Abstract
Introduction
Circadian variability of circulating leptin levels has been well established over the last decade. However, the circadian behavior of leptin in human adipose tissue remains unknown. This also applies to the soluble leptin receptor.
Objective
We investigated the ex vivo circadian behavior of leptin and its receptor expression in human adipose tissue (AT).
Subjects and methods
Visceral and subcutaneous abdominal AT biopsies (n = 6) were obtained from morbid obese women (BMI ≥ 40 kg/m2). Anthropometric variables and fasting plasma glucose, leptin, lipids and lipoprotein concentrations were determined. In order to investigate rhythmic expression pattern of leptin and its receptor, AT explants were cultured during 24-h and gene expression was analyzed at the following times: 08:00, 14:00, 20:00, 02:00 h, using quantitative real-time PCR.
Results
Leptin expression showed an oscillatory pattern that was consistent with circadian rhythm in cultured AT. Similar patterns were noted for the leptin receptor. Leptin showed its achrophase (maximum expression) during the night, which might be associated to a lower degree of fat accumulation and higher mobilization. When comparing both fat depots, visceral AT anticipated its expression towards afternoon and evening hours. Interestingly, leptin plasma values were associated with decreased amplitude of LEP rhythm. This association was lost when adjusting for waist circumference.
Conclusion
Circadian rhythmicity has been demonstrated in leptin and its receptor in human AT cultures in a site-specific manner. This new knowledge paves the way for a better understanding of the autocrine/paracrine role of leptin in human AT.
Keywords: Leptin receptor, Circadian, Rhytmicity, Metabolic syndrome, Obesity
Introduction
Feeding is a crucial physiological function in animals and it responds circadian variability in food availability.1 Leptin and other humoral signals produced in the peripherical tissues are capable of communicating the nutritional state of the organism to the hypothalamic centres that control hunger and satiety, in a circadian-dependent manner.2,3,4 Moreover there is evidence showing the variability of leptin added by factors such as obesity and sex,4 hormonal regulations,5 eating behaviours and sleep patterns.6
The ability of leptin to regulate food intake, body weight, adiposity and insulin sensitivity has been attributed exclusively to its actions in the hypothalamus.7 However, it has been suggested that leptin can play important physiological roles in an autocrine/paracrine way.8 The role of leptin on adipose tissue (AT) has been reported to be essential in modulating the adipocytes’ metabolic function, up-regulating fat oxidation and decreasing lipogenesis.9 Leptin (LEP) functions through a receptor (LEPR) expressed in adipose tissue.10 Therefore, changes in LEP, or its receptor in adipose tissue can be relevant in the development of obesity and other metabolic disorders.4
The existence of an internal circadian clock has been demonstrated in human adipose tissue.11 Moreover, circadian rhythmicity in cortisol-related genes,12 PPAR-gamma12 and adiponectin,13 have been shown with a different circadian behaviour between visceral and subcutaneous AT depots.
It has been discussed that abdominal fat accumulation is related to chronodisruption.14 Given the potentially different roles of different fat depots,15 it is possible that their metabolism is driven by different chronobiological rhythms. Whereas many aspects of leptin metabolism and variability have been intensively studied, less is known about its circadian behavior in human adipose tissue. Moreover the particular role of LEPR in adipocytes is still unclear. Therefore, in this study we examined the circadian behavior of LEP and its receptor in human adipose tissue cultures, and the potential differences between subcutaneous and visceral depots.
Subjects and methods
Subjects
Visceral and subcutaneous abdominal AT biopsies were obtained from morbid obese women (n = 6), aged 51 ± 9 years and BMI: 44.1 ± 5.5 kg/m2, undergoing laparoscopic gastric bypass surgery due to obesity at the General Surgery Service of “Virgen de la Arrixaca” Hospital (Murcia, Spain). The women studied were postmenopausal and were not under hormone replacement therapy. The day before surgery, all patients were synchronized having lunch at 14:30 and dinner at 21:00 hours. The AT biopsies were taken as paired samples from the two AT depots (visceral and subcutaneous) at the beginning of the surgical procedure (estimated time of biopsies sampling at 13:00 hours).
The protocols were approved by the Ethics Committee of the “Virgen de la Arrixaca” University Hospital, and the subjects signed a written informed consent before the biopsies were obtained.
Clinical characteristics
Arterial pressure, BMI, waist and hip circumference were assessed by standard procedures, while skinfolds (biceps, triceps, suprailiac and subscapular) were measured with a Harpenden caliper (Holtain Ltd, Bryberian, Crymmych, Pembrokeshire, UK). Total body fat (%) was evaluated by bioimpedance with a TANITA TBF- 300 (TANITA Corporation of America, Arlington Heights, IL). Sagittal diameter and coronal diameter were measured at the level of the iliac crest (L4–5) using a Holtain Kahn Abdominal Cali skinfold. Those patients with VA/SA > 0.42 were classified as having visceral obesity16. Fasting plasma concentrations of glucose, triacylglycerols, total cholesterol and high-density lipoprotein (HDL) cholesterol were determined with common analytical methods (Roche Diagnostics GmbH, Mannheim, Germany). Basal plasma leptin levels were measured using a a gamma counter (DPC Gambyt, city and country) and RIA kits from Mediagnost Laboratory (Reutlinge, Germany) with a sensitivity of 0.5 ng/ml and intra assay CV of 8.3%. Basal metabolic Rate (BMR) was calculated from the Harris and Benedict equation.17
Adipose tissue culture
Immediately after the surgery, a part of AT biopsies were immediately frozen at −80°C and used for analyzing the basal gene expression. The rest of AT was used for culture, thus 800–1,000 mg AT explants (minimal pieces of 1–2 mm3 in order to allow the maximal contact of adipose tissue with the medium) were transferred to cell culture bottles with membrane filter screw cap to safeguard the viability of the culture, and placed in 5 ml of Dulbecco’s modified Eagles Medium (DMEM) supplemented with 10% fetal bovine serum, and kept at 37° C for 24 hour in a humidified atmosphere containing 7% CO2.
On the next day, the adipose explants were collected to perform gene expression analysis at the following times (T): 0, 6, 12, and 18, T0 being arbitrarily defined as 08:00 h, because this was the usual waking time for patients, T6 as 14:00 h, T12 as 20:00 h and T18 as 02:00 h. Gene expression was measured only for one circadian cycle (24 hours), but in the second day of culture. All cultures were performed in duplicate.
Analysis of gene expression
Total RNA was extracted from AT explants using RNeasy Kit (QIAGEN, Courtabeouf, France). Reverse transcription was performed using random hexamers as primers and Thermoscript® reverse transcriptase (Invitrogen, Cergy Pontoise, France) with 1 g total RNA for each sample. Quantitative real-time PCR was performed using an ABI PRISM 7000 HT Sequence Detection System (Applied Biosystems, CA, USA), using TaqMan® Universal PCR Master Mix and specific TaqMan probes (Applied Biosystems, CA, USA). The primers used in the study are the following references from Applied Biosystems: Human leptin (Hs00174877_m1) and leptin receptor (Hs00174497_ m1). Hs00174497_m1 recognizes the main leptin receptor isoforms (1, 2 and 3).
We used PPIA rRNA (reference Hs99999904_m1 from Applied Biosystems) as internal control. We selected PPIA because this gene showed a lower variance along time compared with the 18S gene. In addition, we carried out a one-way (Zeitgeber time, ZT) analysis of variance (ANOVA) for PPIA and observed no significant difference in any of the adipose depots studied (P > 0.05). Gene mRNA levels were normalized to PPIA using the 2−ΔCt method.18
Rhythm calculation and statistical analysis
Clinical and anthropometric data are presented as means ± SD. The results for gene expression, expressed in arbitrary units, are presented as means ± SEM. Wilcoxon non parametric paired test was used for comparing data from the samples derived from the two adipose depots in each subject. Pearson’s correlations coefficients were used for analyzing associations between the relative expression of LEP and LEPR in both adipose depots.
The single cosinor method was used to analyze for circadian rhythm individually.19 This inferential method involves fitting a curve of a predefined period by the least squares method. The rhythm characteristics and their 95% confidence intervals estimated by this method include the mesor (middle value of the fitted cosine representing a rhythm-adjusted mean), the amplitude (half the difference between the minimum and maximum of the fitted cosine function), the temporal location of maximum value or acrophase (the time at which the peak of a rhythm occurs, expressed in hours) and the Percent Rhythm (PR; the percentage of variability accounted for by cosine curve). Relative amplitude was expressed as a percentage of mesor values (relative amplitude = (amplitude/mesor) × 100). The significance of the rhythms was determined by rejection of the zero amplitude hypotheses with a threshold of 60%.
Mean circadian gene expression of the total population into each fat depot was analyzed by using repeated measures ANOVA test, with a post hoc test of least significant difference (LSD) correction. Pearson’s correlation analyses were used for finding associations between the genetic circadian oscillation (amplitude and percentage of variability) and components of the MetS. All statistical analyses were carried out using SPSS for windows (release 15.0; SPSS Inc, Chicago, US). The level of significance for all statistical tests and hypotheses was set at P < 0.05.
Results
Characteristics of the population
Table I contains baseline characteristics of the women studied. They were morbidly obese (BMI > 40 kg/m2) primarily due to visceral obesity (VA/SA > 0.4). Moreover, they had metabolic syndrome, according to the International Diabetes Federation (IDF) criteria20. The individual and average values for waist circumference, glucose and systolic pressure exceeded the cut off points proposed by IDF.
Table I.
General characteristics of the population studied
| Patients (n = 6) | |
|---|---|
| Age (y) | 51 ± 9 |
| Weight (kg) | 107.9 ± 11.2 |
| Height (cm) | 156 ± 5.0 |
| BMI (Kg/m2) | 44.1 ± 5.5 |
| Body fat (%) | 43 ± 6 |
| WC (cm) | 126 ± 8 |
| HC (cm) | 139 ± 9 |
| WHR | 0.91 ± 0.03 |
| Sagittal Diameter (cm) | 22 ± 4 |
| Coronal Diameter (cm) | 31 ± 4 |
| Bicipital skinfold (mm) | 28 ± 3 |
| Tricipital skinfold (mm) | 28 ± 4 |
| Subscapular skinfold (mm) | 33 ± 4 |
| Suprailliac skinfold (mm) | 31 ± 3 |
| BMR (Kcal) | 1721 ± 120 |
| VA/SApredicted | 0.54 ± 0.28 |
| Leptin (ng/ml) | 40.66 ± 16.14 |
| Glucose (mmol/l) | 6.13 ± 0.82 |
| Cholesterol (mmol/l) | 4.53 ± 1.02 |
| Triglycerides (mmol/l) | 1.02 ± 0.42 |
| HDL-cholesterol (mmol/l) | 1.34 ± 0.22 |
| LDL-cholesterol (mmol/l) | 3.14 ± 0.68 |
| Systolic Pressure (mmHg) | 148. ± 23 |
| Diastolic Pressure (mmHg) | 73 ± 14 |
Data are presented as means ± SD.
BMI: Body Mass Index. WC: Waist circumference. HC: Hip Circumference. WHR: Waist to Hip Ratio. BMR: Basal Metabolic Rate. VA/SApredicted: Visceral Area/Subcutaneous Areapredicted16. HDL: high-density lipoprotein; LDL: low-density lipoprotein.
Basal gene expression
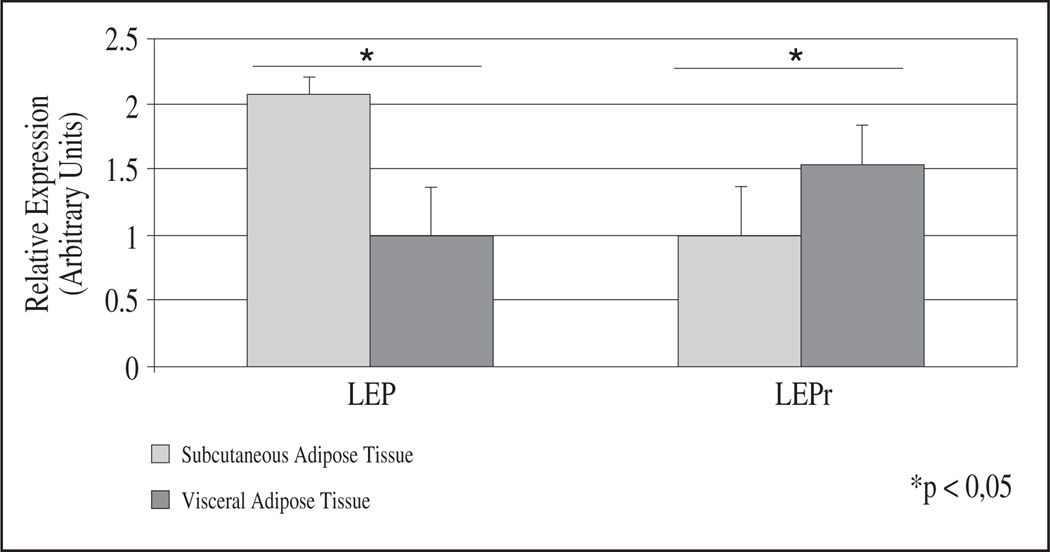
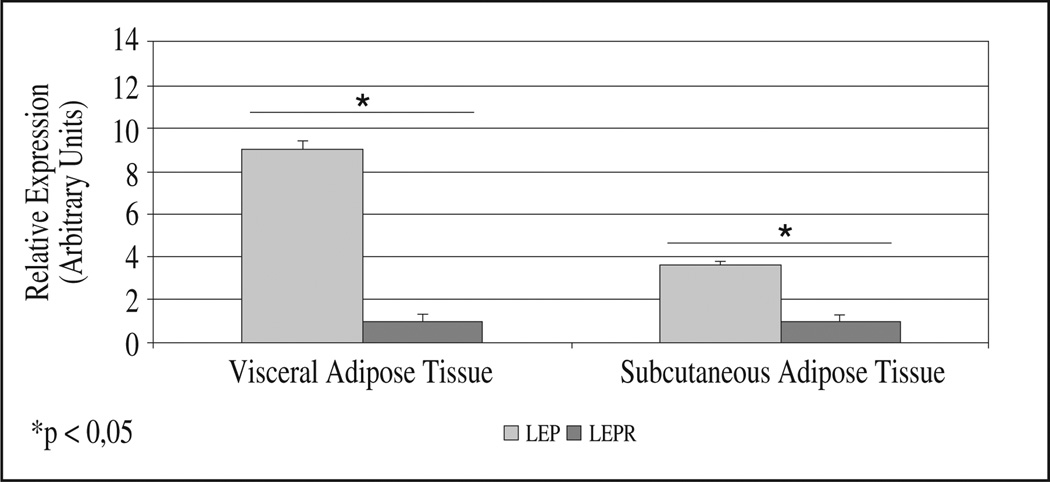
Paired adipose tissue biopsies from the subcutaneous and visceral depots were obtained from each patient. Basal gene expression for LEP and its receptor were significantly different between AT depots (fig. 1). LEP expression was higher in subcutaneous than in visceral fat (P = 0.028), whereas LEPR expression was higher in visceral fat (P = 0.028) (fig. 1). In both fat depots, LEP was much more expressed than its receptor (3.6 times more in visceral and 9.0 times in subcutaneous AT) (fig. 2). Correlation analysis showed a significant negative association between basal LEP expression and percentage of fat-free mass in subcutaneous AT (r = −0.844; P = 0.032) while LEPR was inversely correlated to percent body fat (r = −0.87; P = 0.024), and total fat mass (r = −0.90; P = 0.017). In visceral AT, LEPR expression correlated inversely with BMR (r = −0.90; P = 0.017).
Fig. 1.
Basal gene expression in both adipose depots. Basal relative expression of genes studied (leptin (LEP) and leptin receptor (LEPR)) in subcutaneous (S) and visceral (V) adipose tissue (AT) are showed. mRNA levels were measured with the Real-Time PCR and normalized to PPIA using the ΔΔCt method of relative quantization. Data are reported as means ± SEM (Standard Error of the Mean). For leptin results are presented as percent of VAT relative expression (VAT value = 1) and for LEPR are presented as percent of SAT relative expression (SAT value = 1). Asterisk (*) represents significant differences (Wilcoxon non parametric paired test).
Fig. 2.
Basal gene expression of genes studied within each fat depot. Basal relative expression of genes studied (leptin (LEP) and leptin receptor (LEPR)) within subcutaneous (S) and visceral (V) adipose tissue (AT) are compared. mRNA levels were measured with the Real-Time PCR and normalized to PPIA using the ΔΔCt method of relative quantization. Data are reported as means ± SEM (Standard Error of the Mean). The results are presented as percent of LEPR relative expression in both depots (LEPR value = 1). Asterisk (*) represents significant differences (Wilcoxon non parametric paired test)
LEP and LEPR circadian gene expression
Next, we examined their circadian expression pattern in cultured adipose tissue explants. Parameters imputed from each subject obtained by cosinor analysis, defining the circadian rhythms as mesor, amplitude, relative amplitude, acrophase and percent rhythm are reported in table II. Our data show that the expression of both genes showed circadian patterns.
Table II.
Parameters imputed from cosinor analysis
| Patients | Subcutaneous Adipose Tissue | Visceral Adipose Tissue | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mesor | Amplitude | Relative Amplitude |
Acrophase | % Variance |
Mesor | Amplitude | Relative Amplitude |
Acrophase | % Variance |
|
| LEP | ||||||||||
| P1 | 1.70 | 0.55 | 32.35 | 19.15 | 83.65 | 1.69 | 0.63 | 37.27 | 13.43 | 60.00 |
| P2 | 1.53 | 0.39 | 25.49 | 17.16 | 76.67 | 1.28 | 0.28 | 21.87 | 13.01 | 99.40 |
| P3 | 1.69 | 0.88 | 52.07 | 20.96 | 98.43 | 1.27 | 0.28 | 22.04 | 13.64 | 60.66 |
| P4 | 1.87 | 0.46 | 24.59 | 3.21 | 25.22 | 1.59 | 0.60 | 37.73 | 15.70 | 74.87 |
| P5 | 1.59 | 0.35 | 22.01 | 15.29 | 38.19 | 2.30 | 1.32 | 57.39 | 9.98 | 97.16 |
| P6 | 0.99 | 0.22 | 22.22 | 12.28 | 97.00 | 1.90 | 1.01 | 53.15 | 10.55 | 99.78 |
| LEPR | ||||||||||
| P1 | 2.24 | 2.18 | 97.32 | 17.72 | 67.03 | 9.19 | 10.58 | 115.12 | 23.90 | 47.28 |
| P2 | 1.42 | 0.36 | 25.35 | 1.09 | 38.89 | 1.75 | 1.18 | 67.42 | 18.35 | 79.76 |
| P3 | 1.46 | 0.17 | 11.64 | 16.42 | 8.96 | 2.22 | 1.15 | 51.80 | 21.21 | 83.18 |
| P4 | 2.41 | 2.29 | 95.02 | 6.22 | 77.19 | 1.42 | 0.58 | 40.84 | 12.48 | 87.22 |
| P5 | 1.81 | 1.03 | 56.90 | 3.70 | 90.74 | 1.42 | 0.26 | 18.30 | 2.43 | 31.09 |
| P6 | 2.94 | 3.31 | 112.58 | 0.32 | 74.85 | 2.05 | 1.41 | 68.78 | 1.53 | 80.26 |
Parameters imputed from each subject obtained by cosinor analysis defining the circadian rhythms as mesor, amplitude, relative amplitude, acrophase and percent rhythm are represented in this table. Subjects in which expression of the gene was considered to have circadian rhythmicity are shown in bold.
AU: Arbitrary units, h: hours.
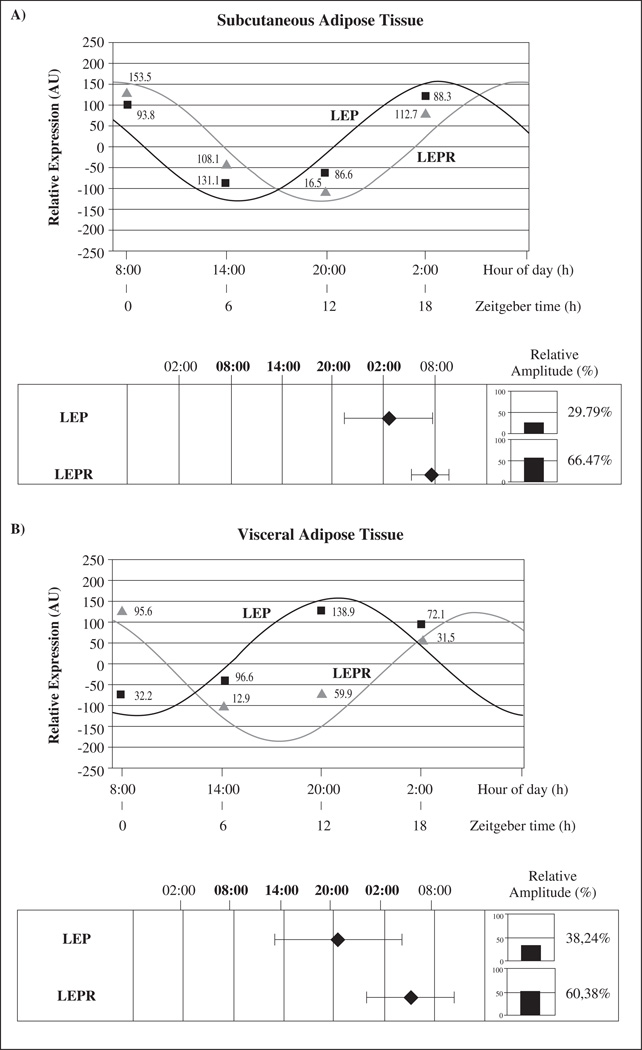
Figure 3 shows mean LEP and LEPR circadian rhythms in subcutaneous (3A) and visceral AT (3B). When relative gene expression among different times was analyzed by using repeated measures ANOVA test, statistical differences were found for LEP in visceral depot (P = 0.004) and the same trend was found in subcutaneous AT (P = 0.067), evidencing the circadian rhythmicity of LEP expression.
Fig. 3.
Circadian rhythms and phase map of all genes studied in total population. Rhythmic expression and phase map of genes studied in human subcutaneous (3A) and visceral adipose tissue (3B). Adipose depots were isolated at 6-h intervals over the course of the day from adipose tissue cultures (time at 0, 6, 12 and 18 hours). Results are presented relative to the lowest basal relative expression for each gene. Data of relative expression are represented as Arbitrary Units (AU). Data are reported as means ± SEM (SEM of ΔCt are represented in parenthesis). The phase map shows the acrophase (time of occurrence of the best-fit maximum value) and relative amplitude of circadian rhythms of genes studied. The mean values of achrophases are plotted ± SEM.
When comparing both AT depots, different circadian patterns were observed. In subcutaneous depot, LEP showed its achrophase (maximum expression) during the night (at 02:00 hours) whereas in visceral AT anticipated its expression in the evening (20:00 hours). On the other hand, LEPR displayed its achrophase during the night in visceral and subcutaneous depots (at 08:00 and 04:00 hours respectively). Similarly to the pattern observed for LEP, the receptor achrophase (LEPR) was advanced in visceral with respect to subcutaneous AT (fig. 3).
Correlation analyses between the genetic circadian oscillation (relative amplitude and percentage of variability) and components of the MetS are shown in table III. We found a positive correlation between obesity (sagittal and coronal diameter, body mass index (BMI), weight, fat mass and body fat), plasma glucose and low HDL-colesterol correlated with the relative amplitude and higher variability of the circadian rhythms for LEP and LEPR. When correlation analysis were performed between the LEP rhythm’s amplitude in visceral AT and leptin plasma values a significant and inverse correlation was observed (r = −0.843; P = 0.035). Interestingly, the significance was lost when data was adjusted for waist circumference. Moreover, we found an interesting negative correlation between the circadian oscillation of LEP expression (relative amplitude) in subcutaneous AT and age (r = −0.855; P = 0.030).
Table III.
Correlations between the gene expression, circadian oscillation [relative amplitude (3A) and percentage of variability (3B)] and components of the metabolic syndrome in visceral (V) and subcutaneous (S) adipose tissues (AT)
| 3A. Relative Amplitude | BMI (kg/m2) | Sagital diameter (cm) | Glucose (mg/dl) | HDL-c (mg/dl) | ||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| LEP (SAT) | −0.841 | 0.036 | −0.925 | 0.008 | −0.638 | 0.247 | −0.484 | 0.409 |
| LEPR (SAT) | −0.518 | 0.292 | −0.727 | 0.102 | −0.889 | 0.044 | −0.094 | 0.881 |
| LEPR (VAT) | −0.197 | 0.708 | −0.198 | 0.707 | −0.312 | 0.610 | −0.954 | 0.025 |
| 3B. Percentage of variability | BMI (kg/m2) | Coronal diameter (cm) | Weight (kg) | Body fat (%) | Fat mass (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | |
| LEP (SAT) | 0.904 | 0.013 | 0.911 | 0.012 | 0.904 | 0.013 | 0.378 | 0.473 | 0.646 | 0.166 |
| LEPR (VAT) | 0.302 | 0.561 | 0.074 | 0.889 | 0.441 | 0.381 | 0.972 | 0.001 | 0.868 | 0.025 |
Significant correlations (P < 0.05) are shown in bold.
Discussion
In this study we have demonstrated that: 1. LEP and LEPR display 24-hr rhythmicity in cultured human adipose tissue. 2 Basal gene expression for LEP and its receptor were significantly different between AT depots. 3. The circadian expression pattern of leptin is site-specific. 4. Leptin plasma values were associated with decreased amplitude of LEP rhythm. This association was influenced by abdominal obesity.
Similarly to our results, previous studies have analyzed LEP expression and shown that LEP mRNA was two- to threefold higher in subcutaneous vs. omental AT.21 The significantly higher expression of LEPR in visceral fat, and of LEP in subcutaneous fat, could be related to a differentiated LEP function in both locations, suggesting an endocrine role in subcutaneous, and an autocrine/paracrine predominance in visceral AT. Moreover, BMR could be linked to obesity-related leptin resistance. This result suggests that an overproduction of LEP, as occurs in obese subjects, could downregulate the expression of LEPR in adipose tissue. As other authors have reported7, high-fat diet induced-obesity induced changes in LEPR expression in a depot and sex-dependent manner in rats. Thus, they found a downregulation of LEPR in retroperitoneal AT in men (not in female) that they associated with a decrease in leptin sensitivity in the obese subjects and a higher tendency to suffer from obesity-related disorders.
In the women studied, AT LEP expression showed circadian rhythmicity. Of note, LEP mRNA levels fluctuated during the day in synchrony with its receptor, suggesting the relevance of this rhythm in adipose tissue.
Ando et al.22, demonstrated the rhythmic mRNA expression of LEP in visceral AT in mice. Conversely, in murine pre-adipocyte 3T3-L1 cell line, Otway et al.23 were not able to observe circadian rhythm of LEP mRNA expression, though LEP accumulation in the culture medium suggested circadian control of LEP secretion from adipocytes.
Our results demonstrated that LEP had its achrophase during the evening and night, with a similar pattern to that formerly shown in plasma, where circulating leptin peaks occurred during the sleep phase between 22:00-03:00 h and nadirs during the morning hours between 08:00 and 17:00.4,24,25 In fact, Chin-Chance et al.26 demonstrated the zenith occurring at 02:00 h, coincident with our own results in AT. Furthermore, the relative amplitudes for leptin were similar for their study (31%) and ours (30%). Although, this amplitude appears to be higher than in other studies using several organs or tissues,27 it was significantly lower to that described in the same population for clock genes,11 glucocorticoid- related genes12 and PPAR-gamma.12
The significance of the daily peaks and valleys in leptin secretion in the hypotalamic regulation of body weight in humans is not fully understood. Flier28 proposes that leptin, rather than an antiobesity hormone, acts as an integrator of neuroendocrine function, signaling energy deficiency. Accordingly, the increase in leptin during the night may mean that it acts as a satiety hormone, favouring fasting and nocturnal rest. Other authors defend that the nocturnal rise of leptin secretion is entrained to mealtime probably due to cumulative hyperinsulinemia of the entire day.29
With respect to AT, the achrophase at night could be associated to a lower degree of fat accumulation and higher mobilization. During the night in humans, there is a predominance of lipolytic activity, responsible of the body fat utilization, which reduces the frequency of hunger signals and, in consequence, reduces the need for food30. Indeed, the autocrine/paracrine role of leptin on AT has been reported to be up-regulating fat oxidation and decreasing lipogenesis.9
When comparing both AT depots, visceral AT anticipated its expression towards afternoon and evening hours (20:00 hours). It has been described that both the sympathetic and parasympathetic division of the autonomous nervous system can discriminate between different compartments of AT, such as the subcutaneous and intraabdominal.31 Previously, it has been reported a difference in the timing of the circadian rhythm in circulating leptin as a function of body fat distribution (android versus gynoid), also showing different association with respect to insulin and cortisol.32 An imbalance between the rhythms of different fat compartments differentially controlled by the autonomous nervous system and in turn by the supraquiasmatic nucleus may be related to Metabolic Syndrome (MetS).33
Aging has been associated with decreased relative amplitude of the circadian rhythm.34 However, one surprising result in the current data is that higher obesity degree correlated with an increase in relative amplitude and with higher variability of the circadian rhythms of both genes studied. A similar situation happened when comparing visceral and subcutaneous AT, with higher relative amplitude in the visceral depot. Although these results should be considered with caution, because multiple comparisons test, the higher circadian response of these genes in visceral AT could be related to the deleterious effect of intraabdominal fat in relation to the risk for diabetes, cardiovascular disease, hypertension, and certain cancers.35 Our data also indicated that Leptin plasma values were associated with a decreased amplitude of LEP rhythm, Interestingly, significance was lost when adjusting for waist circumference suggesting that abdominal fat is related to the decreased in amplitude with leptin.
These results in LEP and its receptor LEPR contrast to those obtained previously for adiponectin13 and for clock genes in human adipose tissue11 and with the current chronodisruption theory, which sustains that obesity alter circadian rhythmicity towards a more flattening pattern.14 Consistent with our findings, Ando et al.,22 using mouse visceral fat reported that for clock genes and different adipokines circadian rhythmicity was flattened with obesity, but not so for leptin. Similarly, Bergman et al.,35 concluded in their previous work performed in plasma that “leptin pulsatility can be preserved in the obese”.
One limitation of the current study was the lack of a control group. Further studies in AT comparing data from obese and lean subjects should be performed to answer this question. However, we could speculate from the current data that in the same way that LEP expression and secretion are augmented with obesity,36 the positive correlation between rhythmicity and obesity obtained in the current study, could be related to leptin resistance.
In conclusion, circadian rhythmicity has been demonstrated in LEP and LEPR in human adipose tissue cultures in a site-specific manner. These discoveries suggest that in AT metabolism it is imperative not only to understand the “what” and the “how” but also the “when” of these metabolic processes. A new observation point should be developed: a chronobiological view of adipose tissue and obesity.
Acknowledgments
This work was supported in part by NIH grants DK075030 and contracts 53-K06-5-10 and 58-1950-9-001 from the US Department of Agriculture Research Service and by the Government of Education, Science and Research of Murcia (Project BIO/FFA 07/01-0004) and by The Spanish Government of Science and Innovation (Projects AGL2008-01655/ALI); the Spanish Ministry of Education and Science (Projects (BFU2007-60658/BFI), by Seneca Foundation (PI/05700/07), by The Institute of Health Carlos III (RETICEF, RD06/0013/0019) and by Línea Especial of University of Navarra (LE/97).
Abbreviations
- AT
Adipose Tissue
- BMI
Body Mass Index
- LEP
Leptin
- LEPR
Leptin Receptor
- VA
Visceral Area
- SA
Subcutaneous Area
- BMR
Basal Metabolic Rate
- DMEM
Dulbecco’s Modified Eagles Medium
- ZT
Zeitgeber Time
- LSD
Least Significant Difference
- IDF
International Diabetes Federation
- MetS
Metabolic Syndrome
References
- 1.Dietrich MO, Horvath TL. Feeding signals and brain circuitry. Eur J Neurosci. 2009;30(9):1688–1696. doi: 10.1111/j.1460-9568.2009.06963.x. [DOI] [PubMed] [Google Scholar]
- 2.Garaulet M, Madrid JA. Chronobiological Aspects of Nutrition, Metabolic Syndrome and Obesity. Adv Drug Deliv Rev. 2010;62(9–10):967–978. doi: 10.1016/j.addr.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Gavrila A, Peng CK, Chan JL, Mietus JE, Goldberger AL, Mantzoros CS. Diurnal and ultradian dynamics of serum adiponectin in healthy men: comparison with leptin, circulating soluble leptin receptor, and cortisol patterns. J Clin Endocrinol Metab. 2003;88(6):2838–2843. doi: 10.1210/jc.2002-021721. [DOI] [PubMed] [Google Scholar]
- 4.Saad MF, Riad-Gabriel MG, Khan A, Sharma A, Michael R, Jinagouda SD, Boyadjian R, Steil GM. Diurnal and ultradian rhythmicity of plasma leptin: effects of gender and adiposity. J Clin Endocrinol Metab. 1998;83(2):453–459. doi: 10.1210/jcem.83.2.4532. [DOI] [PubMed] [Google Scholar]
- 5.White HD, Ahmad AM, Guzder R, Wallace AM, Fraser WD, Vora JP. Gender variation in leptin circadian rhythm and pulsatility in adult growth hormone deficiency: effects of growth hormone replacement. Clin Endocrinol (Oxf) 2003;58(4):482–488. doi: 10.1046/j.1365-2265.2003.01742.x. [DOI] [PubMed] [Google Scholar]
- 6.Mullington JM, Chan JL, Van Dongen HP, Szuba MP, Samaras J, Price NJ, Meier-Ewert HK, Dinges DF, Mantzoros CS. Sleep loss reduces diurnal rhythm amplitude of leptin in healthy men. J Neuroendocrinol. 2003;15(9):851–854. doi: 10.1046/j.1365-2826.2003.01069.x. [DOI] [PubMed] [Google Scholar]
- 7.Friedman JM. The function of leptin in nutrition, weight, and physiology. Nutr Rev. 2002;60(10 Pt 2):S1–S14. doi: 10.1301/002966402320634878. Review. [DOI] [PubMed] [Google Scholar]
- 8.Kulkarni RN, Wang ZL, Wang RM, Hurley JD, Smith DM, Ghatei MA, Withers DJ, Gardiner JV, Bailey CJ, Bloom SR. Leptin rapidly suppresses insulin release from insulinoma cells, rat and human islets and, in vivo, in mice. J Clin Invest. 1997;100:2729–2736. doi: 10.1172/JCI119818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang W, Della-Fera MA, Hartzell DL, Hausman D, Baile CA. Adipose tissue gene expression profiles in ob/ob mice treated with leptin. Life Sci. 2008;83(1–2):35–42. doi: 10.1016/j.lfs.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 10.Kielar D, Clark JS, Ciechanowicz A, Kurzawski G, Sulikowski T, Naruszewicz M. Leptin receptor isoforms expressed in human adipose tissue. Metabolism. 1998;47(7):844–847. doi: 10.1016/s0026-0495(98)90124-x. [DOI] [PubMed] [Google Scholar]
- 11.Gómez-Santos C, Gómez-Abellán P, Madrid JA, Hernández-Morante JJ, Lujan JA, Ordovas JM, Garaulet M. Circadian rhythm of clock genes in human adipose explants. Obesity (Silver Spring) 2009;17(8):1481–1485. doi: 10.1038/oby.2009.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernandez-Morante JJ, Gomez-Santos C, Milagro F, Campión J, Martínez JA, Zamora S, Garaulet M. Expression of cortisol metabolism-related genes shows circadian rhythmic patterns in human adipose tissue. Int J Obes (Lond) 2009;33(4):473–480. doi: 10.1038/ijo.2009.4. [DOI] [PubMed] [Google Scholar]
- 13.Gómez-Abellán P, Gómez-Santos C, Madrid JA, Milagro FI, Campion J, Martínez JA, Ordovás JM, Garaulet M. Circadian expression of adiponectin and its receptors in human adipose tissue. Endocrinology. 2010;151(1):115–122. doi: 10.1210/en.2009-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garaulet M, Gómez-Abellán P, Madrid JA. Chronobiology and obesity: the orchestra out of tune. Clin Lipidology. 2010;5(2):181–188. [Google Scholar]
- 15.Garaulet M, Hernandez-Morante JJ, Lujan J, Tebar FJ, Zamora S. Relationship between fat cell size and number and fatty acid composition in adipose tissue from different fat depots in overweight/obese humans. Int J Obes (Lond) 2006;30:899–905. doi: 10.1038/sj.ijo.0803219. [DOI] [PubMed] [Google Scholar]
- 16.Garaulet M, Hernández-Morante JJ, Tébar FJ, Zamora S, Canteras M. Two-dimensional predictive equation to classify visceral obesity in clinical practice. Obesity (Silver Spring) 2006;14:1181–1191. doi: 10.1038/oby.2006.135. [DOI] [PubMed] [Google Scholar]
- 17.Harris JA, Benedict FG. A Biometric Study of Human Basal Metabolism. Proc Natl Acad Sci USA. 1918;4(12):370–373. doi: 10.1073/pnas.4.12.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schütz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 20.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome a new wold-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 21.Russell CD, Petersen RN, Rao SP, Ricci MR, Prasad A, Zhang Y, Brolin RE, Fried SK. Leptin expression in adipose tissue from obese humans: depot-specific regulation by insulin and dexamethasone. Am J Physiol. 1998;275(3 Pt 1):E507–E515. doi: 10.1152/ajpendo.1998.275.3.E507. [DOI] [PubMed] [Google Scholar]
- 22.Ando H, Yanagihara H, Hayashi Y, Obi Y, Tsuruoka S, Takamura T, Kaneko S, Fujimura A. Rhythmic messenger ribonucleic acid expression of clock genes and adipocytokines in mouse visceral adipose tissue. Endocrinology. 2005;146(12):5631–5636. doi: 10.1210/en.2005-0771. [DOI] [PubMed] [Google Scholar]
- 23.Otway DT, Frost G, Johnston JD. Circadian rhythmicity in murine pre-adipocyte and adipocyte cells. Chronobiol Int. 2009;26(7):1340–1354. doi: 10.3109/07420520903412368. [DOI] [PubMed] [Google Scholar]
- 24.Licinio J, Mantzoros C, Negrão AB, Cizza G, Wong ML, Bongiorno PB, Chrousos GP, Karp B, Allen C, Flier JS, Gold PW. Human leptin levels are pulsatile and inversely related to pituitary-adrenal function. Nat Med. 1998;3:576–579. doi: 10.1038/nm0597-575. [DOI] [PubMed] [Google Scholar]
- 25.Yildiz BO, Suchard MA, Wong ML, McCann SM, Licinio J. Alterations in the dynamics of circulating ghrelin, adiponectin, and leptin in human obesity. Proc Natl Acad Sci USA. 2004;101(28):10434–10439. doi: 10.1073/pnas.0403465101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chin-Chance C, Polonsky KS, Schoeller DA. Twenty-four*hour leptin levels respond to cumulative short-term energy imbalance and predict subsequent intake. J Clin Endocrinol Metab. 2000;85(8):2685–2691. doi: 10.1210/jcem.85.8.6755. [DOI] [PubMed] [Google Scholar]
- 27.Hermida RC, Calvo C, Ayala DE, López JE, Fernández JR, Mojón A, Domínguez MJ, Covelo M. Seasonal variation in plasma fibrinogen in dipper and non-dipper patients with mild-moderate essential hypertension. Med Clin (Barc) 2003;121(1):6–11. doi: 10.1016/s0025-7753(03)74111-6. [DOI] [PubMed] [Google Scholar]
- 28.Flier J. Clinical review 94: what’s in a name? In search of leptin’s physiologic role. J Clin Endocrinol Metab. 1998;83:1407–1413. doi: 10.1210/jcem.83.5.4779. [DOI] [PubMed] [Google Scholar]
- 29.Sinha MK, Caro JF. Clinical aspects of leptin. Vitam Horm. 1998;54:1–30. doi: 10.1016/s0083-6729(08)60919-x. [DOI] [PubMed] [Google Scholar]
- 30.Soler G, Bautista JM, Madrid JA, Salido GM. Circadian rhythms in enzymatic activity of rat liver arginase and glucose 6-phosphate dehydrogenase. Chronobiologia. 1988;15(3):205–212. [PubMed] [Google Scholar]
- 31.Kreier F, Kalsbeek A, Ruiter M, Yilmaz A, Romijn JA, Sauerwein HP, Fliers E, Buijs RM. Central nervous determination of food storage—a daily switch from conservation to expenditure: implications for the metabolic syndrome. Eur J Pharmacol. 2003;480(1–3):51–65. doi: 10.1016/j.ejphar.2003.08.092. [DOI] [PubMed] [Google Scholar]
- 32.Perfetto F, Tarquini R, Cornélissen G, Mello G, Tempestini A, Gaudiano P, Mancuso F, Halberg F. Circadian phase difference of leptin in android versus gynoid obesity. Peptides. 2004;25(8):1297–1306. doi: 10.1016/j.peptides.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Kreier F, Yilmaz A, Kalsbeek A, Romijn JA, Sauerwein HP, Fliers E, Buijs RM. Hypothesis: shifting the equilibrium from activity to food leads to autonomic unbalance and the metabolic syndrome. Diabetes. 2003;52(11):2652–2656. doi: 10.2337/diabetes.52.11.2652. [DOI] [PubMed] [Google Scholar]
- 34.Hofman MA, Swaab DF. Living by the clock: the circadian pacemaker in older people. Ageing Res Rev. 2006;5:33–51. doi: 10.1016/j.arr.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Bergman RN, Kim SP, Catalano KJ, Hsu IR, Chiu JD, Kabir M, Hucking K, Ader M. Why visceral fat is bad: mechanisms of the metabolic syndrome. Obesity (Silver Spring) 2006;14(l):16S–19S. doi: 10.1038/oby.2006.277. 1. [DOI] [PubMed] [Google Scholar]
- 36.Lönnqvist F, Nordfors L, Jansson M, Thörne A, Schalling M, Arner P. Leptin secretion from adipose tissue in women. Relationship to plasma levels and gene expression. J Clin Invest. 1997;99(10):2398–2404. doi: 10.1172/JCI119422. [DOI] [PMC free article] [PubMed] [Google Scholar]