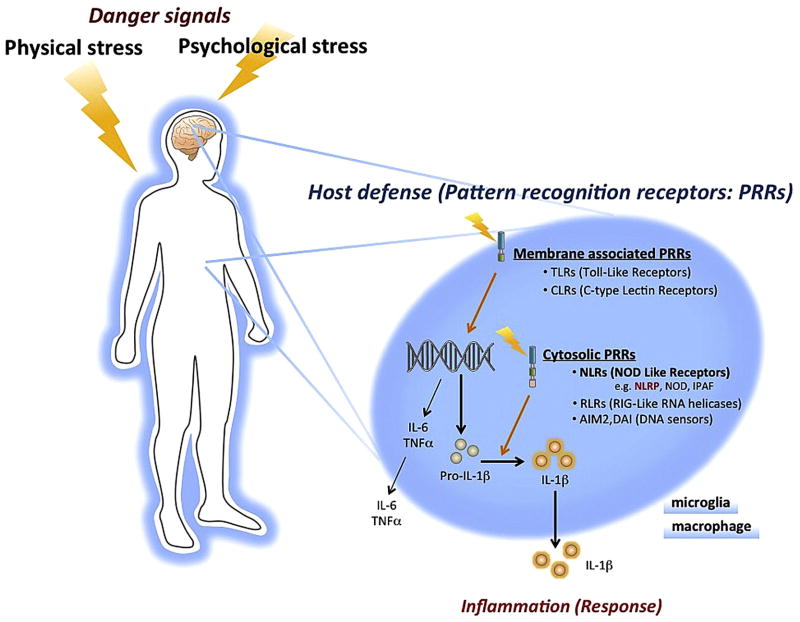
Host survival requires defense mechanisms against external danger substances, including the ability to discriminate “nonself” from “self” and to eliminate or neutralize danger molecules. The mechanisms by which the immune system detects diverse danger signals were only recently elucidated with the discovery of Toll-like receptors (TLRs). TLRs are pattern recognition receptors (PRRs) on the cell-surface of immune cells, characterized by extracellular leucine-rich repeats (LRRs) and an intracellular Toll/IL-1 receptor (TIR) domain (Fig 1). TLRs recognize invariant molecular structures called pathogen-associated molecular patterns (PAMPs) (Schroder and Tschopp, 2010; Tschopp et al., 2003). As a result, there is release of pro-inflammatory “alarm” cytokines, including IL-1β, one of the most potent endogenous pyrogens, as well as tumor necrosis factor-alpha (TNFα) and IL-6 (Tschopp et al., 2003).
Fig. 1.
Danger signals and host defense mechanisms. Danger signals are detected via pattern recognition receptors (PRRs) to prepare defensive responses. PRRs can be classified into two main groups, membrane associated and cytosolic. NOD-like receptors (NLRs) are cytosolic PRRs, and can detect diverse ligands including ATP, fatty acids and amyloid β. NLRP3, one of the best characterized NLRs, forms large multiprotein complexes termed inflammasomes. However, the mechanism by which the NLRP3 inflammasome detects such diverse ligands is unclear. Shown in this figure is the pathway for regulation of IL-1β. Danger signals such as PAMPs are detected by TLRs and induce the synthesis of pro-IL-1β. Pro-IL-1β is processed by the oligomerized NLRP3 complex leading to secretion of IL-1β. Although psychological stress is known to increase plasma levels of IL-1β, the mechanism by which psychological stress is detected by the innate immune system is not known. Abbreviations: AIM, absent in melanoma; DAI, DNA-dependent activator of interferon (IFN)-regulatory factors; IPAF, ICE-protease activating factor; NLRP, NLR family, pyrin domain-containing; NOD, nucleotide-binding oligomerization domain.
Induction of TNFα and IL-6 in response to TLR occurs via activation of gene transcription (Hoshino et al., 1999; Tamandl et al., 2003). The formation of IL-1β also requires TLR4 induction of gene transcription, but requires an additional step, the processing of pro-IL-1β to the mature, active form of IL-1β, which is then released (Bryant and Fitzgerald, 2009) (Fig 1). The processing and release of pro-IL-1β occurs via NLRP3, part of a multiprotein complex referred to as the “inflammasome” (Martinon et al., 2002). A key activator of inflammasome is the ATP purinergic type 2X7 (P2X7) receptor (Ferrari et al., 1997a; Ferrari et al., 2006), although there are examples of NLRP3 independent induction of IL-1β (Bryant and Fitzgerald, 2009; Chae et al., 2011).
4. NLRP3 inflammasome and pattern recognition
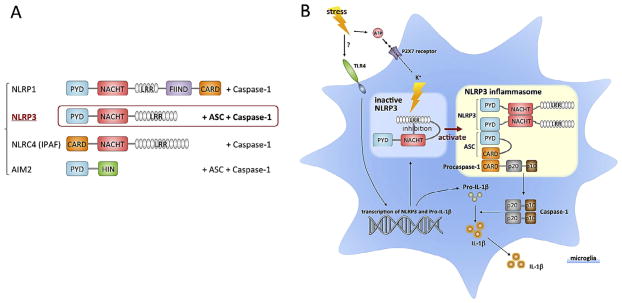
The PRR danger signal receptors are divided into two major types membrane-associated and cytosolic, based on cellular localization (Bryant and Fitzgerald, 2009) (Fig 1). Membrane-associated PRRs include TLRs and C-type lectin receptors (CLR), and cytosolic PRRs include nucleotide-binding oligomerization domain (NOD)-like receptors (NLR), retinoic acid-inducible gene (RIG)-like RNA helicases (RIR), and DNA sensor (absent in melanoma 2: AIM2 etc.). Activation of TLRs leads to increased transcription of pro-IL- 1β, which is subsequently processed by activation of the NLRP3 inflammasome complex (also referred to as cryopyrin or NALP3). NLRP3 has a tripartite structure, consisting of a C-terminal leucine- rich repeat domain (LRR), a central nucleotide-binding oligomerization (NACHT) and N-terminal protein–protein interaction domain, and a Pyrin domain (PYD) (Bryant and Fitzgerald, 2009) (Fig 2). Of all the NLRs, NLRP3 is activated by the most diverse array of danger signals. In addition to PAMPs, NLRP3 also recognizes molecules with damage-associated molecular patterns (DAMPs).
Fig. 2.
Inflammasome subtypes and activation of NLRP3. A. There are four cytosolic pattern recognition receptors that can form inflammasomes that subsequently activate caspase-1 and produce IL-1β. B. The NLRP3 inflammasome undergoes autoinhibition by binding of the LRR domain to the NACHT domain, resulting in blockade of oligomerization and ASC binding. Stimulation relieves this inhibition and enables NLRP3 to bind with pro-caspase-1 through the adaptor protein ASC. Pro-caspase-1 is then cleaved, and the activated caspase-1 cleaves pro-IL-1β, resulting in the release of IL-1β. Interestingly, the NLRP3 inflammasome can detect not only PAMPs, but also DAMPs, which include endogenous danger signals such as ATP. Extracellular ATP binds to the P2X7 receptor ionophore, causing K + efflux, which leads to oligomerization NLRP3. ATP may be associated with stimuli that can cause damage to cells, such as high levels of the excitatory neurotransmitter glutamate, which can cause excitotoxicity. Abbreviations: ASC, apoptosis-associated speck-like protein containing a caspase recruitment domain; CARD, caspase-recruitment domain; FIIND, domain with function to find; LRRs, leucine-rich repeats; NACHT, nucleotide-binding oligomerizetion; NLRP3, NLR family, pyrin domain-containing 3; PYD, Pyrin domain.
In non-stimulated cells, the activity of NLRP3 is inhibited through binding of the LRR to the NACHT domain, thereby blocking oligomerization and binding of the apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) (Fig 2). This auto-inhibition is relieved through the binding of a stimulant molecule to the LRRs. This enables the oligomerization and binding of NLRPs to the adapter protein ASC and pro-caspase-1, making a large multiprotein inflammasome complex, which results in the activation of caspase-1 and cleavage of pro-IL-1β (Tschopp et al., 2003). Four inflammasome complexes have been partially characterized to date: NLRP1, NLRP3, NLRC4 (also called IPAF) and AIM2 (Bryant and Fitzgerald, 2009) (Fig 2).
5. Inflammasome is a key mediator of stress, depression and comorbid illnesses
This review focuses on the role of the inflammasome and IL-1β in depression for several reasons. First, inflammatory cytokines such as IL-1β are elevated in depression, and second, activate the HPA axis, which is dysregulated in depressed patients. Third, chronic inflammatory diseases like rheumatoid arthritis and diabetes that have high rates of comorbidity with depression also activate the NLRP3 inflammasome complex (Evans et al., 2005; Mason et al., 2012). Fourth, we have found that blockade of IL-1β is sufficient to block depressive behavioral and cellular responses resulting from exposure to chronic stress (Koo et al., 2008). Fifth, we hypothesize that IL-1β is the first step in the pro-inflammatory response to psychological stress and results in a cascade of inflammatory cytokine responses. Each of these points is discussed in detail in this section.
5.1. Inflammatory cytokines are elevated in depression
Although the role of the NLRP3 complex in the response to psychological stressors and depression has not been directly examined, there is extensive evidence that the inflammatory cytokines controlled by this inflammasome are increased. There are a large number of studies reporting elevated levels of pro-inflammatory cytokines, including IL-1β, as well as IL-6 and TNFα, in depressed patients (Ader and Cohen, 1993; Dowlati et al., 2010; Hannestad et al., 2011; Howren et al., 2009). Meta-analysis reports the strongest and most consistent evidence for IL-6 and TNFα (Dowlati et al., 2010; Howren et al., 2009). There are several reports that IL-1β is increased in MDD (Leo et al., 2006; Levine et al., 1999; Owen et al., 2001; Thomas et al., 2005), but there has also been a negative meta-analysis (Dowlati et al., 2010). Another study found that antidepressant treatment reduces serum levels of IL-1β, but not TNFα, suggesting a role for IL-1β in treatment response (Hannestad et al., 2011) and as a possible biomarker of depression (Schmidt et al., 2011). Although the pathways and mechanisms underlying elevated IL-1β in depression have not been identified, it is interesting to consider the molecules known to activate the NLRP3 inflammasome (Table 2, Fig. 2).
Table 2.
Cytosolic pattern recognition receptors (PRRs).
| Cytosolic PRRs | Potential ligands |
|---|---|
| CIITA | |
| NALP | Flagellin from Legionella |
| NOD1 | GM-tripeptide, γ-d-Glu-DAP (iEDAP), d-lactyl-l-Ala-γ-Glu-meso-DAP-Gly (FK156), hepatonoyl-γ-Glu-meso-DAP-Ala (FK565) |
| NOD2 | MDP, MurNAc-l-Ala-g-d-Glu-l-Lys (M-TRILys) |
| NLRC3 | NLRC |
| NLRC4 (IPAF) | Flagellin from salmonella, Legionella, Listeria, Pseudomonas |
| NLRC5 | |
| NLRP1 | MDP, Lethal Toxin |
| NLRP2 | |
| NLRP3 | Sendai virus, Influenza virus, Adenovirus, Candida albicans, Saccharomyces cerevisiae, MDP, Nigericin, Maitotoxin, ATP, MSU, Silica, Asbestos, Alum, Amyloid β, Fatty acid, HIV |
| NLRP4–14 | |
| NLRX1 | |
| AIM2 | DNA |
| MNDA | DNA(?) |
| IFI16 | DNA(?) |
| IFIX | DNA(?) |
Listed are the known human cytosolic PRRs, including NLRC4 (IPAF), NLRP1, NLRP3 and AIM2 that assemble to form large multiprotein complexes referred to as inflammasomes (in bold).
Abbreviations: AIM, absent in melanoma; ATP, adenosine triphosphate; CIITA, class II, major histocompatibility complex, transactivator; DNA, deoxyribonucleic acid; HIV, human immunodeficiency virus; IFI, IFN-inducible protein; IPAF, ICE-protease activating factor; MDP, muramyl dipeptide; MNDA, myeloid nuclear differentiation antigen; MSU, monosodium urate crystals; NALP, NACHT, LRR and PYD domains-containing protein; NLRC, NLR family CARD domain-containing protein; NLRP, NLR family, pyrin domain-containing; NLRX, NLR family member X; NOD, nucleotide-binding oligomerization domain.
5.2. Inflammatory cytokines and dysfunction of the HPA axis
Inflammatory cytokines are potent activators of the HPA axis (Berkenbosch et al., 1987; Sapolsky et al., 1987)(Fig 3), which is elevated in depressed patients, possibly due in part to increased levels of IL-1β (Turnbull and Rivier, 1999). A recent large meta-analysis (354 studies, 18,374 individuals) found that approximately 73% of depressed individuals had elevated cortisol values compared to nondepressed individuals (Stetler and Miller, 2011). IL-1β increases the release of hypothalamic corticotropin-releasing hormone (CRH), secretion of pituitary adrenocorticotropic hormone (ACTH), and adrenal steroidogenesis (Fig. 3) (Berkenbosch et al., 1987; Maes et al., 1993; Sapolsky et al., 1987; Tominaga et al., 1991), effects that are thought to occur via indirect mechanisms (Hsieh et al., 2010) (Goshen and Yirmiya, 2009).
Fig. 3.
Pro inflammatory cytokines activate the HPA axis. Hypothalamic CRH stimulates the pituitary, which in turn releases ACTH, leading to stimulation of the adrenal cortex. Released glucocorticoid provides negative feedback on the HPA axis via the hypothalamus and pituitary, as well as hippocampus. Glucocorticoids also suppress pro-inflammatory cytokines under normal conditions, although paradoxically cytokine levels remain high in depressed patients. Cytokine-activation of the HPA axis in the presence of elevated glucocorticoid levels could result from disruption of HPA axis homeostatic mechanisms: that is, inflammatory cytokines activate each step of the HPA axis, including the hypothalamus, pituitary and adrenal cortex. This occurs at the same time that cytokines disrupt glucocorticoid receptor-mediated negative feedback (see text). In this model, inflammatory cytokines in the brain are directly activated by stress. Also, brain pro-inflammatory cytokines can reciprocally affect peripheral cytokines, which can activate the HPA axis and also influence other brain regions via several possible mechanisms (see text). Increased pro-inflammatory cytokines in both brain and periphery disturb negative feedback by glucocorticoids. Abbreviations: ACTH, adrenocorticotropic hormone; CORT, corticosterone; CRH, corticotropin-releasing hormone.
Decreased glucocorticoid receptor (GR) expression and function and loss of negative feedback could also contribute to IL-1β activation of the HPA axis (Hill et al., 1988; Pace and Miller, 2009; Pariante et al., 1999; Verheggen et al., 1996). A role for GR dysfunction is also consistent with the evidence of glucocorticoid resistance (i.e., decreased dexamethasone suppression) that is correlated with increased IL-1 in depressed patients (Maes et al., 1993). This resistance, which could be caused by inflammatory cytokine activation of the HPA axis, is puzzling because glucocorticoids inhibit IL-1β production and release (Goshen and Yirmiya, 2009; Maes et al., 1993; Snyder and Unanue, 1982).
Sustained levels of glucocorticoids can cause atrophy of pyramidal neurons in the hippocampus and medial prefrontal cortex (mPFC), and thereby contribute to depressive symptoms (Sapolsky et al., 2000; Wellman, 2001). These effects could be due to excitotoxicity (Nestler et al., 2002; Sapolsky et al., 2000; Woolley et al., 1990), decreased BDNF expression (Smith et al., 1995; Schaaf et al. 2000), and/or decreased neurogenesis in the adult hippocampus (Chaouloff and Groc, 2011; Muller and Schwarz, 2007; Nestler et al., 2002; Tse et al., 2011). Loss of hippocampal function and negative feedback could also contribute to over activation of the HPA axis (Zunszain et al., 2012).
5.3. NLRP3 inflammasome and comorbidity of depression
Examination of the relationship between depression and comorbid illnesses suggests a role for the NLRP3 inflammasome that is a sensor of a broad range of danger substances (Schroder and Tschopp, 2010). For example, elevated glucose levels can activate the NLRP3 inflammasome, and increase IL-1β, thereby contributing to insulin resistance and the pathology of diabetes (Schroder and Tschopp, 2010; Zhou et al., 2010). The NLRP3 inflammasome is also involved in the innate response to amyloid β, suggesting a role in Alzheimer’s disease (Halle et al., 2008).
The NLRP3 inflammasome is constitutively expressed in macrophages and microglia, the resident macrophages of the central nervous system (CNS). Microglia are monocytic in origin and migrate to the CNS during early embryonic development (Kaushik et al., 2012). Microglia are ubiquitous throughout the brain, with the highest concentration in the hippocampus (Block et al., 2007; Lawson et al., 1990; Perry et al., 1985). Preliminary results from our group have shown that acute restraint stress leads to activation of the NLRP3 inflammasome in the hippocampus (Iwata et al., 2012). We are currently extending this work using NLRP3 knockout mice to examine the functional impact of the NLRP3 inflammasome in the actions of stress. The maturation and release of IL-1β is significantly reduced in NLRP3 null mutant mice (Hanamsagar et al., 2011; Martinon et al., 2006), and we predict that the molecular and behavioral effects of stress will be decreased in these mice. Pharmacological inhibitors of the NLRP3 inflammasome, such as the antidiabetic drug glyburide, may also prove effective in blocking the elevation of IL-1β in response to stress and depression (Lamkanfi et al., 2009).
5.4. Inflammatory cytokines cause depressive behaviors: possible mechanisms
The sickness behavior resulting from IL-1β exposure includes depressive symptoms such as suppression of locomotor activity, social exploration, motivation, and feeding, as well as anhedonia, a core symptom of depression (Anisman et al., 1998; Bluthe et al., 1996; Bonaccorso et al., 2003; Crestani et al., 1991; Hellerstein et al., 1989; Kent et al., 1992; Lacosta et al., 1998; Linthorst and Reul, 1998; McCarthy et al., 1986; Wilhelm et al., 2012). IL- 1β also induces anxiogenic behavior in the open field test and the elevated plus maze, and impaired spatial memory in the Morris water maze (Song et al., 2006). Administration of an IL-1 receptor (IL-1R) antagonist reduces fear conditioning and learned helpless behavior in an inescapable stress/helplessness paradigm (Konsman et al., 2008; Maier and Watkins, 1995), and IL-1R deletion mutant mice have reduced anxiety compared to wild type controls (Koo et al., 2010).
Systemic administration of TNFα also decreases social exploration and causes weight loss (Bluthe et al., 1994; Simen et al., 2006), and central TNFα causes a reduction in social interaction, increased despair behavior, decreased food intake, and a loss of body weight (Bluthe et al., 1994; Palin et al., 2009; Plata-Salaman et al., 1988). The TNFα-induced behavioral alterations may be mediated by endogenous release of IL-1, because pretreatment with an IL-1 receptor inhibitor antagonizes the depressive behaviors caused by TNFα (Bluthe et al., 1994). Central administration of IL-6 decreases wheel-running behavior in rats (Harden et al., 2008), activates the HPA axis, and causes fever (Lenczowski et al., 1999). Infusion of IL-6 into the amygdala or the hippocampus increases immobility in the FST (Wu and Lin, 2008).
IL-1β decreases the expression of brain derived neurotrophic factor (BDNF) and adult hippocampal neurogenesis, which have been linked to susceptibility to depressive behaviors (Banasr and Duman, 2011). IL-1β is also a potent regulator of the serotonin transporter (SERT) (Ramamoorthy et al., 1995; Zhu et al., 2010), and lipopolysaccharide (LPS) stimulation of SERT activity and behavioral despair are blocked by inhibition of IL-1R (Zhu et al., 2010). Interestingly, 30% of depressive people who are resistant to selective serotonin reuptake inhibitors (SSRIs) also have significantly increased levels of pro-inflammatory cytokines (O’Brien et al., 2007).
5.5. Stress activates the inflammasome-IL-1β cascade
The hypothesis that IL-1β is a key factor in the pro-inflammatory response to psychological stress is based on several lines of evidence. First, psychological stressors have been demonstrated to increase pro-inflammatory cytokines such as IL-1β and TNFα in blood and brain tissue (Madrigal et al., 2002; O’Connor et al., 2003; Raison et al., 2006). Second, our preliminary studies demonstrate that acute restraint stress first increases IL-1β in the hippocampus, prior to increasing levels of IL-6 and TNFα (unpublished). Moreover, stress increases ATP levels in the hippocampus resulting in P2X7 receptor dependent elevation of IL-1β, and subsequent release of TNFα and IL-6 (unpublished) (Fig. 2). This is similar to neuropathic pain, which also causes an initial induction of IL-1β that is dependent on ATP-P2X7 receptor activation (Nadeau et al., 2011; Whitehead et al., 2010). In contrast, experimental bacterial infection (i.e., LPS) first increases TNFα (Amiot et al., 1997).
The canonical pathways for induction of IL-1β include both a priming step, mediated by TLR4, as well as P2X7 receptor-activation of the inflammasome to process pro-IL-1β. It is currently not known if stress rapidly increases an endogenous activator of TLR4 that increases transcription. Alternatively, it is possible that there is a basal level of readily available pro-IL-1β that could be converted to IL-1β and released. The latter possibility is supported by studies demonstrating that IL-1β is involved in cellular models of learning and memory (Goshen et al., 2007; Labrousse et al., 2009), indicating pro-IL-1β is present and readily available for processing and release under physiological conditions. Additional studies are required to further characterize the mechanisms underlying the rapid release of IL-1β in response to acute, as well as chronic stress.
6. Effects of anti-inflammatory drugs on depression
Activation of the inflammasome and elevated cytokines in stress and depression suggests that suppression of the inflammatory response could ameliorate depressive symptoms. Interestingly, tricyclic antidepressants (TCA) and SSRIs normalize serum levels of inflammatory cytokines in depressed patients, as well as increase the production of anti-inflammatory cytokines such as IL-10 (Kenis and Maes, 2002; Raedler, 2011). In addition, co-treatment of antidepressants with non-steroidal anti-inflammatory drugs (NSAIDs), such as celecoxib, a cyclooxygenase-2 inhibitor, significantly augments antidepressant efficacy (Muller et al., 2006). Administration of minocycline, an anti-inflammatory drug and selective microglial inhibitor, also augments the clinical efficacy of antidepressants (Pae et al., 2008). Minocycline treatment has neuroprotective effects (i.e., decreases excitotoxicity) by inhibiting microglial proliferation (Tikka et al., 2001). However, a recent study in mice reported that NSAIDs inhibit the beneficial effects of antidepressants on inflammation (Warner-Schmidt et al., 2011).
These findings have led to additional studies examining other approaches that block or normalize pro-inflammatory cytokines. A large-scale survey showed that etanercept, a soluble TNFα receptor that binds to TNFα and inhibits it’s interaction with cell-surface receptors, relieves fatigue and some symptoms of depression in patients being treated for psoriasis (Tyring et al., 2006). 3-hydroxy–3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) decrease cholesterol levels and are used to prevent or treat cardiovascular diseases, but are also reported to have antidepressant effects, possibly as a result of anti-inflammatory actions (Otte et al., 2012). Similarly, a meta-analysis of eicosapentaenoic acid (EPA), an omega-3 fatty acid with anti-inflammatory properties, shows that it has antidepressant actions (Block et al., 2012; Lin and Su, 2007). Ketamine, a rapid onset antidepressant and N-methyl-D-aspartate (NMDA) receptor antagonist, also possesses anti-inflammatory properties, although at doses higher than used for behavioral studies (Adams et al., 2008).
7. New anti-inflammatory approaches for the treatment of depression
7.1. Regulation of IL-1β expression, release, and signaling
We have previously demonstrated that administration of an IL- 1β antagonist blocks the inhibitory effects of stress on neurogenesis, as well as the expression of depressive behaviors (Koo and Duman, 2008). In addition, IL-1R null mice are resistant to the development of depressive behaviors, including anhedonia, caused by chronic stress exposure (Koo and Duman, 2009). These studies indicate that blockade of IL-1R, or the production and release of IL-1β would produce antidepressant effects. We have also reported that blockade of IL-1R downstream signaling molecules, such as IκK, Iκb, and nuclear factor kappa B (NF-κB), blocks the reduction of neurogenesis caused by stress (Koo and Duman, 2008, 2009; Koo et al., 2010). These findings indicate that inhibitors of IL-1β-signaling could be useful for the treatment of depression.
7.2. P2X7 receptors and depression
When considering possible targets for regulation of IL-1β, the purinergic receptor, P2X7, has received attention for several reasons. The processing and release of IL-1β in microglia, as well as peripheral macrophages, are stimulated by ATP-activation of the P2X7 receptor (Block et al., 2012; Ferrari et al., 1997b). The P2X7 receptor is a cell surface K+ ionophore that, when stimulated leads to activation of the NLRP3 inflammasome and release of IL-1β (Fig. 2) (Ferrari et al., 1997b). Moreover, there is a P2X7 receptor gene polymorphism that is associated with major depression (Lucae et al., 2006). P2X7 receptor knockout mice also exhibit an antidepressant- like profile in behavioral models, including the tail suspension and forced swim tests (Basso et al., 2009), and are resistant to stress in certain models of depression (Boucher et al., 2011). Preliminary results from our laboratory demonstrate that a selective, high affinity P2X7 receptor antagonist, A-804598 (Donnelly-Roberts et al., 2009), blocks the down-regulation of neurogenesis caused by acute stress (Iwata et al., 2012). Moreover, we have found that chronic administration of this P2X7 receptor antagonist reverses the depressive behavior caused by chronic unpredictable stress (unpublished observations).
7.3. Peripheral regulation of IL-1β
The induction of IL-1β by stress in the periphery and the brain, and the reciprocal interactions between these systems (Fig. 3) (Dobbin et al., 1991; Nguyen et al., 1998) raises the question as to whether peripheral blockade of IL-1β is sufficient to block the neuronal and behavioral effects of chronic stress. The blood brain barrier (BBB) protects the brain from systemic pathogens, as well as the entry of peripheral peptides and proteins. Inflammatory cytokines, including IL-1β, are large polypeptides and are thus unlikely to cross the BBB (Watkins et al., 1995). However, there are several mechanisms by which the brain and peripheral systems can communicate. The vagus nerve, a cranial nerve connecting the brain to the periphery, has an afferent fiber by which peripheral stimulation can be directly conducted to the central nervous system (Maier et al., 1998). In support of this, vagotomy has been reported to block numerous brain-mediated sickness responses following both LPS and IL-1β administration (Maier et al., 1998; Nguyen et al., 1998; Watkins et al., 1995).
Alternatively, communication between the brain and the periphery could occur via leaky areas (e.g., circumventricular organs), binding to cerebral blood vessel endothelial cells, or active transport of cytokines into the brain (Maier et al., 1998; Moltz, 1993; Watkins et al., 1995). In support of this view, a peripheral injection of etanercept, which neutralizes TNFα and does not cross the BBB, is able to ameliorate sickness behavior produced by a proinflammatory manipulation (Jiang et al., 2008), and can produce antidepressant effects in patients (Krishnan et al., 2007; Tyring et al., 2006). It is possible that peripheral administration of an IL- 1R antagonist, such as Anakinra, which does not cross the BBB, may also reduce IL-1β function in the periphery and subsequently have effects on the brain. Studies are currently underway to determine if blockade or neutralization of IL-1β in the periphery is sufficient to block the effects of stress and/or produce antidepressant effects in cellular and behavioral models of depression.
8. An inflammasome hypothesis of depression: bidirectional pathways between depression and comorbid systemic illnesses
Here we suggest a new hypothesis for depression and comorbid illnesses based on evidence of bidirectional pathways between psychological stress and systemic diseases, and danger substances and depression (Fig. 4). As previously discussed, psychological stress can activate the NLRP3 inflammasome, leading to the release of IL-1β, which can contribute to the pathophysiology of systemic illnesses such as diabetes, cardiovascular, and inflammatory diseases. In addition, danger substances such as fatty acids and allergens can activate the NLRP3 inflammasome and thereby elevate IL-1β and contribute to depression symptoms. Based on this hypothesis, depressed patients may be more susceptible to NLRP3-associated diseases. In parallel, patients with NLRP3-associated systemic diseases are more likely to show depressive symptoms. These two pathways can also work in conjunction: for example, in diabetes and cardiovascular disease, psychological stress, along with fatty acids and cholesterol crystals, could act together to hyper-activate the NLRP3 inflammasome, and thereby increase the susceptibility to depression via elevated release of IL-1β.
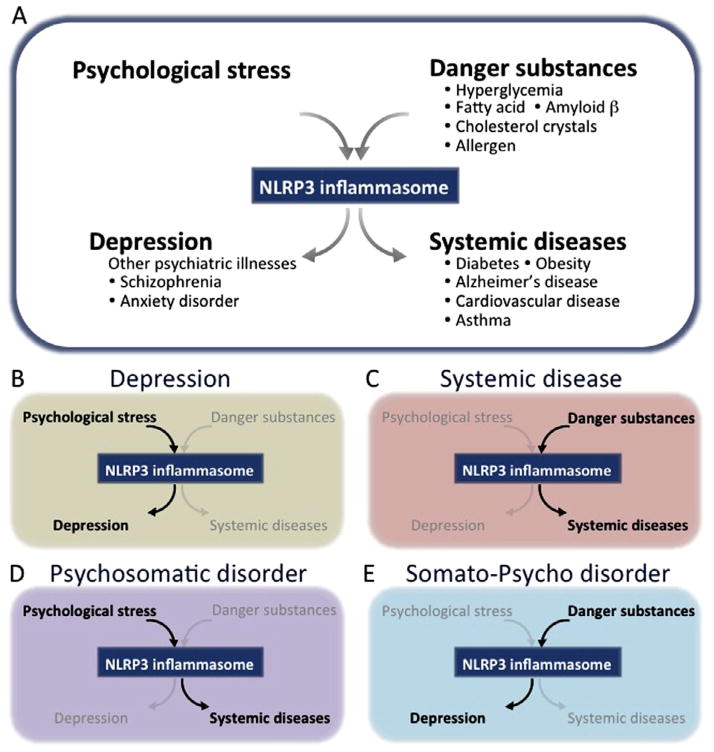
Fig. 4.
NLRP3 inflammasome is a central mediator of depression and comorbid disease. Top panel: (A) Psychological stress could cause activation the NLRP3 inflammasome, which may lead to release of IL-1β and the development of depression. Danger substances also activate the NLRP3 inflammasome and contribute to the pathology of several comorbid diseases. Thus, the NLRP3 could be a central mediator of both psychological and systemic diseases. In support of this, it is well known that psychological stress can affect systemic, peripheral diseases, and these diseases frequently accompany depression. Bottom panel: (B) depression is caused by psychological stress via the NLRP3 inflammasome; (C) systemic diseases can be caused by danger substances via the NLRP3 inflammasome; (D) the NLRP3 inflammasome may also explain how psychological stress can contribute to the development of systemic diseases (psychosomatic disorder); and (E) how intra- and extra- cellular danger substances can lead to the development of depression (somato-psycho disorder). Taken together, it is hypothesized that NLRP3 serves as a bridge between psychological stress and depression, as well as bidirectional pathway between depression and systemic comorbid illnesses.
In summary, the major points of the “inflammasome hypothesis of depression” are: 1) psychological stress activates NLRP3 and causes subsequent development of depression; 2) elevated levels of danger substances such as glucose, fatty acids, and cholesterol crystals, can activate the NLRP3 inflammasome, which could contribute to certain types of comorbid “systemic illnesses”; 3) “psychosomatic diseases” may be caused by psychological stressors that cause systemic diseases via a common NLRP3 inflammasome; and 4) “somato-psycho diseases” may be caused by intra- and extra- cellular danger substances that also activate the NLRP3 inflammasome and subsequently contribute to depression (Fig. 4).
The immune system consists of diverse mechanisms that provide fine control and protection from external hazards and abnormally high levels of danger substances. However, high levels of psychological stress encountered in everyday life can lead to activation of the immune system and could represent a “warning” to activate biological defense mechanisms. If left unchecked, sustained stress and immune responses can lead to full-blown depression, as well as contribute to comorbid systemic illnesses. The NLRP3 inflammasome represents a possible sensor for psychological stress and is a potential target for the treatment of depression.
Acknowledgments
The authors would like to thank Dr. F. Sakaue for helpful discussion and making illustrations in this paper.
References
- Adams SD, Radhakrishnan RS, Helmer KS, Mercer DW. Effects of anesthesia on lipopolysaccharide-induced changes in serum cytokines. J Trauma. 2008;65:170–174. doi: 10.1097/TA.0b013e31805824ca. [DOI] [PubMed] [Google Scholar]
- Ader R, Cohen N. Psychoneuroimmunology: conditioning and stress. Annu Rev Psychol. 1993;44:53–85. doi: 10.1146/annurev.ps.44.020193.000413. [DOI] [PubMed] [Google Scholar]
- Amiot F, Fitting C, Tracey KJ, Cavaillon JM, Dautry F. Lipopolysaccharide-induced cytokine cascade and lethality in LT alpha/TNF alpha-deficient mice. Mol Med. 1997;3:864–875. [PMC free article] [PubMed] [Google Scholar]
- Anisman H, Kokkinidis L, Borowski T, Merali Z. Differential effects of interleukin (IL)-1beta, IL-2 and IL-6 on responding for rewarding lateral hypothalamic stimulation. Brain Res. 1998;779:177–187. doi: 10.1016/s0006-8993(97)01114-1. [DOI] [PubMed] [Google Scholar]
- Bachen EA, Chesney MA, Criswell LA. Prevalence of mood and anxiety disorders in women with systemic lupus erythematosus. Arthritis Rheum. 2009;61:822–829. doi: 10.1002/art.24519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M, Duman RS. Cell growth and survival in the pathophysiology and treatment of depression. Curr Opin Mol Cell Neurosci. 2011;91:333–338. [Google Scholar]
- Basso AM, Bratcher NA, Harris RR, Jarvis MF, Decker MW, Rueter LE. Behavioral profile of P2X7 receptor knockout mice in animal models of depression and anxiety: relevance for neuropsychiatric disorders. Behav Brain Res. 2009;198:83–90. doi: 10.1016/j.bbr.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Berkenbosch F, van Oers J, del Rey A, Tilders F, Besedovsky H. Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1. Science. 1987;238:524–526. doi: 10.1126/science.2443979. [DOI] [PubMed] [Google Scholar]
- Besnard AG, Guillou N, Tschopp J, Erard F, Couillin I, Iwakura Y, Quesniaux V, Ryffel B, Togbe D. NLRP3 inflammasome is required in murine asthma in the absence of aluminum adjuvant. Allergy. 2011;66:1047–1057. doi: 10.1111/j.1398-9995.2011.02586.x. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Block RC, Dier U, Calderonartero P, Shearer GC, Kakinami L, Larson MK, Harris WS, Georas S, Mousa SA. The Effects of EPA+DHA and Aspirin on Inflammatory Cytokines and Angiogenesis Factors. World J Cardiovasc Dis. 2012;2:14–19. doi: 10.4236/wjcd.2012.21003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluthe RM, Michaud B, Kelley KW, Dantzer R. Vagotomy attenuates behavioural effects of interleukin-1 injected peripherally but not centrally. NeuroReport. 1996;7:1485–1488. doi: 10.1097/00001756-199606170-00008. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Pawlowski M, Suarez S, Parnet P, Pittman Q, Kelley KW, Dantzer R. Synergy between tumor necrosis factor alpha and interleukin-1 in the induction of sickness behavior in mice. Psychoneuroendocrinology. 1994;19:197– 207. doi: 10.1016/0306-4530(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Bonaccorso S, Maier SF, Meltzer HY, Maes M. Behavioral changes in rats after acute, chronic and repeated administration of interleukin-1beta: relevance for affective disorders. J Affect Disord. 2003;77:143–148. doi: 10.1016/s0165-0327(02)00118-0. [DOI] [PubMed] [Google Scholar]
- Boucher AA, Arnold JC, Hunt GE, Spiro A, Spencer J, Brown C, McGregor IS, Bennett MR, Kassiou M. Resilience and reduced c-Fos expression in P2X7 receptor knockout mice exposed to repeated forced swim test. Neuroscience. 2011;189:170–177. doi: 10.1016/j.neuroscience.2011.05.049. [DOI] [PubMed] [Google Scholar]
- Bryant C, Fitzgerald KA. Molecular mechanisms involved in inflammasome activation. Trends Cell Biol. 2009;19:455–464. doi: 10.1016/j.tcb.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Cafarella PA, Effing TW, Usmani ZA, Frith PA. Treatments for anxiety and depression in patients with chronic obstructive pulmonary disease: a literature review. Respirology. 2012;17:627–638. doi: 10.1111/j.1440-1843.2012.02148.x. [DOI] [PubMed] [Google Scholar]
- Chaouloff F, Groc L. Temporal modulation of hippocampal excitatory transmission by corticosteroids and stress. Front Neuroendocrinol. 2011;32:25–42. doi: 10.1016/j.yfrne.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Crestani F, Seguy F, Dantzer R. Behavioural effects of peripherally injected interleukin-1: role of prostaglandins. Brain Res. 1991;542:330–335. doi: 10.1016/0006-8993(91)91587-q. [DOI] [PubMed] [Google Scholar]
- Dobbin JP, Harth M, Mccain GA, Martin RA, Cousin K. Cytokine production and lymphocyte-transformation during stress. Brain Behav Immun. 1991;5:339–348. doi: 10.1016/0889-1591(91)90029-a. [DOI] [PubMed] [Google Scholar]
- Donnelly-Roberts DL, Namovic MT, Surber B, Vaidyanathan SX, Perez-Medrano A, Wang Y, Carroll WA, Jarvis MF. [3H]A-804598 ([3H]2- cyano-1-[(1S)-1-phenylethyl]-3-quinolin-5-ylguanidine) is a novel, potent, and selective antagonist radioligand for P2X7 receptors. Neuropharmacology. 2009;56:223–229. doi: 10.1016/j.neuropharm.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner MD, Katz PP, Lactao G, Iribarren C. Impact of depressive symptoms on adult asthma outcomes. Annals Of Allergy, Asthma & Immunology:Official Publication Of The American College Of Allergy, Asthma, & Immunology. 2005;94:566–574. doi: 10.1016/S1081-1206(10)61135-0. [DOI] [PubMed] [Google Scholar]
- Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KR, Nemeroff CB, Bremner JD, Carney RM, Coyne JC, Delong MR, Frasure-Smith N, Glassman AH, Gold PW, Grant I, Gwyther L, Ironson G, Johnson RL, Kanner AM, Katon WJ, Kaufmann PG, Keefe FJ, Ketter T, Laughren TP, Leserman J, Lyketsos CG, McDonald WM, McEwen BS, Miller AH, Musselman D, O’Connor C, Petitto JM, Pollock BG, Robinson RG, Roose SP, Rowland J, Sheline Y, Sheps DS, Simon G, Spiegel D, Stunkard A, Sunderland T, Tibbits P, Jr, Valvo WJ. Mood disorders in the medically ill: scientific review and recommendations. Biol Psychiatry. 2005;58:175–189. doi: 10.1016/j.biopsych.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Melchiorri L, Baricordi OR, Di Virgilio F. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J Immunol. 1997a;159:1451– 1458. [PubMed] [Google Scholar]
- Ferrari D, Chiozzi P, Falzoni S, Hanau S, Di Virgilio F. Purinergic modulation of interleukin-1 beta release from microglial cells stimulated with bacterial endotoxin. J Exp Med. 1997b;185:579–582. doi: 10.1084/jem.185.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, Panther E, Di Virgilio F. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- Fischer A, Otte C, Krieger T, Nicholls RA, Kruger S, Ziegler KJ, Schulz KH, Heesen C, Gold SM. Decreased hydrocortisone sensitivity of T cell function in multiple sclerosis-associated major depression. Psychoneuroendocrinology. 2012;37:1712–1718. doi: 10.1016/j.psyneuen.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Gasse P, Riteau N, Charron S, Girre S, Fick L, Petrilli V, Tschopp J, Lagente V, Quesniaux VF, Ryffel B, Couillin I. Uric acid is a danger signal activating NALP3 inflammasome in lung injury inflammation and fibrosis. Am J Respir Crit Care Med. 2009;179:903–913. doi: 10.1164/rccm.200808-1274OC. [DOI] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ounallah-Saad H, Renbaum P, Zalzstein Y, Ben-Hur T, Levy-Lahad E, Yirmiya R. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology. 2007;32:1106–1115. doi: 10.1016/j.psyneuen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Goshen I, Yirmiya R. Interleukin-1 (IL-1): a central regulator of stress responses. Front Neuroendocrinol. 2009;30:30–45. doi: 10.1016/j.yfrne.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Greden JF. The burden of disease for treatment-resistant depression. J Clin Psychiatry. 2001;62 (Suppl 16):26–31. [PubMed] [Google Scholar]
- Gris D, Ye Z, Iocca HA, Wen H, Craven RR, Gris P, Huang M, Schneider M, Miller SD, Ting JP. NLRP3 plays a critical role in the development of experimental autoimmune encephalomyelitis by mediating Th1 and Th17 responses. J Immunol. 2010;185:974–981. doi: 10.4049/jimmunol.0904145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanamsagar R, Torres V, Kielian T. Inflammasome activation and IL-1beta/ IL-18 processing are influenced by distinct pathways in microglia. J Neurochem. 2011;119:736–748. doi: 10.1111/j.1471-4159.2011.07481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannestad J, DellaGioia N, Bloch M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a metaanalysis. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2011;36:2452–2459. doi: 10.1038/npp.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden LM, du Plessis I, Poole S, Laburn HP. Interleukin (IL)-6 and IL-1 beta act synergistically within the brain to induce sickness behavior and fever in rats. Brain Behav Immun. 2008;22:838–849. doi: 10.1016/j.bbi.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Hellerstein MK, Meydani SN, Meydani M, Wu K, Dinarello CA. Interleukin-1-induced anorexia in the rat. Influence of prostaglandins. J Clin Investig. 1989;84:228–235. doi: 10.1172/JCI114145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MR, Stith RD, McCallum RE. Human recombinant IL-1 alters glucocorticoid receptor function in Reuber hepatoma cells. J Immunol. 1988;141:1522–1528. [PubMed] [Google Scholar]
- Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with Creactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Hsieh CH, Li HY, Chen JC. Nitric oxide and interleukin-1beta mediate noradrenergic induced corticotrophin-releasing hormone release in organotypic cultures of rat paraventricular nucleus. Neuroscience. 2010;165:1191– 1202. doi: 10.1016/j.neuroscience.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Iwata M, Li XY, Banasr M, Ota KT, Sakaue F, Duman RS. Signaling pathways underlying the regulation of IL-1β in response to stress: targets for novel antidepressant agents. The 42nd Annual Meeting of the Society for Neuroscience.2012. [Google Scholar]
- Jiang Y, Deacon R, Anthony DC, Campbell SJ. Inhibition of peripheral TNF can block the malaise associated with CNS inflammatory diseases. Neurobiol dis. 2008;32:125–132. doi: 10.1016/j.nbd.2008.06.017. [DOI] [PubMed] [Google Scholar]
- Kastbom A, Verma D, Eriksson P, Skogh T, Wingren G, Soderkvist P. Genetic variation in proteins of the cryopyrin inflammasome influences susceptibility and severity of rheumatoid arthritis (the Swedish TIRA project) Rheumatology (Oxford) 2008;47:415–417. doi: 10.1093/rheumatology/kem372. [DOI] [PubMed] [Google Scholar]
- Kaushik DK, Gupta M, Kumawat KL, Basu A. NLRP3 inflammasome: key mediator of neuroinflammation in murine Japanese encephalitis. PLoS ONE. 2012;7:e32270. doi: 10.1371/journal.pone.0032270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Gatz M, Gardner CO, Pedersen NL. A Swedish national twin study of lifetime major depression. Am J Psychiatry. 2006;163:109–114. doi: 10.1176/appi.ajp.163.1.109. [DOI] [PubMed] [Google Scholar]
- Kenis G, Maes M. Effects of antidepressants on the production of cytokines. Int J Neuropsychopharmacol. 2002;5:401–412. doi: 10.1017/S1461145702003164. [DOI] [PubMed] [Google Scholar]
- Kent S, Bluthe RM, Kelley KW, Dantzer R. Sickness behavior as a new target for drug development. Trends Pharmacol Sci. 1992;13:24–28. doi: 10.1016/0165-6147(92)90012-u. [DOI] [PubMed] [Google Scholar]
- Kessler RC. The effects of stressful life events on depression. Annu Rev Psychol. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) J Am Med Assoc. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSMIII- R psychiatric disorders in the United States. Results from the National comorbidity survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Veeneman J, Combe C, Poole S, Luheshi GN, Dantzer R. Central nervous action of interleukin-1 mediates activation of limbic structures and behavioural depression in response to peripheral administration of bacterial lipopolysaccharide. Eur J Neurosci. 2008;28:2499–2510. doi: 10.1111/j.1460-9568.2008.06549.x. [DOI] [PubMed] [Google Scholar]
- Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Nat Acad Sci USA. 2008;105:751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Duman RS. Interleukin-1 receptor null mutant mice show decreased anxiety-like behavior and enhanced fear memory. Neurosci Lett. 2009;456:39–43. doi: 10.1016/j.neulet.2009.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. Nuclear factorkappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Nat Acad Sci USA. 2010;107:2669–2674. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan R, Cella D, Leonardi C, Papp K, Gottlieb AB, Dunn M, Chiou CF, Patel V, Jahreis A. Effects of etanercept therapy on fatigue and symptoms of depression in subjects treated for moderate to severe plaque psoriasis for up to 96 weeks. Br J Dermatol. 2007;157:1275–1277. doi: 10.1111/j.1365-2133.2007.08205.x. [DOI] [PubMed] [Google Scholar]
- Labrousse VF, Costes L, Aubert A, Darnaudery M, Ferreira G, Amedee T, Laye S. Impaired interleukin-1beta and c-Fos expression in the hippocampus is associated with a spatial memory deficit in P2X(7) receptor-deficient mice. PLoS ONE. 2009;4:e6006. doi: 10.1371/journal.pone.0006006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacosta S, Merali Z, Anisman H. Influence of interleukin-1beta on exploratory behaviors, plasma ACTH, corticosterone, and central biogenic amines in mice. Psychopharmacology. 1998;137:351–361. doi: 10.1007/s002130050630. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Mueller JL, Vitari AC, Misaghi S, Fedorova A, Deshayes K, Lee WP, Hoffman HM, Dixit VM. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J cell biol. 2009;187:61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult-mouse brain. Neuroscience. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- Lenczowski MJ, Bluthe RM, Roth J, Rees GS, Rushforth DA, van Dam AM, Tilders FJ, Dantzer R, Rothwell NJ, Luheshi GN. Central administration of rat IL-6 induces HPA activation and fever but not sickness behavior in rats. Am J Physiol. 1999;276:R652–658. doi: 10.1152/ajpregu.1999.276.3.R652. [DOI] [PubMed] [Google Scholar]
- Leo R, Di Lorenzo G, Tesauro M, Razzini C, Forleo GB, Chiricolo G, Cola C, Zanasi M, Troisi A, Siracusano A, Lauro R, Romeo F. Association between enhanced soluble CD40 ligand and proinflammatory and prothrombotic states in major depressive disorder: pilot observations on the effects of selective serotonin reuptake inhibitor therapy. J clin Psychiatry. 2006;67:1760–1766. doi: 10.4088/jcp.v67n1114. [DOI] [PubMed] [Google Scholar]
- Levine J, Barak Y, Chengappa KN, Rapoport A, Rebey M, Barak V. Cerebrospinal cytokine levels in patients with acute depression. Neuropsychobiology. 1999;40:171–176. doi: 10.1159/000026615. [DOI] [PubMed] [Google Scholar]
- Lin PY, Su KP. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J clin Psychiatry. 2007;68:1056–1061. doi: 10.4088/jcp.v68n0712. [DOI] [PubMed] [Google Scholar]
- Linthorst AC, Reul JM. Brain neurotransmission during peripheral inflammation. Ann N Y Acad Sci. 1998;840:139–152. doi: 10.1111/j.1749-6632.1998.tb09558.x. [DOI] [PubMed] [Google Scholar]
- Lucae S, Salyakina D, Barden N, Harvey M, Gagne B, Labbe M, Binder EB, Uhr M, Paez-Pereda M, Sillaber I, Ising M, Bruckl T, Lieb R, Holsboer F, Muller-Myhsok B. P2RX7, a gene coding for a purinergic ligand-gated ion channel, is associated with major depressive disorder. Hum Mol Genet. 2006;15:2438–2445. doi: 10.1093/hmg/ddl166. [DOI] [PubMed] [Google Scholar]
- Madrigal JL, Hurtado O, Moro MA, Lizasoain I, Lorenzo P, Castrillo A, Bosca L, Leza JC. The increase in TNF-alpha levels is implicated in NF-kappaB activation and inducible nitric oxide synthase expression in brain cortex after immobilization stress. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2002;26:155–163. doi: 10.1016/S0893-133X(01)00292-5. [DOI] [PubMed] [Google Scholar]
- Maes M. Evidence for an immune response in major depression: a review and hypothesis. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19:11–38. doi: 10.1016/0278-5846(94)00101-m. [DOI] [PubMed] [Google Scholar]
- Maes M, Bosmans E, Meltzer HY, Scharpe S, Suy E. Interleukin-1 beta: a putative mediator of HPA axis hyperactivity in major depression? Am J Psychiatry. 1993;150:1189–1193. doi: 10.1176/ajp.150.8.1189. [DOI] [PubMed] [Google Scholar]
- Maier SF, Goehler LE, Fleshner M, Watkins LR. The role of the vagus nerve in cytokine-to-brain communication. Ann N Y Acad Sci. 1998;840:289–300. doi: 10.1111/j.1749-6632.1998.tb09569.x. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Intracerebroventricular interleukin-1 receptor antagonist blocks the enhancement of fear conditioning and interference with escape produced by inescapable shock. Brain Res. 1995;695:279–282. doi: 10.1016/0006-8993(95)00930-o. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR, Fleshner M. Psychoneuroimmunology. The interface between behavior, brain, and immunity. Am Psychol. 1994;49:1004–1017. doi: 10.1037//0003-066x.49.12.1004. [DOI] [PubMed] [Google Scholar]
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- Mason DR, Beck PL, Muruve DA. Nucleotide-binding oligomerization domain-like receptors and inflammasomes in the pathogenesis of nonmicrobial inflammation and diseases. J Innate Immun. 2012;4:16–30. doi: 10.1159/000334247. [DOI] [PubMed] [Google Scholar]
- McCarthy DO, Kluger MJ, Vander AJ. Effect of centrally administered interleukin-1 and endotoxin on food intake of fasted rats. Physiol Behav. 1986;36:745–749. doi: 10.1016/0031-9384(86)90363-x. [DOI] [PubMed] [Google Scholar]
- Mitroulis I, Skendros P, Ritis K. Targeting IL-1beta in disease; the expanding role of NLRP3 inflammasome. Eur J Int Med. 2010;21:157–163. doi: 10.1016/j.ejim.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Moltz H. Fever: causes and consequences. Neurosci Biobehav Rev. 1993;17:237– 269. doi: 10.1016/s0149-7634(05)80009-0. [DOI] [PubMed] [Google Scholar]
- Muller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol psychiatry. 2007;12:988– 1000. doi: 10.1038/sj.mp.4002006. [DOI] [PubMed] [Google Scholar]
- Muller N, Schwarz MJ, Dehning S, Douhe A, Cerovecki A, Goldstein-Muller B, Spellmann I, Hetzel G, Maino K, Kleindienst N, Moller HJ, Arolt V, Riedel M. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol psychiatry. 2006;11:680–684. doi: 10.1038/sj.mp.4001805. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global burden of disease study. Lancet. 1997;349:1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- Nadeau S, Filali M, Zhang J, Kerr BJ, Rivest S, Soulet D, Iwakura Y, de Rivero Vaccari JP, Keane RW, Lacroix S. Functional recovery after peripheral nerve injury is dependent on the pro-inflammatory cytokines IL-1beta and TNF: implications for neuropathic pain. J Neurosci. 2011;31:12533–12542. doi: 10.1523/JNEUROSCI.2840-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nery FG, Borba EF, Hatch JP, Soares JC, Bonfa E, Neto FL. Major depressive disorder and disease activity in systemic lupus erythematosus. Compr Psychiatry. 2007;48:14–19. doi: 10.1016/j.comppsych.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Nguyen KT, Deak T, Owens SM, Kohno T, Fleshner M, Watkins LR, Maier SF. Exposure to acute stress induces brain interleukin-1beta protein in the rat. J Neurosci. 1998;18:2239–2246. doi: 10.1523/JNEUROSCI.18-06-02239.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien SM, Scully P, Fitzgerald P, Scott LV, Dinan TG. Plasma cytokine profiles in depressed patients who fail to respond to selective serotonin reuptake inhibitor therapy. J Psychiatr Res. 2007;41:326–331. doi: 10.1016/j.jpsychires.2006.05.013. [DOI] [PubMed] [Google Scholar]
- O’Connor KA, Johnson JD, Hansen MK, Frank JLW, Maksimova E, Watkins LR, Maier SF. Peripheral and central proinflammatory cytokine response to a severe acute stressor. Brain Res. 2003;991:123–132. doi: 10.1016/j.brainres.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Otte C, Zhao S, Whooley MA. Statin use and risk of depression in patients with coronary heart disease: longitudinal data from the heart and soul study. J Clin Psychiatry. 2012;73:610–615. doi: 10.4088/JCP.11m07038. [DOI] [PubMed] [Google Scholar]
- Owen BM, Eccleston D, Ferrier IN, Young AH. Raised levels of plasma interleukin-1beta in major and postviral depression. Acta Psychiatr Scand. 2001;103:226–228. doi: 10.1034/j.1600-0447.2001.00162.x. [DOI] [PubMed] [Google Scholar]
- Pace TW, Miller AH. Cytokines and glucocorticoid receptor signaling. Relevance to major depression. Ann N Y Acad Sci. 2009;1179:86–105. doi: 10.1111/j.1749-6632.2009.04984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pae CU, Marks DM, Han C, Patkar AA. Does minocycline have antidepressant effect? Biomed & pharma Biomed & pharmaco. 2008;1179:86–105. doi: 10.1016/j.biopha.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Palin K, Bluthe RM, McCusker RH, Levade T, Moos F, Dantzer R, Kelley KW. The type 1 TNF receptor and its associated adapter protein, FAN, are required for TNFalpha-induced sickness behavior. Psychopharmacology. 2009;201:549–556. doi: 10.1007/s00213-008-1331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante CM, Pearce BD, Pisell TL, Sanchez CI, Po C, Su C, Miller AH. The proinflammatory cytokine, interleukin-1alpha, reduces glucocorticoid receptor translocation and function. Endocrinology. 1999;140:4359–4366. doi: 10.1210/endo.140.9.6986. [DOI] [PubMed] [Google Scholar]
- Perry VH, Hume DA, Gordon S. Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience. 1985;15:313–326. doi: 10.1016/0306-4522(85)90215-5. [DOI] [PubMed] [Google Scholar]
- Plata-Salaman CR, Oomura Y, Kai Y. Tumor necrosis factor and interleukin-1 beta: suppression of food intake by direct action in the central nervous system. Brain Res. 1988;448:106–114. doi: 10.1016/0006-8993(88)91106-7. [DOI] [PubMed] [Google Scholar]
- Pontillo A, Silva LT, Oshiro TM, Finazzo C, Crovella S, Duarte AJ. HIV-1 induces NALP3-inflammasome expression and interleukin-1beta secretion in dendritic cells from healthy individuals but not from HIV-positive patients. AIDS. 2012;26:11–18. doi: 10.1097/QAD.0b013e32834d697f. [DOI] [PubMed] [Google Scholar]
- Raedler TJ. Inflammatory mechanisms in major depressive disorder. Curr Opin Psychiatry. 2011;24:519–525. doi: 10.1097/YCO.0b013e32834b9db6. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy S, Ramamoorthy JD, Prasad PD, Bhat GK, Mahesh VB, Leibach FH, Ganapathy V. Regulation of the human serotonin transporter by interleukin-1 beta. Biochem Biophys Res Commun. 1995;216:560–567. doi: 10.1006/bbrc.1995.2659. [DOI] [PubMed] [Google Scholar]
- Sapolsky R, Rivier C, Yamamoto G, Plotsky P, Vale W. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science. 1987;238:522–524. doi: 10.1126/science.2821621. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schaaf MJ, De Kloet ER, Vreugdenhil E. Corticosterone effects on BDNF expression in the hippocampus. Implications for memory formation. Stress. 2000;3:201–208. doi: 10.3109/10253890009001124. [DOI] [PubMed] [Google Scholar]
- Schiepers OJ, Wichers MC, Maes M. Cytokines and major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:201–217. doi: 10.1016/j.pnpbp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Shelton RC, Duman RS. Functional biomarkers of depression: diagnosis, treatment, and pathophysiology. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2011;36:2375–2394. doi: 10.1038/npp.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Sheehy C, Murphy E, Barry M. Depression in rheumatoid arthritisunderscoring the problem. Rheumatology (Oxford) 2006;45:1325–1327. doi: 10.1093/rheumatology/kel231. [DOI] [PubMed] [Google Scholar]
- Shin MS, Kang Y, Lee N, Kim SH, Kang KS, Lazova R, Kang I. U1-small nuclear ribonucleoprotein activates the NLRP3 inflammasome in human monocytes. J Immunol. 2012;188:4769–4775. doi: 10.4049/jimmunol.1103355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simen BB, Duman CH, Simen AA, Duman RS. TNFalpha signaling in depression and anxiety: behavioral consequences of individual receptor targeting. Biol Psychiatry. 2006;59:775–785. doi: 10.1016/j.biopsych.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995;15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder DS, Unanue ER. Corticosteroids inhibit murine macrophage Ia expression and interleukin 1 production. J Immunol. 1982;129:1803–1805. [PubMed] [Google Scholar]
- Song C, Horrobin DF, Leonard BE. The comparison of changes in behavior, neurochemistry, endocrine, and immune functions after different routes, doses and durations of administrations of IL-1beta in rats. Pharmacopsychiatry. 2006;39:88–99. doi: 10.1055/s-2006-941557. [DOI] [PubMed] [Google Scholar]
- Stetler C, Miller GE. Depression and hypothalamic–pituitary–adrenal activation: a quantitative summary of four decades of research. Psychosom Med. 2011;73:114–126. doi: 10.1097/PSY.0b013e31820ad12b. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Tamandl D, Bahrami M, Wessner B, Weigel G, Ploder M, Furst W, Roth E, Boltz-Nitulescu G, Spittler A. Modulation of toll-like receptor 4 expression on human monocytes by tumor necrosis factor and interleukin-6: tumor necrosis factor evokes lipopolysaccharide hyporesponsiveness, whereas interleukin-6 enhances lipopolysaccharide activity. Shock. 2003;20:224–229. doi: 10.1097/00024382-200309000-00005. [DOI] [PubMed] [Google Scholar]
- Tecoma ES, Huey LY. Psychic distress and the immune response. Life Sci. 1985;36:1799–1812. doi: 10.1016/0024-3205(85)90152-3. [DOI] [PubMed] [Google Scholar]
- Thomas AJ, Davis S, Morris C, Jackson E, Harrison R, O’Brien JT. Increase in interleukin-1beta in late-life depression. Am J Psychiatry. 2005;162:175–177. doi: 10.1176/appi.ajp.162.1.175. [DOI] [PubMed] [Google Scholar]
- Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci. 2001;21:2580–2588. doi: 10.1523/JNEUROSCI.21-08-02580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga T, Fukata J, Naito Y, Usui T, Murakami N, Fukushima M, Nakai Y, Hirai Y, Imura H. Prostaglandin-dependent in vitro stimulation of adrenocortical steroidogenesis by interleukins. Endocrinology. 1991;128:526–531. doi: 10.1210/endo-128-1-526. [DOI] [PubMed] [Google Scholar]
- Tschopp J, Martinon F, Burns K. NALPs: a novel protein family involved in inflammation. Nat Rev Mol Cell Biol. 2003;4:95–104. doi: 10.1038/nrm1019. [DOI] [PubMed] [Google Scholar]
- Tse YC, Bagot RC, Hutter JA, Wong AS, Wong TP. Modulation of synaptic plasticity by stress hormone associates with plastic alteration of synaptic NMDA receptor in the adult hippocampus. PLoS ONE. 2011;6:e27215. doi: 10.1371/journal.pone.0027215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull AV, Rivier CL. Regulation of the hypothalamic–pituitary–adrenal axis by cytokines: actions and mechanisms of action. Physiol Rev. 1999;79:1–71. doi: 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, Lalla D, Woolley M, Jahreis A, Zitnik R, Cella D, Krishnan R. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheggen MM, van Hal PT, Adriaansen-Soeting PW, Goense BJ, Hoogsteden HC, Brinkmann AO, Versnel MA. Modulation of glucocorticoid receptor expression in human bronchial epithelial cell lines by IL-1 beta, TNFalpha and LPS. Eur Respir J. 1996;9:2036–2043. doi: 10.1183/09031936.96.09102036. [DOI] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Vanover KE, Chen EY, Marshall JJ, Greengard P. Antidepressant effects of selective serotonin reuptake inhibitors (SSRIs) are attenuated by antiinflammatory drugs in mice and humans. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:9262–9267. doi: 10.1073/pnas.1104836108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Goehler LE, Relton JK, Tartaglia N, Silbert L, Martin D, Maier SF. Blockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: evidence for vagal mediation of immune-brain communication. Neurosci Lett. 1995;183:27–31. doi: 10.1016/0304-3940(94)11105-r. [DOI] [PubMed] [Google Scholar]
- Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol. 2001;49:245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead KJ, Smith CG, Delaney SA, Curnow SJ, Salmon M, Hughes JP, Chessell IP. Dynamic regulation of spinal pro-inflammatory cytokine release in the rat in vivo following peripheral nerve injury. Brain Behav Immun. 2010;24:569–576. doi: 10.1016/j.bbi.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Wilhelm CJ, Murphy-Crews A, Menasco DJ, Huckans MS, Loftis JM. Corticotropin releasing factor-1 receptor antagonism alters the biochemical, but not behavioral effects of repeated interleukin-1beta administration. Neuropharmacology. 2012;62:313–321. doi: 10.1016/j.neuropharm.2011.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- Wu TH, Lin CH. IL-6 mediated alterations on immobile behavior of rats in the forced swim test via ERK1/2 activation in specific brain regions. Behav Brain Res. 2008;193:183–191. doi: 10.1016/j.bbr.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- Zhu CB, Lindler KM, Owens AW, Daws LC, Blakely RD, Hewlett WA. Interleukin-1 receptor activation by systemic lipopolysaccharide induces behavioral despair linked to MAPK regulation of CNS serotonin transporters. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35:2510–2520. doi: 10.1038/npp.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunszain PA, Anacker C, Cattaneo A, Choudhury S, Musaelyan K, Myint AM, Thuret S, Price J, Pariante CM. Interleukin-1beta: a new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2012;37:939–949. doi: 10.1038/npp.2011.277. [DOI] [PMC free article] [PubMed] [Google Scholar]