Abstract
Anandamide, an endogenous eicosanoid derivative (arachidonoylethanolamide), binds to the cannabinoid receptor, a member of the G protein-coupled superfamily. It also inhibits both adenylate cyclase and N-type calcium channel opening. The enzymatic synthesis of anandamide in bovine brain tissue was examined by incubating brain membranes with [14C]ethanolamine and arachidonic acid. Following incubation and extraction into toluene, a radioactive product was identified which had the same Rf value as authentic anandamide in several thin-layer chromatographic systems. When structurally similar fatty acid substrates were compared, arachidonic acid exhibited the lowest EC50 and the highest activity for enzymatic formation of the corresponding ethanolamides. The concentration-response curve of arachidonic acid exhibited a steep slope, and at higher concentrations arachidonate inhibited enzymatic activity. When brain homogenates were separated into subcellular fractions by sucrose density gradient centrifugation, anandamide synthase activity was highest in fractions enriched in synaptic vesicles, myelin, and microsomal and synaptosomal membranes. When several areas of brain were examined, anandamide synthase activity was found to be highest in the hippocampus, followed by the thalamus, cortex, and striatum, and lowest in the cerebellum, pons, and medulla. The ability of brain tissue to enzymatically synthesize anandamide and the existence of specific receptors for this eicosanoid suggest the presence of anandamide-containing (anandaergic) neurons.
Full text
PDF
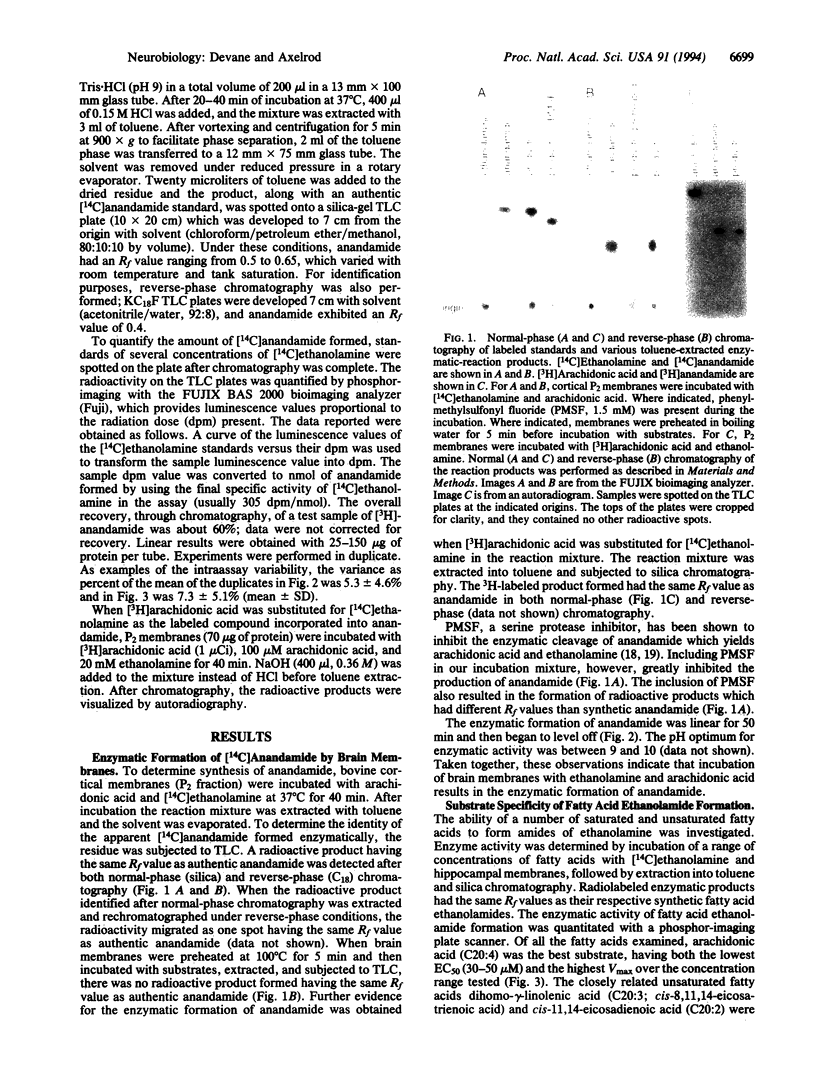
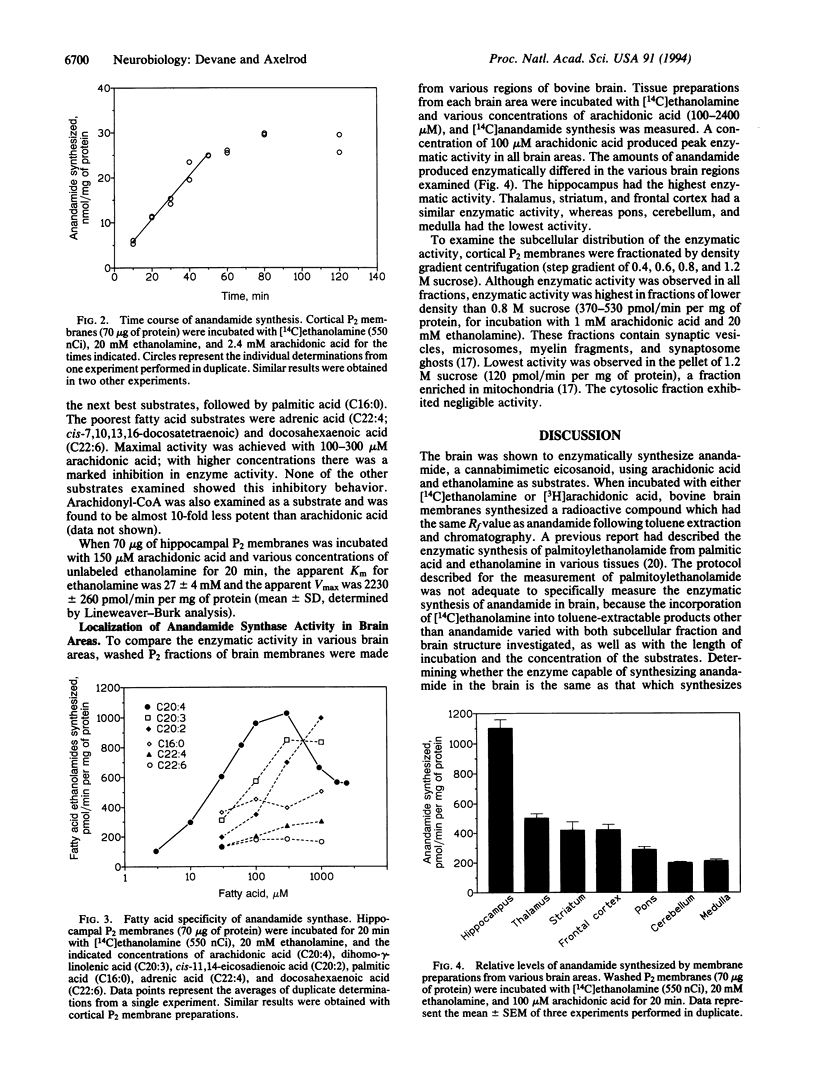

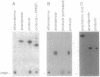
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachur N. R., Udenfriend S. Microsomal synthesis of fatty acid amides. J Biol Chem. 1966 Mar 25;241(6):1308–1313. [PubMed] [Google Scholar]
- Caulfield M. P., Brown D. A. Cannabinoid receptor agonists inhibit Ca current in NG108-15 neuroblastoma cells via a pertussis toxin-sensitive mechanism. Br J Pharmacol. 1992 Jun;106(2):231–232. doi: 10.1111/j.1476-5381.1992.tb14321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childers S. R., Sexton T., Roy M. B. Effects of anandamide on cannabinoid receptors in rat brain membranes. Biochem Pharmacol. 1994 Feb 11;47(4):711–715. doi: 10.1016/0006-2952(94)90134-1. [DOI] [PubMed] [Google Scholar]
- Crawley J. N., Corwin R. L., Robinson J. K., Felder C. C., Devane W. A., Axelrod J. Anandamide, an endogenous ligand of the cannabinoid receptor, induces hypomotility and hypothermia in vivo in rodents. Pharmacol Biochem Behav. 1993 Dec;46(4):967–972. doi: 10.1016/0091-3057(93)90230-q. [DOI] [PubMed] [Google Scholar]
- Deutsch D. G., Chin S. A. Enzymatic synthesis and degradation of anandamide, a cannabinoid receptor agonist. Biochem Pharmacol. 1993 Sep 1;46(5):791–796. doi: 10.1016/0006-2952(93)90486-g. [DOI] [PubMed] [Google Scholar]
- Devane W. A., Dysarz F. A., 3rd, Johnson M. R., Melvin L. S., Howlett A. C. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988 Nov;34(5):605–613. [PubMed] [Google Scholar]
- Devane W. A., Hanus L., Breuer A., Pertwee R. G., Stevenson L. A., Griffin G., Gibson D., Mandelbaum A., Etinger A., Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992 Dec 18;258(5090):1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Felder C. C., Briley E. M., Axelrod J., Simpson J. T., Mackie K., Devane W. A. Anandamide, an endogenous cannabimimetic eicosanoid, binds to the cloned human cannabinoid receptor and stimulates receptor-mediated signal transduction. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7656–7660. doi: 10.1073/pnas.90.16.7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder C. C., Veluz J. S., Williams H. L., Briley E. M., Matsuda L. A. Cannabinoid agonists stimulate both receptor- and non-receptor-mediated signal transduction pathways in cells transfected with and expressing cannabinoid receptor clones. Mol Pharmacol. 1992 Nov;42(5):838–845. [PubMed] [Google Scholar]
- Fride E., Mechoulam R. Pharmacological activity of the cannabinoid receptor agonist, anandamide, a brain constituent. Eur J Pharmacol. 1993 Feb 9;231(2):313–314. doi: 10.1016/0014-2999(93)90468-w. [DOI] [PubMed] [Google Scholar]
- Gérard C. M., Mollereau C., Vassart G., Parmentier M. Molecular cloning of a human cannabinoid receptor which is also expressed in testis. Biochem J. 1991 Oct 1;279(Pt 1):129–134. doi: 10.1042/bj2790129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanus L., Gopher A., Almog S., Mechoulam R. Two new unsaturated fatty acid ethanolamides in brain that bind to the cannabinoid receptor. J Med Chem. 1993 Oct 1;36(20):3032–3034. doi: 10.1021/jm00072a026. [DOI] [PubMed] [Google Scholar]
- Herkenham M., Lynn A. B., Little M. D., Johnson M. R., Melvin L. S., de Costa B. R., Rice K. C. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollister L. E. Health aspects of cannabis. Pharmacol Rev. 1986 Mar;38(1):1–20. [PubMed] [Google Scholar]
- Howlett A. C., Bidaut-Russell M., Devane W. A., Melvin L. S., Johnson M. R., Herkenham M. The cannabinoid receptor: biochemical, anatomical and behavioral characterization. Trends Neurosci. 1990 Oct;13(10):420–423. doi: 10.1016/0166-2236(90)90124-s. [DOI] [PubMed] [Google Scholar]
- Howlett A. C. Cannabinoid inhibition of adenylate cyclase. Biochemistry of the response in neuroblastoma cell membranes. Mol Pharmacol. 1985 Apr;27(4):429–436. [PubMed] [Google Scholar]
- Johnson D. E., Heald S. L., Dally R. D., Janis R. A. Isolation, identification and synthesis of an endogenous arachidonic amide that inhibits calcium channel antagonist 1,4-dihydropyridine binding. Prostaglandins Leukot Essent Fatty Acids. 1993 Jun;48(6):429–437. doi: 10.1016/0952-3278(93)90048-2. [DOI] [PubMed] [Google Scholar]
- Kato K., Clark G. D., Bazan N. G., Zorumski C. F. Platelet-activating factor as a potential retrograde messenger in CA1 hippocampal long-term potentiation. Nature. 1994 Jan 13;367(6459):175–179. doi: 10.1038/367175a0. [DOI] [PubMed] [Google Scholar]
- Mackie K., Devane W. A., Hille B. Anandamide, an endogenous cannabinoid, inhibits calcium currents as a partial agonist in N18 neuroblastoma cells. Mol Pharmacol. 1993 Sep;44(3):498–503. [PubMed] [Google Scholar]
- Mackie K., Hille B. Cannabinoids inhibit N-type calcium channels in neuroblastoma-glioma cells. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3825–3829. doi: 10.1073/pnas.89.9.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda L. A., Lolait S. J., Brownstein M. J., Young A. C., Bonner T. I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990 Aug 9;346(6284):561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Vogel Z., Barg J., Levy R., Saya D., Heldman E., Mechoulam R. Anandamide, a brain endogenous compound, interacts specifically with cannabinoid receptors and inhibits adenylate cyclase. J Neurochem. 1993 Jul;61(1):352–355. doi: 10.1111/j.1471-4159.1993.tb03576.x. [DOI] [PubMed] [Google Scholar]
- Whittaker V. P., Michaelson I. A., Kirkland R. J. The separation of synaptic vesicles from nerve-ending particles ('synaptosomes'). Biochem J. 1964 Feb;90(2):293–303. doi: 10.1042/bj0900293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. H., Errington M. L., Lynch M. A., Bliss T. V. Arachidonic acid induces a long-term activity-dependent enhancement of synaptic transmission in the hippocampus. Nature. 1989 Oct 26;341(6244):739–742. doi: 10.1038/341739a0. [DOI] [PubMed] [Google Scholar]