Abstract
The ROMK (Kir1.1; Kcnj1) gene is believed to encode the apical small conductance K+ channels (SK) of the thick ascending limb (TAL) and cortical collecting duct (CCD). Loss-of-function mutations in the human ROMK gene cause Bartter’s syndrome with renal Na+ wasting, consistent with the role of this channel in apical K+ recycling in the TAL that is crucial for NaCl reabsorption. However, the mechanism of renal K+ wasting and hypokalemia that develop in individuals with ROMK Bartter’s syndrome is not apparent given the proposed loss of the collecting duct SK channel. Thus, we generated a colony of ROMK null mice with ~25% survival to adulthood that provides a good model for ROMK Bartter’s syndrome. The remaining 75% of null mice die in less than 14 days after birth. The surviving ROMK null mice have normal gross renal morphology with no evidence of significant hydronephrosis, whereas non-surviving null mice exhibit marked hydronephrosis. ROMK protein expression was absent in TAL and CCD from null mice but exhibited normal abundance and localization in wild-type littermates. ROMK null mice were polyuric and natriuretic with an elevated hematocrit consistent with mild extracellular volume depletion. SK channel activity in TAL and CCD was assessed by patch clamp analysis in ROMK wild-type ROMK(+/+), heterozygous ROMK(+/−), and null ROMK(−/−) mice. In 313 patches with successful seals from the three ROMK genotypes, SK channel activity in ROMK (+/+ and +/−) exhibited normal single channel kinetics. The expression frequencies are as follows: 67 (TAL) and 58% (CCD) in ROMK(+/+); about half that of the wild-type in ROMK(+/−), being 38 (TAL) and 25% (CCD); absent in both TAL or CCD in ROMK(−/−) between 2 and 5 weeks in 15 mice (61 and 66 patches, respectively). The absence of SK channel activity in ROMK null mice demonstrates that ROMK is essential for functional expression of SK channels in both TAL and CCD. Despite loss of ROMK expression, the normokalemic null mice exhibited significantly increased kaliuresis, indicating alternative mechanisms for K+ absorption/secretion in the nephron.
The kidney small conductance K+ channel (SK)1 expressed in the apical membranes of thick ascending limb (TAL) and cortical collecting duct (CCD) cells mediates K+ secretion in the distal nephron, thereby playing an essential role in K+ balance (1). In the TAL, the SK channel mediates K+ recycling across the apical membrane that is essential for maintaining the supply of luminal K+ for Na-K-2Cl cotransport (2–5). In the principal cells of the CCD, the SK channel provides a major pathway for apical K+ secretion.
ROMK (Kir1.1; gene locus, Kcnj1), cloned from rat kidney outer medulla (6), is a member of the inward rectifier (Kir) family of K+ channels (7). Several lines of evidence have suggested that ROMK may encode the SK channel. First, there are several NH2-terminal alternative splice variants of rodent (6, 8–10) and human (11) ROMK that are differentially expressed along the distal nephron segments where SK channels have been observed (9, 10). Second, ROMK protein has been immunolocalized to apical membranes of the nephron segments expressing the various ROMK transcripts (12–14). Finally, ROMK shares many functional and regulatory properties with native SK channels (15, 16). They have similar single channel kinetics including low channel conductance, high open probability, one open time and one closed time (6, 14, 16). Moreover, they are similarly regulated by channel phosphorylation and dephosphorylation processes (17, 18), cytosolic pH (19, 20), and tyrosine kinases that are modulated by dietary K+ intake (21–23).
Bartter’s syndrome comprises a set of genetically heterogeneous disorders characterized by salt wasting and polyuria-associated low blood pressure and hypokalemic alkalosis (24–27). Molecular genetic studies have identified loss-of-function mutations in any one of four genes encoding transporters mediating salt absorption by the TAL (28–31). Mutations in the apical Na-K-2Cl cotransporter (NKCC2) (32) and basolateral Cl− channel α- (CLCNKB (33)) or β-subunits (barttin (34, 35)) result in Bartter’s syndrome by disrupting the pathway for NaCl transport in the TAL. In addition, null mutations in ROMK also give rise to the Bartter’s phenotype consistent with a role for this K+ channel in TAL function (36–38). The Bartter’s mutations in ROMK have been shown to reduce K+ channel expression or function (39–42), consistent with the necessity of this channel for normal salt reabsorption in the TAL. However, the characteristic hypokalemia in individuals with ROMK Bartter’s is difficult to explain on the basis of loss of ROMK function if this channel encodes the SK channel in CCD.
In the present study, we assessed the expression of SK channel by patch clamping in TAL and CCD from ROMK null mice. Given the low survival and hydronephrosis of the original ROMK null mice (69), which would have presented difficulties in obtaining tubules for patch clamping, we developed a ROMK null mouse with high survival and normal histology by crossing surviving ROMK null mutants with heterozygotes from litters in which there were surviving null mutants. These mice exhibited characteristics of Bartter’s syndrome including polyuria, Na+ and K+ wasting. Kidney morphology in the ROMK null mice was normal without finding of hydronephrosis, and ROMK protein expression in TAL and CCD was absent.
K+ channel activity was absent in apical membranes from either TAL or CCD from ROMK(−/−) mice, whereas wild-type ROMK(+/+) littermates exhibited normal SK activity. The percent of successful patches in TAL or CCD showing SK channel activity in heterozygous ROMK(+/−) mice was ~50% of that in ROMK(+/+) littermates. These results demonstrate that ROMK encodes the SK channel in apical membranes of both TAL and CCD principal cells.
EXPERIMENTAL PROCEDURES
Breeding and Genotyping
The generation of the ROMK null mice is described in the accompanying article (69). Initially, surviving null ROMK(−/−) males were bred with heterozygous females to enhance survival. ROMK(+/−) heterozygous breeding pairs from these survivors were intercrossed for several generations to develop a new colony. All mice were maintained on standard mouse chow and tap water. Pups were genotyped 7 days after birth by PCR using DNA extracted from tail biopsies. The wild-type gene was amplified using a forward primer (5′-GTGACAGAACAGTGTGCC-3′) corresponding to codons 149–154 and a reverse primer (5′-CTCCTTCAGGTGTGATGG-3′) corresponding to anticodons 240–234. The mutant gene was amplified using the reverse primer from the ROMK gene and a primer (5′-CTGACTAGGGGAGGAGTAGAAGG-3′) complementary to sequences in the 5′-untranslated region of the neo gene.
Immunocytochemistry and Morphology Studies
ROMK(+/+), ROMK(+/−), and ROMK(−/−) mice were anesthetized by pentobarbital (0.1 mg/g body wt) and perfused via the aorta with Hanks’ solution, with drainage from the inferior vena, until the kidneys blanched. The mice were perfusion-fixed with 2% paraformaldehyde, 75 mm lysine, 10 mm sodium periodate (PLP). The kidneys were removed and transferred into 10% sucrose buffer overnight before cutting. Frozen sections (1 or 10 µm) were processed for immunofluorescence histochemistry with antibody labeling (43, 44). Sections were incubated for 16–18 h at 4 °C with primary antibody (rabbit anti-rat ROMK produced by amino acids 370–391 in ROMK1 (45)) diluted 1:250 in phosphate-buffered saline, 0.3% Triton X-100, 0.1% bovine serum albumin, 10% goat serum. After washing 3 times with Tris-buffered saline, the sections were incubated for 1–2 h with goat anti-rabbit Alexa Fluor 488 (IgG (1:200) from Molecular Probes, Eugene, OR). Controls with omission of primary antibody showed no significant fluorescence.
Metabolic Balance
ROMK(+/+) and ROMK(−/−) mice were housed in metabolic cages obtained from Lab Products Inc, Seaford, DE. Two mice from same litter of similar genotype were housed in a single cage to ensure normal eating and drinking behavior. After 2 days of training in the cage, 24-h food and water intake and urine output were measured and recorded. All data represent the average of three 24-h values. Urinary Na+ and K+ concentrations were measured by a flame photometer, and daily Na+ and K+ excretion was calculated as mEq/24 h. Na+, Cl−, and K+ concentrations were also measured in plasma from retro-orbital bleeds by a Corning Blood Gas analyzer (46). Blood gas analysis was performed on freshly drawn blood and measured by a Corning Blood Gas Analyzer.
Patch Clamping
Experiments were performed in mice between 2 and 5 weeks after birth. The left kidney was removed following anesthesia by intraperitoneal injection of pentobarbital sodium (0.1 mg/g body wt). 4–5 TAL and CCD tubules were microdissected from each mouse for apical patch clamping as described previously (47). All experiments were carried out at room temperature (22–24 °C).
Bath and tubule dissection solutions contained (mm) 140 NaCl, 5 KCl, 1.8 MgCl2, 1.8 CaCl2, and 10 HEPES (pH 7.4 adjusted with NaOH). For the inside-out patch configuration, the bath solution was the same as the dissection solution except for 0.8 MgCl2, 0 CaCl2, 1 EGTA. 0.5 mm MgATP (Sigma) was added to the bath to keep channels from running down. The pipette solution contained (mm) 140 KCl, 1.8 MgCl2 and 10 HEPES (pH 7.4 adjusted with KOH).
Single channel activity was recorded in both cell-attached and insideout configurations as reported previously (47). For analysis, current recordings were from inside-out patches with a pipette holding potential (−V) of −40 mV. Channel open time (To), closed time (Tc), and open probability (Po) were calculated over 4 s with a filter frequency of 250 Hz using pCLAMP software, version 6.0.5 of Fetchan and pSTAT (Anxon Instrument, Inc.). Channel conductance was calculated from current-voltage (I–V) curves between −40 and −80 mV.
Statistics
Data are presented as means ± S.E. Two-way Student’s t test was used to compare control and experimental groups. The difference between the mean values of ROMK(+/+) and ROMK(−/−) groups was considered significant at p < 0.05.
RESULTS
ROMK-deficient Mice with Increased Survival without Severe Hydronephrosis
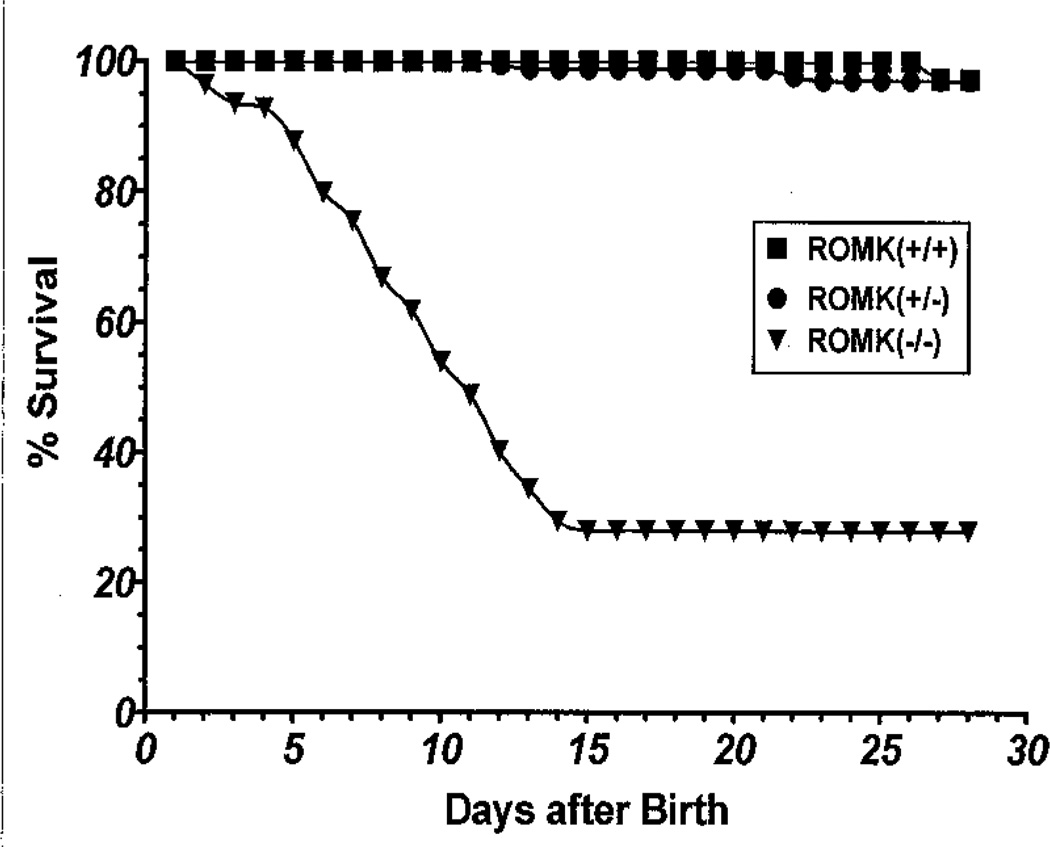
Companion studies by Lorenz et al. (69) showed that the mortality of ROMK-deficient mice was very high with less than 5% survival to weaning at 21 days. These ROMK(−/−) mice had renal insufficiency with profound hydronephrosis. We developed a colony with increased survival by selectively crossing surviving ROMK null mice with heterozygotes from litters in which there were surviving null mutants. As shown in Fig. 1, 25% of ROMK(−/−) mice survived to adulthood, and the remaining mice died before 14 days. There was no difference in survival rate between the wild-type ROMK(+/+) and heterozygous ROMK(+/−) mice.
Fig. 1. Survival rates of ROMK wild-type ROMK(+/+), heterozygous ROMK(+/−), and null ROMK(−/−) mice.
The percentage of survival was analyzed by the ratio of the animal numbers at birth (day 1) and the number of survivors at subsequent days.
Fig. 2 shows the gross morphology of kidneys obtained from wild-type ROMK(+/+), heterozygous (ROMK(+/−), and null (ROMK(−/−) mice that either survived or did non-survive beyond 14 days. In non-surviving ROMK(−/−) mice, the size of the kidney was about one-third of normal (Fig. 2, E and F); the renal cortex was considerably thinner than in wild-type (Fig. 2, A and B) or heterozygous (Fig. 2, C and D) mice, and the renal pelvis surrounding the renal papilla and the pelvic fornices at the level of the outer medulla were extensively dilated. These changes indicated that significant hydronephrosis was present in the non-surviving null mice, but this was still somewhat milder than that seen in the original ROMK-deficient mice (69). In contrast, the surviving null mice did not have significant hydronephrosis, and the kidneys were only slightly smaller than those from wild-type (Fig. 2, A and B) or heterozygous (Fig. 2, C and D) mice. We cannot exclude the possibly that mild hydronephrosis may be present in a small number of our ROMK(−/−) mice given the limited number of animals examined in this study. There were no gross histological difference among the ROMK(+/+), ROMK(+/−) and surviving ROMK(−/−) mice (Fig. 2).
Fig. 2. Kidney morphology in ROMK(+/+) wild-type (A and B), ROMK(+/−) heterozygous (C and D), ROMK(−/−) null non-survivor (E and F), and survivor (G and H) mice.
Whole kidney images (A, C, E, and G) are at ×10, and detailed images (B, D, F, and H) are at ×400. Sections are 10 µm thick and stained with Richardson solution containing a 1:1 mixture of Azure II and methylene blue. * in E indicates extensively dilated pelvic fornices at the level of the outer medulla.
Expression of ROMK Protein in TAL and CCD Is Absent in ROMK(−/−) Mice
To confirm the absence of ROMK expression in kidney in our ROMK(−/−) genotype, we examined the expression and localization of ROMK in wild-type and null mouse kidneys by immunofluorescence using a polyclonal rabbit anti-rat antibody directed against a COOH-terminal peptide (45). Fig. 3 shows paired phase and immunofluorescence images of ROMK staining in 1-µm cryosections of kidney cortex from ROMK(+/+) and ROMK(−/−) mice. ROMK was clearly expressed at apical borders of the TAL and CCD segments in cortical medullary rays. In contrast, there was no immunostaining of ROMK in null mice in either the TAL or the collecting duct, confirming the ROMK(−/−) genotype generated as described in the accompanying article (69).
Fig. 3. ROMK is absent in ROMK(−/−) null mice.
Matching phase (A and C) and immunofluorescence images (B and D) of ROMK(+/+) wild-type (A and B) and ROMK(−/−) null (C and D) mice are shown. 1-µm sections were incubated with rabbit anti-ROMK polyclonal antibody and Alexa Fluor 488 goat anti-rabbit IgG. All images are at ×400 magnification. PT, proximal tubule.
ROMK-deficient Mice Have Polyuria, Increased Na+ and K+ Excretion with Mild Volume Depletion, but No Hypokalemia and a Normal Acid-base State
Table I shows the plasma Na+, Cl−, and K+ concentrations measured in adult ROMK(+/+) and ROMK(−/−) mice. Plasma Na+, Cl−, and K+ in ROMK null mice were within the physiological range and similar to wild-type values. Table II shows the acid-base status in ROMK wild-type, heterozygous, and knockout mice from ~2-week-old pups and adult mice. The 2-week-old animals included both surviving and non-surviving pups. A slight metabolic acidosis was seen in the ROMK null pups compared with either ROMK(+/+) of ROMK(+/−) mice. However, no significant abnormality in acid-base status was observed in adult ROMK null mice.
Table I.
Plasma Na+, K+, CI− and hematocrit in ROMK wild-type and knockout mice
| Genotype | n | PNa | PK | PCI | HCT |
|---|---|---|---|---|---|
| mEq/liter | mEq/liter | mEq/liter | % | ||
| ROMK (+/+) | 15 | 153.2 ± 1.8 | 4.9 ± 0.1 | 108.0 ± 1.4 | 45.6 ± 1.1 |
| ROMK (−/−) | 17 | 149.0 ± 2.0 | 4.7 ± 0.1 | 102.0 ± 1.2 | 52.7 ± 1.1a |
Values are means ± S.E. from each group. n, number of animals. PNa, plasma Na+; PK, plasma K+; PCl, plasma Cl; HCT, hematocrit.
Significantly different from control (p < 0.05).
Table II.
Acid-base Status in ROMK wild-type and mutant mice
| Genotype | n | pH | PCO2 | HCO3 |
|---|---|---|---|---|
| mmHg | mm | |||
| Pups | ||||
| ROMK (+/+) | 6 | 7.28 ± 0.02 | 55.5 ± 2.04 | 25.46 ± 1.44 |
| ROMK (+/−) | 6 | 7.27 ± 0.02 | 53.6 ± 4.24 | 24.27 ± 2.31 |
| ROMK (−/−) | 6 | 7.13 ± 0.08 | 44.1 ± 3.63 | 15.75 ± 3.41a |
| Adult | ||||
| ROMK (+/+) | 18 | 7.33 ± 0.01 | 48.4 ± 2.23 | 24.41 ± 0.74 |
| ROMK (+/−) | 21 | 7.30 ± 0.01 | 50.5 ± 0.93 | 24.30 ± 0.55 |
| ROMK (−/−) | 23 | 7.31 ± 0.01 | 52.1 ± 1.31 | 25.92 ± 1.05 |
Values are means ± S.E. from each group. Pups, 2-week-old ROMK mutant mice; adult, mice are 3 weeks or older.
Significant difference from control (p < 0.05).
The results of metabolic studies, shown in Tables III and IV, demonstrate that the 24-h urine volumes and urinary Na+ and K+ excretion rates were significantly higher in ROMK null mice compared with wild-type mice. The polyuria in the ROMK(−/−) mice was associated with a significant reduction in urine osmolality compared with the ROMK(+/+) mice (Table III). Daily water and food intake were also increased in the ROMK(−/−) mice. The hematocrit was significantly higher in ROMK null mice (Table I) suggesting mild hypovolemia consistent with the polyuria and natriuresis. The magnitude of the increase in hematocrit in the ROMK(−/−) mice was far less than seen in the NKCC2(−/−) knockout reported previously by Takahashi et al. (48), consistent with the less severe polyuria and dehydration in our ROMK knockout. Thus, the ROMK(−/−) mice exhibited polyuria and salt wasting, consistent with a Bartter’s type TAL tubulopathy.
Table III.
Urine volume, ENa, EK, and osmolality in ROMK knock-out and wild-type mice
| Genotype | n | UV | ENa | EK | Osmolality |
|---|---|---|---|---|---|
| ml/24 h | mEq/24 h | mmol/kg | |||
| ROMK (+/+) | 22 | 1.18 ± 0.12 | 188.9 ± 30.1 | 173.0 ± 24.1 | 1986.7 ± 35.2 |
| ROMK (−/−) | 21 | 5.61 ± 0.36a | 297.9 ± 26.3a | 289.4 ± 11.8a | 678.9 ± 35.3a |
Values are means ± S.E. from each group. n, number of animals. UV, urine volume; ENa, excretion of Na+; EK, excretion of K+.
Significantly different from control (p < 0.05).
Table IV.
Body weight, food, and water intake in ROMK knockout and wild-type mice
| Genotype | n | BW | Food intake | Water intake |
|---|---|---|---|---|
| g | g/24 h | ml/24 h | ||
| ROMK (+/+) | 22 | 25.3 ± 0.8 | 1.95 ± 0.16 | 3.04 ± 0.16 |
| ROMK (−/−) | 21 | 24.5 ± 1.0 | 3.11 ± 0.19a | 9.20 ± 0.39a |
Values are means ± S.E. from each group. n, number of animals. BW, body weight
Significantly different from control (p < 0.05).
SK Channel Activity Is Absent in ROMK(−/−) Mice
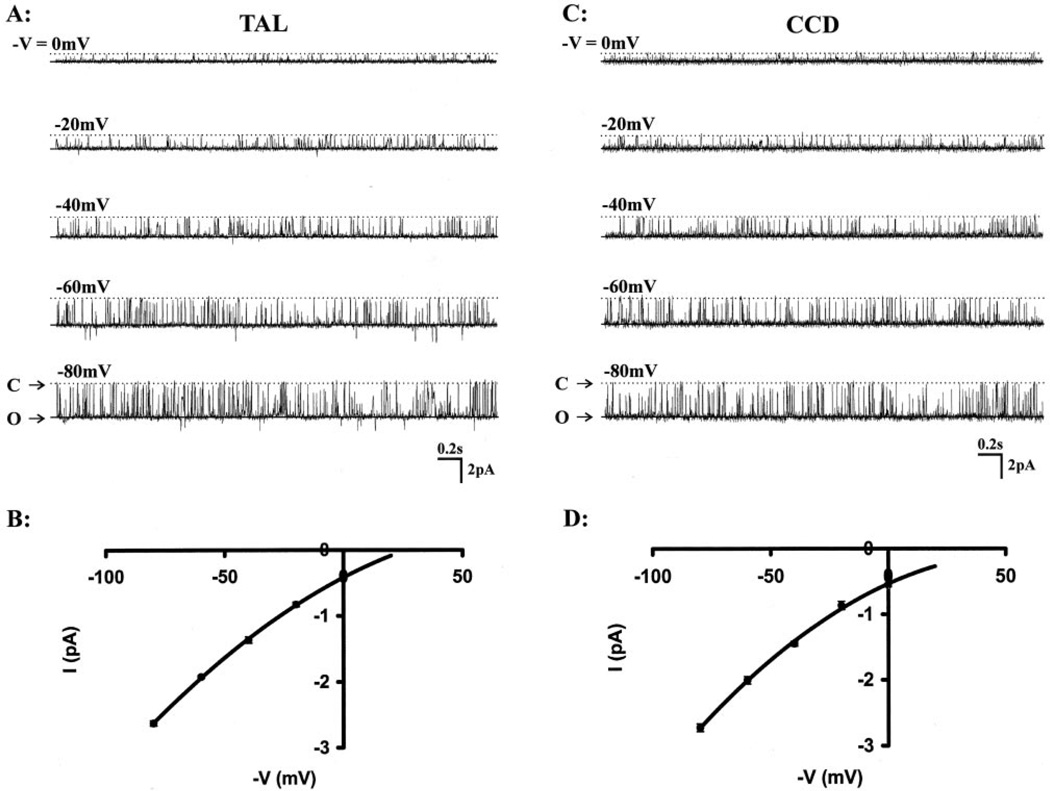
Typical SK channels were observed in 11 wild-type and 19 heterozygous mice between 2 and 5 weeks after birth. Fig. 4, A and C, shows representative single SK channel recordings in wild-type TAL and CCD using the inside-out configuration at different pipette holding voltages. With 140 mm KCl in the pipette and 140 mm NaCl + 5 mm KCl in bath, I–V plots showed slightly inward-rectified currents (Fig. 4, B and D). Channel conductance (G = ~31 pS) was calculated from the I–V curve between −40 and −80 mV in the inside-out configuration. There were no differences in the channel conductance between SK channels in the TAL or CCD obtained from wild-type or heterozygous mice. SK channels had a high open probability (Po = 0.88~0.89) in both TAL and CCD with one open time and one closed time in insideout configuration at a pipette holding potential (−V) of −40 mV. The data are summarized in Table V.
Fig. 4. Representative single channel currents were recorded from wild-type mice TAL (A) and CCD (C) in inside-out configuration with 140 mm KCl in the pipette and 140 mm NaCl, 5 mm KCl in the bath.
The different pipette holding potentials (−V) were indicated on each left side of tracings. B and D are the SK channel I–V curves in TAL and CCD in the same experimental conditions in which the channel showed slightly inward-rectified current, respectively. O and C represent the channel open and closed states.
Table V.
SK channel kinetics in ROMK wild-type (+/+) and heterozygous (+/−) mice
| Mouse | CCD (+/+) | TAL (+/+) | CCD (+/−) | TAL (+/−) |
|---|---|---|---|---|
| Po | 0.89 ± 0.07 | 0.88 ± 0.02 | 0.88 ± 0.17 | 0.89 ± 0.01 |
| To, ms | 28.43 ± 0.28 | 28.28 ± 0.23 | 24.49 ± 0.29 | 25.36 ± 0.27 |
| Tc, ms | 1.72 ± 0.30 | 1.21 ± 0.13 | 1.84 ± 0.17 | 1.25 ± 0.35 |
| G, pS | 31.86 ± 0.50 | 31.5 ± 0.23 | 31.13 ± 0.58 | 31.87 ± 0.37 |
Po = channel open probability; To = channel open time; Tc = channel closed time; G = channel conductance calculated between −40 and −80 mV. All the data were calculated from inside-out configuration with 140 mm KCl in the pipette and 140 mm NaCl, 5 mm KCl in the bath. Pipette holding potential (−V) was −40 mV. Channel conductance (G) was calculated between 0 and −80 mV.
A total 313 of patches with successful seals was obtained in the three ROMK genotypes. Wild-type ROMK(+/+) mice showed SK channel activity in 18 of 27 patches (66.67%) in TAL and 14 of 24 patches (58.33%) in CCD. This is consistent with the lower density of SK channels in the mouse compared with that in the rat (49). Functional expression of SK channels in TAL and CCD in wild-type ROMK(+/+) mice was observed as early as 2 weeks. SK channel activity in ROMK heterozygous mice was about half that of the wild-type, 38% (20 of 52 patches) in TAL and 25% (21 of 83 patches) in CCD. In contrast, no SK channel activity was observed at any age in ROMK null mice (n = 15 mice, total of 61 and 66 patches) in either TAL or CCD (data summarized in Table VI). Neither intermediate (70 pS) nor large conductance K+ channels (~150 pS) were observed in our wild-type mice using our current patch conditions.
Table VI.
Patches showing SK channel activity in ROMK wild-type (+/+), heterozygous (+/−), and null (−/−) mice
| Mouse | CCD (+/+) | TAL (+/+) | CCD (+/−) | CTAL (+/−) | CCD (−/−) | TAL (−/−) |
|---|---|---|---|---|---|---|
| No. of mice | 20 | 20 | 20 | 19 | 15 | 15 |
| No. of patches showing channels | 14 | 18 | 21 | 20 | 0 | 0 |
| Patch success | 24 | 27 | 83 | 52 | 66 | 61 |
| % | 58.3 | 66.7 | 25.5 | 38.5 | 0.0 | 0.0 |
DISCUSSION
Selective breeding of ROMK-deficient mice resulted in development of a colony without (or significantly reduced) hydronephrosis but displaying some of the characteristics of human Bartter’s syndrome including polyuria with increased urinary Na+ loss and mild extracellular fluid volume depletion. The increased urinary Na+ loss and low urine osmolality in these ROMK-deficient mice are consistent with dysfunction of NaCl handling by the TAL as demonstrated in the accompanying article (69) and in human Bartter’s syndrome (50, 51).
The ROMK-deficient mice did not develop hypokalemia (Table I) as seen in human Bartter’s syndrome and in NKCC2-deficient mice (48), but did, however, exhibit a high 24-h urinary K+ excretion in the steady state (Table II). Increased fluid and Na+ delivery to the rat CCD, as may occur by reducing NaCl reabsorption by furosemide-mediated inhibition of NKCC2 in the TAL, promotes K+ secretion and urinary K+ loss (52, 53). However, the loss of SK activity in principal cells may have limited the magnitude of kaliuresis in ROMK-deficient mice despite increased Na+ and fluid delivery to the CCD. In addition, the adult ROMK null mice did not develop metabolic alkalosis as is also typical for human Bartter’s syndrome (50, 51). This may reflect the fact that ROMK(−/−) mice did not develop hypokalemia which would contribute to the development and maintenance of hypokalemia. The 2-week-old ROMK null pups had a mild metabolic acidosis because of the inclusion of both pups with and without hydronephrosis (Fig. 2). The pups with hydronephrosis would ultimately die before weaning with the metabolic acidosis most likely being due to the loss of functioning renal mass. Metabolic acidosis was also observed in the original ROMK(−/−) mice exhibiting hydronephrosis reported in the accompanying article (69).
By using the patch clamp technique, we showed that the biophysical properties of SK channels in TAL and principal cells from our mouse colony (Table IV) are similar to that of SK channels observed previously in the rat (1, 16, 54–56) and mouse (47) and to ROMK expressed in Xenopus oocytes (8, 16, 57). In the rat, postnatal ROMK transcript and protein expression have been observed as early as 1 week in the medullary TAL and is clearly expressed by 3 weeks in cortical TAL and CCD (58). If a similar post-natal expression pattern occurs in the mouse, then the absence of SK channel activity in TAL and CCD from ROMK-deficient mice at 3–5 weeks (Table V) establishes that ROMK is essential for functional expression of SK channels involved in K+ recycling in the TAL and in K+ secretion in principal cells. Heterozygous ROMK(+/−) mice expressed about 50% of the frequency of active channels compared with the wild-type ROMK(+/+) controls (Table V) consistent with one active allele.
Two types of K+ channels have been described in apical membranes of the rat (59) and mouse (47, 49) TAL: a small conductance K+ channel (SK, ~30 pS) and an intermediate conductance K+ channel (~70 pS). A third type of calcium-activated large conductance K+ channel (~150 pS) has also been observed in mouse TAL cells and in cultured rabbit TAL cells (60). It is generally believed that the 30- and 70-pS channels are involved in K+ recycling in the TAL, whereas the large conductance K+ channel may be involved in cell volume regulation (15, 49). In the rat TAL, 60–80% of the total apical K+ conductance is contributed by the 70-pS K+ channel (49, 61, 62), although the contribution of this intermediate conductance K+ channel to total apical K+ conductance in the mouse appears to be somewhat lower (49). We do not know if human TAL cells express the 70-pS K+ channel or the potential contribution of this channel to total apical membrane K+ conductance in this TAL segment. However, by assuming that 60–80% of apical conductance is mediated by the 70-pS K+ channel in the human TAL, loss of the SK channel should only modestly affect TAL function unless ROMK were necessary for functional expression of the intermediate conductance K+ channel (15, 49). In our experiments, we did not observe the intermediate conductance K+ channel in TAL from wild-type or ROMK-deficient mice. It should be noted, however, that our experimental conditions were optimized for observation of the SK channel in the wild-type mice. Additional studies will be required to address the role of ROMK in the 70-pS channel where we have optimized the activity of the 70-pS channel in wild-type mouse controls (e.g. high K+ diet).
Two types of K+ channels have also been observed in apical membranes of principal cells in rat (63, 64) and mouse2 CCD as follows: a small conductance K+ channel (SK, 30 pS) and a calcium-activated large conductance K+ channel (150 pS). The large conductance K+ channel has been suggested to mediate flow-dependent K+ secretion by principal cells (65, 66). Under our experimental conditions, we did not observe the large conductance K+ channel in principal cells from ROMK-deficient mice. Again, it is possible that our patch conditions were not conducive to observe the Ca2+-activated large conductance K+ channel because the bath solution did not contain Ca2+.
One of the phenotypic characteristics of Bartter’s syndrome is hypokalemia. In our ROMK(−/−) mice the plasma potassium concentration was normal (Table I), yet K+ excretion rate was significantly elevated (Table II) and accompanied by increased food intake (Table III). Interestingly, this enhanced potassium excretion in ROMK(−/−) mice occurred in the absence of SK channel activity in the CCD, indicating that alternative mechanisms for urinary K+ excretion are present. Several factors may contribute to the K+ loss seen in ROMK null mice. These include the following: 1) diminished reabsorption of K+ in the TAL due to loss K+ recycling required for Na-K-2Cl function, for example, administration of furosemide leads to a reduction in K+ reabsorption by the TAL, significant increase of K+ delivery into the distal convoluted tubule, and kaliuresis (67); 2) K+ secretion by flow-dependent, Ca2+-activated K+ channels in the CCD (65, 66); 3) K+ secretion via apical KCl cotransport in the CCD (68); 4) augmented paracellular K+ back-leak into the CCD by increased aldosterone and the consequent increase in lumen negativity; and 5) the presence of other K+ transporters or channels. The ROMK-deficient mice characterized in this study should provide an excellent model for determining which mechanisms account for urinary K+ loss in the absence of SK channels as well as for understanding the other pathophysiology of renal K+ and Na+ handling in Bartter’s syndrome.
Acknowledgments
We thank Drs. Michael Kashgarian, DeRen Shao, and Sue-Ann Mentone for the technical assistance with kidney morphology and antibody staining.
Footnotes
This work was supported by National Institutes of Health Grants DK54999 (to S. C. H.), DK54998 (to G. G.), DK54983 (to W. W.), and DK50594 (to G. S.).
The abbreviations used are: SK, small conductance K+ channel; CCD, cortical collecting duct; TAL, thick ascending limb; NKCC2, Na-K-2Cl cotransporter.
M. Lu, W.-H. Wang, G. Giebisch, and S. C. Hebert, unpublished observations.
REFERENCES
- 1.Giebisch G. Am. J. Physiol. 1998;274:F817–F833. doi: 10.1152/ajprenal.1998.274.5.F817. [DOI] [PubMed] [Google Scholar]
- 2.Greger R, Schlatter E. Pfluegers Arch. 1981;392:92–94. doi: 10.1007/BF00584588. [DOI] [PubMed] [Google Scholar]
- 3.Greger R, Bleich M, Schlatter E. Kidney Int. 1991;40(Suppl. 33):119–124. [PubMed] [Google Scholar]
- 4.Hebert SC, Andreoli TE. Am. J. Physiol. 1984;246:F745–F756. doi: 10.1152/ajprenal.1984.246.6.F745. [DOI] [PubMed] [Google Scholar]
- 5.Hebert SC, Andreoli TE. J. Gen. Physiol. 1986;87:567–590. doi: 10.1085/jgp.87.4.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ho K, Nichols CG, Lederer WJ, Lytton J, Vassilev PM, Kanazirska MV, Hebert SC. Nature. 1993;362:31–38. doi: 10.1038/362031a0. [DOI] [PubMed] [Google Scholar]
- 7.Nichols CG, Lopatin AN. Annu. Rev. Physiol. 1997;59:171–191. doi: 10.1146/annurev.physiol.59.1.171. [DOI] [PubMed] [Google Scholar]
- 8.Zhou H, Tate SS, Palmer LG. Am. J. Physiol. 1994;266:C809–C824. doi: 10.1152/ajpcell.1994.266.3.C809. [DOI] [PubMed] [Google Scholar]
- 9.Boim MA, Ho K, Shuck ME, Bienkowski MJ, Block JH, Slightom JL, Yang Y, Brenner BM, Hebert SC. Am. J. Physiol. 1995;268:F1132–F1140. doi: 10.1152/ajprenal.1995.268.6.F1132. [DOI] [PubMed] [Google Scholar]
- 10.Lee W-S, Hebert SC. Am. J. Physiol. 1995;268:F1124–F1131. doi: 10.1152/ajprenal.1995.268.6.F1124. [DOI] [PubMed] [Google Scholar]
- 11.Shuck ME, Block JH, Benjamin CW, Tsai T-D, Lee KS, Slightom JL, Bienkowski MJ. J. Biol. Chem. 1994;269:24261–24270. [PubMed] [Google Scholar]
- 12.Xu JZ, Hall AE, Peterson LN, Bienkowski MJ, Eessalu TE, Hebert SC. Am. J. Physiol. 1997;273:F739–F748. doi: 10.1152/ajprenal.1997.273.5.F739. [DOI] [PubMed] [Google Scholar]
- 13.Kohda Y, Ding W, Phan E, Housini I, Wang J, Star RA, Huang CL. Kidney Int. 1998;54:1214–1223. doi: 10.1046/j.1523-1755.1998.00120.x. [DOI] [PubMed] [Google Scholar]
- 14.Mennitt PA, Wade JB, Ecelbarger CA, Palmer LG, Frindt G. J. Am. Soc. Nephrol. 1997;8:1823–1830. doi: 10.1681/ASN.V8121823. [DOI] [PubMed] [Google Scholar]
- 15.Wang W, Hebert SC, Giebisch G. Annu. Rev. Physiol. 1997;59:413–436. doi: 10.1146/annurev.physiol.59.1.413. [DOI] [PubMed] [Google Scholar]
- 16.Palmer LG, Choe H, Frindt G. Am. J. Physiol. 1997;273:F404–F410. doi: 10.1152/ajprenal.1997.273.3.F404. [DOI] [PubMed] [Google Scholar]
- 17.Xu ZC, Yang Y, Hebert SC. J. Biol. Chem. 1996;271:9313–9319. doi: 10.1074/jbc.271.16.9313. [DOI] [PubMed] [Google Scholar]
- 18.McNicholas CM, Wang W, Ho K, Hebert SC, Giebisch G. Proc. Natl. Acad. Sci. U. S. A. 1994;91:8077–8081. doi: 10.1073/pnas.91.17.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choe H, Zhou H, Palmer LG, Sackin H. Am. J. Physiol. 1997;273:F516–F529. doi: 10.1152/ajprenal.1997.273.4.F516. [DOI] [PubMed] [Google Scholar]
- 20.Fakler B, Schultz JH, Yang J, Schulte U, Brändle U, Zenner HP, Jan LY, Ruppersberg JP. EMBO J. 1996;15:4093–4099. [PMC free article] [PubMed] [Google Scholar]
- 21.Moral Z, Dong K, Wei Y, Sterling H, Deng H, Ali S, Gu R, Huang XY, Hebert SC, Giebisch G, Wang WH. J. Biol. Chem. 2001;276:7156–7163. doi: 10.1074/jbc.M008671200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei Y, Bloom P, Gu R, Wang W. J. Biol. Chem. 2000;275:20502–20507. doi: 10.1074/jbc.M000783200. [DOI] [PubMed] [Google Scholar]
- 23.Wang W, Lerea KM, Chan M, Giebisch G. Am. J. Physiol. 2000;278:F165–F171. doi: 10.1152/ajprenal.2000.278.1.F165. [DOI] [PubMed] [Google Scholar]
- 24.Bartter FC, Pronove P, Gill JR, Jr, MacCardle RC, Diller E. Am. J. Med. 1962;33:811–828. doi: 10.1016/0002-9343(62)90214-0. [DOI] [PubMed] [Google Scholar]
- 25.Gill JR., Jr Annu. Rev. Med. 1980;31:405–419. doi: 10.1146/annurev.me.31.020180.002201. [DOI] [PubMed] [Google Scholar]
- 26.Stein JH. Kidney Int. 1985;28:85–93. doi: 10.1038/ki.1985.123. [DOI] [PubMed] [Google Scholar]
- 27.Gordon JA, Stokes JBI. Hosp. Pract. 1994;29:103–108. doi: 10.1080/21548331.1994.11443023. [DOI] [PubMed] [Google Scholar]
- 28.Guay-Woodford LM. Am. J. Med. 1998;105:151–161. doi: 10.1016/s0002-9343(98)00196-x. [DOI] [PubMed] [Google Scholar]
- 29.Karolyi L, Koch MC, Grzeschik KH, Seyberth HW. J. Mol. Med. 1998;76:317–325. doi: 10.1007/s001090050223. [DOI] [PubMed] [Google Scholar]
- 30.Asteria C. Eur. J. Endocrinol. 1997;137:613–615. doi: 10.1530/eje.0.1370613. [DOI] [PubMed] [Google Scholar]
- 31.Simon DB, Lifton RP. Curr. Opin. Cell Biol. 1998;10:450–454. doi: 10.1016/s0955-0674(98)80057-4. [DOI] [PubMed] [Google Scholar]
- 32.Simon DB, Karet FE, Hamdan JM, DiPietro A, Sanjad SA, Lifton RP. Nat. Genet. 1996;13:183–188. doi: 10.1038/ng0696-183. [DOI] [PubMed] [Google Scholar]
- 33.Simon DB, Bindra RS, Mansfield TA, Nelson-Williams C, Mendonça E, Stone R, Schurman S, Nayir A, Alpay H, Bakkaloglu A, Rodriguez-Soriano J, Morales JM, Sanjad SA, Taylor CM, Pilz D, Brem A, Trachtman H, Griswold W, Richard GA, John E, Lifton RP. Nat. Genet. 1997;17:171–178. doi: 10.1038/ng1097-171. [DOI] [PubMed] [Google Scholar]
- 34.Estevez R, Boettger T, Stein V, Birkenhager R, Otto E, Hildebrandt F, Jentsch TJ. Nature. 2001;414:558–561. doi: 10.1038/35107099. [DOI] [PubMed] [Google Scholar]
- 35.Birkenhager R, Otto E, Schurmann MJ, Vollmer M, Ruf EM, Maier-Lutz I, Beekmann F, Fekete A, Omran H, Feldmann D, Milford DV, Jeck N, Konrad M, Landau D, Knoers NV, Antignac C, Sudbrak R, Kispert A, Hildebrandt F. Nat. Genet. 2001;29:310–314. doi: 10.1038/ng752. [DOI] [PubMed] [Google Scholar]
- 36.Simon DB, Karet FE, Rodriguez-Soriano J, Hamdan JH, DiPietro A, Trachtman H, Sanjad SA, Lifton RP. Nat. Genet. 1996;14:152–156. doi: 10.1038/ng1096-152. [DOI] [PubMed] [Google Scholar]
- 37.Karolyil L, Konrad M, Kockerling A, Ziegler A, Zimmermann DK, Roth B, Wieg C, Grzeschik K-H, Koch MC, Seyberth HW, Vargus R, Forestier L, Jean G, Deschaux M, Rizzoni GF, Niaudet P, Antignac C, Feldman D, Lorridon F, Cougoureux E, Laroze F, Alessandri J-L, David L, Saunier P, Deschenes G, Hildebrandt F, Vollmer M, Proesmans W, Brandis M, van den Heuvell LPWJ, Lemmink HH, Nillesen W, Monnens LAH, Knoers NVAM, Guay-Woodford LM, Wright CJ, Madrigal G, Hebert SC. Hum. Mol. Genet. 1997;6:17–26. [Google Scholar]
- 38.Vollmer M, Koehrer M, Topaloglu R, Strahm B, Omran H, Hildebrandt F. Pediatr. Nephrol. 1998;12:69–71. doi: 10.1007/s004670050408. [DOI] [PubMed] [Google Scholar]
- 39.Schwalbe RA, Bianchi L, Accili EA, Brown AM. Hum. Mol. Genet. 1998;7:975–980. doi: 10.1093/hmg/7.6.975. [DOI] [PubMed] [Google Scholar]
- 40.Derst C, Konrad M, Köckerling A, Karschin A, Daut J, Seyberth HW. Biochem. Biophys. Res. Commun. 1997;230:641–645. doi: 10.1006/bbrc.1996.6024. [DOI] [PubMed] [Google Scholar]
- 41.Derst C, Wischmeyer E, Preisig-Muller R, Spauschus A, Konrad M, Hensen P, Jeck N, Seyberth HW, Daut J, Karschin A. J. Biol. Chem. 1998;273:23884–23891. doi: 10.1074/jbc.273.37.23884. [DOI] [PubMed] [Google Scholar]
- 42.Flagg TP, Tate M, Merot J, Welling PA. J. Gen. Physiol. 1999;114:685–700. doi: 10.1085/jgp.114.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu J, Hall AE, Peterson LN, Bienkowski MJ, Essalu TE, Hebert SC. Am. J. Physiol. 1997;273:F739–F748. doi: 10.1152/ajprenal.1997.273.5.F739. [DOI] [PubMed] [Google Scholar]
- 44.Biemesderfer D, Rutherford PA, Nagy T, Pizzonia JH, Abu-Alfa AK, Aronson PS. Am. J. Physiol. 1997;273:F289–F299. doi: 10.1152/ajprenal.1997.273.2.F289. [DOI] [PubMed] [Google Scholar]
- 45.Ecelbarger CA, Kim GH, Knepper MA, Liu J, Tate M, Welling PA, Wade JB. J. Am. Soc. Nephrol. 2001;12:10–18. doi: 10.1681/ASN.V12110. [DOI] [PubMed] [Google Scholar]
- 46.Wang T, Yang CL, Abbiati T, Schultheis PJ, Shull GE, Giebisch G, Aronson PS. Am. J. Physiol. 1999;277:F298–F302. doi: 10.1152/ajprenal.1999.277.2.F298. [DOI] [PubMed] [Google Scholar]
- 47.Lu M, MacGregor GG, Wang W, Giebisch G. J. Gen. Physiol. 2000;116:299–310. doi: 10.1085/jgp.116.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takahashi N, Chernavvsky DR, Gomez RA, Igarashi P, Gitelman HJ, Smithies O. Proc. Natl. Acad. Sci. U. S. A. 2000;97:5434–5439. doi: 10.1073/pnas.090091297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu M, Wang W. Kidney Blood Press. Res. 2000;23:75–82. doi: 10.1159/000025957. [DOI] [PubMed] [Google Scholar]
- 50.Gill JR, Jr, Bartter FC. Am. J. Med. 1978;65:766–772. doi: 10.1016/0002-9343(78)90794-5. [DOI] [PubMed] [Google Scholar]
- 51.Tsukamoto T, Kobayashi T, Kawamoto K, Fukase M, Chihara K. Am. J. Kidney Dis. 1995;25:637–641. doi: 10.1016/0272-6386(95)90137-x. [DOI] [PubMed] [Google Scholar]
- 52.Velazquez H, Wright FS. Am. J. Physiol. 1986;250:F1013–F1023. doi: 10.1152/ajprenal.1986.250.6.F1013. [DOI] [PubMed] [Google Scholar]
- 53.Good DW, Wright FS. Am. J. Physiol. 1979;236–5:F192–F205. doi: 10.1152/ajprenal.1979.236.2.F192. [DOI] [PubMed] [Google Scholar]
- 54.Wang W, Sackin H, Giebisch G. Annu. Rev. Physiol. 1992;54:81–96. doi: 10.1146/annurev.ph.54.030192.000501. [DOI] [PubMed] [Google Scholar]
- 55.Wang W, Giebisch G. J. Gen. Physiol. 1991;98:35–61. doi: 10.1085/jgp.98.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frindt G, Palmer LG. Am. J. Physiol. 1989;256:F143–F151. doi: 10.1152/ajprenal.1989.256.1.F143. [DOI] [PubMed] [Google Scholar]
- 57.Hebert TE, Loisel TP, Adam L, Ethier N, Onge SS, Bouvier M. Biochem. J. 1998;330:287–293. doi: 10.1042/bj3300287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zolotnitskaya A, Satlin LM. Am. J. Physiol. 1999;276:F825–F836. doi: 10.1152/ajprenal.1999.276.6.F825. [DOI] [PubMed] [Google Scholar]
- 59.Wang W. Am. J. Physiol. 1994;267:F599–F605. doi: 10.1152/ajprenal.1994.267.4.F599. [DOI] [PubMed] [Google Scholar]
- 60.Guggino SE, Guggino WB, Green N, Sacktor B. Am. J. Physiol. 1987;252:C121–C127. doi: 10.1152/ajpcell.1987.252.2.C121. [DOI] [PubMed] [Google Scholar]
- 61.Lu M, Zhu Y, Balazy M, Reddy KM, Falck JR, Wang W-H. J. Gen. Physiol. 1996;108:537–547. doi: 10.1085/jgp.108.6.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang W, Lu M. J. Gen. Physiol. 1995;106:727–743. doi: 10.1085/jgp.106.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Frindt G, Palmer LG. Am. J. Physiol. 1987;252:F458–F467. doi: 10.1152/ajprenal.1987.252.3.F458. [DOI] [PubMed] [Google Scholar]
- 64.DeJongh KS, Warner C, Catterall WA. J. Biol. Chem. 1990;265:14738–14741. [PubMed] [Google Scholar]
- 65.Woda CB, Bragin A, Kleyman TR, Satlin LM. Am. J. Physiol. 2001;280:F786–F793. doi: 10.1152/ajprenal.2001.280.5.F786. [DOI] [PubMed] [Google Scholar]
- 66.Taniguchi J, Imai M. J. Membr. Biol. 1998;164:35–45. doi: 10.1007/s002329900391. [DOI] [PubMed] [Google Scholar]
- 67.Hropot M, Fowler N, Karlmark B, Giebisch G. Kidney Int. 1985;28:477–489. doi: 10.1038/ki.1985.154. [DOI] [PubMed] [Google Scholar]
- 68.Velazquez H, Ellison DH, Wright FS. Am. J. Physiol. 1987;253:F555–F562. doi: 10.1152/ajprenal.1987.253.3.F555. [DOI] [PubMed] [Google Scholar]
- 69.Lorenz JN, Baird NR, Judd LM, Noonan WT, Andringa A, Doetschman T, Manning PA, Liu LH, Miller ML, Shull GE. J. Biol. Chem. 2002;277:37871–37880. doi: 10.1074/jbc.M205627200. [DOI] [PubMed] [Google Scholar]